The Influence of Lanthanum Admixture on Microstructure and Electrophysical Properties of Lead-Free Barium Iron Niobate Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Technological Process
2.2. Characterization
3. Results and Discussion
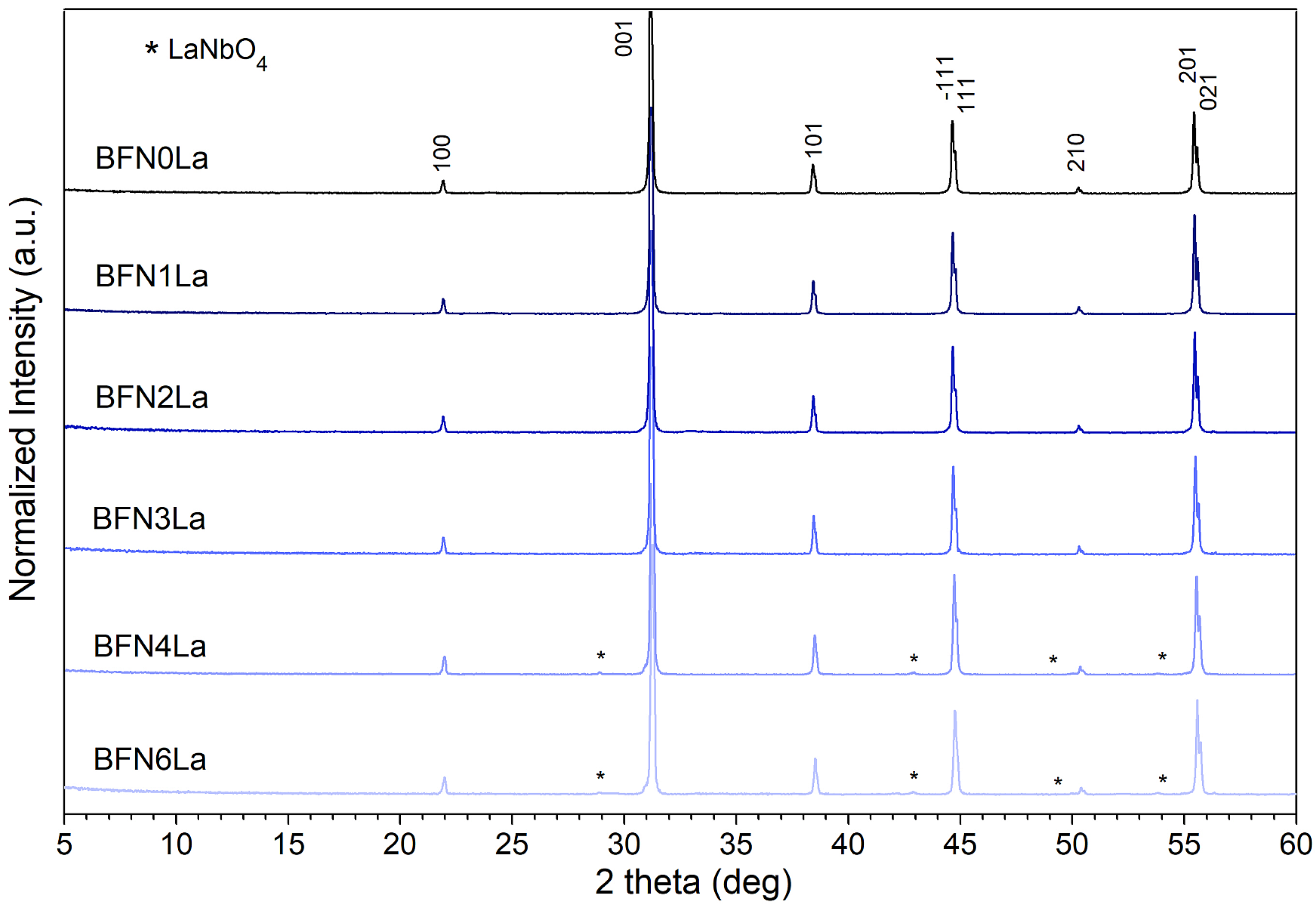
3.1. Crystal Structure
3.2. Microstructure
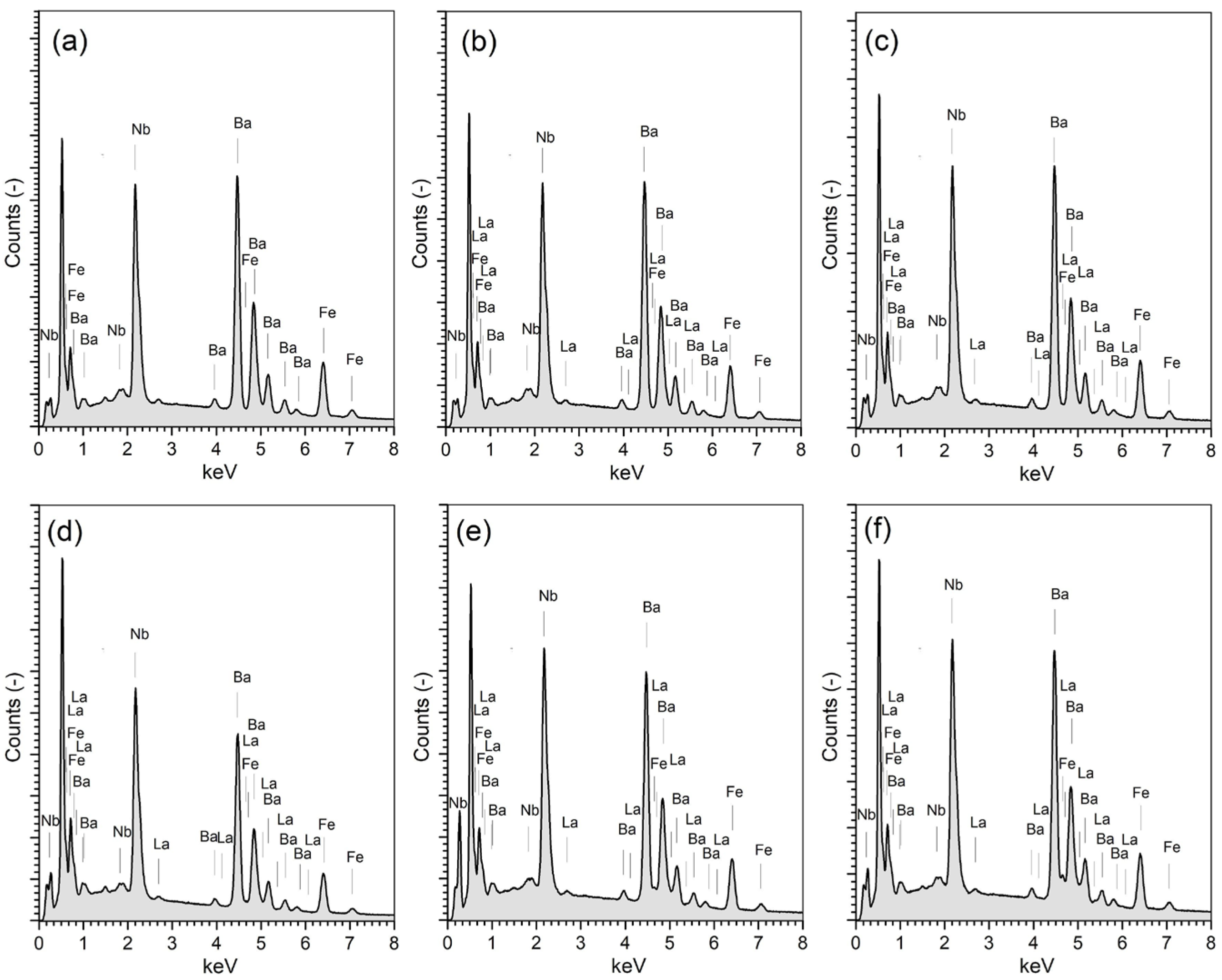
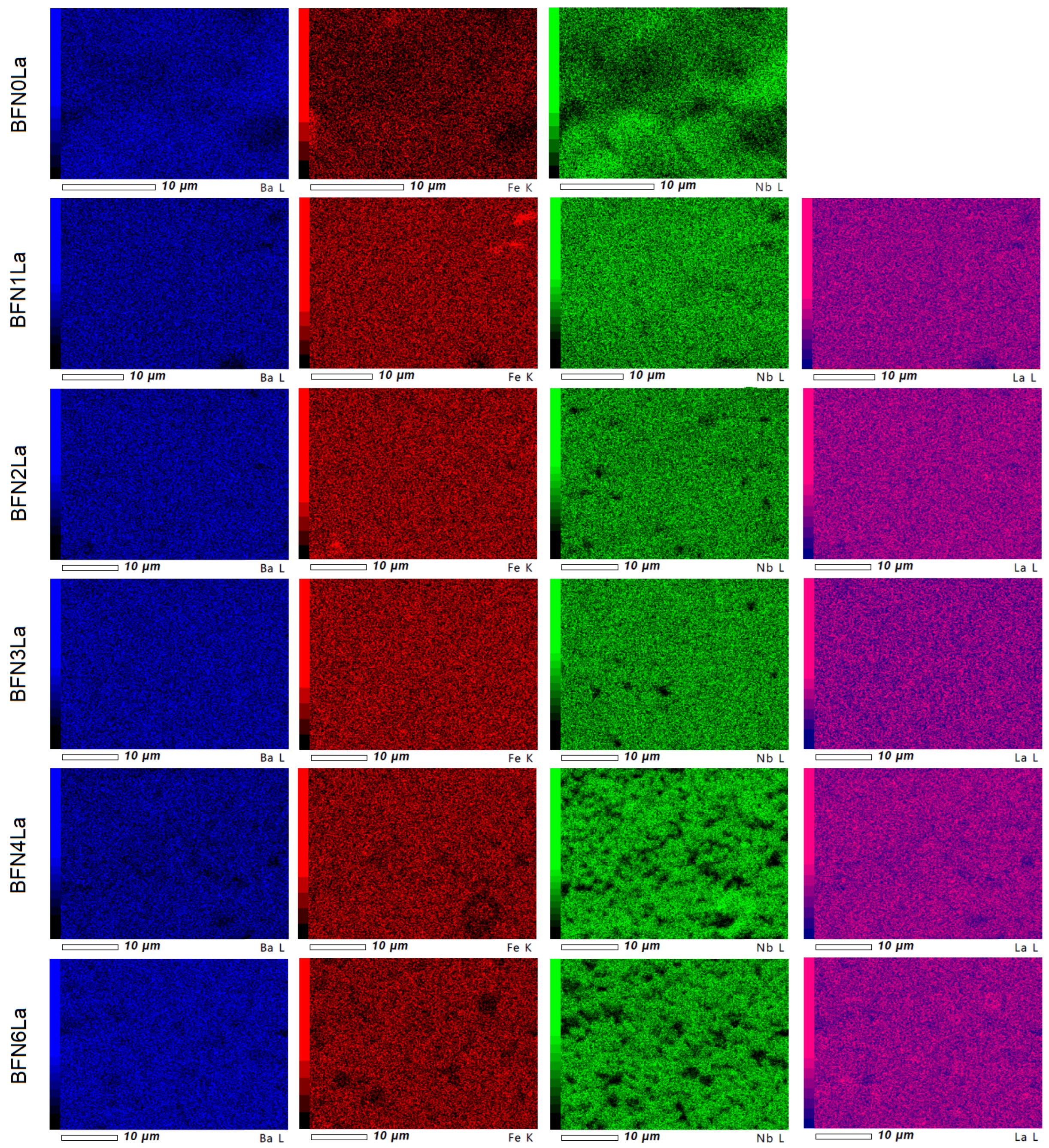
3.3. EDS and EPMA Tests
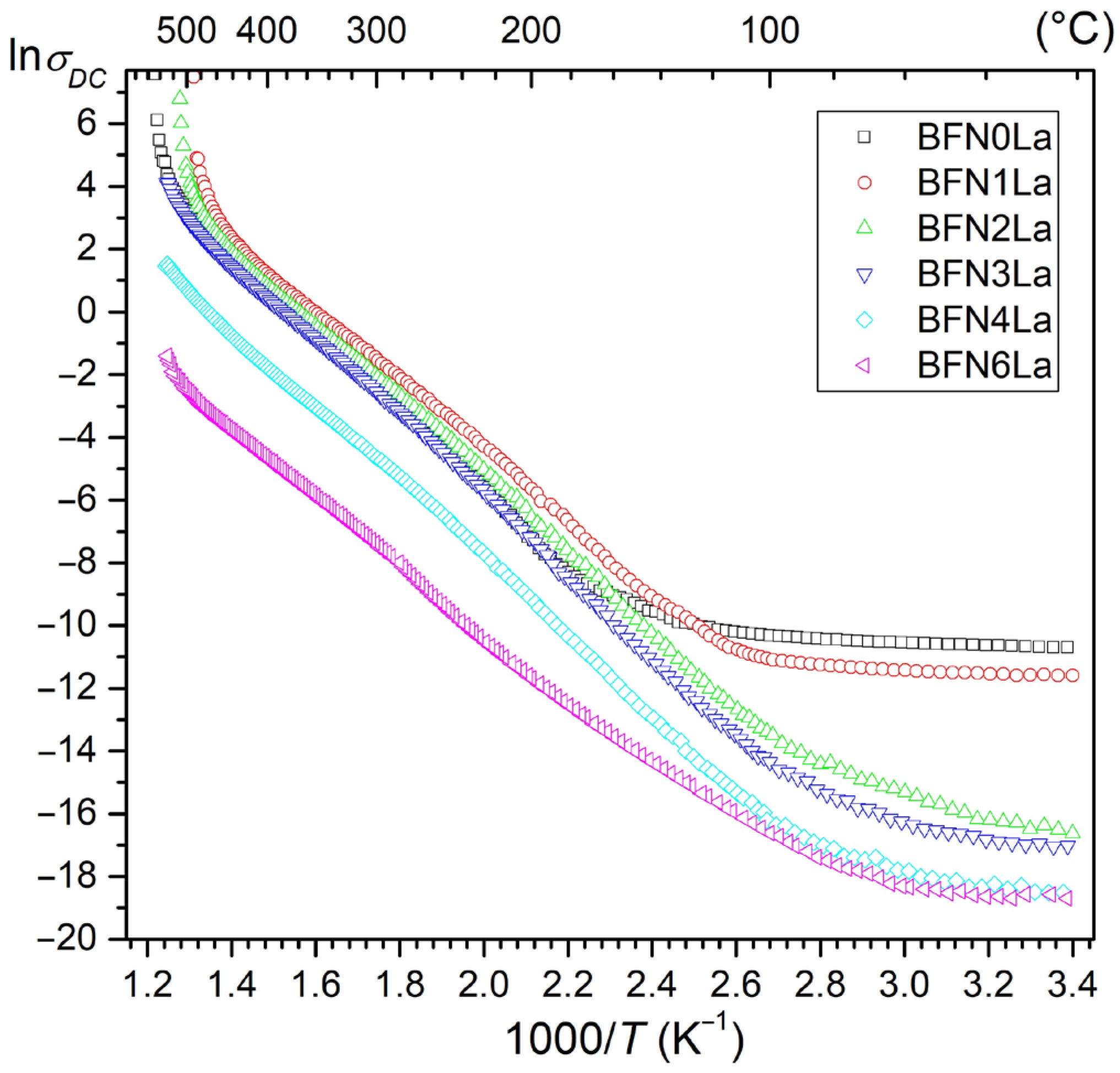
3.4. DC Electric Conductivity
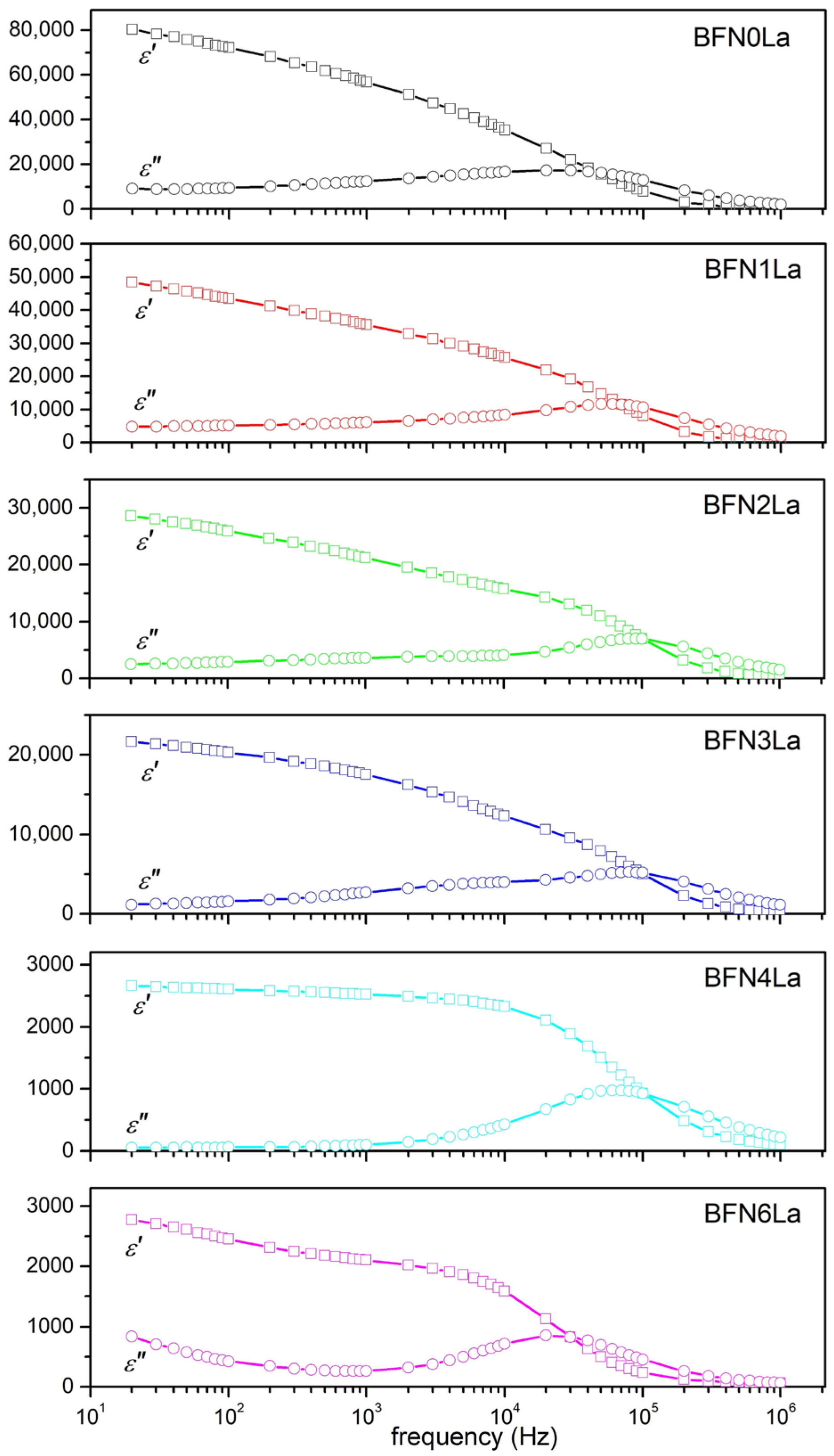
3.5. Dielectric Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmid, H. Some symmetry aspects of ferroics and single phase multiferroics. J. Phys. Condens. Matter. 2008, 20, 434201. [Google Scholar] [CrossRef]
- Boucher, E.; Guiffard, B.; Lebrun, L.; Guyomar, D. Effects of Zr/Ti ratio on structural, dielectric and piezoelectric properties of Mn and (Mn, F) doped lead zirconate titanate ceramics. Ceram. Int. 2006, 32, 479–485. [Google Scholar] [CrossRef]
- Surowiak, Z.; Bochenek, D. Multiferroic materials for sensors, transducers and memory devices. Arch. Acoust. 2008, 33, 243–260. Available online: https://acoustics.ippt.pan.pl/index.php/aa/article/view/531/pdf_237 (accessed on 22 July 2024).
- Scott, J.F. Applications of magnetoelectrics. J. Mater. Chem. 2012, 22, 4567–4574. [Google Scholar] [CrossRef]
- Khacheba, M.; Abdessalem, N.; Handi, A.; Khemakhem, H. Effect of acceptor and donor dopants (Na, Y) on the microstructure and dielectric characteristics of high Curie point PZT-modified ceramics. J. Mater. Sci. Mater. El. 2020, 31, 361–372. [Google Scholar] [CrossRef]
- Allamraju, K.V.; Srikanth, K. Modal analysis of PZT discs for uniaxial impact loaded energy harvesters. Mater. Today-Proc. 2017, 4, 2682–2686. [Google Scholar] [CrossRef]
- Dumitru, A.I.; Pintea, J.; Patroi, D.; Dumitru, T.-G.; Matekovits, L.; Peter, I. Investigation of multiferroic properties of Fe3+ and (La3+, Fe3+) doped PbZr0.53Ti0.47O3 ceramics. In Proceedings of the 2020 IEEE International Conference on Electrical Engineering and Photonics (EExPolytech), St. Petersburg, Russia, 15–16 October 2020. [Google Scholar] [CrossRef]
- Karapuzha, A.S.; James, N.K.; Khanbareh, H.; van der Zwaag, S.; Groen, W.A. Structure, dielectric and piezoelectric properties of donor doped PZT ceramics across the phase diagram. Ferroelectrics 2016, 504, 160–171. [Google Scholar] [CrossRef]
- Fiebig, M.; Lottermoser, T.; Meier, D.; Trassin, M. The evolution of multiferroics. Nat. Rev. Mater. 2016, 1, 16046. [Google Scholar] [CrossRef]
- Spaldin, N.A. Multiferroics: Past, present, and future. MRS Bull. 2017, 42, 385–390. [Google Scholar] [CrossRef]
- Wang, K.F.; Liu, J.-M.; Renc, Z.F. Multiferroicity: The coupling between magnetic and polarization orders. Adv. Phys. 2009, 58, 321–448. [Google Scholar] [CrossRef]
- Suchanicz, J.; Sitko, D.; Stanuch, K.; Swierczek, K.; Jagło, G.; Kruk, A.; Kluczewska-Chmielarz, K.; Konieczny, K.; Czaja, P.; Aleksandrowicz, J.; et al. Temperature and e-poling evolution of structural, vibrational, dielectric, and ferroelectric properties of Ba1−xSrxTiO3 ceramics (x = 0, 0.1, 0.2, 0.3, 0.4 and 0.45). Materials 2023, 16, 6316. [Google Scholar] [CrossRef] [PubMed]
- Suchanicz, J.; Kluczewska-Chmielarz, K.; Nowakowska-Malczyk, M.; Jagło, G.; Stachowski, G.; Kruzina, T.V. Thermal and electric field induced phase transitions of Na0.5Bi0.5TiO3 single crystals. J. Alloys Compd. 2022, 911, 165104. [Google Scholar] [CrossRef]
- Bochenek, D.; Osińska, K.; Mankiewicz, M.; Niemiec, P.; Dercz, G. Technology and dielectric properties of the KNLN doped with Nd3+ and Pr3+ ions. Arch. Metall. Mater. 2020, 65, 1183–1188. [Google Scholar] [CrossRef]
- Kocoń, N.; Dzik, J.; Szalbot, D.; Pikula, T.; Adamczyk-Habrajska, M.; Wodecka-Duś, B. Synthesis and dielectric properties of Nd doped Bi5Ti3FeO15 ceramics. Arch. Metall. Mater. 2021, 66, 359–365. [Google Scholar] [CrossRef]
- Czaja, P.; Szostak, E.; Hetmańczyk, J.; Zachariasz, P.; Majda, D.; Suchanicz, J.; Karolus, M.; Bochenek, D.; Osińska, K.; Jędryka, J.; et al. Thermal stability and non-linear optical and dielectric properties of lead-free K0.5Bi0.5TiO3 ceramics. Materials 2024, 17, 2089. [Google Scholar] [CrossRef] [PubMed]
- Dzik, J.; Pikula, T.; Szalbot, D.; Adamczyk-Habrajska, M.; Wodecka-Duś, B.; Panek, R. Microstructure, XRD and Mössbauer spectroscopy study of Gd doped BiFeO3. Process. Appl. Ceram. 2020, 14, 134–140. [Google Scholar] [CrossRef]
- Eitssayeam, S.; Intatha, U.; Pengpat, K.; Tunkasiri, T. Preparation and characterization of barium iron niobate (BaFe0.5Nb0.5O3) ceramics. Curr. Appl. Phys. 2006, 6, 316–318. [Google Scholar] [CrossRef]
- Mishra, A.; Khatua, D.K.; De, A.; Majumdar, B.; Frömling, T.; Ranjan, R. Structural mechanism behind piezoelectric enhancement in off-stoichiometric Na0.5Bi0.5TiO3 based lead-free piezoceramics. Acta Mater. 2019, 164, 761–775. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.G.; Xiao, M.; Xiao, D.Q.; Huang, T.; Wu, B.; Li, F.X.; Zhu, J.G. Phase transition and electrical properties of (1−x)K0.5Na0.5NbO3–xBi0.5Na0.5Zr0.8Ti0.2O3 lead-free piezoceramics. Ceram. Int. 2014, 40, 9165–9169. [Google Scholar] [CrossRef]
- Hadouch, Y.; Ben Moumen, S.; Mezzourh, H.; Mezzane, D.; Amjoud, M.; Asbani, B.; Razumnaya, A.G.; Gagou, Y.; Rožič, B.; Kutnjak, Z.; et al. Electrocaloric effect and high energy storage efficiency in lead-free Ba0.95Ca0.05Ti0.89Sn0.11O3 ceramic elaborated by sol–gel method. J. Mater. Sci. Mater. Electron. 2022, 33, 2067–2079. [Google Scholar] [CrossRef]
- Wei, J.; Xue, D.; Wu, C.; Li, Z. Enhanced ferromagnetic properties of multiferroic Bi1−xSrxMn0.2Fe0.8O3 synthesized by sol-gel process. J. Alloys Compd. 2008, 453, 20–23. [Google Scholar] [CrossRef]
- Chaiyo, N.; Muanghlua, R.; Niemcharoen, S.; Boonchom, B.; Vittayakorn, N. Solution combustion synthesis and characterization of lead-free piezoelectric sodium niobate (NaNbO3) powders. J. Alloys Compd. 2011, 509, 2445–2449. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Farooq, S. Enhancement of electrical resistivity of Sr0.5Ba0.5Fe12O19 nanomaterials by doping with lanthanum and nickel. Mater. Chem. Phys. 2009, 118, 308–313. [Google Scholar] [CrossRef]
- Jeon, J.-H. Mechanochemical synthesis and mechanochemical activation-assisted synthesis of alkaline niobate-based lead-free piezoceramic powders. Curr. Opin. Chem. Eng. 2014, 3, 30–35. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Kenzhina, I.E.; Zdorovets, M.V.; Saiymova, M.; Tishkevich, D.I.; Trukhanove, S.V.; Trukhanov, A.V. Synthesis, phase composition and structural and conductive properties of ferroelectric microparticles based on ATiOx (A = Ba, Ca, Sr). Ceram. Int. 2019, 45, 17236–17242. [Google Scholar] [CrossRef]
- Barick, B.K.; Mishra, K.K.; Arora, A.K.; Choudhary, R.N.P.; Pradhan, D.K. Impedance and Raman spectroscopic studies of (Na0.5Bi0.5)TiO3. J. Phys. D Appl. Phys. 2011, 44, 355402. [Google Scholar] [CrossRef]
- Bahanurdin, F.K.; Mohamed, J.J.; Ahmad, Z.A. Effect of Sintering temperature on structure and dielectric properties of lead free K0.5Na0.5NbO3 prepared via hot isostatic pressing. Mater. Sci. Forum 2017, 888, 42–46. [Google Scholar] [CrossRef]
- Maiwa, H. Piezoelectric properties of BaTiO3 ceramics prepared by hot isostatic pressing. J. Ceram. Soc. Jpn. 2013, 121, 655–658. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Thong, H.-C.; Cheng, Y.-Y.-S.; Li, J.-W.; Wang, K. Defect-mediated domain-wall motion and enhanced electric-field-induced strain in hot-pressed K0.5Na0.5NbO3 lead-free piezoelectric ceramics. J. Appl. Phys. 2021, 129, 024102. [Google Scholar] [CrossRef]
- Bijalwan, V.; Prajzler, V.; Erhart, J.; Tan, H.; Roupcová, P.; Sobola, D.; Tofel, P.; Maca, K. Rapid pressure-less and spark plasma sintering of (Ba0.85Ca0.15Zr0.1T0.9)O3 lead-free piezoelectric ceramics. J. Eur. Ceram. Soc. 2021, 41, 2514–2523. [Google Scholar] [CrossRef]
- Lan, J.-J.; Chen, X.-M.; Liu, L.-N.; Lian, H.-L.; He, Y.-R.; Song, Y.-C.; Zhu, L.-J.; Liu, P. Low-temperature synthesis of K0.5Na0.5NbO3 ceramics in a wide temperature window via cold-sintering assisted sintering method and enhanced electrical properties. J. Eur. Ceram. Soc. 2023, 43, 73–81. [Google Scholar] [CrossRef]
- Venkata Ramana, M.; Roopas Kiran, S.; Ramamanohar Reddy, N.; Siva Kumar, K.V.; Murthy, V.R.K.; Murty, B.S. Synthesis of lead free sodium bismuth titanate (NBT) ceramic by conventional and microwave sintering methods. J. Adv. Dielectr. 2011, 1, 71–77. [Google Scholar] [CrossRef]
- Saha, S.; Sinha, T.P. Structural and dielectric studies of BaFe0.5Nb0.5O3. J. Phys. Condens. Matter. 2002, 14, 249–258. [Google Scholar] [CrossRef]
- Hinatsu, Y.; Masaki, N.M. Magnetic susceptibilities and mossbauer spectra of perovskites A2FeNbO6 (A = Sr, Ba). J. Solid State Chem. 2000, 154, 591. [Google Scholar] [CrossRef]
- Intatha, U.; Eitssayeam, S.; Wang, J.; Tunkasiri, T. Impedance study of giant dielectric permittivity in BaFe0.5Nb0.5O3 perovskite ceramic. Curr. Appl. Phys. 2010, 10, 21. [Google Scholar] [CrossRef]
- Bochenek, D.; Surowiak, Z.; Poltierova-Vejpravova, J. Producing the lead-free BaFe0. 5Nb0. 5O3 ceramics with multiferroic properties. J. Alloys Compd. 2009, 487, 572–576. [Google Scholar] [CrossRef]
- Patel, P.K.; Rani, J.; Adhlakha, N.; Singh, H.; Yadav, K.L. Enhanced dielectric properties of doped barium titanate ceramics. J. Phys. Chem. Solids 2013, 4, 545. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.M.; Ni, L.; Liu, X.Q. Dielectric abnormities of complex perovskite Ba(Fe1∕2Nb1∕2)O3 ceramics over broad temperature and frequency range. Appl. Phys. Lett. 2007, 90, 022904. [Google Scholar] [CrossRef]
- Auromun, K.; Choudhary, R.N.P. Structural, dielectric and electrical characteristics of lead-free scandium modified barium iron niobate: Ba(Fe0.5−xScxNb0.5)O3. Phys. B 2020, 594, 412291. [Google Scholar] [CrossRef]
- Kantha, P.; Pisitpipathsin, N.; Pengpat, K.; Eitssayeam, S.; Rujijanagul, G.; Gua, R.; Bhalla, A.S. Effects of GeO2 addition on physical and electrical properties of BaFe0.5Nb0.5O3 ceramic. Mater. Res. Bull. 2012, 47, 2867. [Google Scholar] [CrossRef]
- Bochenek, D.; Niemiec, P.; Adamczyk, M. Lead-free BFN ceramics doped by chromium, lithium or manganese. Eur. Phys. J. B 2015, 88, 278. [Google Scholar] [CrossRef]
- Köferstein, R.; Oehler, F.; Ebbinghaus, S.G. Magnetic, optical, dielectric, and sintering properties of nano-crystalline BaFe0.5Nb0.5O3 synthesized by a polymerization method. J. Mater. Sci. 2017, 53, 1024–1034. [Google Scholar] [CrossRef]
- Bochenek, D.; Niemiec, P.; Szafraniak-Wiza, I.; Adamczyk, M.; Skulski, R. Preparation and dielectric properties of the lead-free BaFe1/2Nb1/2O3 ceramics obtained from mechanically triggered powder. Eur. Phys. J. B 2015, 88, 277. [Google Scholar] [CrossRef]
- Bochenek, D.; Bartkowska, J.A.; Kozielski, L.; Szafraniak-Wiza, I. Mechanochemical activation and spark plasma sintering of the lead-free Ba(Fe1/2Nb1/2)O3 ceramics. Materials 2021, 14, 2254. [Google Scholar] [CrossRef]
- Mahfoz Kotb, H.; Saber, O.; Ahmad, M.M. Colossal relative permittivity and low dielectric loss in BaFe0.5Nb0.5O3 ceramics prepared by spark plasma sintering. Results Phys. 2020, 19, 103607. [Google Scholar] [CrossRef]
- Charoenthai, N.; Traiphol, R.; Rujijanagul, G. Microwave synthesis of barium iron niobate and dielectric properties. Mater. Lett. 2008, 62, 4446–4448. [Google Scholar] [CrossRef]
- Kumar, R.; Narayan, A.; Pradhan, L.K.; Kar, M.; Singh, N.K. Structural, dielectric, impedance and conductivity studies of Ba(Fe0.5Nb0.5)O3 nanomaterial prepared by the mechanochemical method. Ferroelectrics 2018, 537, 198–213. [Google Scholar] [CrossRef]
- Intatha, U.; Eitssayeam, S.; Pengpat, K.; MacKenzie, K.J.; Tunkasiri, T. Dielectric properties of low temperature sintered LiF doped BaFe0.5Nb0.5O3. Mater. Lett. 2007, 61, 196–200. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Chang, Y.-S.; Chen, G.-J.; Chung, C.-C.; Huang, T.-W. Effects of bismuth doping on the dielectric properties of Ba(Fe0.5Nb0.5)O3 ceramic. Solid State Commun. 2008, 145, 212–217. [Google Scholar] [CrossRef]
- Negi, R.R.; Chandrasekhar, M.; Kumara, P.; Kar, J.P.; Prakash, C. Synthesis and characterizations of BT-BFN ceramics for capacitor applications. Ferroelectrics 2017, 517, 34–40. [Google Scholar] [CrossRef]
- Chen, K.; Yin, S.; Xue, D. Active La–Nb–O compounds for fast lithium-ion energy storage. Tungsten 2019, 1, 287–296. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Chang, Y.-H.; Chen, G.-J. Effects of lanthanum doping on the dielectric properties of BaFe0.5Nb0.5O3 ceramic. J. Appl. Phys. 2004, 96, 6624–6628. [Google Scholar] [CrossRef]
- Raj, A.; Gupta, P.; Choudhary, R.N.P. Structural and electrical properties of La3+ modified Ba(Fe0.5Nb0.5)O3 ceramics. J. Phys. Chem. Solids 2021, 148, 109676. [Google Scholar] [CrossRef]
- Shankar, S.; Thakur, O.P.; Jayasimhadri, M. Strong enhancement in structural, dielectric, impedance and magnetoelectric properties of NdMnO3-BaTiO3 multiferroic composites. Mat. Chem. Phys. 2021, 270, 124856. [Google Scholar] [CrossRef]
- Raymond, O.; Font, R.; Suárez-Almodovar, N.; Portelles, J.; Siqueiros, J.M. Frequency-temperature response of ferroelectromagnetic PbFe1/2Nb1/2O3 ceramics obtained by different precursors. Part I. Structural and thermo-electrical characterization. J. Appl. Phys. 2005, 97, 084107. [Google Scholar] [CrossRef]
- Wójcik, K.; Zieleniec, K.; Milata, M. Electrical properties of lead iron niobate PFN. Ferroelectrics 2003, 289, 107–120. [Google Scholar] [CrossRef]
- Shannigrahi, S.R.; Tay, F.E.H.; Yao, K.; Choudhary, R.N.P. Effect of rare earth (La, Nd, Sm, Eu, Gd, Dy, Er and Yb) ion substitutions on the microstructural and electrical properties of sol-gel grown PZT ceramics. J. Eur. Ceram. Soc. 2004, 24, 163–170. [Google Scholar] [CrossRef]
- Liu, J.; Duan, C.-G.; Mei, W.N.; Smith, R.W.; Hardy, J.R. Dielectric properties and Maxwell-Wagner relaxation of compounds ACu3Ti4O12 (A=Ca, Bi2/3, Y2/3, La2/3). J. Appl. Phys. 2005, 98, 093703. [Google Scholar] [CrossRef]
- Wang, C.C.; Lu, H.B.; Jin, K.J.; Yang, G.Z. Temperature-dependent dielectric strength of a Maxwell-Wagner type relaxation. Mod. Phys. Lett. B 2008, 22, 1297–1305. [Google Scholar] [CrossRef]
- Koops, C.G. On the dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. Phys. Rev. 1951, 83, 121–124. [Google Scholar] [CrossRef]
- Du, C.L.; Zhang, S.T.; Cheng, G.X.; Lu, M.H.; Gu, Z.B.; Wang, J.; Chen, Y.F. Composition-dependent structures and properties of Bi4Ti3xZrxO12 ceramics. Phys. B 2005, 368, 157–162. [Google Scholar] [CrossRef]
- Nayak, P.; Badapanda, T.; Singh, A.K.; Panigrahi, S. An approach for correlating the structural and electrical properties of Zr4+-modified SrBi4Ti4O15/SBT ceramic. RSC Adv. 2017, 7, 16319–16331. [Google Scholar] [CrossRef]
- Patel, P.K.; Yadav, K.L.; Singh, H.; Yadav, A.K. Origin of giant dielectric constant and magnetodielectric study in Ba(Fe0.5Nb0.5)O3 nanoceramics. J. Alloys Compd. 2014, 591, 224–229. [Google Scholar] [CrossRef]
- Rayssi, C.; Rhouma, F.I.H.; Dhahri, J.; Khirouni, K.; Zaidi, M.; Belmabrouk, H. Structural, electric and dielectric properties of Ca0.85Er0.1Ti1-xCo4x/3O3 (0 ≤ x ≤ 0.1). Appl. Phys. A Mater. Sci. Process. 2017, 123, 778. [Google Scholar] [CrossRef]
- Verma, K.; Kumar, A.; Varshney, D. Dielectric relaxation behavior of AxCo1−xFe2O4 (A = Zn, Mg) mixed ferrites. J. Alloys Compd. 2012, 526, 91–97. [Google Scholar] [CrossRef]
- Ganguly, M.; Parida, S.; Sinha, E.; Rout, S.K.; Simanshu, A.K.; Hussain, A.; Kim, I.W. Structural, dielectric and electrical properties of BaFe0.5Nb0.5O3 ceramic prepared by solid-state reaction technique. Mater. Chem. Phys. 2011, 131, 535–539. [Google Scholar] [CrossRef]
- Rayssi, C.; Kossi, S.E.; Dhahri, J.; Khirouni, K. Frequency and temperature-dependence of dielectric permittivity and electric modulus studies of the solid solution Ca0.85Er0.1Ti1-xCo4x/3O3 (0 ≤ x ≤ 0.1). RSC Adv. 2018, 8, 17139–17150. [Google Scholar] [CrossRef]
- Fang, B.; Shan, Y.; Imoto, H. Charge compensation mechanism decreases dielectric loss in manganese-doped Pb(Fe1/2Nb1/2)O3 ceramics. Jpn. J. Appl. Phys. 2004, 43, 2568. [Google Scholar] [CrossRef]


| Parameter | BFN0La | BFN1La | BFN2La | BFN3La | BFN4La | BFN6La |
|---|---|---|---|---|---|---|
| γ | 0.8367 | 0.8364 | 0.8360 | 0.8356 | 0.8355 | 0.8326 |
| ρ (Ωm) | 1.82 × 106 | 2.14 × 106 | 4.60 × 106 | 5.92 × 106 | 6.64 × 106 | 6.85 × 106 |
| (μm) | 5.67 | 4.96 | 4.48 | 2.42 | 1.77 | 1.67 |
| ε′ at RT | 56,750 | 35,550 | 21,226 | 17,550 | 2523 | 2104 |
| ε″ at RT | 12,485 | 6400 | 3609 | 2632 | 101 | 168 |
| tanδ at RT | 0.22 | 0.18 | 0.17 | 0.15 | 0.04 | 0.08 |
| Ea at I (eV) | 0.048 | 0.064 | 0.302 | 0.153 | 0.160 | 0.096 |
| Ea at II (eV) | 1.075 | 0.968 | 1.042 | 1.108 | 1.059 | 0.898 |
| Element | BFN0La | BFN1La | BFN2La | BFN3La | BFN4La | BFN6La | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TH | EX | TH | EX | TH | EX | TH | EX | TH | EX | TH | EX | |
| Ba | 52.88 | 52.61 | 52.35 | 52.25 | 51.82 | 51.24 | 51.28 | 51.02 | 50.75 | 51.11 | 49.69 | 49.46 |
| Fe | 10.75 | 10.40 | 10.75 | 10.57 | 10.75 | 10.53 | 10.75 | 10.64 | 10.75 | 10.32 | 10.75 | 10.54 |
| Nb | 17.88 | 18.75 | 17.89 | 18.25 | 17.88 | 18.56 | 17.88 | 18.13 | 17.88 | 17.95 | 17.88 | 18.01 |
| La | - | - | 0.53 | 0.62 | 1.07 | 1.29 | 1.61 | 1.8 | 2.14 | 2.23 | 3.21 | 3.66 |
| O | 18.48 | 18.24 | 18.48 | 18.31 | 18.48 | 18.38 | 18.48 | 18.41 | 18.48 | 18.39 | 18.47 | 18.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochenek, D.; Brzezińska, D.; Niemiec, P.; Kozielski, L. The Influence of Lanthanum Admixture on Microstructure and Electrophysical Properties of Lead-Free Barium Iron Niobate Ceramics. Materials 2024, 17, 3666. https://doi.org/10.3390/ma17153666
Bochenek D, Brzezińska D, Niemiec P, Kozielski L. The Influence of Lanthanum Admixture on Microstructure and Electrophysical Properties of Lead-Free Barium Iron Niobate Ceramics. Materials. 2024; 17(15):3666. https://doi.org/10.3390/ma17153666
Chicago/Turabian StyleBochenek, Dariusz, Dagmara Brzezińska, Przemysław Niemiec, and Lucjan Kozielski. 2024. "The Influence of Lanthanum Admixture on Microstructure and Electrophysical Properties of Lead-Free Barium Iron Niobate Ceramics" Materials 17, no. 15: 3666. https://doi.org/10.3390/ma17153666





