A Study on the Surface Oxidation Pretreatment and Nickel Plating Mechanism of Carbon Fiber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods and Procedures
2.3. Testing and Characterization
3. Results and Discussion
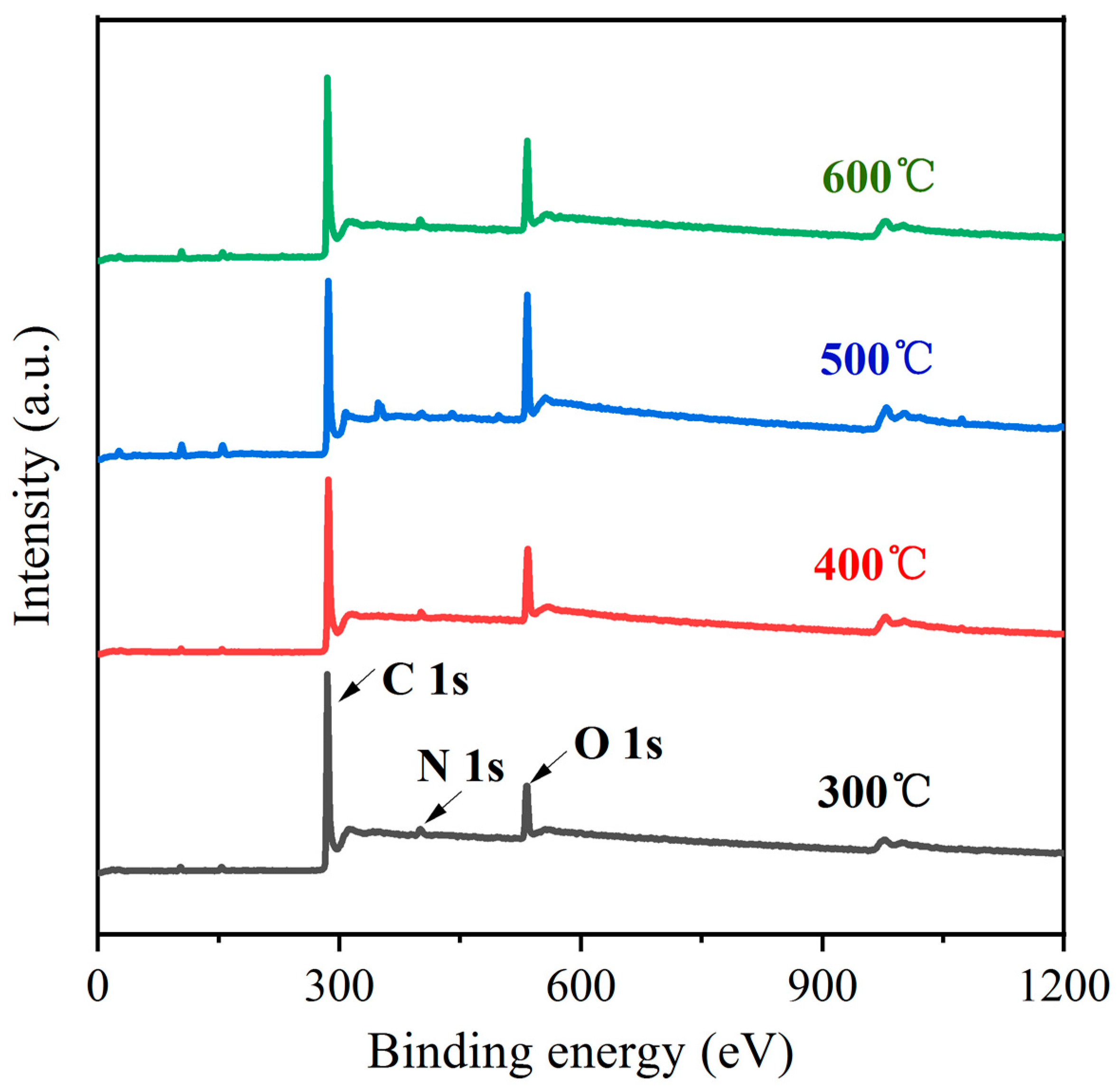
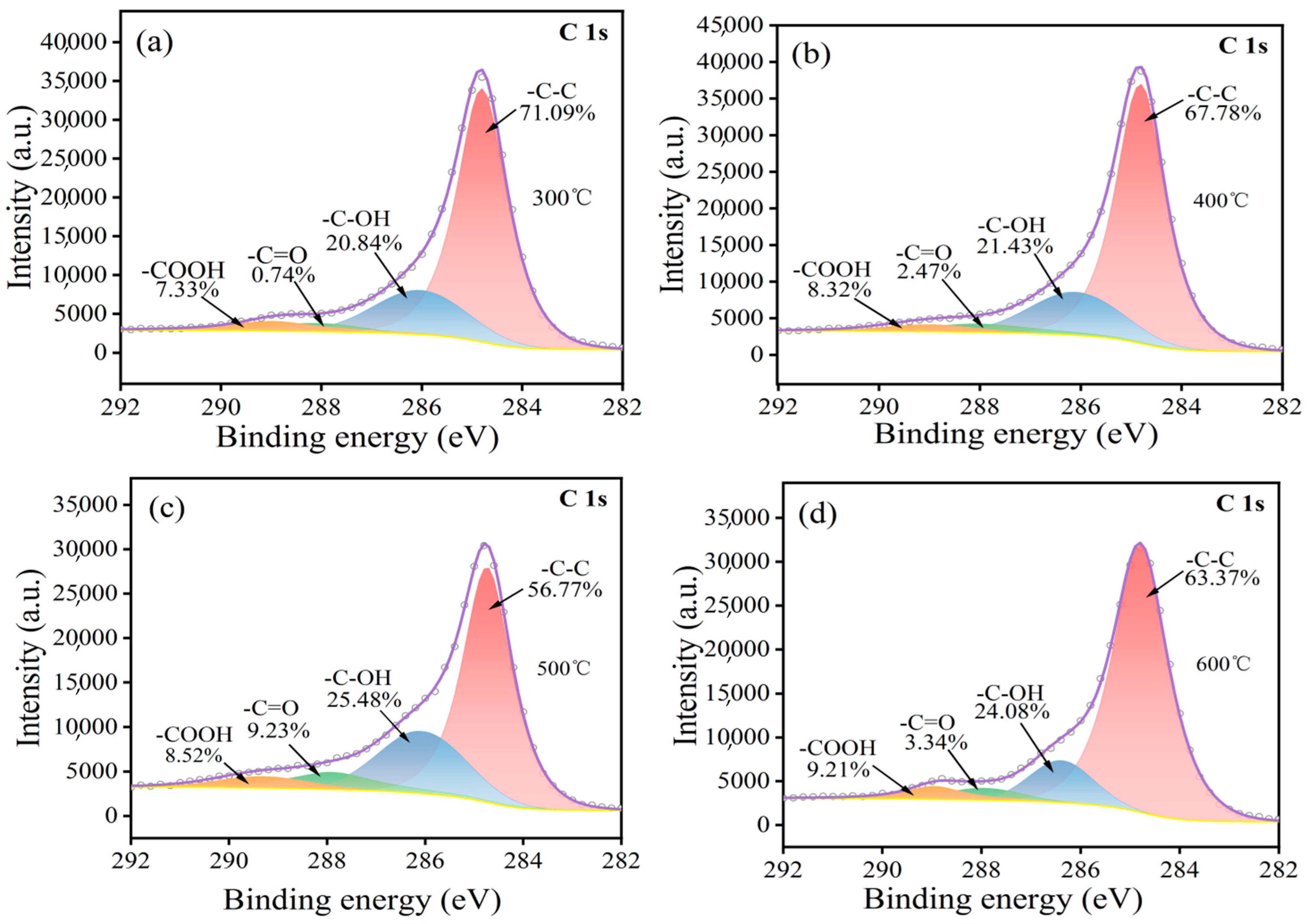
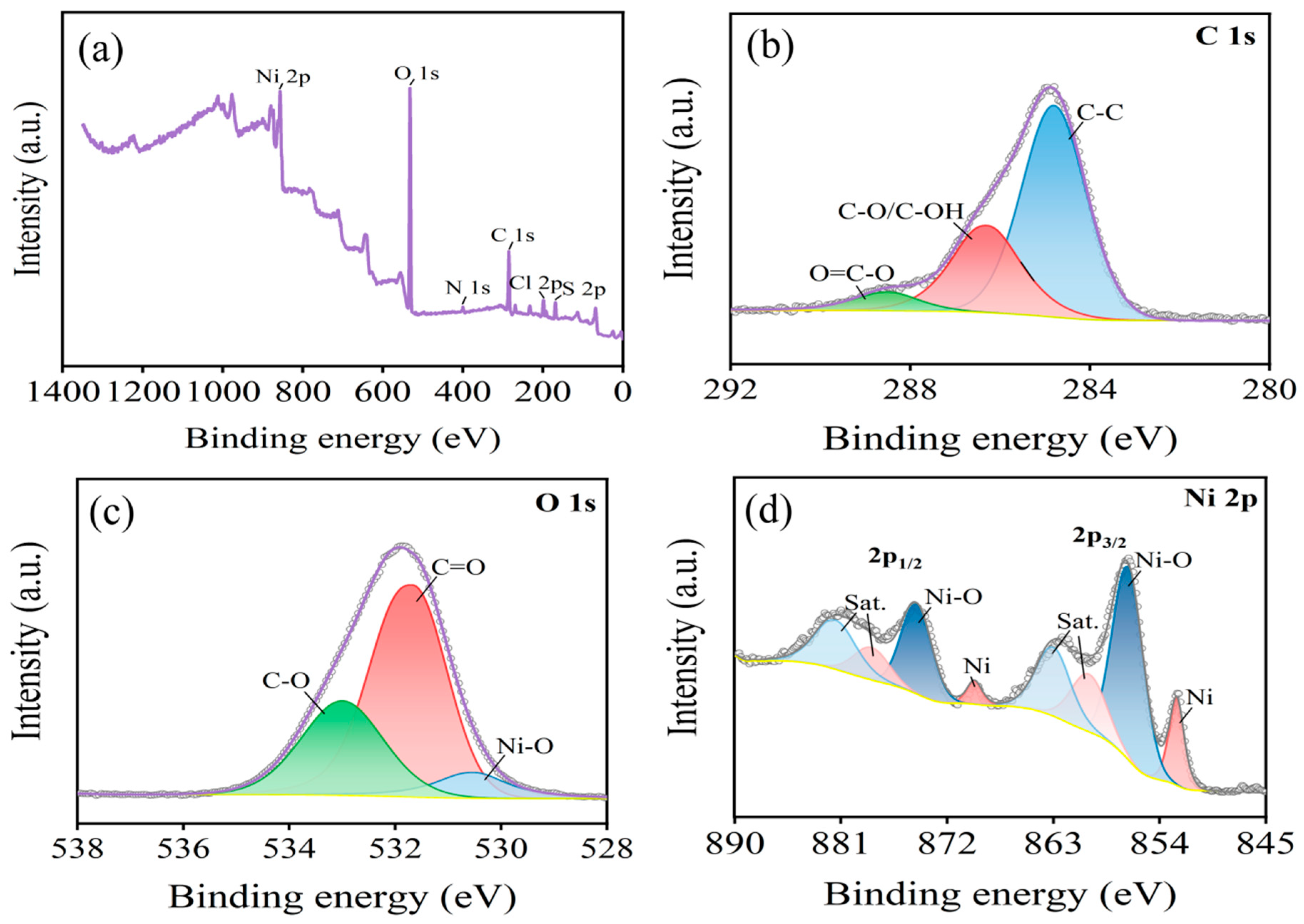
3.1. Surface Modification of the Carbon Fibers
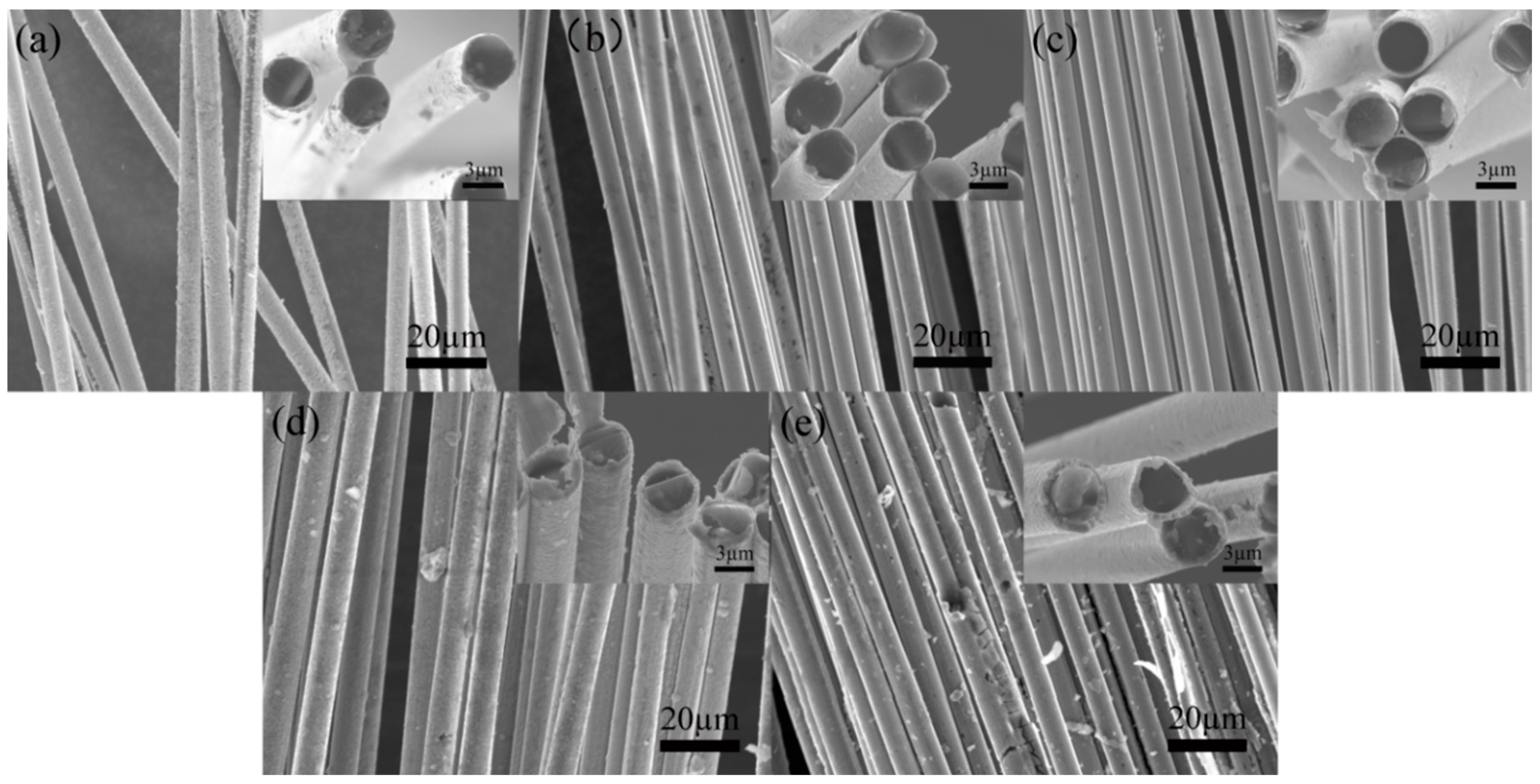
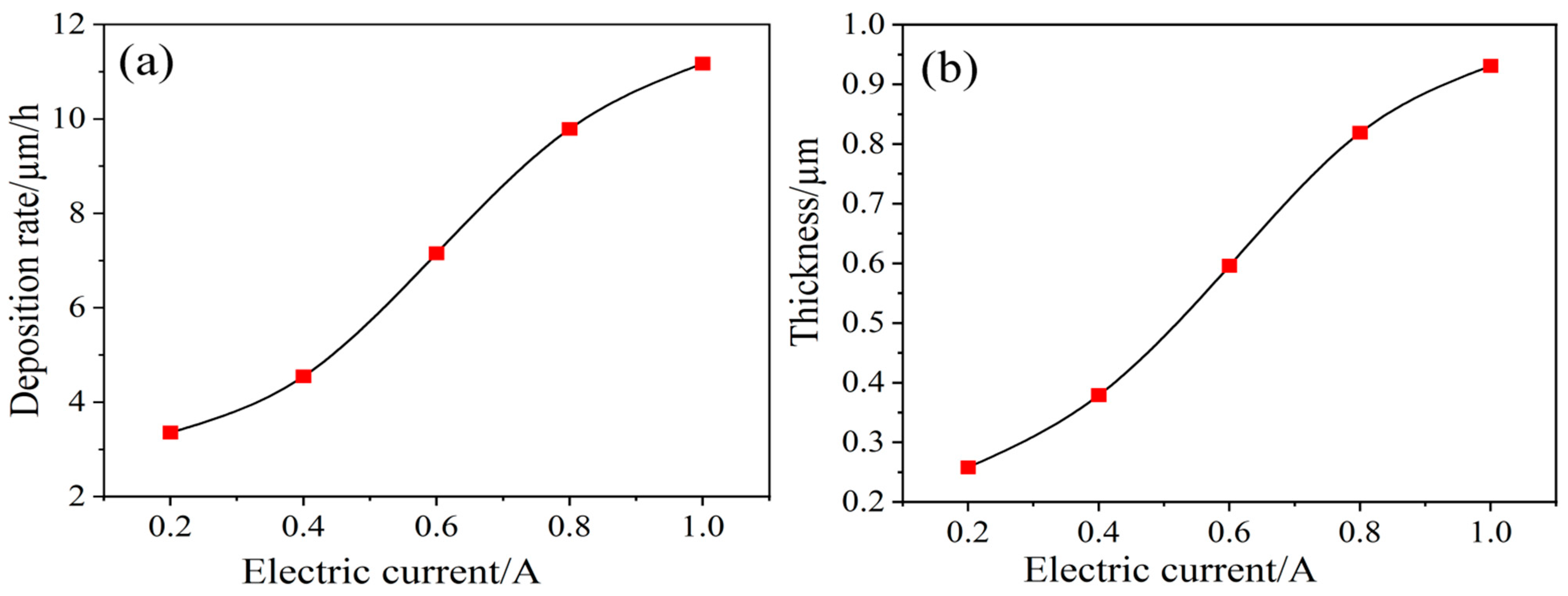
3.2. Ni Electrodeposition on Carbon Fiber Surfaces
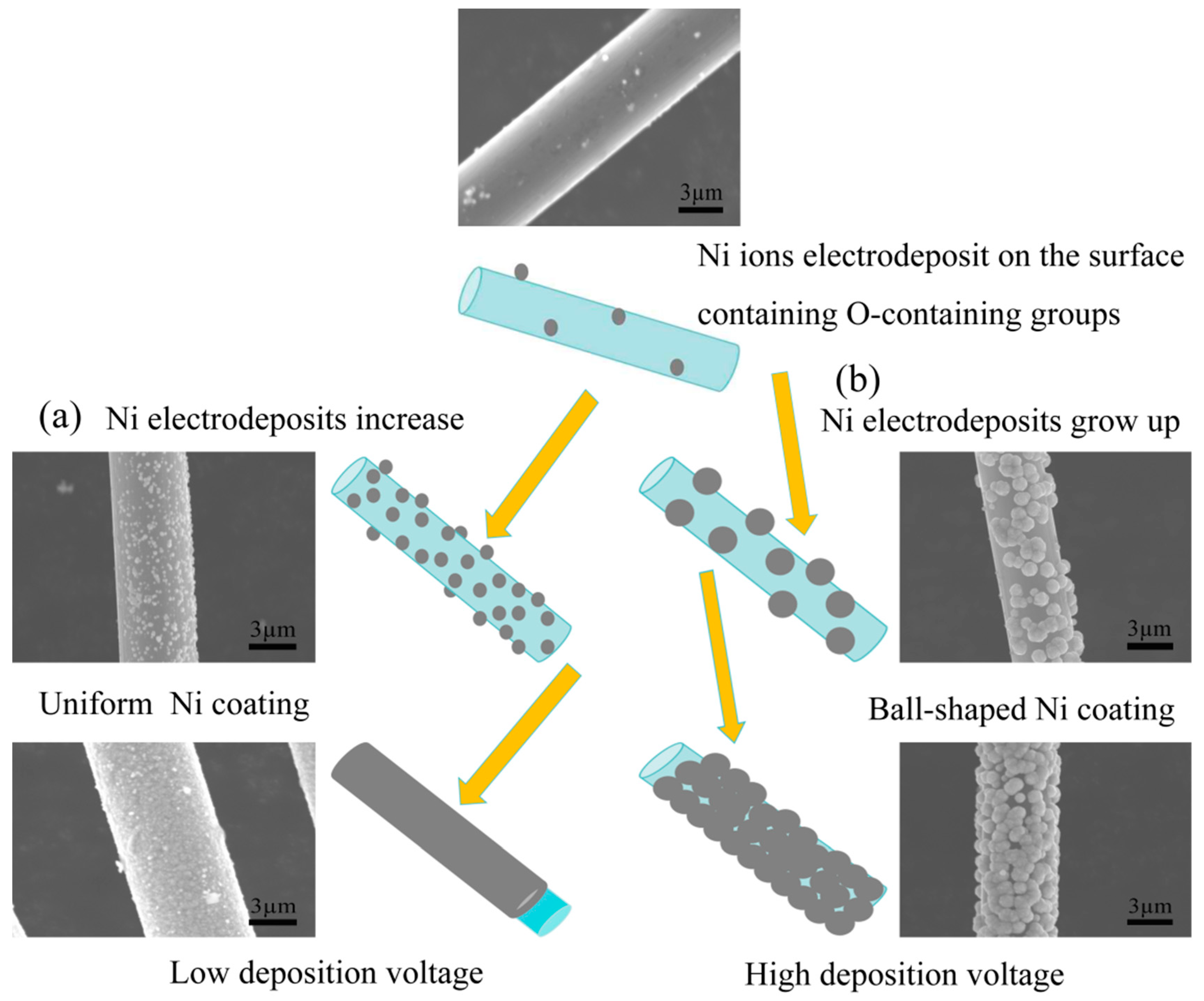
3.3. Mechanism of Ni Electrodeposition on Carbon Fiber Surfaces
4. Conclusions
- (1)
- High-temperature oxidation has been demonstrated to be an effective method for modifying the surface of carbon fibers, leading to improved adhesion properties. This study has presented that a temperature of 500 °C exhibits the highest concentration of functional groups such as -OH, -C=O, and -COOH, resulting in the most effective modification. It is crucial to note that exceeding this temperature threshold leads to a significant loss of carbon fiber mass, thereby compromising its strengthening capabilities.
- (2)
- In the process of the heating and oxidation of carbon fiber, its surface functional groups constantly change. At a low heating temperature, the surface is mainly dominated by -OH groups and -C=O groups. With an increase in temperature, a large number of -OH groups are oxidized into -COOH groups, and the continuous oxidation of some -C=O groups leads to the formation of -COOH groups or CO2. This causes loss of the C element on the carbon fiber surface.
- (3)
- Further modification through Ni electrodeposition under stable voltage conditions indicates that a low voltage yields a Ni-plated layer on the carbon fibers that is both smooth and uniform. Application of an insufficient voltage results in incomplete Ni deposition, while excessive voltage leads to surface roughness and the undesirable presence of particle deposits in the plated layer. Specifically, at a high voltage (15 V), a spherical Ni-plated layer forms on the carbon fiber surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yalovega, G.E. Interfacial interaction in NiOx/MWNTs and CuOx/MWNTs nanostructures as efficient electrode materials for high-performance supercapacitors. Nanomaterials 2024, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.G.; Zhang, J.; Xia, S.M. Progress in the Preparation and Application of Nickel-Plated Carbon Fibers. Shandong Chem. Ind. 2019, 48, 41–42. [Google Scholar]
- Ouyang, W.J.; Jia, J.G.; Ma, Q. Preparation and Characterization of Cf/Cu Composite Materials Based on the Process of Uniformly Coating Shortcut Carbon Fibers with Cu Layers. J. Compos. Mater. 2018, 33, 2824–2830. [Google Scholar]
- Shirvanimoghaddam, K.; Hamim, S.U.; Akbari, M.K.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Shahzad Munir, K.; Naebe, M.; et al. Carbon Fiber Reinforced Metal Matrix Composites: Fabrication Processes and Properties. Compos. Part A Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Huang, Y.D.; Wang, T.Y.; Liu, L. Influence of fibre surface oxidation-reduction followed by silsesquioxane coating treatment on interfacial mechanical propertiesof carbon fibre/polyarylacetylene composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 936–944. [Google Scholar] [CrossRef]
- Pamula, E.; Rouxhet, P.G. Bulk and surface chemical functionalities of type IIIPAN-based carbon fibres. Carbon 2003, 41, 1905–1915. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, Q.; Zhao, X.; Sun, H.; Wang, S.; Zhu, Y.; Liu, Z.; Wang, C.; He, X. Grafting carbon nanotubes densely on carbon fibers by poly(propylene imine) for interfacial enhancement of carbon fiber composites. Carbon 2020, 158, 704–710. [Google Scholar] [CrossRef]
- Li, Y.B.; Peng, Q.Y.; He, X.D.; Hu, P.; Wang, C.; Shang, Y.; Wang, R.; Jiao, W.; Lv, H. Synthesis and characterization of a new hierarchical reinforcement by chemically grafting graphene oxide onto carbon fibers. J. Mater. Chem. 2012, 22, 18748–18752. [Google Scholar] [CrossRef]
- Wu, Q.; Wan, Q.; Yang, X.; Wang, F.; Bai, H.; Zhu, J. Remarkably improved interfacial adhesion of pitch-based carbon fiber composites by constructing a synergistic hybrid network at interphase. Compos. Sci. Technol. 2021, 205, 108648. [Google Scholar] [CrossRef]
- Barton, B.E.; Behr, M.J.; Patton, J.T.; Hukkanen, E.J.; Landes, B.G.; Wang, W.; Horstman, N.; Rix, J.E.; Keane, D.; Derstine, C. High-Modulus Low-Cost Carbon Fibers from Polyethylene Enabled by Boron Catalyzed Graphitization. Small 2017, 13, 1701926. [Google Scholar] [CrossRef]
- Newcomb, B.A. Processing, Structure, and Properties of Carbon Fibers. Compos. Part A Appl. Sci. Manuf. 2019, 91, 262–282. [Google Scholar] [CrossRef]
- Li, N. Practical Technology of Electroless Plating; Chemical Industry Press: Beijing, China, 2004; pp. 245–288. [Google Scholar]
- Huo, C.H.; He, W.; Fan, Z.X. Study on the Metallization Process of Carbon Fiber Surfaces. Surf. Technol. 2003, 32, 40–42. [Google Scholar]
- Daoushb, W.M.; Lima, B.K.; Moa, C.B.; Nama, D.H.; Honga, S.H. Electrical and mechanical properties of carbon nanotube reinforced copper nanocomposites fabricated by electroless deposition process. Mater. Sci. Eng. A 2009, 513–514, 247–253. [Google Scholar] [CrossRef]
- Hu, F.Z. Interface of Polymers and Their Composites; China Light Industry Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
- Xu, X.F.; Hong, L.L.; Xiao, P. Study on Nickel Electroplating and Chemical Nickel Plating on Carbon Fiber Surface. Funct. Mater. 2013, 44, 264–267. [Google Scholar]
- Sun, Y.; Wan, X.W.; Jiang, J.X. Study on the Pretreatment Process of Chemical Nickel Plating on Carbon Fiber Surfaces. China Surf. Eng. 2007, 20, 41–45. [Google Scholar]
- Cui, K.F.; Zhong, L. New Palladium-free Activation Process for Chemical Copper Plating on Alumina Ceramic Surfaces. Electroplat. Environ. Prot. 2019, 39, 39–41. [Google Scholar]
- Qin, T.N.; Ma, L.Q.; Liu, M.J. Improvements and Development Directions in the Activation Process of Chemical Plating on Non-metallic Material Surfaces. China Surf. Eng. 2010, 23, 69–74. [Google Scholar]
- He, W.; Tang, X.Z.; Chi, L.Z. Study on the Chemical Nickel Plating Process on Carbon Fiber Surfaces. Electroplat. Finish. 2003, 22, 8–11. [Google Scholar]
- Mangun, C.L.; Benak, K.R.; Economy, J.; Foster, K.L. Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 2001, 39, 1809–1820. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Mishakov, I.V.; Bauman, Y.I.; Shubin, Y.V.; Maksimova, T.A.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water purification from chlorobenzenes using heteroatom-functionalized carbon nanofibers produced on self-organizing Ni-Pd catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Belenkov, E.A.; Greshnyakov, V.A.; Yalovega, G.E.; Bashkin, I.O. New aspects in the study of carbon-hydrogen interaction in hydrogenated carbon nanotubes for energy storage applications. J. Alloys Compd. 2019, 792, 713–720. [Google Scholar] [CrossRef]
- Barinov, A.; Gregoratti, L.; Dudin, P.; La Rosa, S.; Kiskinova, M. Imaging and Spectroscopy of Multiwalled Carbon Nanotubes during Oxidation: Defects and Oxygen Bonding. Adv. Mater. 2009, 21, 1916–1920. [Google Scholar] [CrossRef]
- Terzyk, A.P. The Influence of Activated Carbon Surface Chemical Composition on the Adsorption of Acetaminophen (Paracetamol) in Vitro: Part II. TG, FTIR, and XPS Analysis of Carbons and the Temperature Dependence of Adsorption Kinetics at the Neutral pH. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 23–45. [Google Scholar] [CrossRef]
- Liu, L. Improvement of the thermal conductivity and friction performance of poly (ether ether ketone)/carbon fiber laminates by addition of graphene. RSC Adv. 2015, 5, 57853–57859. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Besedina, N.A.; Brzhezinskaya, M.; Malkov, M.N.; Stolyarova, D.Y.; Arutyunyan, A.F.; Struchkov, N.S.; Saveliev, S.D.; Brunkov, P.N.; et al. Guiding graphene derivatization for covalent immobilization of aptamers. Carbon 2022, 196, 264–279. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J. The Characterization of Activated Carbons with Oxygen and Nitrogen Surface Groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Lee, W.H.; Lee, J.G.; Reucroft, P.J. XPS study of carbon fiber surfaces treated by thermal oxidation in a gas mixture of O2/(O2 + N2). Appl. Surf. Sci. 2001, 171, 136–142. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Lopez-Ramon, M.; Carrasco-Marin, F. Changes in Surface Chemistry of Activated Carbons by Wet Oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Y.L.; Chen, Y.J.; Liang, J. Interfacial and mechanical properties of carbon fibers modified by electrochemical oxidation in (NH4HCO3)/(NH4)2C2O4·H2O aqueous compound solution. Appl. Surf. Sci. 2010, 256, 6199–6204. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, R.L.; Gao, F.C.; He, M.; Wang, C.; Liu, L.; Zhao, L.; Cui, H. Interfacial Microstructure and Enhanced Mechanical Properties of Carbon Fiber Composites Caused by Growing Generation 1-4 Dendritic Poly(amidoamine) on a Fiber Surface. Langmuir 2016, 32, 8339–8349. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.R.; Jiang, W.; Wang, L.; Gardner, S.D.; Pittman, C.U., Jr. Surface Characterization of Electrochemically Oxidized Carbon Fibers. Carbon 1999, 37, 1785–1796. [Google Scholar] [CrossRef]
- Wang, C.Z.; Yang, X.P.; Yu, Y.H. XPS, AFM Study on the Mechanism of Electrochemical Surface Treatment Process of Pitch-based Carbon Fibers. Acta Mater. Compos. Sin. 2002, 19, 28–32. [Google Scholar]
- Lu, Y.X.; Liang, Q.; Xue, L.L. Palladium-free Catalytic Electroless Copper Deposition on Bamboo Fabric: Preparation, Morphology, and Electromagnetic Properties. Appl. Surf. Sci. 2012, 258, 4782–4787. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.B.; Ren, S.B.; Zhang, C.; Liu, T.T.; Qu, X.H. Thermophysical Properties and Microstructure of Graphite Flake/Copper Composites Processed by Electroless Copper Coating. J. Alloys Compd. 2014, 587, 255–259. [Google Scholar] [CrossRef]
- Zou, G.-Z.; Cao, M.S.; Zhang, L.; Li, J.G.; Xu, H.; Chen, Y.J. A nanoscale core-shell of β-SiCP–Ni prepared by electroless plating at lower temperature. Surf. Coat. Technol. 2006, 201, 108–112. [Google Scholar] [CrossRef]
- Matsubara, H.; Yonekawa, T.; Ishino, Y.; Nishiyama, H.; Saito, N.; Inoue, Y. Observation of initial deposition process of electroless nickel plating by quartz crystal microbalance method and microscopy. Electrochim. Acta 2002, 47, 4011–4018. [Google Scholar] [CrossRef]
- Hua, Z.S.; Yao, G.C.; Ma, J. XPS Analysis of Nickel Plating Layers on Carbon Fiber Surfaces. Chin. J. Nonferrous Met. 2011, 21, 165–170. [Google Scholar]
- Han, X.; Zhou, Y.X.; Wang, Z.J. The Effect of Electroplating Time on Continuous Nickel Plating on Carbon Fiber Surfaces. Electroplat. Finish. 2014, 33, 363–365. [Google Scholar]
- Fukunaga, A.; Ueda, S.; Nagumo, M. Air-oxidation and Anodization of Pitch-based Carbon Fibers. Carbon 1999, 37, 1081–1085. [Google Scholar] [CrossRef]
- Nikolic, N.; Popov, K.; Pavlovic, L.; Pavlović, M.G. Morphologies of Copper Deposits Obtained by the Electrodeposition at High Overpotentials. Surf. Coat. Technol. 2006, 201, 560–566. [Google Scholar] [CrossRef]






| Carbon Content (%) | Tensile Strength (GPa) | Modulus (GPa) | Density (g/cm3) | Diameter (µm) | Elongation (%) |
|---|---|---|---|---|---|
| ≥93 | ≥3.0 | 210–240 | 1.77 | 6–8 | ≥1.5 |
| NiSO4·6H2O | NiCl2·6H2O | H3BO3 | (CH3(CH2)11OSO3Na | Temperature | Time |
|---|---|---|---|---|---|
| 260 g/L | 60 g/L | 40 g/L | 0.1 g/L | 25 °C | 5 min |
| Sample Name | C1s/% | O1s/% | O1s/C1s/% |
|---|---|---|---|
| 300 °C CFs | 86.78 | 13.22 | 15.23 |
| 400 °C CFs | 84.40 | 15.60 | 18.48 |
| 500 °C CFs | 74.12 | 25.88 | 34.92 |
| 600 °C CFs | 81.38 | 18.62 | 22.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Li, X.; Zhu, D. A Study on the Surface Oxidation Pretreatment and Nickel Plating Mechanism of Carbon Fiber. Materials 2024, 17, 3650. https://doi.org/10.3390/ma17153650
Wang Q, Li X, Zhu D. A Study on the Surface Oxidation Pretreatment and Nickel Plating Mechanism of Carbon Fiber. Materials. 2024; 17(15):3650. https://doi.org/10.3390/ma17153650
Chicago/Turabian StyleWang, Qinghui, Xuesong Li, and Dongdong Zhu. 2024. "A Study on the Surface Oxidation Pretreatment and Nickel Plating Mechanism of Carbon Fiber" Materials 17, no. 15: 3650. https://doi.org/10.3390/ma17153650






