Characterization of Iron Oxide Nanotubes Obtained by Anodic Oxidation for Biomedical Applications—In Vitro Studies
Abstract
:1. Introduction
2. Materials and Methods
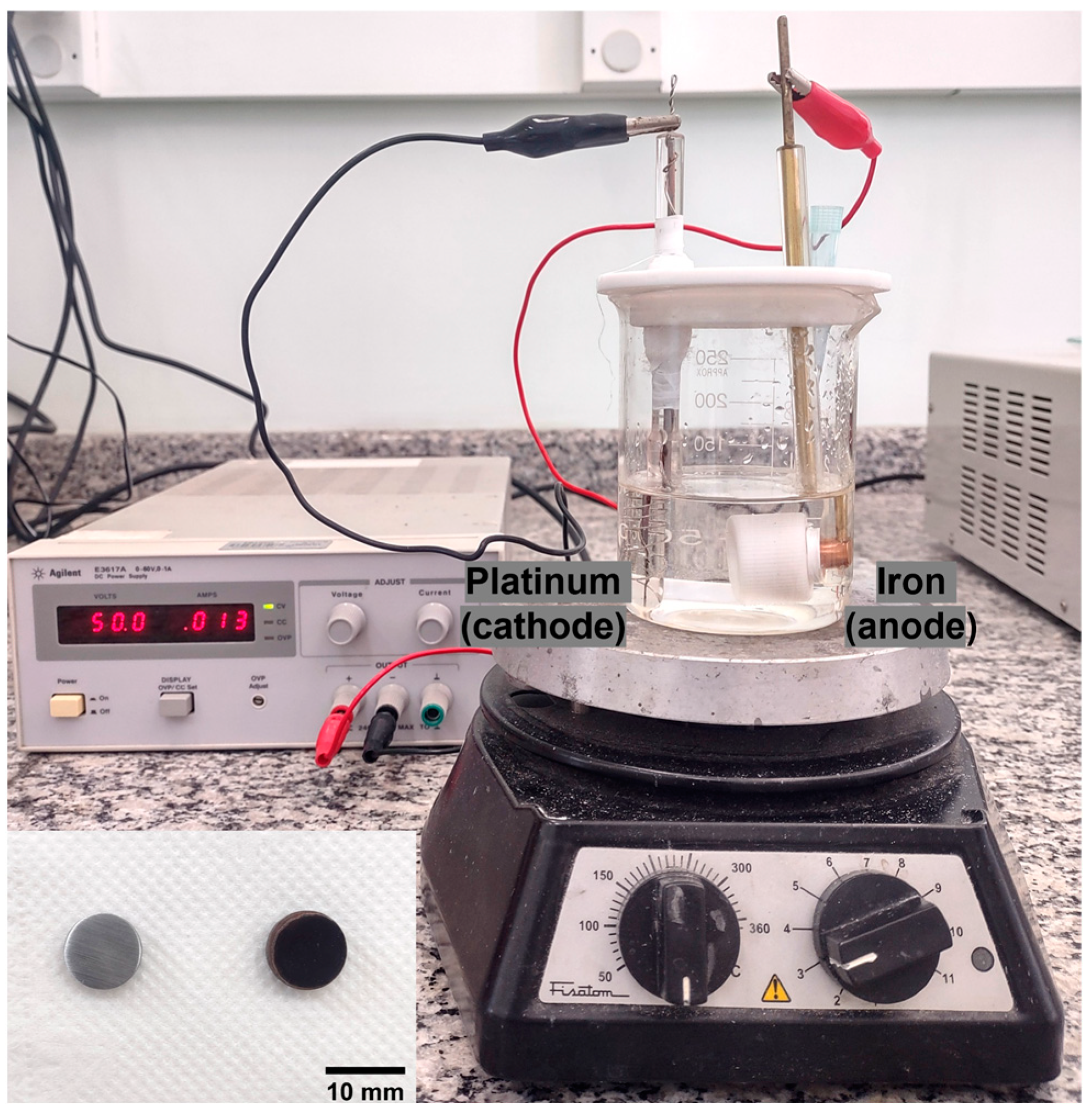
2.1. Experimental Procedure
2.2. Surface Characterization
2.3. In Vitro Cell Studies
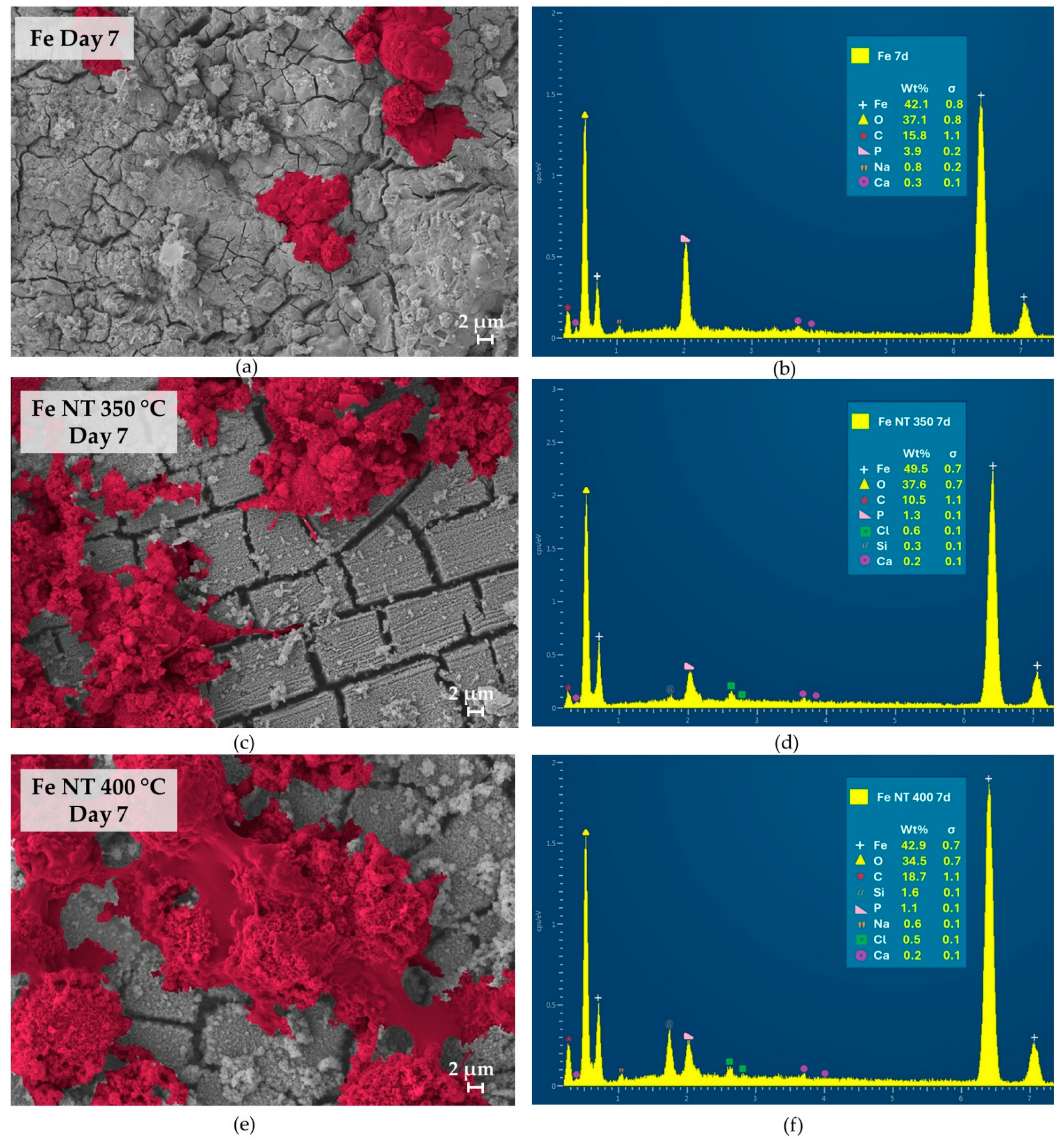
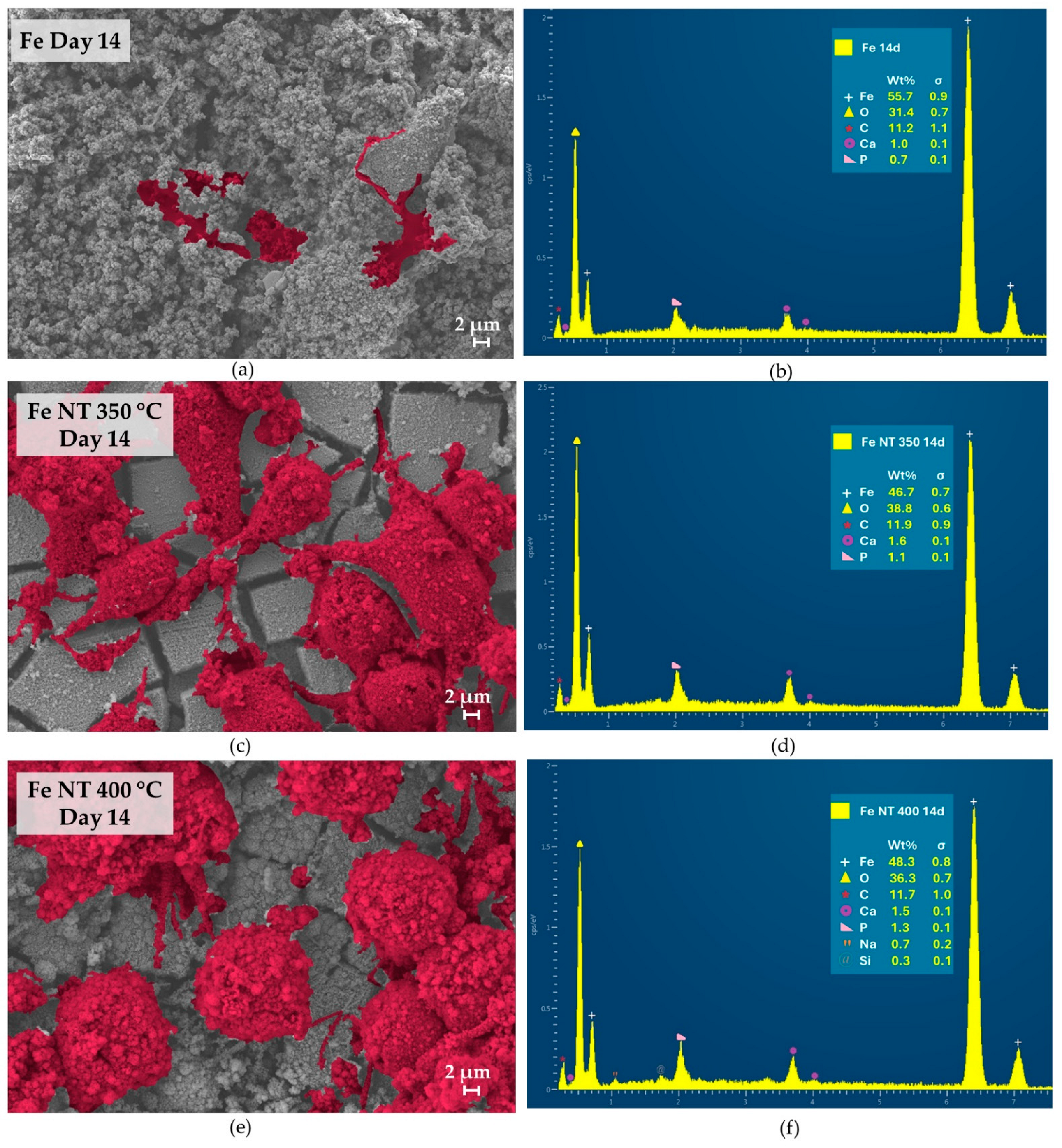
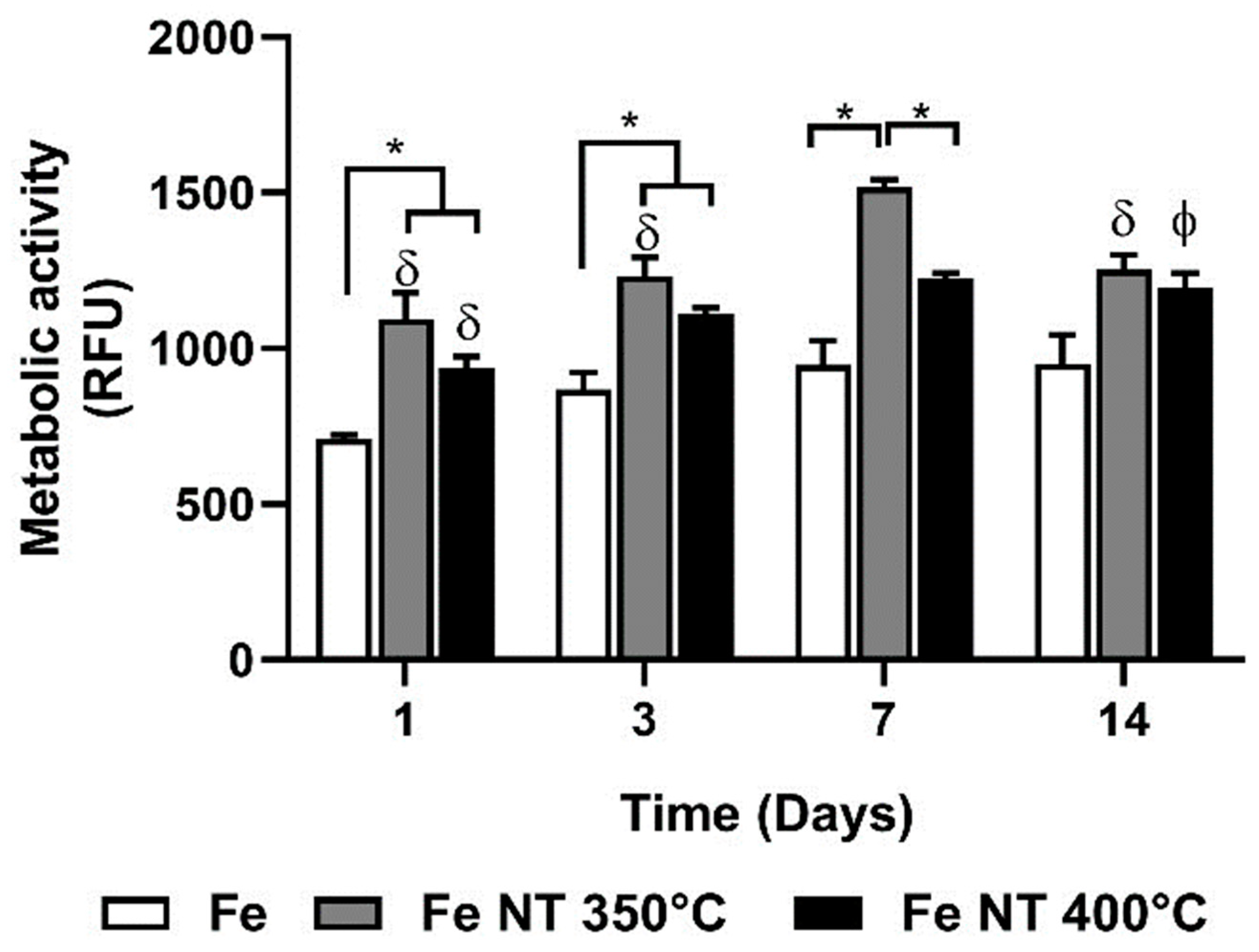
3. Results
4. Conclusions
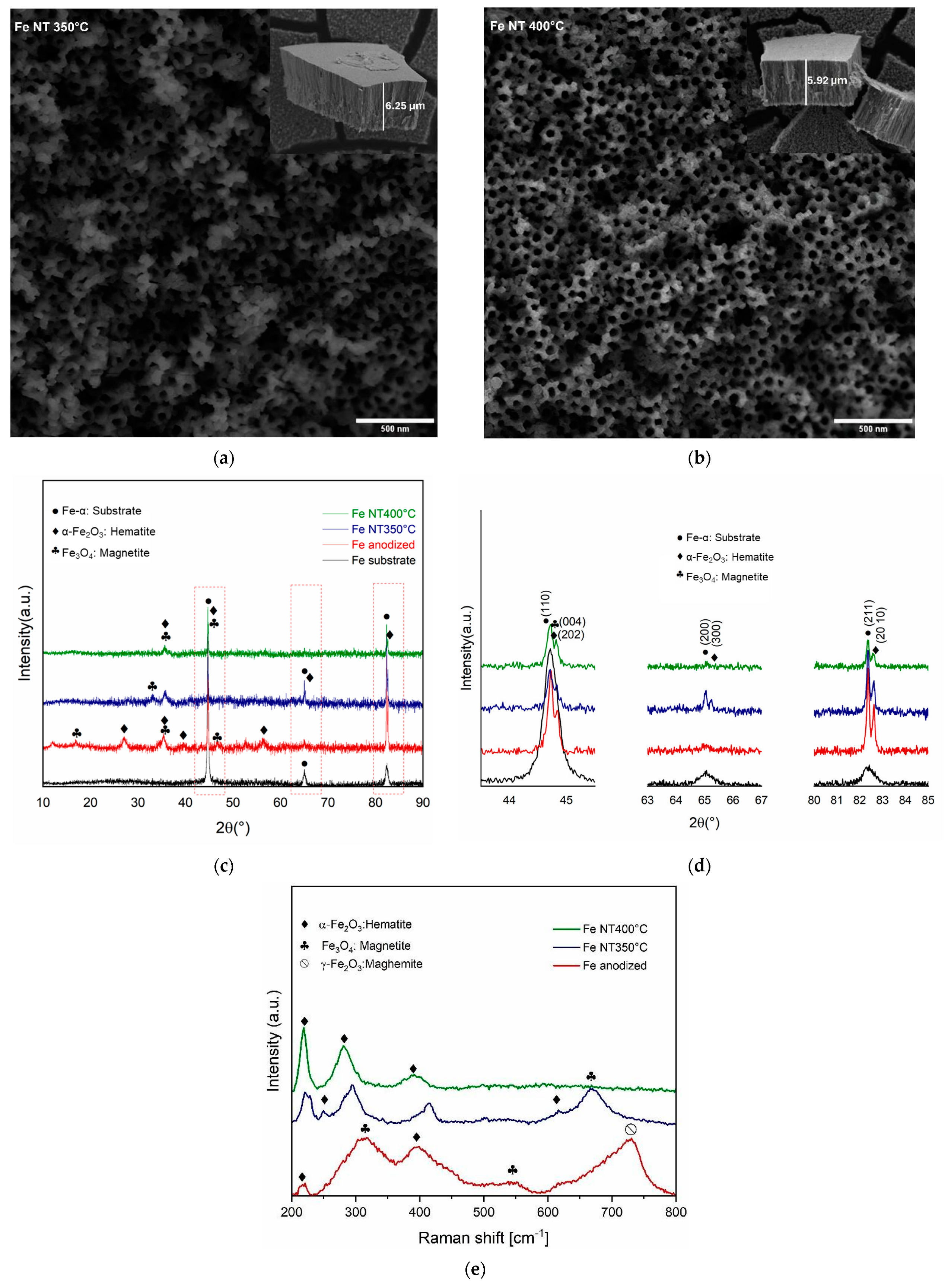
- It is possible to develop an ordered and uniform tubular nanostructure layer on iron oxide on the surface of pure iron. Under anodized and annealed conditions, the mean pore diameters and wall thicknesses were estimated to be 70.02 nm and 6.25 µm for Fe NT 350 °C and 60.46 nm and 5.92 µm for Fe NT 400 °C. The surface morphology of the Fe substrate before and after heat treatment revealed significant changes in surface roughness and height deviations.
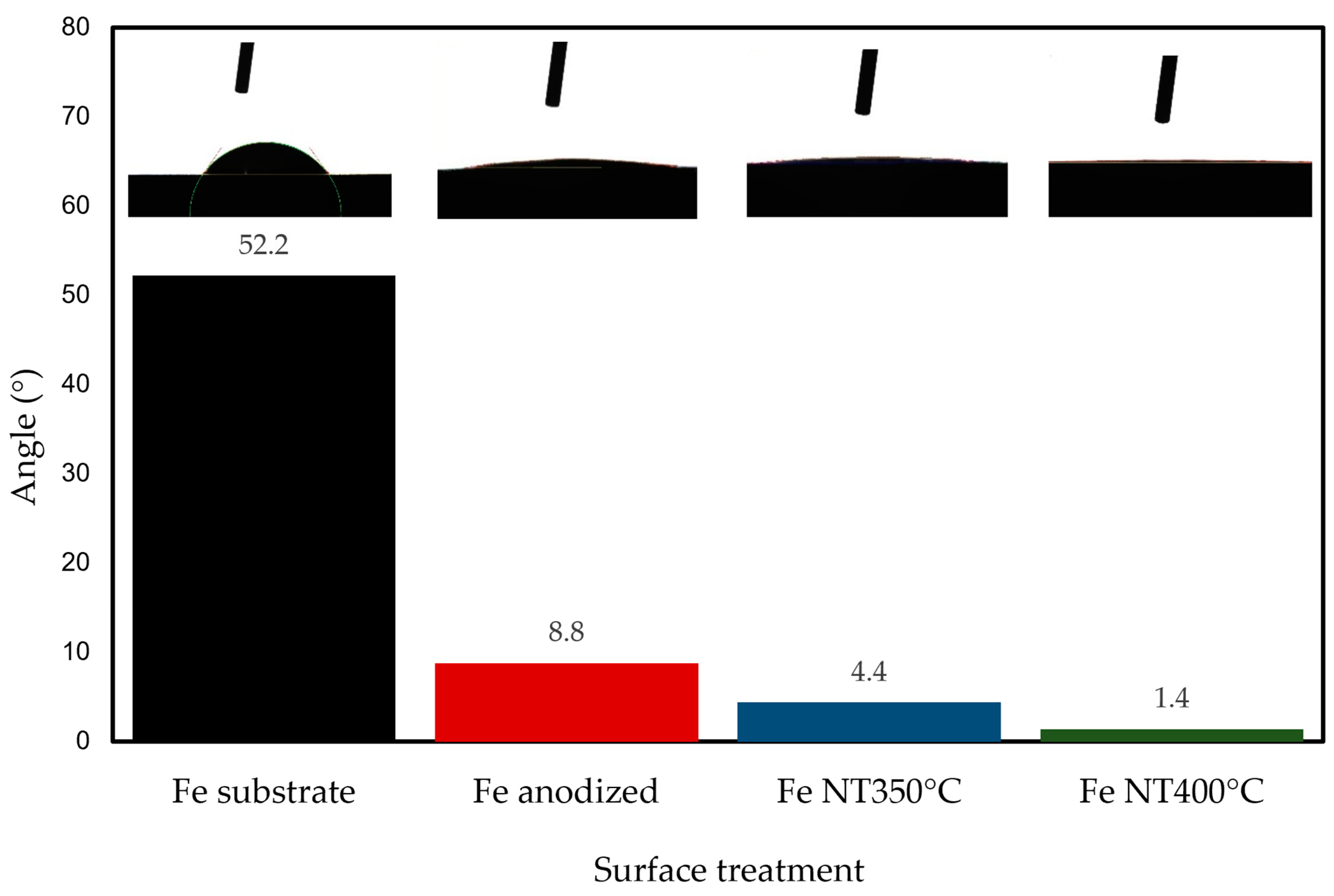
- Structural characterization confirmed the predominant presence of hematite and magnetite phases in the anodized and annealed samples. The increased wettability, as evidenced by contact angle measurements, demonstrated the effect of surface modification.
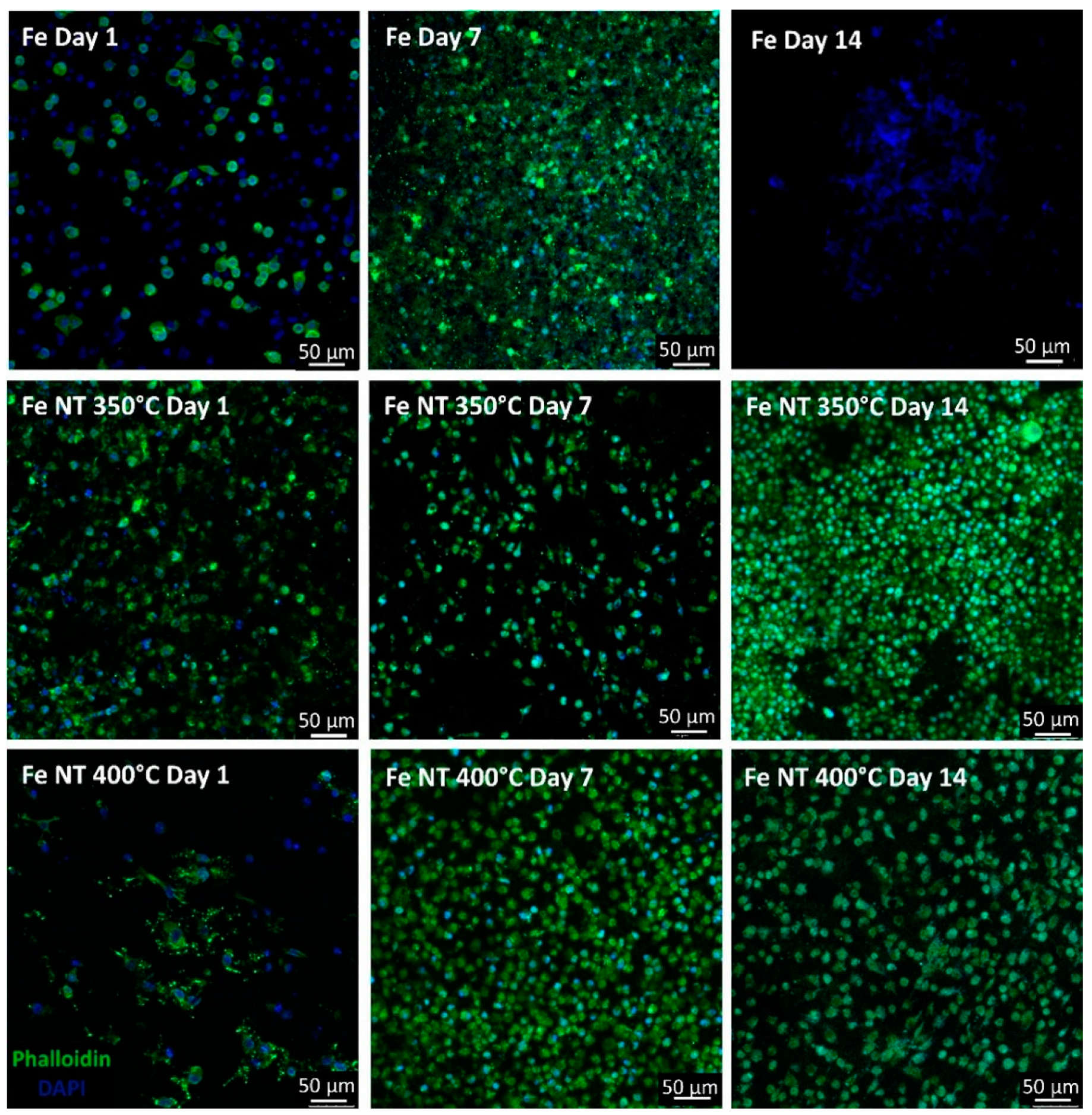
- Surface modifications promoted substantial changes in cell adhesion and proliferation; therefore, when we compared the surfaces containing NTs, it was observed that on day 7, the number of cells present on the surface of Fe NT 400 °C was already greater than on the surface of Fe NT 350 °C, showing that this was the best condition, since, for the Fe NT 350 °C sample, this similarity in cell quantity was only achieved on day 14. On the other hand, the surfaces of Fe NT 350 °C showed biomineralization on Day 1, an important factor to be considered. Therefore, in vivo studies seeking to evaluate the contribution of these surfaces to cell degradation and proliferation will be decisive for choosing the calcination temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Zheng, Y.; Gu, X.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Vojtěch, D.; Kubásek, J.; Čapek, J. Comparative mechanical and corrosion studies on magnesium, zinc and iron alloys as biodegradable metals. Mater. Tehnol. 2015, 49, 877–882. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Hayes, B. Degradable, absorbable or resorbable—What is the best grammatical modifier for an implant that is eventually absorbed by the body? Sci. China Mater. 2017, 60, 377–391. [Google Scholar] [CrossRef]
- Hermawan, H. Biodegradable Metals; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Dong, H.; Lin, F.; Boccaccini, A.R.; Virtanen, S. Corrosion behavior of biodegradable metals in two different simulated physiological solutions: Comparison of Mg, Zn and Fe. Corros. Sci. 2021, 182, 109278. [Google Scholar] [CrossRef]
- Moravej, M.; Mantovani, D. Biodegradable Metals for Cardiovascular Stent Application: Interests and New Opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef]
- Asgari, M.; Hang, R.; Wang, C.; Yu, Z.; Li, Z.; Xiao, Y. Biodegradable Metallic Wires in Dental and Orthopedic Applications: A Review. Metals 2018, 8, 212. [Google Scholar] [CrossRef]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A.R. Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015, 26, 138. [Google Scholar] [CrossRef]
- Gąsior, G.; Szczepański, J.; Radtke, A. Biodegradable Iron-Based Materials—What Was Done and What More Can Be Done? Materials 2021, 14, 3381. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.; Qin, L. Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef]
- Witte, F. Biodegradable Metals. In Biomaterials Science, 4th ed.; Elsevier: San Diego, CA, USA, 2020; pp. 271–287. [Google Scholar] [CrossRef]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Peuster, M. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal—Results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Huang, T.; Zheng, Y. Uniform and accelerated degradation of pure iron patterned by Pt disc arrays. Sci. Rep. 2016, 6, 23627. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, S.; Paternoster, C.; Mantovani, D. Iron-Based Degradable Implants. In Encyclopedia of Biomedical Engineering; Elsevier: Quebec City, QC, Canada, 2019; pp. 374–385. [Google Scholar] [CrossRef]
- Feng, Y.P.; Blanquer, A.; Fornell, J.; Zhang, H.; Solsona, P.; Baró, M.D.; Suriñach, S.; Ibáñez, E.; García-Lecina, E.; Wei, X.; et al. Novel Fe–Mn–Si–Pd alloys: Insights into mechanical, magnetic, corrosion resistance and biocompatibility performances. J. Mater. Chem. B 2016, 4, 6402–6412. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, Y. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomater. 2011, 7, 1407–1420. [Google Scholar] [CrossRef]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Mantovani, D. Fe–Mn alloys for metallic biodegradable stents: Degradation and cell viability studies. Acta Biomater. 2010, 6, 1852–1860. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable FeMnC(Pd) alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Mouzou, E.; Paternoster, C.; Tolouei, R.; Purnama, A.; Chevallier, P.; Dubé, D.; Prima, F.; Mantovani, D. In vitro degradation behavior of Fe–20Mn–1.2C alloy in three different pseudo-physiological solutions. Mater. Sci. Eng. C 2016, 61, 564–573. [Google Scholar] [CrossRef]
- Liu, R.-Y.; He, R.-G.; Xu, L.-Q.; Guo, S.-F. Design of Fe–Mn–Ag Alloys as Potential Candidates for Biodegradable Metals. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 584–590. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Liu, W.; Zheng, Z.; Zhao, M. Grain Growth upon Annealing and Its Influence on Biodegradation Rate for Pure Iron. Materials 2022, 15, 8030. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.L.; Zheng, Y.F.; Wei, S.C.; Hu, C.; Yang, G. In vitro corrosion, cytotoxicity and hemocompatibility of bulk nanocrystalline pure iron. Biomed. Mater. 2010, 5, 065015. [Google Scholar] [CrossRef] [PubMed]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Alamdari, H.; Mantovani, D.; Dubé, D. Iron–manganese: New class of metallic degradable biomaterials prepared by powder metallurgy. Powder Metall. 2008, 51, 38–45. [Google Scholar] [CrossRef]
- Huang, T.; Cheng, J.; Zheng, Y.F. In vitro degradation and biocompatibility of Fe–Pd and Fe–Pt composites fabricated by spark plasma sintering. Mater. Sci. Eng. C 2014, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Msallamová, Š.; Jablonská, E.; Lipov, J.; Vojtěch, D. A novel high-strength and highly corrosive biodegradable Fe-Pd alloy: Structural, mechanical and in vitro corrosion and cytotoxicity study. Mater. Sci. Eng. C 2017, 79, 550–562. [Google Scholar] [CrossRef]
- Drevet, R.; Zhukova, Y.; Malikova, P.; Dubinskiy, S.; Korotitskiy, A.; Pustov, Y.; Prokoshkin, S. Martensitic Transformations and Mechanical and Corrosion Properties of Fe-Mn-Si Alloys for Biodegradable Medical Implants. Metall. Mater. Trans. A 2018, 49, 1006–1013. [Google Scholar] [CrossRef]
- Xie, K.; Guo, M.; Huang, H.; Liu, Y. Fabrication of iron oxide nanotube arrays by electrochemical anodization. Corros. Sci. 2014, 88, 66–75. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Lei, Y.; Sun, H.; Yao, Y. Biocompatibility of Fe–O films synthesized by plasma immersion ion implantation and deposition. Surf. Coat. Technol. 2008, 203, 1523–1529. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, T.; Zheng, Y.F. Microstructure, mechanical property, biodegradation behavior, and biocompatibility of biodegradable Fe-Fe2O3 composites. J. Biomed. Mater. Res. A 2014, 102, 2277–2287. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Jiang, C.; Li, Y.; Fu, Y. In vitro corrosion behavior and cytocompatibility of pure Fe implanted with Ta. Surf. Coat. Technol. 2017, 320, 201–205. [Google Scholar] [CrossRef]
- Huang, T.; Zheng, Y.; Han, Y. Accelerating degradation rate of pure iron by zinc ion implantation. Regen. Biomater. 2016, 3, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, J.; Detsch, R.; Taccardi, N.; Heise, S.; Virtanen, S.; Boccaccini, A.R. Biodegradable nanostructures: Degradation process and biocompatibility of iron oxide nanostructured arrays. Mater. Sci. Eng. C 2018, 85, 203–213. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Toker, S.M.; ÖZBULUT, E.; Kolçak, Z.; Güner, E. A preliminary Investigation of Surface Micro Modification Effects on the Biocompatibility of 316L Stainless Steel. Eur. Mech. Sci. 2021, 5, 109–115. [Google Scholar] [CrossRef]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 20, 2791–2808. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.; Zheng, Y.; Guan, Y.; Lu, X.; Luo, J.-L. Nanotubular surface modification of metallic implants via electrochemical anodization technique. Int. J. Nanomed. 2014, 9, 4421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Frank, M.A.; Yang, Y.; Boccaccini, A.R.; Virtanen, S. A novel local drug delivery system: Superhydrophobic titanium oxide nanotube arrays serve as the drug reservoir and ultrasonication functions as the drug release trigger. Mater. Sci. Eng. C 2018, 82, 277–283. [Google Scholar] [CrossRef]
- Lucas-Granados, B.; Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-Antón, J. Controlled hydrodynamic conditions on the formation of iron oxide nanostructures synthesized by electrochemical anodization: Effect of the electrode rotation speed. Appl. Surf. Sci. 2017, 392, 503–513. [Google Scholar] [CrossRef]
- Rangaraju, R.R.; Raja, K.; Panday, A.; Misra, M. An investigation on room temperature synthesis of vertically oriented arrays of iron oxide nanotubes by anodization of iron. Electrochim. Acta 2009, 55, 785–793. [Google Scholar] [CrossRef]
- Sarma, B.; Jurovitzki, A.L.; Smith, Y.R.; Ray, R.S.; Misra, M. Influence of annealing temperature on the morphology and the supercapacitance behavior of iron oxide nanotube (Fe-NT). J. Power Sources 2014, 272, 766–775. [Google Scholar] [CrossRef]
- Pawlik, A.; Hnida, K.; Socha, R.P.; Wiercigroch, E.; Małek, K.; Sulka, G.D. Effects of anodizing conditions and annealing temperature on the morphology and crystalline structure of anodic oxide layers grown on iron. Appl. Surf. Sci. 2017, 426, 1084–1093. [Google Scholar] [CrossRef]
- Obayi, C.S.; Tolouei, R.; Mostavan, A.; Paternoster, C.; Turgeon, S.; Okorie, B.A.; Obikwelu, D.O.; Mantovani, D. Effect of grain sizes on mechanical properties and biodegradation behavior of pure iron for cardiovascular stent application. Biomatter 2014, 6, e959874. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Y.; Liu, J.; Jiang, C.; Li, Y. In vitro corrosion properties and cytocompatibility of Fe-Ga alloys as potential biodegradable metallic materials. Mater. Sci. Eng. C 2017, 71, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Ghicov, A.; Schmuki, P. High aspect ratio, self-ordered iron oxide nanopores formed by anodization of Fe in ethylene glycol/NH4F electrolytes. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2009, 3, 64–66. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Sinha-Ray, S.; Takoudis, C.; Mathew, M.T.; Sukotjo, C.; Yarin, A.L.; Shokuhfar, T. Fabrication of drug eluting implants: Study of drug release mechanism from titanium dioxide nanotubes. J. Phys. D Appl. Phys. 2015, 48, 275401. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Zan, R.; Ni, J.; Zhang, X. Cellular different responses to different nanotube inner diameter on surface of pure tantalum. Mater. Sci. Eng. C 2019, 109, 110520. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, X.; Jia, Z.; Shi, Y.; Cheng, Y.; Zheng, Y. Rapamycin-loaded nanoporous α-Fe2O3 as an endothelial favorable and thromboresistant coating for biodegradable drug-eluting Fe stent applications. J. Mater. Chem. B 2017, 5, 1182–1194. [Google Scholar] [CrossRef]
- Rangel, A.L.; Chaves, J.A.M.; Escada, A.L.; Konatu, R.T.; Popat, K.C.; Claro, A.P.R.A. Modification of the Ti15Mo alloy surface through TiO2 nanotube growth—An in vitro study. J. Appl. Biomater. Funct. Mater. 2018, 16, 222–229. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared- and Raman-Active Phonons of Magnetite, Maghemite, and Hematite: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef]
- Lu, J.-F.; Tsai, C.-J. Hydrothermal phase transformation of hematite to magnetite. Nanoscale Res. Lett. 2014, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- de Faria, D.L.A.; Silva, S.V.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Jubb, A.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
- Lucas-Granados, B.; Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-Antón, J. Influence of electrolyte temperature on the synthesis of iron oxide nanostructures by electrochemical anodization for water splitting. Int. J. Hydrogen Energy 2018, 43, 7923–7937. [Google Scholar] [CrossRef]
- Can, M.M.; Coşkun, M.; Fırat, T. A comparative study of nanosized iron oxide particles; magnetite (Fe3O4), maghemite (γ-Fe2O3) and hematite (α-Fe2O3), using ferromagnetic resonance. J. Alloys Compd. 2012, 542, 241–247. [Google Scholar] [CrossRef]
- Kim, H.; Seo, D.-H.; Kim, H.; Park, I.; Hong, J.; Park, K.-Y.; Kang, K. Multicomponent Effects on the Crystal Structures and Electrochemical Properties of Spinel-Structured M 3 O 4 (M = Fe, Mn, Co) Anodes in Lithium Rechargeable Batteries. Chem. Mater. 2012, 24, 720–725. [Google Scholar] [CrossRef]
- Xu, X.; Shi, C.; Li, Q.; Chen, R.; Chen, T. Fe-N-Doped carbon foam nanosheets with embedded Fe2O3 nanoparticles for highly efficient oxygen reduction in both alkaline and acidic media. RSC Adv. 2017, 7, 14382–14388. [Google Scholar] [CrossRef]
- Srivastava, V.; Kumar, S. Formation of hierarchical structures of Fe2O3 by the liquid-liquid interface technique. CrystEngComm 2014, 16, 11122–11126. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe5C2 nanoparticles: A facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef]
- Lin, T.-C.; Seshadri, G.; Kelber, J.A. A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Hu, X.; Bi, L.; Cai, P.; Jia, J.; Chai, G.; Wei, S.; Dai, L.; Wen, Z. Fe Vacancies Induced Surface FeO6 in Nanoarchitectures of N-Doped Graphene Protected β-FeOOH: Effective Active Sites for pH-Universal Electrocatalytic Oxygen Reduction. Adv. Funct. Mater. 2018, 28, 1803330. [Google Scholar] [CrossRef]
- Qian, X.; Wu, Y.; Kan, M.; Fang, M.; Yue, D.; Zeng, J.; Zhao, Y. FeOOH quantum dots coupled g-C3N4 for visible light driving photo- Fenton degradation of organic pollutants. Appl. Catal. B 2018, 237, 513–520. [Google Scholar] [CrossRef]
- Kačiulis, S.; Mattogno, G.; Pandolfi, L.; Cavalli, M.; Gnappi, G.; Montenero, A. XPS study of apatite-based coatings prepared by sol–gel technique. Appl. Surf. Sci. 1999, 151, 1–5. [Google Scholar] [CrossRef]
- Ruiz, F.; Benzo, Z.; Quintal, M.; Garaboto, Á.; Albornoz, A.; Brito, J.L. X-ray photoelectron spectroscopy study of pyrolytically coated graphite platforms submitted to simulated electrothermal atomic absorption spectrometry conditions. Appl. Surf. Sci. 2006, 252, 8695–8701. [Google Scholar] [CrossRef]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of Oxygen Plasma Nanotexturing of Organic Polymer Surfaces: From Stable Super Hydrophilic to Super Hydrophobic Surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, A.; Shchukarev, A.; Štenclová, P.; Wågberg, T.; Segervald, J.; Jia, X.; Kromka, A. Reference XPS spectra of amino acids. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1050, 012001. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-Light-Driven N−F−Codoped TiO2 Photocatalysts. 1. Synthesis by Spray Pyrolysis and Surface Characterization. Chem. Mater. 2005, 17, 2588–2595. [Google Scholar] [CrossRef]
- Hao, P.; Zhu, W.; Lei, F.; Ma, X.; Xie, J.; Tan, H.; Li, L.; Liu, H.; Tang, B. Morphology and electronic structure modulation induced by fluorine doping in nickel-based heterostructures for robust bifunctional electrocatalysis. Nanoscale 2018, 10, 20384–20392. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Lee, J.Y. Plasmonic Oxygen-Deficient TiO2-x Nanocrystals for Dual-Band Electrochromic Smart Windows with Efficient Energy Recycling. Adv. Mater. 2020, 32, 2004686. [Google Scholar] [CrossRef]
- Celik-Kucuk, A.; Abe, T. Electrochemical behavior of CuF2 as reversible cathode in an organic liquid electrolyte for room-temperature fluoride-shuttle batteries. J. Power Sources 2021, 496, 229828. [Google Scholar] [CrossRef]
- Escada, A.L.D.A.; Camargo, S.E.A.; de Vasconcellos, L.M.R.; Milhan, N.V.M.; Claro, A.P.R.A. Cytotoxicity Analysis of Ti-7.5Mo Alloy After Biomimetic Surface Treatment to Use as Dental Materials. Mater. Res. 2017, 20, 1614–1621. [Google Scholar] [CrossRef]
- Ahmad, D.; van den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, B.; Wu, Y.; Zheng, Y. Comparative in vitro Study on Pure Metals (Fe, Mn, Mg, Zn and W) as Biodegradable Metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Narayanan, R.; Kwon, T.-Y.; Kim, K.-H. TiO2 nanotubes from stirred glycerol/NH4F electrolyte: Roughness, wetting behavior and adhesion for implant applications. Mater. Chem. Phys. 2009, 117, 460–464. [Google Scholar] [CrossRef]
- Minagar, S.; Wang, J.; Berndt, C.C.; Ivanova, E.P.; Wen, C. Cell response of anodized nanotubes on titanium and titanium alloys. J. Biomed. Mater. Res. A 2013, 101A, 2726–2739. [Google Scholar] [CrossRef] [PubMed]
- Capellato, P.; Smith, B.S.; Popat, K.C.; Claro, A.P.A. Fibroblast functionality on novel Ti 30Ta nanotube array. Mater. Sci. Eng. C 2012, 32, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Rangel, A.L.R.; Falentin-Daudré, C.; da Silva Pimentel, B.N.A.; Vergani, C.E.; Migonney, V.; Claro, A.P.R.A. Nanostructured titanium alloy surfaces for enhanced osteoblast response: A combination of morphology and chemistry. Surf. Coat. Technol. 2019, 383, 125226. [Google Scholar] [CrossRef]
- Rabeeh, V.P.M.; Hanas, T. Enhancing biointerfacial properties of porous pure iron by gold sputtering for degradable implant applications. Mater. Today Commun. 2022, 31, 103492. [Google Scholar] [CrossRef]
- Pavel, M.; Renna, M.; Park, S.J.; Menzies, F.M.; Ricketts, T.; Füllgrabe, J.; Ashkenazi, A.; Frake, R.A.; Lombarte, A.C.; Bento, C.F.; et al. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun. 2018, 9, 2961. [Google Scholar] [CrossRef]


| Fe (%) | Fe NT 350 °C (%) | Fe NT 400 °C (%) | |
|---|---|---|---|
| F 1s | - | 2.88 ± 0.16 | 0.85 ± 0.12 |
| Cu 2p | - | 1.78 ± 0.50 | 1.74 ± 0.50 |
| O 1s | 19.64 ± 0.40 | 49.53 ± 0.68 | 50.51± 0.66 |
| Fe 2p | 1.67 ± 0.17 | 18.37 ± 0.52 | 16.55 ± 0.56 |
| C 1s | 78.69 ± 0.44 | 27.45 ± 0.75 | 30.36 ± 0.69 |
| Binding Energy (eV) and Atomic Concentration (%) | |||||
|---|---|---|---|---|---|
| Fe 2p | O 1s | C 1s | F 1s | Cu 2p | |
| 711.2 (0.09) | |||||
| 718.6 (0.78) | 529.9 (6.97) | 284.8 (64.38) | |||
| Fe | 718.8 (0.20) | 531.2 (10.41) | 286.0 (9.74) | - | - |
| 719.9 (0.09) | 532.2 (2.24) | 288.5 (4.64) | |||
| 724.6 (0.47) | |||||
| 711.2 (2.75) | 935.3 (0.18) | ||||
| 718.6 (7.25) | 283.7 (0.86) | 936.9 (0.30) | |||
| 718.8 (1.94) | 529.9 (33.21) | 285.1 (15.07) | 683.5 (0.23) | 941.4 (0.24) | |
| Fe NT 350 °C | 719.5 (1.15) | 531.2 (15.13) | 286.6 (8.87) | 683.7 (1.78) | 945.6 (0.28) |
| 719.9 (1.30) | 532.2 (1.27) | 288.5 (2.71) | 685.3 (0.88) | 950.0 (0.22) | |
| 724.6 (3.91) | 951. 0 (0.20) | ||||
| 952.7 (0.16) | |||||
| 711.2 (2.42) | 933.5 (0.23) | ||||
| 718.6 (6.31) | 529.9 (35.86) | 284.8 (20.06) | 683.5 (0.08) | 935.2 (0.26) | |
| Fe NT 400 °C | 718.8 (1.71) | 531.2 (8.11) | 286.0 (8.25) | 683.7 (0.42) | 940.4 (0.29) |
| 719.5 (1.05) | 532.2 (6.73) | 288.5 (2.16) | 685.3 (0.36) | 946.0 (0.22) | |
| 719.9 (0.95) | 952.1 (0.23) | ||||
| 724.6 (4.12) | 952.9 (0.17) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, R.d.C.R.; Rangel, A.L.R.; da Silva, K.B.; Escada, A.L.d.A.; Chaves, J.A.M.; Maia, F.R.; Pina, S.; Reis, R.L.; Oliveira, J.M.; Rosifini Alves, A.P. Characterization of Iron Oxide Nanotubes Obtained by Anodic Oxidation for Biomedical Applications—In Vitro Studies. Materials 2024, 17, 3627. https://doi.org/10.3390/ma17153627
Rangel RdCR, Rangel ALR, da Silva KB, Escada ALdA, Chaves JAM, Maia FR, Pina S, Reis RL, Oliveira JM, Rosifini Alves AP. Characterization of Iron Oxide Nanotubes Obtained by Anodic Oxidation for Biomedical Applications—In Vitro Studies. Materials. 2024; 17(15):3627. https://doi.org/10.3390/ma17153627
Chicago/Turabian StyleRangel, Rita de Cássia Reis, André Luiz Reis Rangel, Kerolene Barboza da Silva, Ana Lúcia do Amaral Escada, Javier Andres Munoz Chaves, Fátima Raquel Maia, Sandra Pina, Rui L. Reis, Joaquim M. Oliveira, and Ana Paula Rosifini Alves. 2024. "Characterization of Iron Oxide Nanotubes Obtained by Anodic Oxidation for Biomedical Applications—In Vitro Studies" Materials 17, no. 15: 3627. https://doi.org/10.3390/ma17153627








