Middle Triassic Limestones as a Source of Trace Elements and REY
Abstract
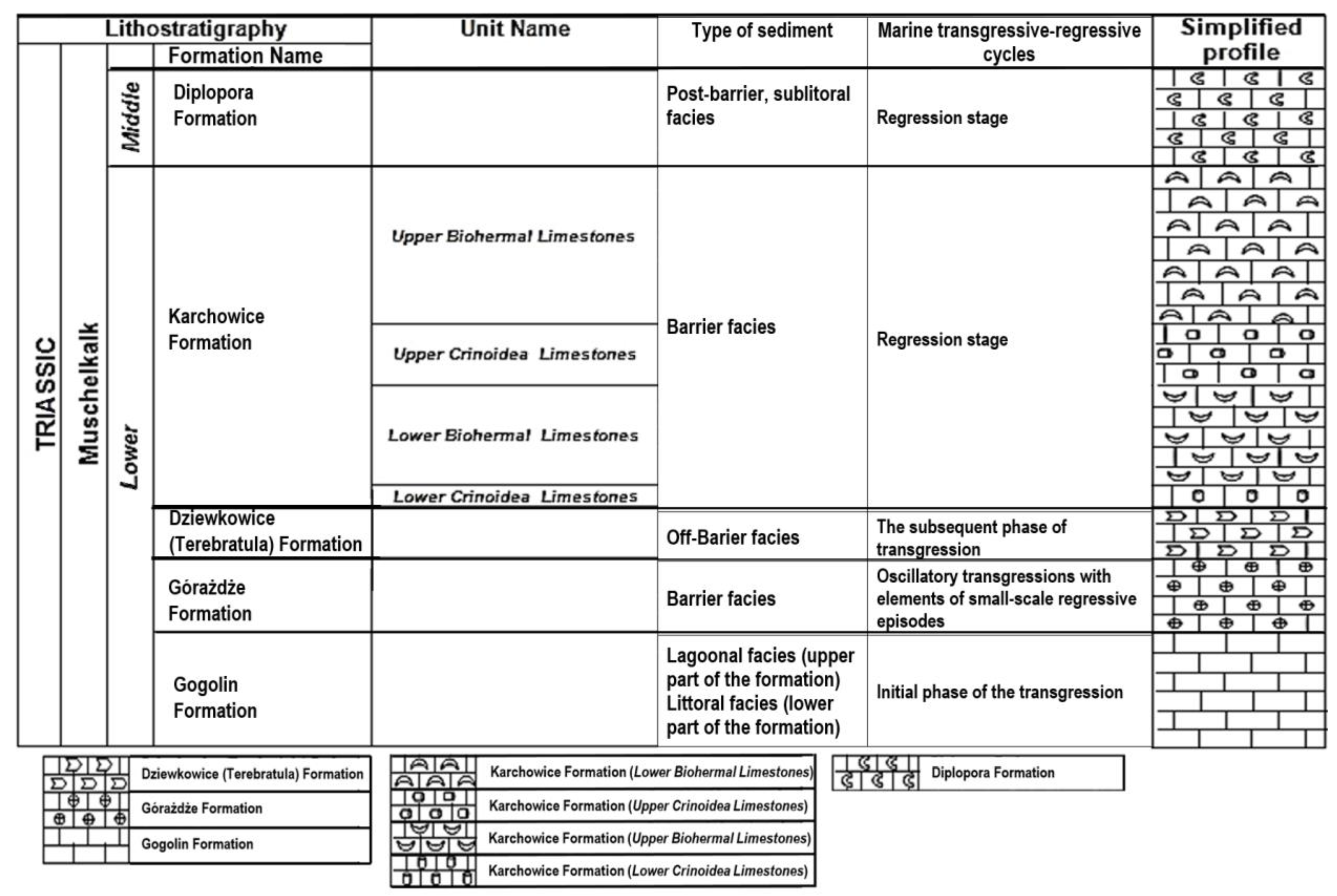
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
- Analysis of average chemical composition:
- –
- ICP-MS: The measurements were made by applying a ZSX Primus II Rigaku spectrometer, Rigaku, Tokyo, Japan, equipped with the 4 kW, 60 kV Rh anode and wavelength dispersion detection system.
- –
- Spectrometry X-ray fluorescence (XRF): The measurements were made using the wave-dispersive X-ray fluorescence spectrometer, ZSX PRIMUS RIGAKU, Rigaku, Tokyo, Japan, equipped with a rhodium X-ray tube with the possibility of smooth setting of the voltage of 20–60 kV, analytical crystals LiF, Ge and several synthetic crystals.
- Point analysis of chemical composition in a micro area:
- –
- Electron microprobe analysis (EPMA): The measurements were made using a JXA-8230 X-ray micro-analyzer manufactured by JOEL, Santa Monica, CA, USA. The examinations were performed on polished sections which were sputtered with a carbon coat. The analysis with the application of WDS spectrometers was carried out in micro-areas of two samples—G1 and LD11.
3. Results
3.1. ICP-MS
3.2. X-ray Fluorescence (XRF)
3.3. Electron Probe Microanalysis
3.3.1. Sample G1
3.3.2. Sample LD11
4. Discussion
5. Conclusions
- The results of the research showed that, in the examined limestones, an increased content of some analyzed elements was measured. Increased amounts were determined in the case of S, Sr, Ba, Cr, Ni, and Mo, and in some samples of Karchowice limestones and limestones from the contact zone with Paleogene-Neogene basalts. Lower contents were determined for Pb, Zn, and Cu and were the lowest for Nb, Cd, Zr, Y, Hf, and Rb.
- The increased content of trace elements was determined in the limestones from the Karchowice Beds. The amounts of trace elements determined in the rocks from the Gogolin Beds, from the Górażdże Beds, and from the Terebratula Beds are comparable. The higher amount of some trace elements determined in the limestones of the Karchowice Beds may be related to the low sea level during marine regressions. The increased contents of some trace elements determined in the limestones from the contact zone with basalts may be related to the influence of magma activity.
- Trace elements like Zn, Pb, Cu, Mo, and Ni are probably bound in sulfides. Ti and Cr are probably bound in oxides.
- Sulfur is an ingredient of Fe, Zn, Pb, and Cu sulfides or is bound in sulfates. P is a component of organic matter.
- Sr and Ba are common elements in some carbonate minerals. The presence of Sr and Ba indicates that primary calcium carbonate material included aragonite. Aragonite was transformed into low-magnesium calcite during the diagenetic processes; therefore, today Sr and Ba are present in calcite.
- Ni is bound in clay minerals or in sulfides. Cadmium is an ingredient of Zn sulfide. Zr can be delivered with fresh water to a basin where carbonate sedimentation takes place. It may also be bound in non-carbonate autogenic minerals. Hf usually coexists with Zr. Nb can occur as an admixture in the Ti and Zr minerals or Mn concretions.
- Chromium is dispersed in rock-forming minerals. Mo occurs in clay minerals. In content up to 0.1%, it may coexist with Mn concretions that are situated in the deep zones of the sea. Rb coexists with K in feldspars or feldspathoids. Small amounts of Rb could be found in seawater.
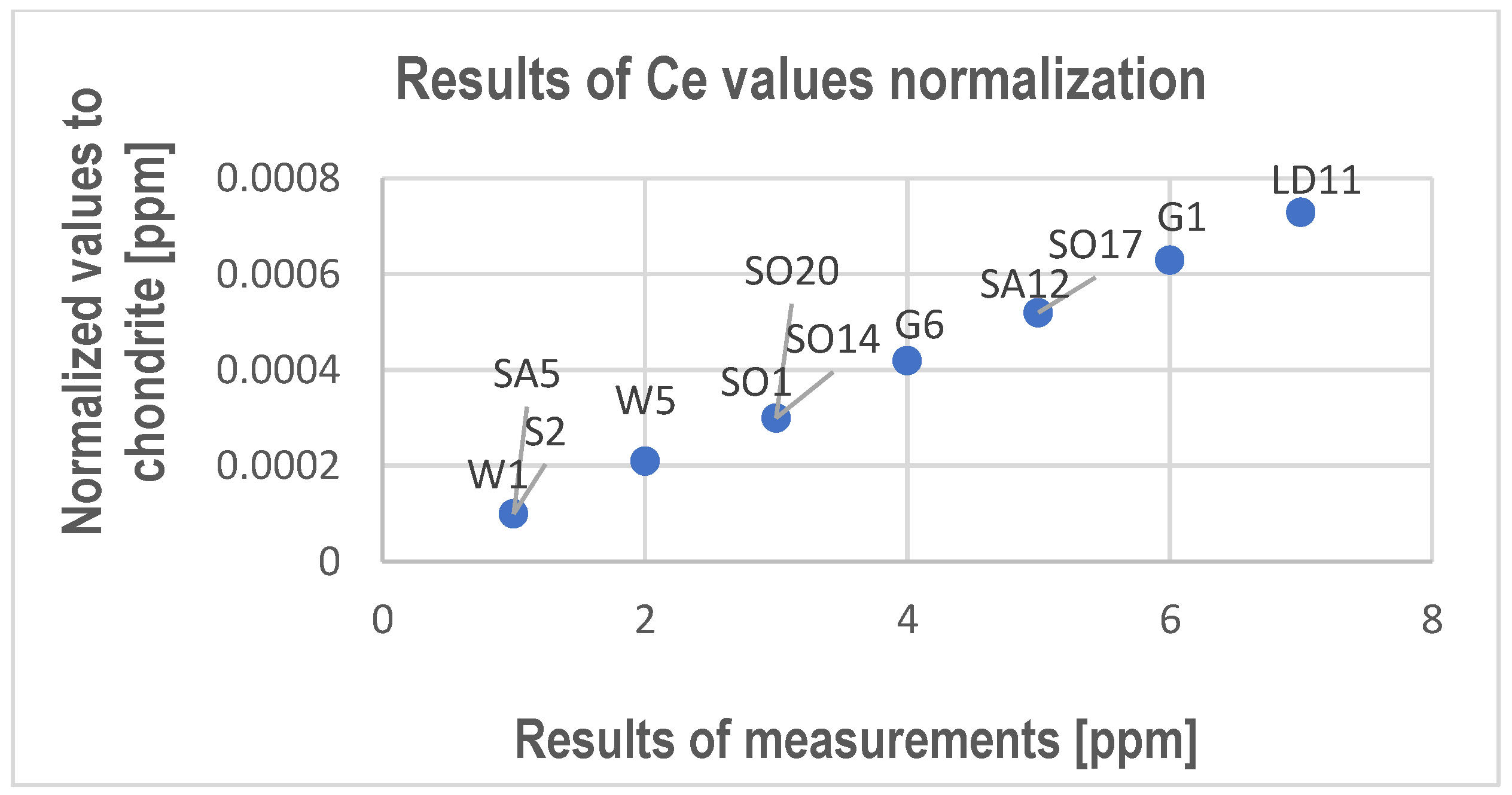
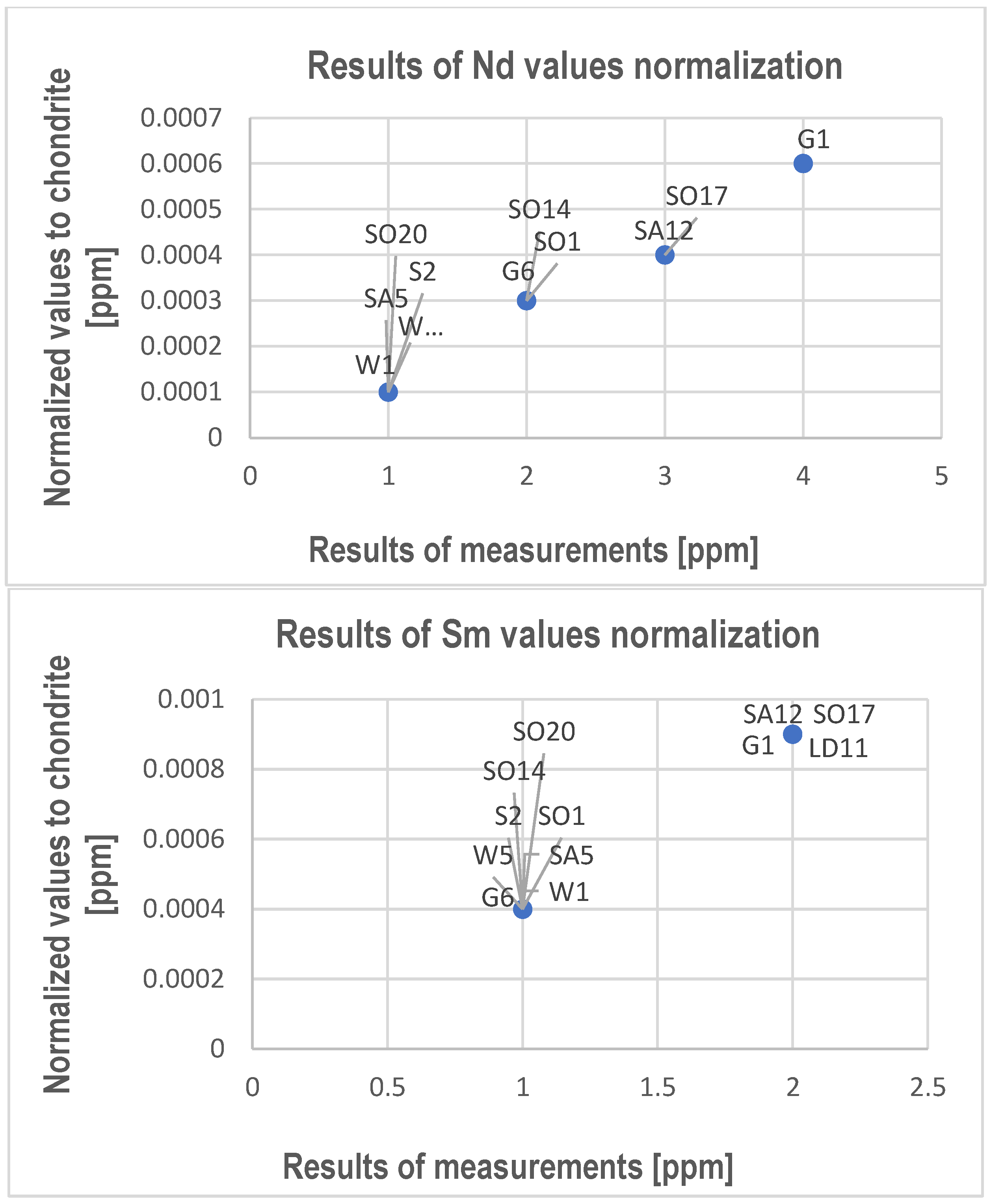
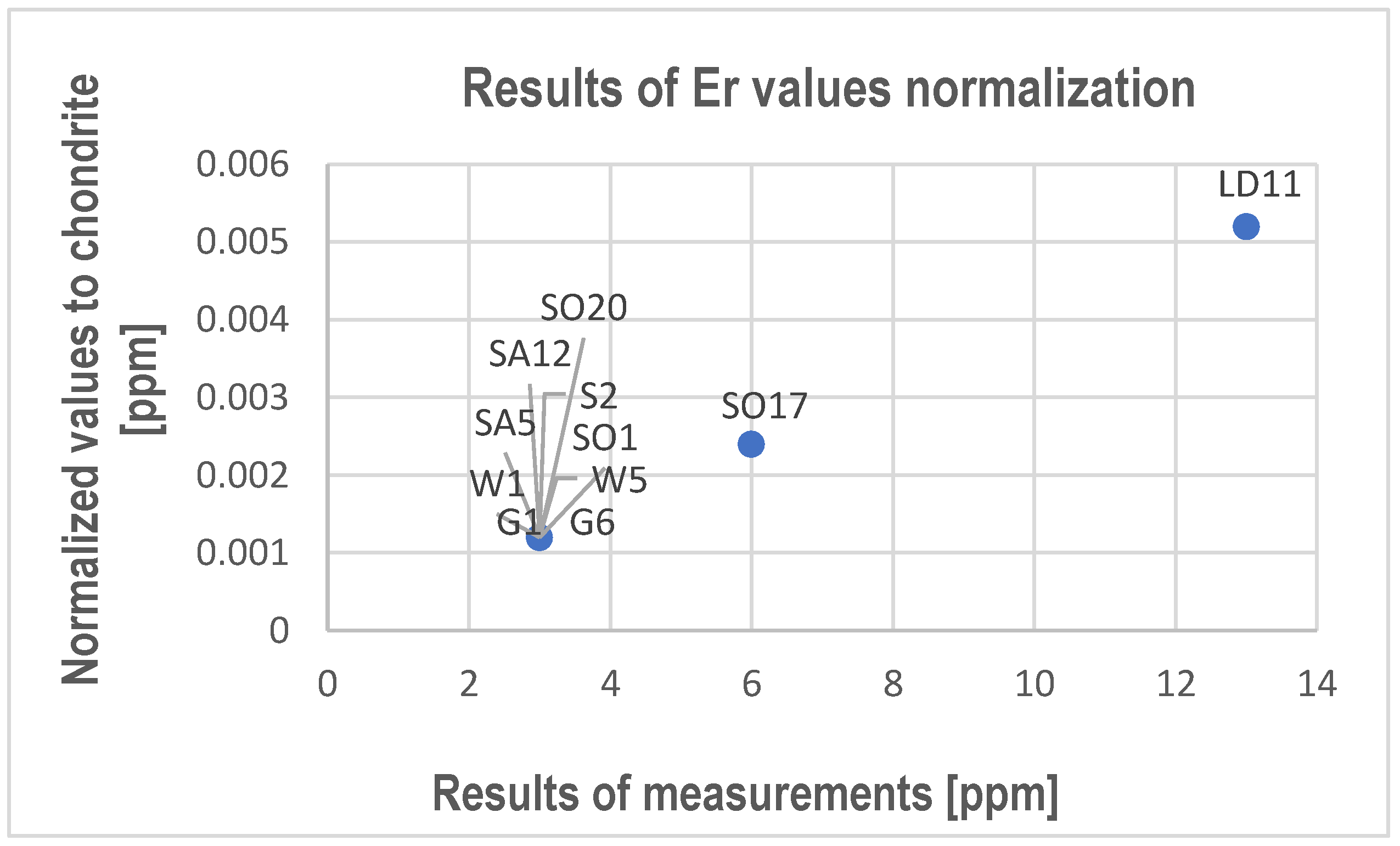
- REY and Sc substitute Ca usually in calcite and sometimes in dolomite. The tests allowed us to determine only Ce, Nd, Sm, Dy, Ga, Er, Y, and Sc.
- Though they have been determined in the studied limestones, with even higher values measured in points using electron probe microanalysis, the average content measured in rock samples is too low to be possible to recover these elements from limestone, and the products of the processing of these rocks do not have a harmful impact on the environment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pozzi, M.; Stanienda, K. The influence of the contents of magnesium of the Triassic limestones from ‘Tarnów Opolski’ Deposit on their sorption properties in desulphurization technology. Scientific notebooks of the Silesian University of Technology (Zeszyty Naukowe Politechniki Śląskiej). Ser. Min. 2000, 246, 427–437. [Google Scholar]
- Stanienda, K. Carbonates in the Triassic rocks from the area of Tarnów Opolski. Miner. Resour. Manag. 2006, 22, 243–251. [Google Scholar]
- Stanienda, K. Effects of Dolomitization Processes in the Triassic Limestone of ‘Tarnów Opolski’ Deposit; Silesian University of Technology Press: Gliwice, Poland, 2011. [Google Scholar]
- Stanienda, K. Diagenesis of the Triassic Limestone from the Opole Silesia in the Aspect of Magnesian Calcite Presence; Silesian University of Technology Press: Gliwice, Poland, 2013. [Google Scholar]
- Stanienda, K. Possibility of Huntite presence in the Triassic limestones of Opole Silesia. Miner. Resour. Manag. 2013, 29, 79–98. [Google Scholar] [CrossRef]
- Stanienda, K. Strontium and barium in the Triassic limestone of the Opole Silesia deposits. Arch. Min. Sci. 2016, 61, 29–45. [Google Scholar] [CrossRef][Green Version]
- Stanienda, K. Carbonate phases rich in magnesium in the Triassic limestones of the eastern part of the Germanic Basin. Carbonates Evaporites 2016, 31, 387–405. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K. Magnesium calcite in Muschelkalk limestones of the Polish part of the Germanic Basin. Carbonates Evaporites 2018, 33, 801–821. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K. Selected trace elements in the triassic limestones of the Opole Silesia in Poland. In Proceedings of the 19th Conference with International Participation Waste Management—GzO’19 Urban Mining and 14th Conference with International Participation 46th Jump over the Leather Skin, Ljubljana, Slovenia, 12–13 April 2019. [Google Scholar]
- Banner, J.L. Application of the trace element and isotope geochemistry of strontium to studies of carbonate diagenesis. Sedimentology 1995, 42, 805–824. [Google Scholar] [CrossRef]
- Barrat, J.A.; Boulégue, J.; Tiercelin, J.J.; Lesourd, M. Strontium isotopes and rare-earth element geochemistry of hydrothermal carbonate deposits from Lake Tanganyika, East Africa. Geochim. Cosmochim. Acta 2000, 64, 287–298. [Google Scholar] [CrossRef]
- Bouazza, N.; Mrihi, A.; Maâte, A. Geochemical assessment of limestone for cement manufacturing. Procedia Technol. 2016, 22, 211–218. [Google Scholar] [CrossRef][Green Version]
- Elzinga, E.J.; Reeder, R.J.; Withers, S.H.; Peale, R.E.; Mason, R.A.; Beck, K.M.; Hess, W.P. EXAFS study of rare-earth element coordination in calcite. Geochim. Cosmochim. Acta 2002, 66, 2875–2885. [Google Scholar] [CrossRef]
- Hua, G.; Yuansheng, D.; Lian, Z.; Jianghai, Y.; Hu, H. Trace and rare earth elemental geochemistry of carbonate succession in the Middle Gaoyuzhuang Formation, Pingquan Section: Implications for Early Mesoproterozoic ocean redox conditions. J. Palaeogeogr. 2013, 2, 209–221. [Google Scholar] [CrossRef]
- Jiang, L.; Cai, C.; Worden, R.H.; Li, K.; Xiang, L.; Chu, X.; Shen, A.; Li, W. Rare earth element and yttrium (REY) geochemistry in carbonate reservoirs during deep burial diagenesis: Implications for REY mobility during thermochemical sulfate reduction. Chem. Geol. 2015, 415, 87–101. [Google Scholar] [CrossRef]
- Mori, Y. X-ray fluorescence analysis of major and trace elements in carbonate rocks using glass bead samples. Bull. Kitakyushu Mus. Nat. Hist. Hum. Hist. Ser. A (Nat. Hist.) 2007, 5, 1–12. [Google Scholar]
- Ostrom, M.E. Trace Elements in Illinois Pennsylvanian Limestones; Urbana Publisher: Champaign, IL, USA, 1957; Publication Link: Circular 243. [Google Scholar]
- Tanaka, K.; Miura, N.; Asahara, Y.; Kawabe, I. Rare earth element and strontium isotopic study of seamount-type limestones in Mesozoic accretionary complex of Southern Chichibu Terrane, central Japan: Implication for incorporation process of seawater REE into limestones. Geochem. J. 2003, 37, 163–180. [Google Scholar] [CrossRef]
- Boggs, S., Jr. Petrology of Sedimentary Rocks, 2nd ed.; Cambridge University Press: London, UK, 2010. [Google Scholar]
- Mackenzie, F.T.; Andersson, A.J. The Marine carbon system and ocean acidification during Phanerozoic Time. Geochem. Perspect. 2013, 2, 225. [Google Scholar] [CrossRef]
- Morse, J.W.; Mackenzie, F.T. Geochemistry of Sedimentary Carbonates; Elsevier: Amsterdam, The Netherlands, 1990; Volume 33, p. 707. [Google Scholar]
- Polański, A. Fundamentals of Geochemistry (Podstawy Geochemii); Geological Publishers (Wydawnictwa Geologiczne): Warszawa, Poland, 1988. [Google Scholar]
- Cherniak, D.J. REE diffusion in calcite. Earth Planet. Sci. Lett. 1998, 160, 273–287. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, Y.; Shimizu, H. Determination of the host phase of rare earth elements in natural carbonate using X-ray absorption near-edge structure. Geochem. J. 2009, 43, 143–149. [Google Scholar] [CrossRef]
- Szulc, J. Middle Triassic evolution of the Northern Peri-Tethys area as influenced by early opening of the Tethys Ocean. Ann. Soc. Geol. Pol. 2000, 70, 1–48. [Google Scholar]
- Gałkiewicz, T. A theory of orthohydrothermal origin oj the Silesian-Cracovian zinc-lead deposits. Ann. Soc. Gelogique Pol. 1971, XLI, 565–570. [Google Scholar]
- Pieczonka, J. Polymetallic mineralization in Triassic strata of the NW part of the Kraków-Częstochowa Monocline. Mineralogia 2010, 41, 35–53. [Google Scholar] [CrossRef]
- Niedźwiedzki, R. Lithostratigraphy of the Górażdże and the Dziewkowice Formations in Opole Silesia. Prace Geol. Mineral. Uniw. Wroc. 2000, 71, 1–72. [Google Scholar]
- Bodzioch, A. Biogeochemical Diagenesis of the Lower Muschelkalk of Opole Region; UAM Scientific Publishing House: Poznań, Poland, 2005. [Google Scholar]
- Szulc, J.; Beker, A. International Workshop on the Triassic of Southern Poland; Field Trip Guide, Polish Geological Society, Polish Geological Institute, Institute of Geological Sciences, Jagiellonian University: Cracow, Poland, 2007. [Google Scholar]
- McLennan, S.M. Relationship between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosyst. 2001, 2, 1525–2027. [Google Scholar] [CrossRef]
- Zhang, K.; Shields, G.A. Sedimentary Ce anomalies: Secular change and implications for paleoenvironmental evolution. Earth-Sci. Rev. 2022, 229, 104015. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution. Geol. Mag. 1985, 122, 673–674. [Google Scholar] [CrossRef]
- McLennan, S.M.; Xiao, G. Composition of the upper continental crust revisited: Insights from sedimentary rocks. Mineral. Mag. 1998, 62, 983–984. [Google Scholar] [CrossRef]
- Stanienda, K. Mineral phases in carbonate rocks of the Gogolin Beds from the area of Opole Silesia. Miner. Resour. Manag. 2014, 30, 17–42. [Google Scholar] [CrossRef]
- Parker, R. Composition of the Earth’s Crust. Chapter D in the “Data of Geochemistry”; PAPER 440-D; United States Government Printing Office: Washington, DC, USA, 1967. Available online: https://pubs.usgs.gov/pp/0440d/report.pdf (accessed on 10 January 2024).
- McDonough, W.F.; Sun, S. The composition of Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Haldar, S.K. Introduction to Mineralogy and Petrology; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128205853. [Google Scholar]
- Kim, H.S.; Park, J. Effects of limestone on the dissolution of phosphate from sediments under anaerobic condition. Environ. Technol. 2008, 29, 375–380. [Google Scholar] [CrossRef]
- Morse, J.W.; Andersson, A.J.; Mackenzie, F.T. Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and ‘‘ocean acidification’’: Role of high Mg-calcites. Geochim. Cosmochim. Acta 2006, 70, 5814–5830. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K. (Silesian University of Technology, Gliwice, Poland). Influence of Paleogene and Neogene basalt volcanism on changes in geochemical properties and isotopic composition of carbonate rocks from the contact zone of Triassic limestones and basalts from the area of Saint Anne Mountains. 2022; unpublished. [Google Scholar]
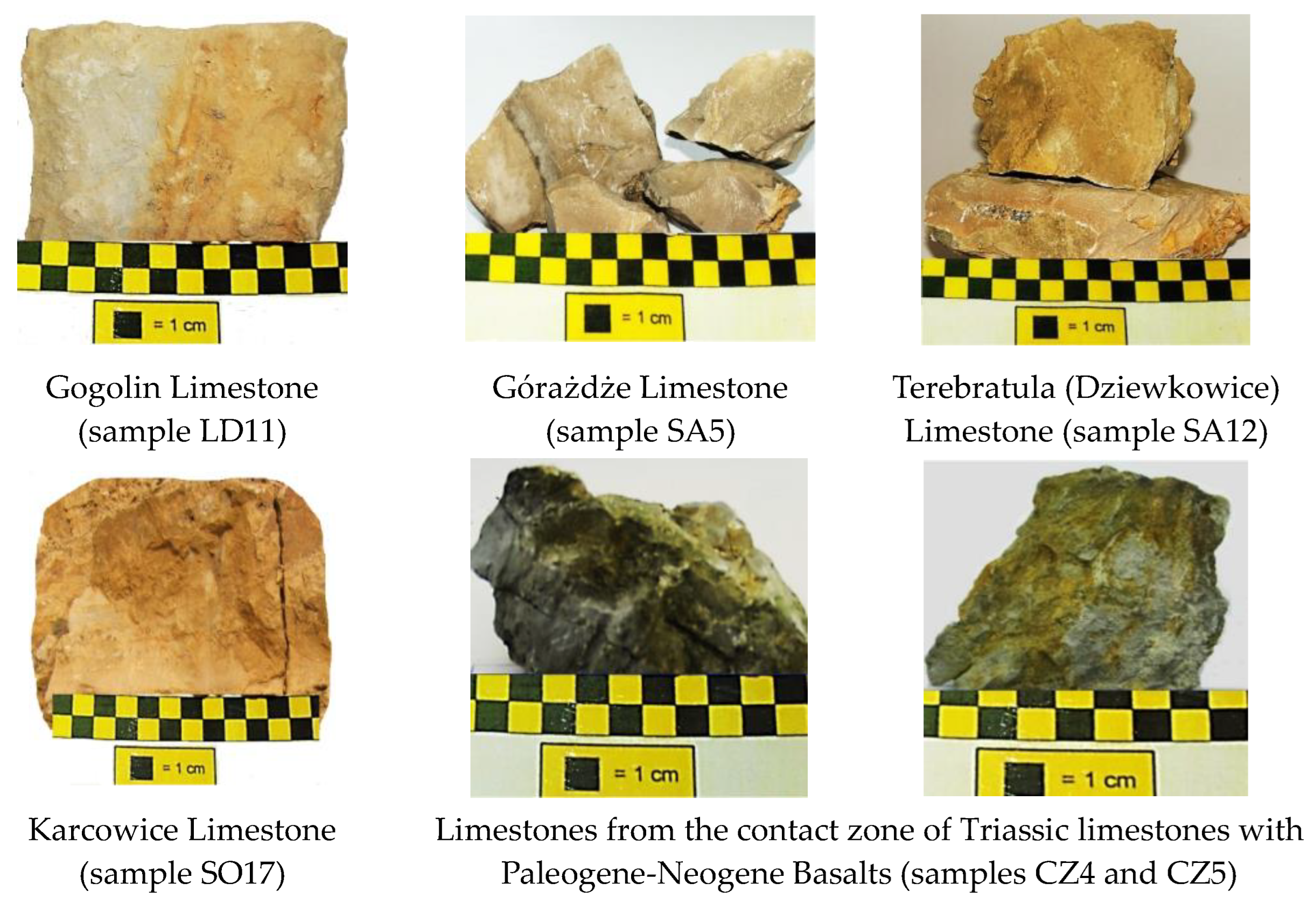
 —areas of sampling.
—areas of sampling.
 —areas of sampling.
—areas of sampling.

| Points Marked on the Map in Figure 1 | Location Name | Geographical Coordinates (GPS Data) |
|---|---|---|
| 3 | Szymiszów | 50°32′11″ N 18°13′35″ E |
| 4 | Saint Anne Mountain (amphitheater) | 50°27′18″ N 18°9′38″ E |
| 4 | Saint Anne Mountain (nephelinite quarry) | 50°27′12″ N 18°9′59″ E |
| 4 | Wysoka (near Saint Anne Mountain) | 50°28′32″ N 18°10′12″ E |
| 5 | Ligota Dolna and Kamienna | 50°29′14″ N 18°7′27″ E |
| 12 | Strzelce Opolskie | 50°31′53″ N 18°18′41″ E |
| 13 | Gogolin | 50°30′10″ N 18°1′59″ E |
| No. | Element | ICP-MS Method | XRF Method | ||
|---|---|---|---|---|---|
| LOD [ppm] | LOQ [ppm] | LOD [ppm] | LOQ [ppm] | ||
| 1 | Ti | 3 | 4 | 19 | 22 |
| 2 | Cr | 3 | 4 | 10 | 12 |
| 3 | Ni | 15 | 16 | 6 | 8 |
| 4 | Cu | 10 | 12 | 5 | 6 |
| 5 | Zn | 15 | 16 | 5 | 6 |
| 6 | Rb | 1 | 4 | 3 | 4 |
| 7 | Sr | 3 | 4 | 4 | 5 |
| 8 | Zr | 1 | 2 | 19 | 21 |
| 9 | Nb | 1 | 2 | 7 | 9 |
| 10 | Mo | 3 | 4 | 6 | 8 |
| 11 | Cd | 1 | 2 | - | - |
| 12 | Ba | 3 | 4 | - | - |
| 13 | Hf | 1 | 2 | - | - |
| 14 | Pb | 3 | 4 | 8 | 9 |
| 15 | Ce | 1 | 2 | - | - |
| 16 | Nd | 1 | 2 | - | - |
| 17 | Sm | 1 | 2 | - | - |
| 18 | Dy | 1 | 2 | - | - |
| 19 | Y | 1 | 2 | 3 | 4 |
| 20 | Sc | 1 | 2 | - | - |
| 21 | Er | 1 | 2 | 3 | 4 |
| No. | Element | Sample Numbers (Element Content in ppm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G6 | LD11 | W1 | W5 | SA5 | SA12 | S2 | SO1 | SO14 | SO17 | SO20 | ||
| 1 | Ti | 250 | 100 | 330 | 90 | 110 | 100 | 270 | 100 | 110 | 190 | 380 | 1700 |
| 2 | Cr | 320 | 260 | 200 | 260 | 460 | 210 | 1200 | 1300 | 260 | 210 | 220 | 9400 |
| 3 | Ni | 350 | 340 | 330 | 380 | 750 | 340 | 310 | 1600 | 400 | 350 | 360 | 18,000 |
| 4 | Cu | <10 | <10 | 290 | <10 | 30 | <10 | 10 | 10 | 10 | 30 | 60 | 60 |
| 5 | Zn | 20 | 110 | 30 | 50 | 70 | 110 | 30 | 70 | 20 | 40 | 50 | 240 |
| 6 | Rb | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| 7 | Sr | 260 | 120 | 450 | 240 | 370 | 240 | 32 | 230 | 360 | 210 | 540 | 160 |
| 8 | Zr | 20 | 10 | 10 | 10 | 20 | <1 | 10 | 30 | <10 | <10 | 20 | 10 |
| 9 | Nb | 40 | <1 | <1 | <1 | <1 | <1 | 10 | 10 | 10 | 10 | 10 | 40 |
| 10 | Mo | 120 | 120 | 120 | 130 | 210 | 140 | 120 | 1100 | 220 | 170 | 160 | 9000 |
| 11 | Cd | <1 | <1 | 1 | 1 | 1 | <1 | <1 | 2 | <1 | <1 | <1 | 120 |
| 12 | Ba | 24 | 15 | 29 | 10 | 20 | 16 | 28 | 260 | 20 | 20 | 34 | 35 |
| 13 | Hf | 2 | <1 | <1 | 2 | 2 | 1 | <1 | 1 | <1 | <1 | 1 | <1 |
| 14 | Pb | 25 | 9 | 79 | 24 | 39 | 20 | 27 | 22 | 10 | 20 | 20 | 62 |
| Element | Sample Numbers (Element Content in ppm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G6 | LD11 | W1 | W5 | SA5 | SA12 | S2 | SO1 | SO14 | SO17 | SO20 | |
| Ce | 6 | 4 | 7 | 1 | 2 | <1 | 5 | 1 | 3 | 3 | 5 | 3 |
| Nd | 4 | 2 | 4 | 1 | 1 | <1 | 3 | <1 | 2 | 2 | 3 | 1 |
| Sm | 2 | 1 | 2 | 1 | 1 | <1 | 2 | <1 | <1 | <1 | 2 | <1 |
| Dy | 1 | <1 | 1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Ʃ REE | ~14 | ~7 | ~14 | ~3 | ~4 | <1 | ~10 | ~1 | ~5 | ~5 | ~10 | ~4 |
| Y | 2 | 2 | 2 | 1 | 1 | <1 | 2 | 1 | 3 | 3 | 3 | 2 |
| Sc | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | 10 | 10 | <10 | <10 |
| No. | Element | Element Content in ppm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G6 | LD11 | W1 | W5 | SA5 | SA12 | S2 | SO1 | SO14 | SO17 | SO20 | ||
| 1 | P | 48 | 41 | 73 | 39 | 56 | 38 | 60 | 93 | 54 | 201 | 331 | 150 |
| 2 | Ti | 114 | 25 | 235 | 31 | 60 | 24 | 173 | 44 | 65 | 155 | 293 | 87 |
| 3 | Cr | 320 | 260 | 200 | 260 | 460 | 210 | 13 | 20 | 15 | 9076 | 40 | 14 |
| 4 | Ni | 20 | 17 | 17 | 22 | 20 | 23 | 20 | 23 | 21 | 1304 | 27 | 24 |
| 5 | Cu | 23 | 21 | 20 | 23 | 19 | 17 | 20 | 17 | 21 | 731 | 25 | 21 |
| 6 | Zn | 16 | 15 | 25 | 44 | 50 | 90 | 10 | 54 | 19 | 66 | 47 | 44 |
| 7 | Rb | 4 | <3 | 7 | <3 | <3 | <3 | 6 | <3 | <3 | <3 | 5 | 5 |
| 8 | Sr | 436 | 185 | 676 | 395 | 517 | 471 | 533 | 234 | 504 | 651 | 675 | 230 |
| 9 | S | 473 | 235 | 462 | 221 | 179 | 436 | 280 | 294 | 292 | 1854 | 564 | 328 |
| 10 | Y | 5 | 4 | 2 | 3 | 3 | 1 | 4 | 1 | 2 | 9 | 3 | 1 |
| 11 | Zr | <3 | 3 | <3 | 5 | 5 | <3 | - | - | - | - | - | - |
| 12 | Nb | - | - | - | - | - | - | <7 | <7 | <7 | 13 | <7 | <7 |
| 13 | Pb | <8 | <8 | 58 | <8 | 33 | 22 | <8 | 18 | <8 | <8 | 19 | 47 |
| 14 | Mo | - | - | - | - | - | - | <6 | <6 | <6 | 343 | <6 | 0 |
| 15 | Er | <3 | <3 | 13 | <3 | <3 | <3 | <3 | <3 | <3 | <3 | 6 | 3 |
| No. | Element | Element Content in ppm | ||||
|---|---|---|---|---|---|---|
| CZ1 | CZ2 | CZ3 | CZ4 | CZ5 | ||
| 1 | P | 291 | 194 | 1024 | 72 | 1182 |
| 2 | Ti | 357 | 295 | 3056 | 71 | 404 |
| 3 | Cr | 39 | <10 | 78 | <10 | 211 |
| 4 | Ni | 30 | 33 | 56 | 21 | 39 |
| 5 | Cu | 50 | 38 | 40 | 47 | 35 |
| 6 | Zn | 213 | 102 | 167 | 51 | 416 |
| 7 | Rb | 5 | <3 | <3 | <3 | <3 |
| 8 | Sr | 250 | 245 | 108 | 203 | 228 |
| 9 | Ba | <65 | <65 | 114 | 192 | 197 |
| 10 | S | 337 | 241 | 220 | 146 | 224 |
| 11 | Y | <3 | <3 | 9 | <3 | 5 |
| 12 | Zr | 29 | 20 | 41 | <3 | 25 |
| 13 | Nb | <7 | <7 | 18 | <7 | <7 |
| 14 | Pb | 103 | 29 | 29 | 81 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanienda-Pilecki, K.; Nowińska, K.; Nowrot, A.; Szewczenko, J. Middle Triassic Limestones as a Source of Trace Elements and REY. Materials 2024, 17, 3668. https://doi.org/10.3390/ma17153668
Stanienda-Pilecki K, Nowińska K, Nowrot A, Szewczenko J. Middle Triassic Limestones as a Source of Trace Elements and REY. Materials. 2024; 17(15):3668. https://doi.org/10.3390/ma17153668
Chicago/Turabian StyleStanienda-Pilecki, Katarzyna, Katarzyna Nowińska, Andrzej Nowrot, and Janusz Szewczenko. 2024. "Middle Triassic Limestones as a Source of Trace Elements and REY" Materials 17, no. 15: 3668. https://doi.org/10.3390/ma17153668
APA StyleStanienda-Pilecki, K., Nowińska, K., Nowrot, A., & Szewczenko, J. (2024). Middle Triassic Limestones as a Source of Trace Elements and REY. Materials, 17(15), 3668. https://doi.org/10.3390/ma17153668










