Improved Energy Density at High Temperatures of FPE Dielectrics by Extreme Low Loading of CQDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CQDs
2.3. Preparation of Composites
2.4. Characterizations
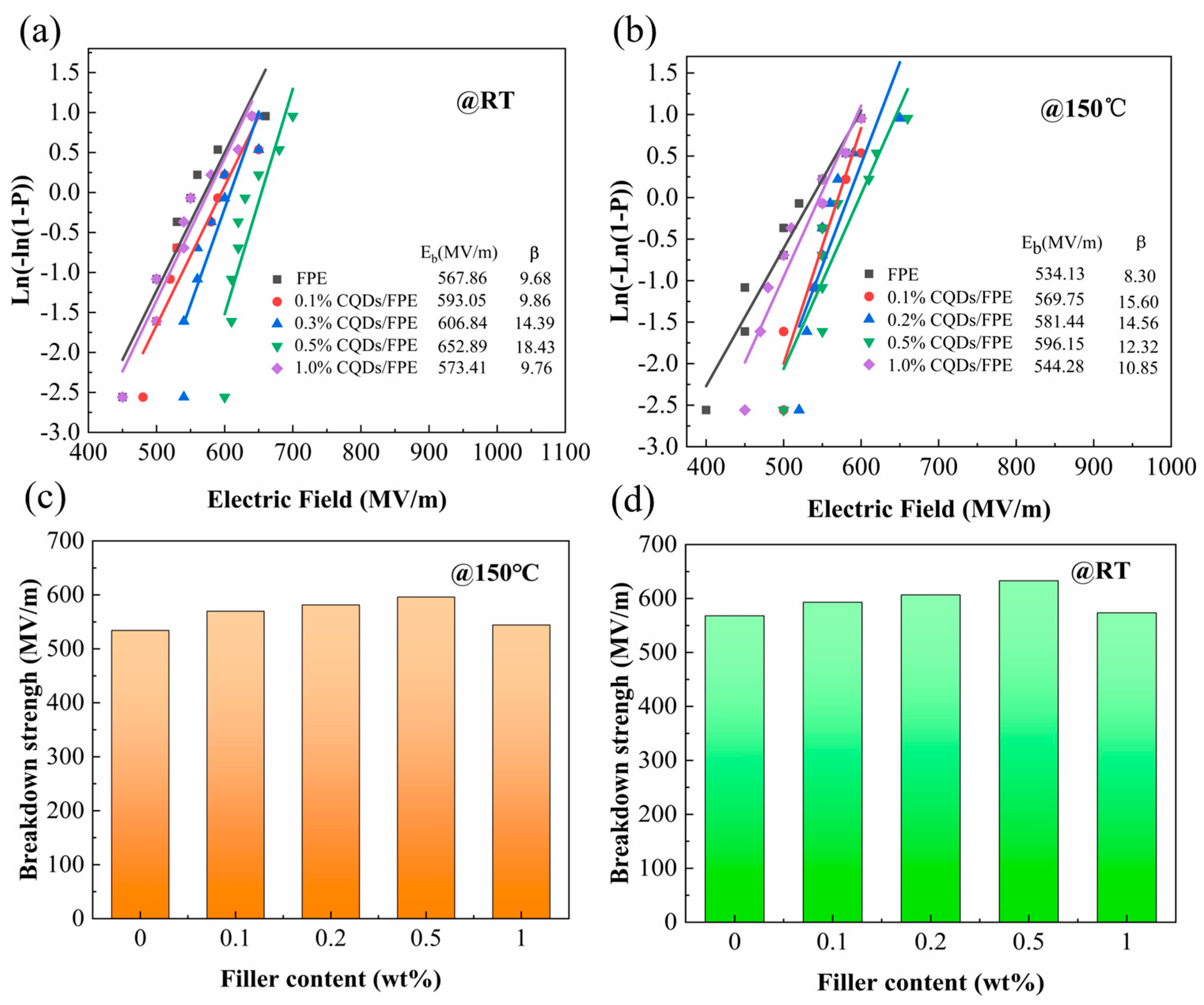
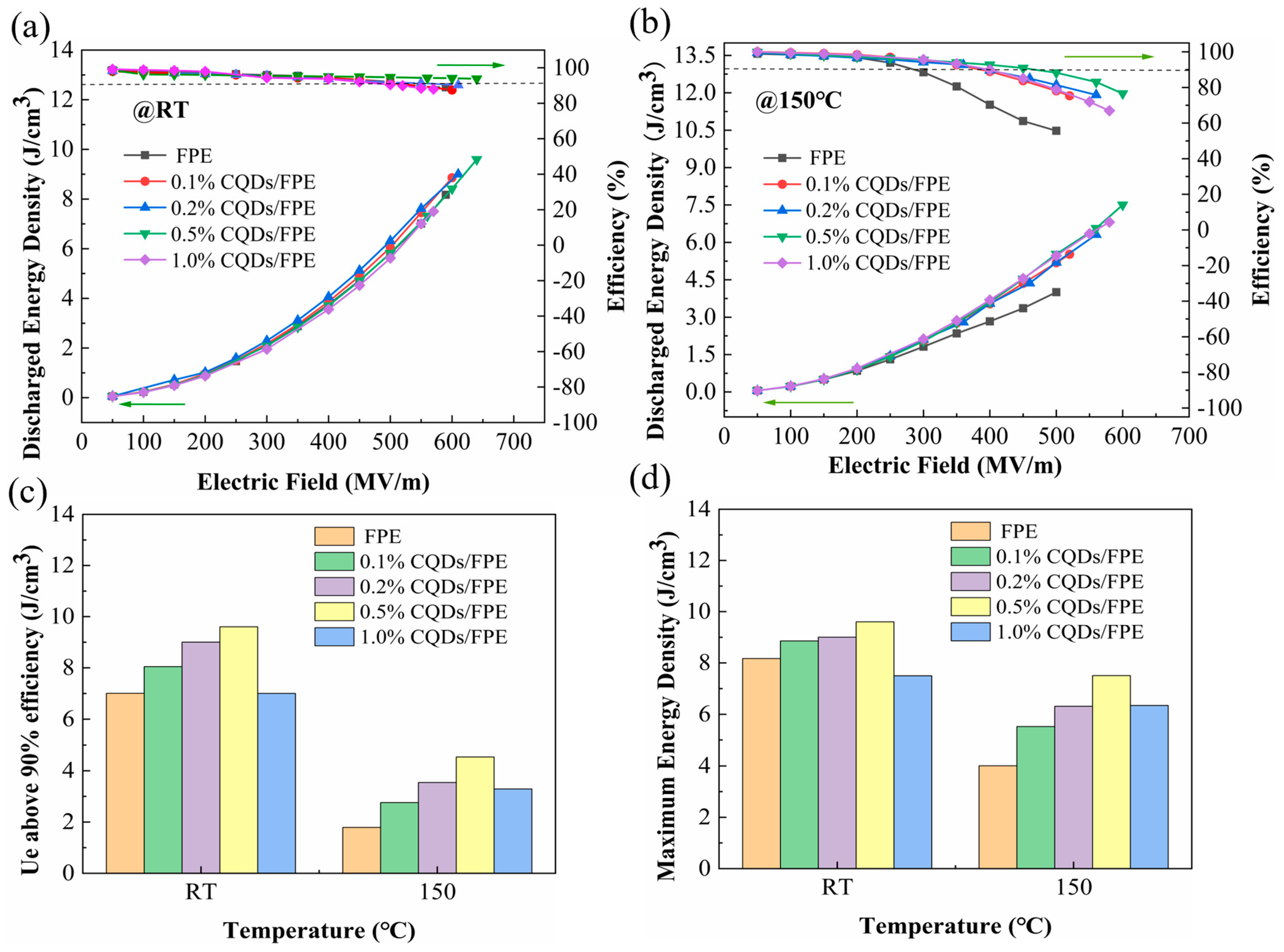
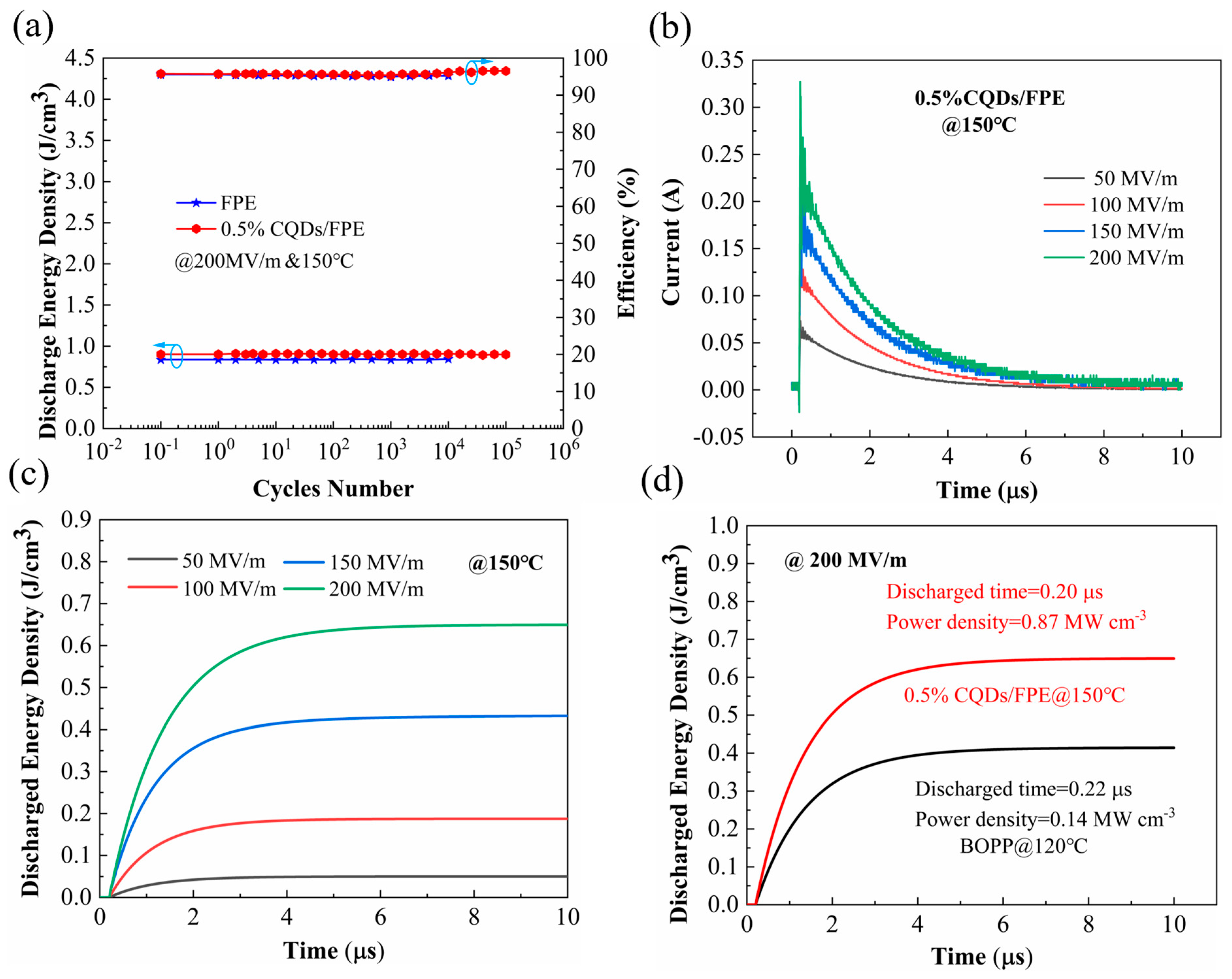
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, F.Y.; Li, Y.T.; Zhang, J.Y.; He, L.; Li, J.L.; Sun, N.; Li, G.L.; Jiang, Y.; Zhou, K.; Liang, Q.Q.; et al. Scalable Dual In Situ Synthesis of Polyester Nanocomposites for High-Energy Storage. Small 2024, 2401308. [Google Scholar] [CrossRef]
- Li, X.N.; Luo, H.; Yang, C.C.; Wang, F.; Jiang, X.; Guo, R.; Zhang, D. Enhancing High-Temperature Energy Storage Performance of PEI-Based Dielectrics by Incorporating ZIF-67 with a Narrow Bandgap. ACS Appl. Mater. Interfaces 2023, 15, 41828–41838. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, J.M.; Yang, C.C.; Luo, H.; Li, X.A.; Hou, H.S.; Zou, G.Q.; Zhang, D. Improved Capacitive Energy Storage Nanocomposites at High Temperature Utilizing Ultralow Loading of Bimetallic MOF. Small 2023, 19, 2300510. [Google Scholar] [CrossRef]
- Xie, Z.L.; Le, K.; Li, H.; Pang, X.; Xu, T.L.; Altoe, V.; Klivansky, L.M.; Wang, Y.F.; Huang, Z.Y.; Shelton, S.W.; et al. Interfacial Engineering Using Covalent Organic Frameworks in Polymer Composites for High-Temperature Electrostatic Energy Storage. Adv. Funct. Mater. 2024, 34, 2314910. [Google Scholar] [CrossRef]
- He, G.H.; Liu, Y.; Wang, C.; Chen, S.; Luo, H.; Zhang, D. All-organic polymer dielectrics prepared via optimization of sequential structure of polystyrene-based copolymers. Chem. Eng. J. 2022, 446, 137106. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, H.; Xie, H.; Xiao, Z.; Wang, F.; Jiang, X.; Zhou, X.; Zhang, D. Trilayer PVDF nanocomposites with significantly enhanced energy density and energy efficiency using 0.55Bi0.5Na0.5TiO3-0.45(Sr0.7Bi0.2)TiO3 nanofibers. Microstructures 2023, 3, 2023008. [Google Scholar]
- Ren, L.L.; Yang, L.J.; Zhang, S.Y.; Li, H.; Zhou, Y.; Ai, D.; Xie, Z.L.; Zhao, X.T.; Peng, Z.R.; Liao, R.J.; et al. Largely enhanced dielectric properties of polymer composites with HfO2nanoparticles for high-temperature film capacitors. Compos. Sci. Technol. 2021, 201, 108528. [Google Scholar] [CrossRef]
- Chen, J.; Pei, Z.T.; Liu, Y.J.; Shi, K.M.; Zhu, Y.K.; Zhang, Z.C.; Jiang, P.K.; Huang, X.Y. Aromatic-Free Polymers Based All-Organic Dielectrics with Breakdown Self-Healing for High-Temperature Capacitive Energy Storage. Adv. Mater. 2023, 35, 2306562. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Zhou, Y.; Zhu, Y.J.; Liang, J.J.; Wang, S.J.; Peng, S.M.; Li, Y.S.; Cheng, S.; Yang, M.C.; Hu, J.; et al. Polymer/molecular semiconductor all-organic composites for high-temperature dielectric energy storage. Nat. Commun. 2020, 11, 3919. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Zhou, C.Y.; Ji, W.H.; Zhang, Y.H.; Jiang, Z.H.; Bertram, F.; Shang, Y.S.; Zhang, H.B.; Shen, C. Anisotropic Semicrystalline Homopolymer Dielectrics for High-Temperature Capacitive Energy Storage. Angew. Chem.-Int. Edit. 2024, 63, e202319766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Ma, Z.Y.; Deng, H.; Fu, Q. Improving high-temperature energy storage performance of PI dielectric capacitor films through boron nitride interlayer. Adv. Compos. Hybrid Mater. 2022, 5, 238–249. [Google Scholar] [CrossRef]
- Zha, J.W.; Xiao, M.Y.; Wan, B.Q.; Wang, X.M.; Dang, Z.M.; Chen, G. Polymer dielectrics for high-temperature energy storage: Constructing carrier traps. Prog. Mater. Sci. 2023, 140, 101208. [Google Scholar] [CrossRef]
- Xiong, J.; Fan, X.; Long, D.J.; Zhu, B.F.; Zhang, X.; Lu, J.Y.; Xie, Y.C.; Zhang, Z.C. Significant improvement in high-temperature energy storage performance of polymer dielectrics via constructing a surface polymer carrier trap layer. J. Mater. Chem. A 2022, 10, 24611–24619. [Google Scholar] [CrossRef]
- Li, H.; Gadinski, M.R.; Huang, Y.Q.; Ren, L.L.; Zhou, Y.; Ai, D.; Han, Z.B.; Yao, B.; Wang, Q. Crosslinked fluoropolymers exhibiting superior high-temperature energy density and charge-discharge efficiency. Energy Environ. Sci. 2020, 13, 1279–1286. [Google Scholar] [CrossRef]
- Luo, H.; Wang, F.; Guo, R.; Zhang, D.; He, G.H.; Chen, S.; Wang, Q. Progress on Polymer Dielectrics for Electrostatic Capacitors Application. Adv. Sci. 2022, 9, 2202438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.C.; Guan, F.; Jiang, M.; Li, Y.P.; Zhu, C.C.; Yue, D.; Li, J.L.; Liu, X.X.; Feng, Y. Enhanced energy storage performance of all-organic sandwich structured dielectrics with FPE and P(VDF-HFP). Compos. Part A-Appl. Sci. Manuf. 2022, 159, 107018. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, W.C.; Guan, F.; Yao, Y.H.; Yue, D.; Feng, Y. The simple synthesis of all-organic polymer bilayer films: Achieving simultaneous enhancements in energy storage density and efficiency. J. Appl. Polym. Sci. 2023, 140, e53991. [Google Scholar] [CrossRef]
- Meng, Z.T.; Zhang, T.D.; Zhang, C.H.; Shang, Y.N.; Lei, Q.Q.; Chi, Q.G. Advances in Polymer Dielectrics with High Energy Storage Performance by Designing Electric Charge Trap Structures. Adv. Mater. 2023, e2310272. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Li, H.; Zhou, Y.; Ren, L.L.; Han, Z.B.; Yao, B.; Zhou, W.; Zhao, L.; Xu, J.M.; Wang, Q. Tuning Nanofillers in In Situ Prepared Polyimide Nanocomposites for High-Temperature Capacitive Energy Storage. Adv. Energy Mater. 2020, 10, 1903881. [Google Scholar] [CrossRef]
- Liu, X.J.; Zheng, M.S.; Chen, G.; Dang, Z.M.; Zha, J.W. High-temperature polyimide dielectric materials for energy storage: Theory, design, preparation and properties. Energy Environ. Sci. 2022, 15, 56–81. [Google Scholar] [CrossRef]
- Wang, P.J.; Guo, Y.; Zhou, D.; Li, D.; Pang, L.X.; Liu, W.F.; Su, J.Z.; Shi, Z.Q.; Sun, S.K. High-Temperature Flexible Nanocomposites with Ultra-High Energy Storage Density by nanostructured MgO fillers. Adv. Funct. Mater. 2022, 32, 2204155. [Google Scholar] [CrossRef]
- Ren, L.L.; Li, H.; Xie, Z.L.; Ai, D.; Zhou, Y.; Liu, Y.; Zhang, S.Y.; Yang, L.J.; Zhao, X.T.; Peng, Z.R.; et al. High-Temperature High-Energy-Density Dielectric Polymer Nanocomposites Utilizing Inorganic Core-Shell Nanostructured Nanofillers. Adv. Energy Mater. 2021, 11, 2101297. [Google Scholar]
- Liu, H.X.; Zhong, X.; Pan, Q.; Zhang, Y.; Deng, W.T.; Zou, G.Q.; Hou, H.S.; Ji, X.B. A review of carbon dots in synthesis strategy. Coord. Chem. Rev. 2024, 498, 215468. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Liang, F.Y.; Sun, J.L.; Sun, R.; Liu, C.; Deng, C.; Seidi, F. Synthesis Strategies, Optical Mechanisms, and Applications of Dual-Emissive Carbon Dots. Nanomaterials 2023, 13, 2869. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.H.; Liang, K.; Zhu, J.J.; Yang, B.; Zhao, D.Y.; Kong, B. Hetero-atom-doped carbon dots: Doping strategies, properties and applications. Nano Today 2020, 33, 100879. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.T.; Ye, Y.; Guo, R.T.; Wang, A.N.; Zou, G.Q.; Hou, H.S.; Ji, X.B. Kilogram-Scale Synthesis and Functionalization of Carbon Dots for Superior Electrochemical Potassium Storage. ACS Nano 2021, 15, 6872–6885. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, J.F.; Niu, Y.J.; Li, L.; Wang, F.; Hu, R.C.; Cheng, J.; Sun, L.; Pan, Z.Z.; Xu, X.W.; et al. Enhanced high-temperature energy storage properties of polymer composites by interlayered metal nanodots. J. Mater. Chem. A 2022, 10, 18773–18781. [Google Scholar] [CrossRef]
- Cai, H.C.; Wang, R.; Gou, B.; Fu, J.; Zhu, Y.J.; Yang, H.; Zhou, J.A.; Li, M.X.; Zhong, A.; Zhang, D.M.; et al. Significantly improved high-temperature capacitive performance in polymer dielectrics utilizing ultra-small carbon quantum dots with Coulomb-blockade effect. Chem. Eng. J. 2023, 476, 146672. [Google Scholar] [CrossRef]
- Wang, L.W.; Huang, X.Y.; Zhu, Y.K.; Jiang, P.K. Enhancing electrical energy storage capability of dielectric polymer nanocomposites via the room temperature Coulomb blockade effect of ultra-small platinum nanoparticles. Phys. Chem. Chem. Phys. 2018, 20, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.R.; Luo, H.; Liu, Y.; Guo, R.; Ji, X.B.; Hou, H.S.; Zhang, D. Extremely low loading of carbon quantum dots for high energy density in polyetherimide nanocomposites. Chem. Eng. J. 2022, 433, 133601. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, H.; Wang, F.; Li, X.A.; Xie, H.R.; Liu, Y.; Zou, G.Q.; Ji, X.B.; Hou, H.S.; Zhang, D. Ultralow loading of carbon quantum dots leading to significantly improved breakdown strength and energy density of P(VDF-TrFE-CTFE). J. Mater. Chem. A 2023, 11, 16127–16137. [Google Scholar] [CrossRef]
- Ding, J.L.; Zhou, Y.; Xu, W.H.; Yang, F.; Zhao, D.Y.; Zhang, Y.H.; Jiang, Z.H.; Wang, Q. Ultraviolet-Irradiated All-Organic Nanocomposites with Polymer Dots for High-Temperature Capacitive Energy Storage. Nano-Micro Lett. 2024, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Lin, Y.; Wang, Y.; Yang, H. Sandwich-structured SrTiO3/PEI composite films with high-temperature energy storage performance. J. Power Sources 2024, 609, 234677. [Google Scholar] [CrossRef]
- Zhang, S.C.; Chen, D.; Liu, Z.F.; Ruan, M.N.; Guo, Z.A. Novel strategy for efficient water splitting through pyro-electric and pyro-photo-electric catalysis of BaTiO3 by using thermal resource and solar energy. Appl. Catal. B-Environ. 2021, 284, 119686. [Google Scholar] [CrossRef]
- Ding, J.L.; Xu, W.H.; Zhu, X.B.; Liu, Z.; Zhang, Y.H.; Jiang, Z.H. All-organic nanocomposite dielectrics contained with polymer dots for high-temperature capacitive energy storage. Nano Res. 2023, 16, 10183–10190. [Google Scholar] [CrossRef]
- Wang, Q.L.; Tanaka, Y.; Miyake, H.; Endo, K.; An, Y.; Du, H.S.; Chen, X.R.; Paramane, A. Enhanced high-temperature electrical properties and charge dynamics of inorganic/organic silicone elastomer nanocomposites via nanostructure grafting and molecular trap construction. Polym. Compos. 2024. [Google Scholar] [CrossRef]
- Lin, Q.; Hou, Y.F.; Zheng, Y.Q.; Tan, Y.N.; Du, P.; Luo, L.H.; Li, W.P. Poly(ether imide)-Based Nanocomposites with Low Fraction of Hierarchical Ag@AO Nanofiber for High-Temperature Energy Storage. ACS Appl. Energ. Mater. 2022, 5, 2329–2338. [Google Scholar] [CrossRef]
- Fan, M.Z.; Hu, P.H.; Dan, Z.K.; Jiang, J.Y.; Sun, B.Z.; Shen, Y. Significantly increased energy density and discharge efficiency at high temperature in polyetherimide nanocomposites by a small amount of Al2O3 nanoparticles. J. Mater. Chem. A 2020, 8, 24536–24542. [Google Scholar] [CrossRef]
- Hassan, Y.A.; Hu, H.L. Current status of polymer nanocomposite dielectrics for high-temperature applications. Compos. Part A-Appl. Sci. Manuf. 2020, 138, 106064. [Google Scholar] [CrossRef]
- Ren, W.B.; Pan, J.Y.; Dan, Z.K.; Zhang, T.; Jiang, J.Y.; Fan, M.Z.; Hu, P.H.; Li, M.; Lin, Y.H.; Nan, C.W.; et al. High-temperature electrical energy storage performances of dipolar glass polymer nanocomposites filled with trace ultrafine nanoparticles. Chem. Eng. J. 2021, 420, 127614. [Google Scholar] [CrossRef]
- Feng, M.J.; Feng, Y.; Zhang, C.H.; Zhang, T.D.; Chen, Q.G.; Chi, Q.G. Ultrahigh energy storage performance of all-organic dielectrics at high-temperature by tuning the density and location of traps. Mater. Horiz. 2022, 9, 3002–3012. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ai, D.; Ren, L.L.; Yao, B.; Han, Z.B.; Shen, Z.H.; Wang, J.J.; Chen, L.Q.; Wang, Q. Scalable Polymer Nanocomposites with Record High-Temperature Capacitive Performance Enabled by Rationally Designed Nanostructured Inorganic Fillers. Adv. Mater. 2019, 31, e1900875. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, R.; Shen, G.Y.; Sun, K.X.; Lv, F.C.; Fan, S.D. Tailoring anisotropic thermal conductivity of 2D aramid nanoribbon-based dielectrics with potential high-temperature capacitive energy storage. J. Mater. Chem. C 2024, 12, 7338–7350. [Google Scholar] [CrossRef]
- Yang, M.H.; Zhao, Y.L.; Wang, Z.P.; Yan, H.R.; Liu, Z.R.; Li, Q.; Dang, Z.M. Surface ion-activated polymer composite dielectrics for superior high-temperature capacitive energy storage. Energy Environ. Sci. 2024, 17, 1592–1602. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, X.H.; Ni, X.Y.; Qian, J.; Zuo, P.Y.; Zhuang, Q.X. Excellent high-temperature energy storage capacity for polyetherimide nanocomposites with hierarchically structured nanofillers. Compos. Part A-Appl. Sci. Manuf. 2023, 175, 107791. [Google Scholar] [CrossRef]
- Yan, J.J.; Wang, J.; Zeng, J.Y.; Shen, Z.H.; Li, B.W.; Zhang, X.; Zhang, S.J. Modulating the charge trapping characteristics of PEI/BNNPs dilute nanocomposite for improved high-temperature energy storage performance. J. Mater. Chem. C 2022, 10, 13157–13166. [Google Scholar] [CrossRef]
- Zhi, J.P.; Wang, J.; Shen, Z.H.; Li, B.W.; Zhang, X.; Nan, C.W. Nanofiber-reinforced polymer nanocomposite with hierarchical interfaces for high-temperature dielectric energy storage applications. Sci. China-Mater. 2023, 66, 2652–2661. [Google Scholar] [CrossRef]
- Fan, Z.H.; Gao, S.B.; Chang, Y.F.; Wang, D.W.; Zhang, X.; Huang, H.T.; He, Y.B.; Zhang, Q.F. Ultra-superior high-temperature energy storage properties in polymer nanocomposites via rational design of core-shell structured inorganic antiferroelectric fillers. J. Mater. Chem. A 2023, 11, 7227–7238. [Google Scholar] [CrossRef]
- Li, Z.W.; Qin, H.M.; Song, J.H.; Liu, M.; Zhang, X.L.; Wang, S.; Xiong, C.X. Polyimide Nanodielectrics Doped with Ultralow Content of MgO Nanoparticles for High-Temperature Energy Storage. Polymers 2022, 14, 2918. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.P.; Pan, Z.B.; Zhang, Y.; Shi, S.H.; Cheng, Y.; Chen, H.X.; Li, Z.C.; Fan, X.; Liu, J.J.; Yu, J.H.; et al. Regulation of Interfacial Polarization and Local Electric Field Strength Achieved Highly Energy Storage Performance in Polyetherimide Nanocomposites at Elevated Temperature via 2D Hybrid Structure. Adv. Mater. Interfaces 2022, 9, 2201100. [Google Scholar] [CrossRef]
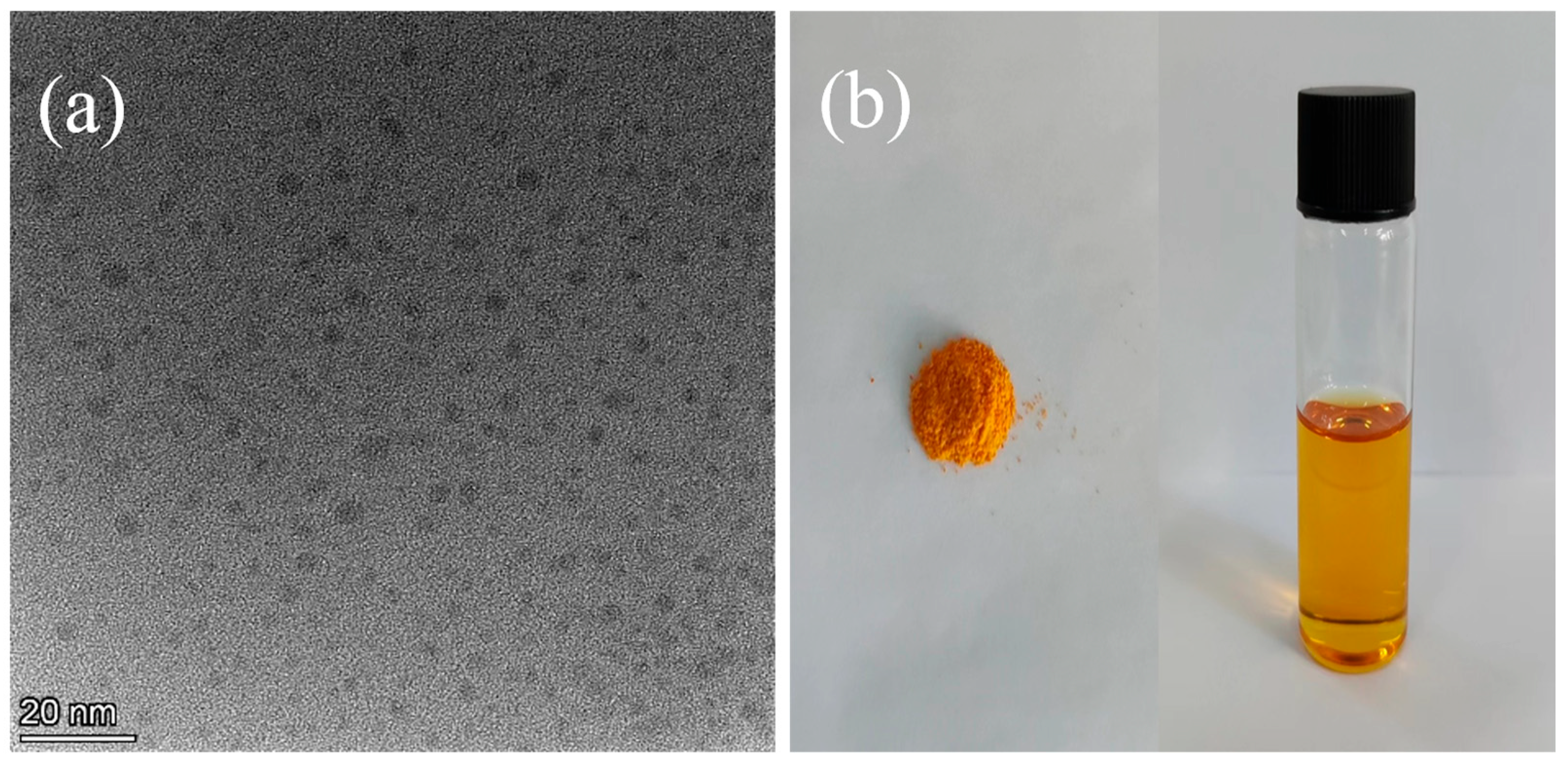
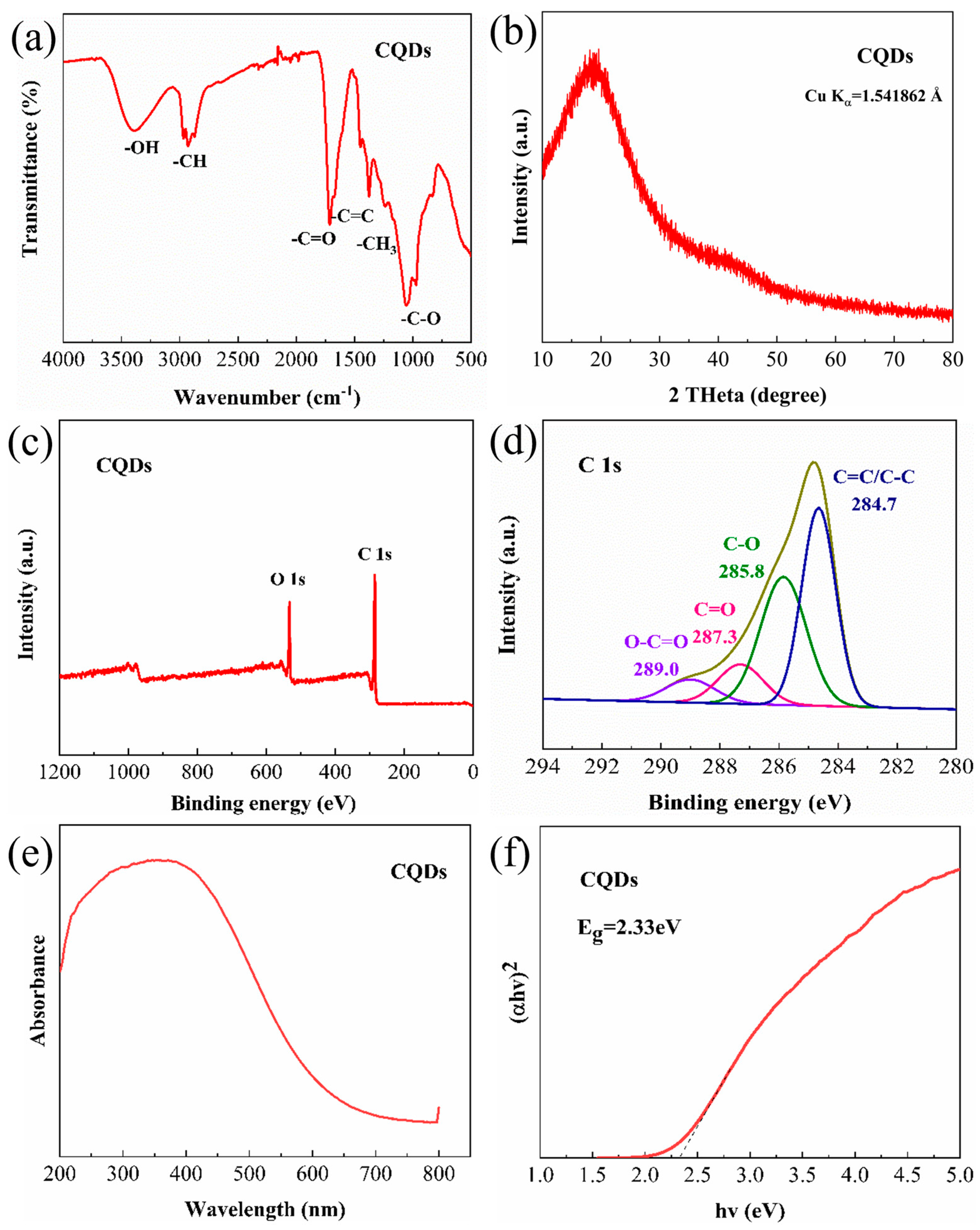
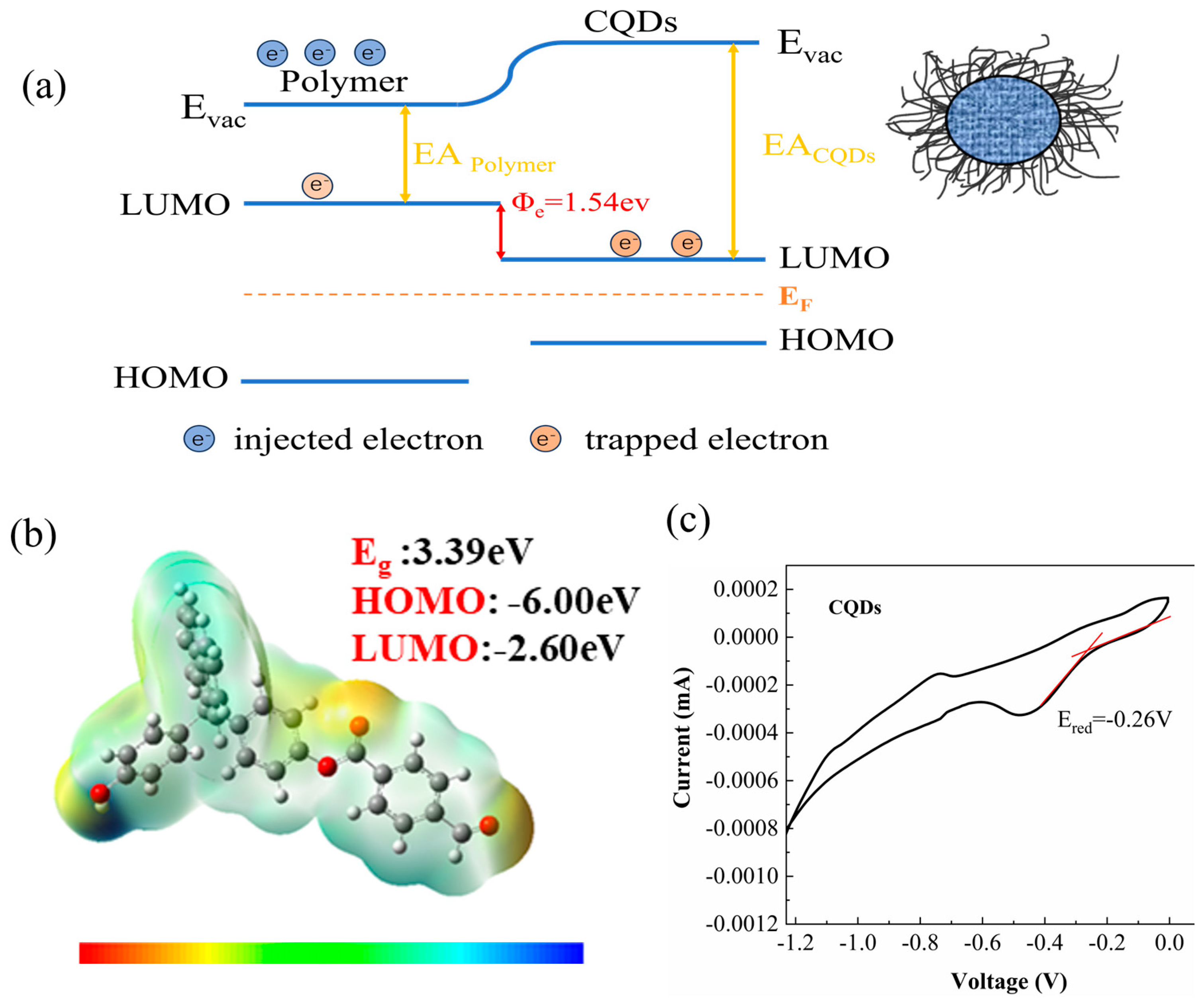
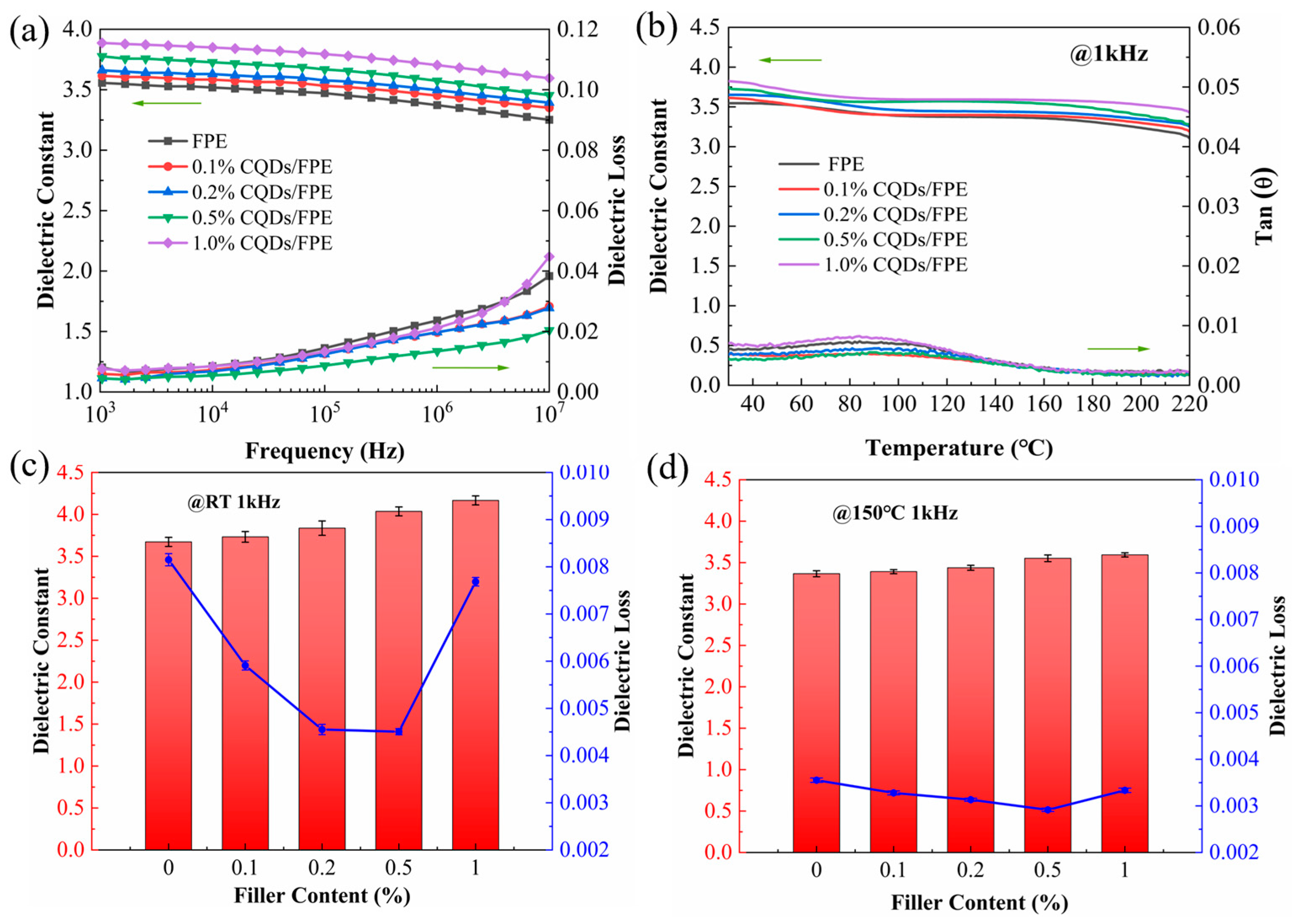

| Samples | Dialysis Time (h) | Na Content (μg/mL) |
|---|---|---|
| Undialyzed stock solution | 0 | 1.056 |
| Solution outside dialysis bag | 9 | 0.474 |
| Solution outside dialysis bag | 18 | 0.535 |
| Solution outside dialysis bag | 30 | 0.506 |
| Solution outside dialysis bag | 36 | 0.501 |
| Dialyzed 36 h stock solution | 36 | 1.034 |
| Matrix | Filler | Eb (MV/m) | Ue (J/cm3) | η (%) | References |
|---|---|---|---|---|---|
| PEI | BNNS@ST | 460 | 4.29 | >80 | Compos. Pt. A-Appl. Sci. Manuf. 2023, 175, 9, 107791 [11] |
| PC | Au | 518 | 4.84 | >90 | Mater. Chem. A 2022, 10, 18773 [27] |
| PEI | ZIF 8–67 | 564 | 2.96 | >90 | Small 2023, 19, 12 [3] |
| PEI | BNNPs | 580 | 4.2 | >90 | Mater. Chem. C 2022, 10, 13157 [46] |
| PEI | BN/BaTiO3 | 507 | 5.23 | >90 | Sci. China-Mater. 2023, 66, 2652 [47] |
| PEI | PLZST@Al2O3 | 489 | 10.2 | 83.5 | Mater. Chem. A 2023, 11, 7227 [48] |
| PI | MgO | 450 | 2.6 | 89 | Polymers 2022, 14, 10, 2918 [49] |
| PI | BNNS | 489 | 2.58 | >90 | Adv. Compos. Hybrid Mater. 2022, 5, 238 [50] |
| FPE | CQDs | 596 | 4.53 | >90 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Luo, H.; Liu, Y.; Wang, F.; Peng, B.; Li, X.; Hu, D.; He, G.; Zhang, D. Improved Energy Density at High Temperatures of FPE Dielectrics by Extreme Low Loading of CQDs. Materials 2024, 17, 3625. https://doi.org/10.3390/ma17143625
Wang H, Luo H, Liu Y, Wang F, Peng B, Li X, Hu D, He G, Zhang D. Improved Energy Density at High Temperatures of FPE Dielectrics by Extreme Low Loading of CQDs. Materials. 2024; 17(14):3625. https://doi.org/10.3390/ma17143625
Chicago/Turabian StyleWang, Huan, Hang Luo, Yuan Liu, Fan Wang, Bo Peng, Xiaona Li, Deng Hu, Guanghu He, and Dou Zhang. 2024. "Improved Energy Density at High Temperatures of FPE Dielectrics by Extreme Low Loading of CQDs" Materials 17, no. 14: 3625. https://doi.org/10.3390/ma17143625






