1. Introduction
Autogenous bone has the highest bone regenerative capacity among bone replacement materials for treating bone defects [
1]. Autogenous bone contains many components important for bone formation, such as calcium phosphate, collagen, bone morphogenetic proteins (BMPs), and cells associated with bone formation. However, the amount of autogenous bone that can be harvested is limited, so artificial bone made of calcium phosphate is widely used as an alternative material because it has inorganic components and a crystal structure similar to natural bone [
2,
3]. However, the ability of this material to regenerate bone is inferior to that of autogenous bone. Also, in dental clinical practice, granule-type artificial bone with a particle diameter of several hundred µm is used. The resorption of artificial bone to replace natural bone occurs more rapidly with fine particles than with larger granules, resulting in the faster healing of bone defects. However, fine particles induce inflammation [
4]. In addition, when they enter blood vessels, they may move into the bloodstream and accumulate in the body’s organs. Thus, granular artificial bones currently on the market have a particle diameter of several hundred µm to several mm because they have been developed based on products that have obtained regulatory approval.
Nonetheless, new medical technology cannot advance by merely following predecessors. Different approaches from existing products are required to develop materials with novel functions. For this purpose, a collaboration with different fields is essential. Therefore, we used a strategy to develop bioabsorbable polymers that adhere to bone based on basic research on tooth adhesion. Here, we describe a novel bone replacement material with bone-adhesive properties and enhanced resorbability in the body.
2. Current Artificial Bone
There are various types of artificial bones made of calcium phosphate, such as granules, blocks, and hardened pastes. Granular artificial bones are the most common in dental clinical practice. Block-type artificial bone is intended to maintain its shape but could be slow to be absorbed in the body. Hardened pastes should be slightly resorbed because areas in contact with blood are insufficiently hardened, but the hardened body is basically nonabsorbable. Initially, non-absorbable hydroxyapatite was used for granular artificial bone. However, as the demand for resorption and bone replacement increased for artificial bone, β-tricalcium·phosphate (β-TCP) granules were introduced into clinical practice as an absorbable artificial bone. Subsequently, artificial bones that consisted mainly of apatite carbonate—the same inorganic component of natural bone [
5]—and octacalcium phosphate (OCP), a precursor of apatite carbonate [
6,
7], were developed.
2.1. Hydroxyapatite
Hydroxyapatite (Ca
10(PO
4)
6(OH)) has a composition similar to that of bone. Therefore, it is highly biocompatible and can adhere directly to bone tissue during bone formation when used as a bone replacement material [
8]. Nevertheless, simply synthesized hydroxyapatite does not contain trace elements such as Na
+ and Mg
2+, which are found in real bone. Over the past few decades, many researchers have demonstrated that adding various ionic substituents to synthetic hydroxyapatite can produce a mineral composition similar to that of natural bone tissue [
9,
10,
11,
12]. Furthermore, synthetic hydroxyapatite cannot reproduce the porosity that distinguishes it from natural bone. Synthesized hydroxyapatite is characterized by very slow or no absorption, due to a high Ca/P rate and crystallinity [
13]. A limitation of using hydroxyapatite as a bone replacement material is its low mechanical strength and fragility. Nanotechnology is an important solution to this fragility. Reducing the size of the crystalline grain of hydroxyapatite to a smaller or nano-sized one can decrease internal pores and defects. Moreover, material plasticity can be improved by increasing the number of grain boundaries [
14]. Zhao et al. successfully improved carbon nanofiber-reinforced hydroxyapatite by reducing the size of the hydroxyapatite grain using spark plasma sintering [
15]. Zeng et al. also reduced the grain size of hydroxyapatite and improved its mechanical properties by creating hydroxyapatite using a two-step sintering process in the digital light processing of 3D printing technology [
16].
2.2. β-Tricalcium Phosphate (β-TCP)
β-TCP (β-Ca
3(PO
4)
2) has been widely used as a bone replacement material for more than 30 years [
17,
18]. It has good osteoconductivity due to its porosity, which promotes vascular fiber growth and osteogenic cell adhesion [
13,
19]. β-TCP also has low immunogenicity in the body and high biosafety [
13]. As described earlier, it possesses excellent properties as a bone replacement material. In their study of dog alveolar bone, Nakajima et al. found that the bone regenerative potential of β-TCP was comparable with that of freeze-dried heterologous bone, autogenous bone, demineralized freeze-dried bone, and inorganic bovine bone [
20]. Galois et al. considered the use of β-TCP an optimal choice for moderate bone defects [
18]. β-TCP is considered to be absorbed by osteoclasts, and its Ca/P rate is lower than that of hydroxyapatite, resulting in faster degradation and absorption in the body than hydroxyapatite [
21]. However, its absorption rate is said to be unpredictable [
22]. Furthermore, the interconnected porous structures of β-TCP can improve vascularization; however, the mechanical strength is reduced [
23], thereby limiting applications and sites of use [
17]. Recent studies have attempted to improve the mechanical strength of β-TCP using liquid phase sintering to increase the density of the material [
24,
25].
2.3. Carbonate Apatite
Carbonate apatite is the inorganic composition of bone, and the material was developed based on the idea that a material with a composition similar to that of bone would be best suited for use as a bone replacement material. Nevertheless, it was initially difficult to develop carbonate apatite because it pyrolyzes and therefore cannot be sintered. When calcium carbonate blocks are immersed in a phosphate solution, carbonate apatite precipitates on the block surface. This reaction progresses over time, transforming the entire block into carbonate apatite [
5]. Using this technique, carbonate apatite can be produced in a clinically usable form. Carbonate apatite dissolves in the weak acid produced in the osteoclast Howship’s lacuna, similar to bone, and is absorbed faster than hydroxyapatite [
26]. Clinical trials have shown that carbonate apatite is an effective bone substitute in human sinus lifts [
27]. Carbonate apatite also possesses an excellent bone-conducting ability. It has been reported that carbonate apatite has better bone formation around it in beagle dogs than hydroxyapatite and possesses a superior osteoconductive ability [
28]. This superior osteoconductivity is due to intercellular signaling from osteoclasts. In other words, osteoclasts that absorb carbonate apatite release liquid factors known as clastokines that activate osteoblasts [
29]. Hydroxyapatite is not absorbed or is absorbed slowly, causing this phenomenon to rarely occur. Moreover, osteoblasts are activated in carbonate apatite through information transfer between the material and the cells. When bone marrow cells are seeded on the surface of carbonate apatite and analyzed for differentiation markers to osteoblasts, they show higher values than those on the hydroxyapatite surface [
30]. In recent years, carbonate apatite has been verified to be more porous in order to improve its osteogenic potential [
31].
2.4. Octacalcium Phosphate (OCP)
OCP (Ca
8H
2(PO
4)6-5H
2O) was suggested as a precursor for hydroxyapatite formation from an aqueous solution [
6] and for apatite crystal formation during mineralization [
7]. OCP is composed of a repetitive apatite layer, similar to that in Ca-deficient hydroxyapatite, and a hydrated layer containing large amounts of water molecules, such as dicalcium phosphate dihydrate (DCPD) [
7]. It was anticipated that OCP could be used as a bone replacement material because of its potential to function as a precursor phase, as observed in the formation of OCP before hydroxyapatite deposition in bone collagen matrices in natural bone [
32]. When implanted in the mouse calvaria, OCP demonstrated earlier bone formation than hydroxyapatite and Ca-deficient hydroxyapatite. Furthermore, bone apatite crystals are bonded by OCP crystals during bone formation [
33]. The degradation of OCP might be primarily caused by the phagocytosis of osteoclasts because OCP induces osteoclast formation in an in vitro coculture system of osteoblasts and osteoclast precursor cells [
34]. The degradation rate of OCP is faster than that of hydroxyapatite and tricalcium phosphate [
35]. Based on these characteristics of OCP, a human clinical trial was conducted using a mixture of OCP and collagen, which demonstrated effective results in cases of sinus floor elevation in the 1st and 2nd stages, socket preservation, cyst, and alveolar cleft procedures [
36]. Various forms of OCP, including granules [
37] and blocks [
38], and various additives, such as biodegradable polymers [
36,
39,
40] and ions [
41,
42], are now considered to improve the effectiveness of OCP.
3. Problems and Solutions of Existing Artificial Bone
These artificial bones made of calcium phosphate are osteoconductive [
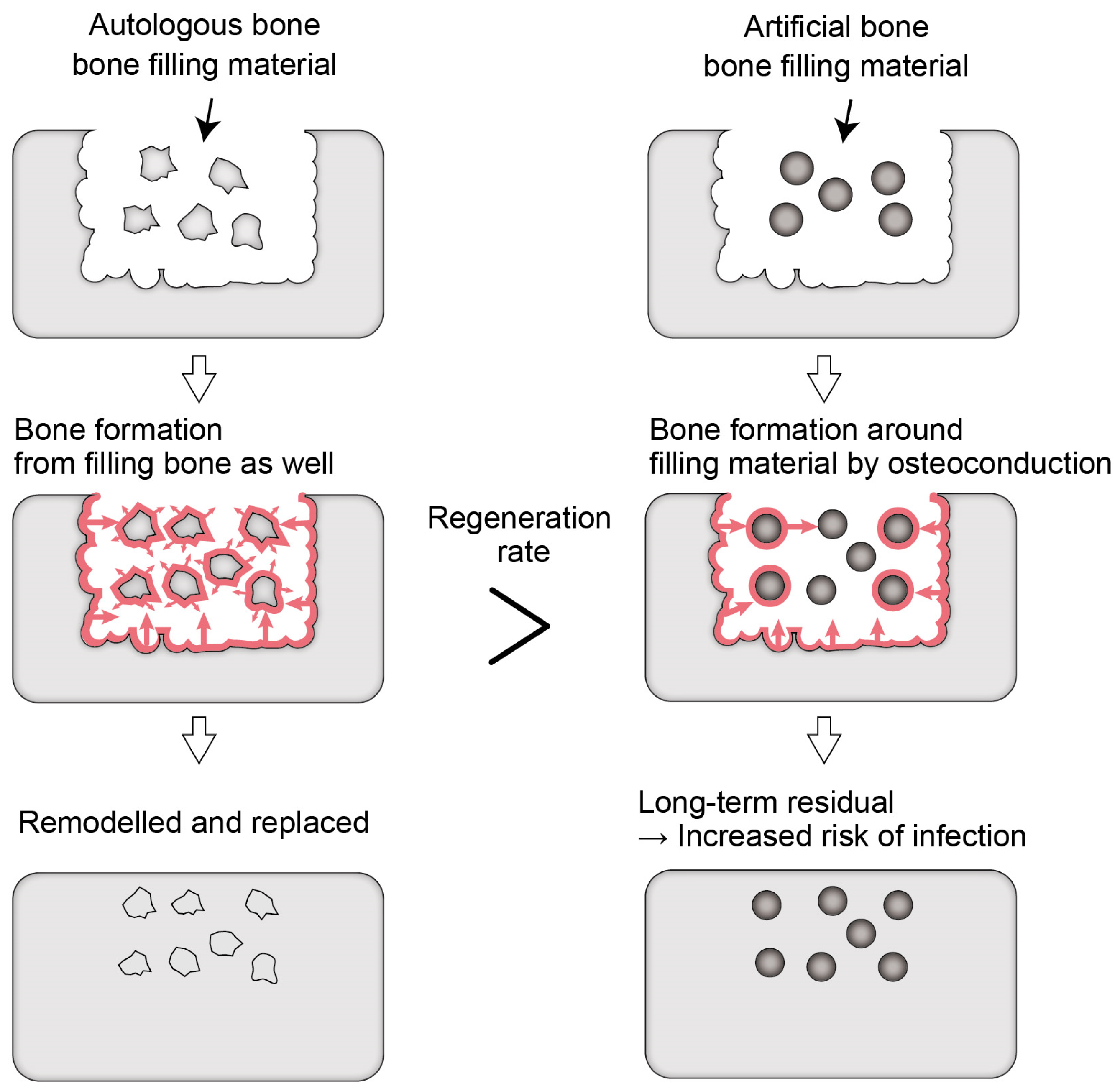
43], as mentioned above. Bone regeneration occurs around granules with a size of several µm before they are resorbed, and the artificial bone remains in the bone. Therefore, it is suggested that even β-TCP, carbonate apatite, and OCP also take a long time to be completely absorbed and replaced by natural bone in the human body. The larger the particle size of the resorbable artificial bone, the longer it takes for its resorption and replacement by natural bone in the human body. This may help reduce bone resorption but is a major problem for controlling infection because the implanted material that remains in the body increases the risk of infection (
Figure 1). For example—as is evident in catheter infections—it is challenging to control infection when an implanted artificial material is exposed to the outside of the body and becomes infected. The same happens to artificial bones. When the infection on artificial bones cannot be controlled, they must be removed by curettage.
Furthermore, granular artificial bones are difficult to handle. Mixing with saline or blood improves handling but only slightly. The size of bone defects in dentistry is smaller than in orthopedics, and the particle size of the granular artificial bone used in dentistry is also smaller. Besides the oral cavity being a site of easy infection, osteogenesis of the alveolar ridge requires placing a granular artificial bone to fit the defect, which increases the procedure’s difficulty. In addition, a smaller granular artificial bone results in greater risks of dispersal, migration, and leakage through the incision.
4. Necessity of Bioabsorbable Polymers that Adhere to Calcium Phosphate
A bioabsorbable material that can adhere to bone and artificial bone components is desirable because it would allow the artificial bone to be easily placed in a bone defect by mixing the artificial bone with it and making it patty-like. In addition to the improved handling due to enhanced formability and adhesion to the bone surface, the stronger sealing of the natural bone–artificial bone interface is expected to improve the treatment outcome. Furthermore, if the material that adheres to calcium phosphate can maintain its gel state in the body, and if fine powdered artificial bone can be used, it leads to the development of new artificial bone able to be quickly absorbed and replaced with natural bone. The rapid resorption of bone replacement materials and their substitution with natural bone is ideal for reducing the risk of infection.
5. Problems with Existing Bioabsorbable Polymers
For the above reasons, the development of absorbable polymers that can adhere to calcium phosphate, which is an inorganic component of bone and is used as artificial bone, has been long awaited. If such materials are available, innovative materials that contribute to implant therapy, such as putty-like artificial bones with short-term resorption and replacement by mixing a bioabsorbable polymer and calcium phosphate fine powder, are expected to be developed. However, materials that can be absorbed and replaced with tissue in the body are challenging to put to practical use. Collagen, hyaluronic acid, polyglycolic acid, and polylactic acid are still widely used as absorbable polymers that can be implanted in the body, but none of them can adhere to bone or tooth.
6. Development of Non-Animal-Derived Bioabsorbable Polymers
Phosphorylated pullulan was developed to solve problems with the above existing artificial bones. The raw material, pullulan, is a natural polysaccharide produced by fermentation with black yeast; it has water-soluble properties among polysaccharides and can be processed into powder and film. Pullulan has been used in the food industry for many years and was introduced as a non-gelatin capsule material in pharmaceuticals because its firm had excellent gas barrier properties when bovine spongiform encephalopathy became a major problem in Japan [
44].
We hypothesized that introducing numerous phosphate groups into pullulan would yield a bioabsorbable material that adheres to bone (
Table 1).
7. Design Concept of Phosphorylated Pullulan
Mechanical mating between the adherend and the material and chemical bonding at the interface are important factors when considering adhesive materials. Mechanical mating is considered to affect the adhesive strength in tooth bonding, whereas chemical bonding contributes to bond durability [
45]. It is also a concept agreed upon by all those involved in adhesive bonding that adhesive materials need to have adequate wettability to achieve a high adhesive strength. Pullulan is easily soluble in water, and the viscosity of a pullulan aqueous solution is very low among polysaccharides. Using pullulan as a backbone reduces viscosity, compared to other polysaccharides, leading to the creation of a bioabsorbable polymer with adequate wettability.
Phosphorylated pullulan was created based on the tooth adhesion theory, using pullulan as a backbone. In the dental field, the progress of tooth adhesion is particularly remarkable. It is well known that adhesive techniques and materials are applied to most current dental treatments, such as aesthetic restorations, including resin fillings, posts and cores, crown restorations, orthodontics, fixing mobile teeth, and bonding fractured teeth. Many dental adhesives have been developed and applied in clinical practice with repeated improvements and development. Nowadays, two types of adhesives (glass ionomer cement and resin cement) are commonly used in dental treatment.
Glass ionomer cement is cured by an acid-base reaction between polycarboxylic acid and the cations leached from aluminosilicate glass and can chemically bond well to untreated enamel and dentin. Thus, glass ionomer cements have the ability to chemically bond to the tooth [
46]. Compared to resin-based tooth-bonding systems, glass ionomer cement can be used even in areas with insufficient moisture protection, which is thought to be largely due to the characteristics of polycarboxylic acid (the main component). Polycarboxylic acid has many carboxy groups that chemically bond to teeth. In addition, many of these carboxyl groups are considered to make glass ionomer cements somewhat hydrophilic and not as hydrophobic as resin-based tooth-bonding systems.
Furthermore, we previously compared three adhesive monomers: 4-methacryloxyethyl trimellitic acid (4-MET), 2-methacryloxyethyl phenyl hydrogen phosphate (phenyl-P), and 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP) and found that 10-MDP had the best chemical bonding ability [
47]. Since this report, the effectiveness of the divalent phosphate group has become clear, and 10-MDP has been used in various tooth-bonding systems. These results of basic research on tooth adhesion [
48,
49,
50,
51,
52] allowed for the development of phosphorylated pullulan, based on the idea of “introducing a large number of phosphate groups into a polysaccharide with high water solubility” (
Figure 2).
8. Efficacy of Phosphorylated Pullulan as a Bone Replacement Material
Phosphorylated pullulan has been applied in various ways, due to its characteristics, such as a coating agent for implants [
53,
54,
55,
56] and as a direct pulp-capping material [
57,
58,
59]. Takahata et al. [
60] and Morimoto et al. [
61] reported the efficacy of phosphorylated pullulan as a bone replacement material (
Table 2).
Takahata et al. [
60] implanted a mixture of phosphorylated pullulan and β-TCP in the medullary cavity of a mouse femur, a rabbit ulnar, and a porcine vertebral body bone and evaluated bone formation. A histological evaluation of the medullary cavity of a mouse femur revealed that only phosphorylated pullulan remained 2 weeks after implantation. Five weeks after implantation, bone marrow replacement was observed in the group implanted with only phosphorylated pullulan, and bone formation was observed in the group implanted with a mixture of pullulan phosphate and β-TCP. After 8 weeks, additional new bone was observed in the group implanted with a mixture of phosphorylated pullulan and β-TCP, but no bone formation was observed in the group implanted with only phosphorylated pullulan. The effects of Biopex-R and a mixture of phosphorylated pullulan and β-TCP in the rabbit ulnar were compared. Eight weeks after implantation, a micro-CT showed that Biopex-R separated from the existing bone in the group implanted with only Biopex-R, but the bone defect was completely amended in the group implanted with a mixture of phosphorylated pullulan and β-TCP. Histologically, the type of this bone formation is classified as endochondral ossification. On the other hand, the formation of new bone in the porcine vertebral body bone in the group with no treatment for the defect and the group implanted with Biopex-R was not confirmed by micro-CT, even after 8 weeks. By contrast, a mixture of phosphorylated pullulan and β-TCP implanted in the bone defect of the porcine vertebral body bone resulted in new bone formation at 8 weeks. Histologically, only fibrotic changes were observed in the untreated group, even at 8 weeks. However, in the group implanted with a mixture of phosphorylated pullulan and β-TCP, the new bone began to form at 4 weeks, and trabecular bone formation began at 8 weeks. Based on this, Takahata et al. concluded that phosphorylated pullulan alone cannot induce bone formation, but when used together with β-TCP, it promotes bone remodeling.
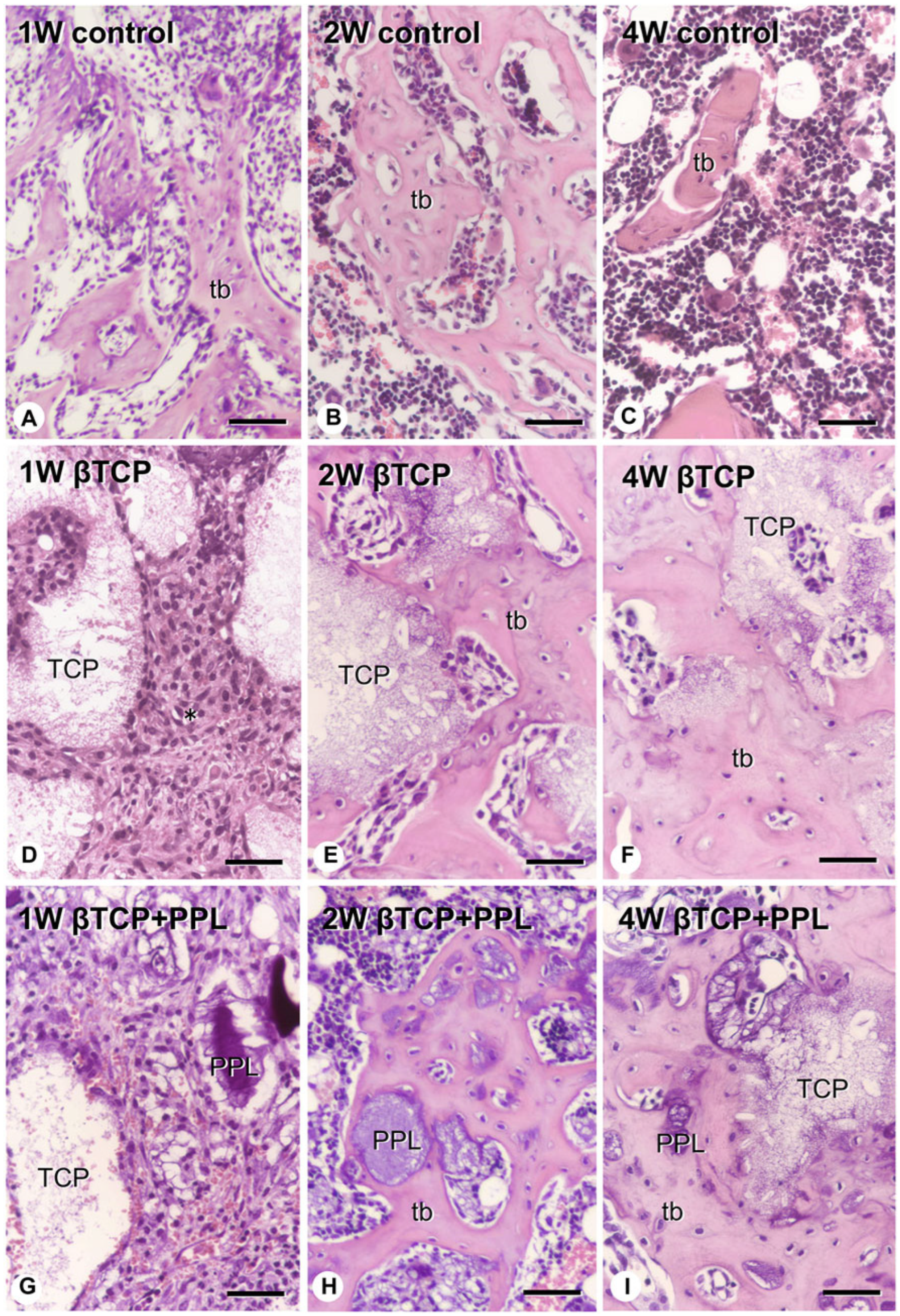
Morimoto et al. [
61] implanted a mixture of phosphorylated pullulan and β-TCP in rats with a tibia bone defect, analyzed the bone regeneration process histologically and microstructurally, and reported that phosphorylated pullulan may have osteoconductive and calcification retention properties. These authors created a cylindrical bone defect with a diameter of 2.0 mm in the tibia of 10-week-old rats, and two materials, namely β-TCP (mean granule diameter = 100–250 μm) and a mixture of pullulan phosphate and β-TCP (4:6,
w/
w), were implanted in the animals and compared. A group with the bone defect but without an implant was used as the control. In the control group, a large amount of new bone was observed in the bone defect after 1 week, but these new bones decreased 4 weeks after implantation. By contrast, in the β-TCP implantation group, β-TCP granules were surrounded by numerous fibroblast-like cells 1 week after implantation; then, new bone was formed at 2 to 4 weeks, using β-TCP as a scaffold. This new bone remained in the bone defect 4 weeks after implantation. In the group implanted with a mixture of phosphorylated pullulan and β-TCP, numerous fibroblast-like cells were observed around phosphorylated pullulan and β-TCP 1 week after implantation, and new bone was observed on the phosphorylated pullulan and β-TCP granules after 2 weeks (
Figure 3 and
Figure 4).
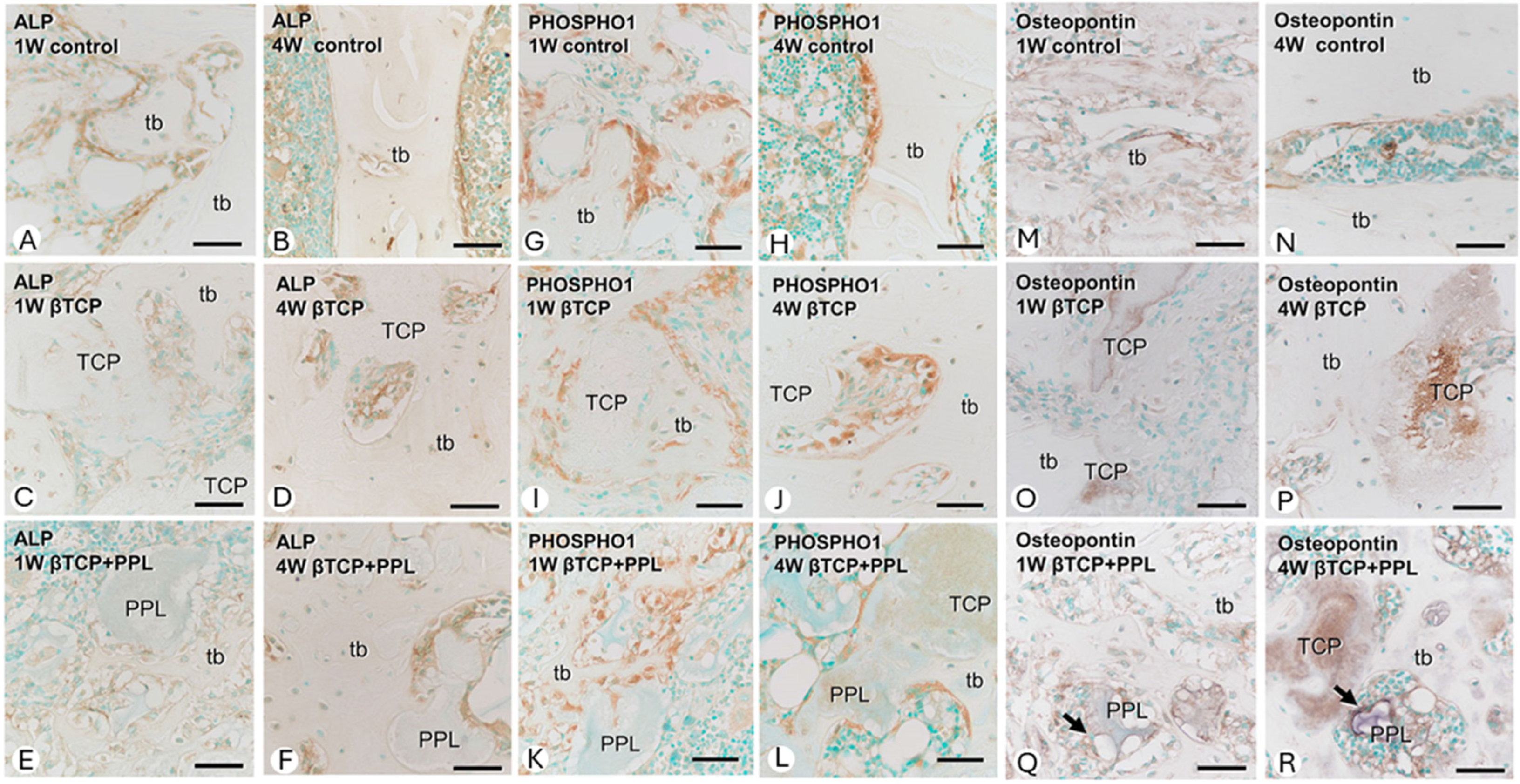
Osteoblast lineage cells and bone formation at the bone regeneration site were evaluated by immunohistochemistry for alkaline phosphatase (ALP) (
Figure 5A–F), PHOSPHO1 (
Figure 5G–L), and osteopontin (
Figure 5M–R). In the control group (without implantation), numerous ALP-positive osteoblast lineage cells (osteoblasts and preosteoblasts) were present on the surface of new bone during all postoperative periods. Moreover, PHOSPHO1-positive reactions—expressed on osteoblasts responsible for matrix vesicular calcification—were also observed on the bone surface. In the β-TCP implantation group, ALP-positive osteoblast lineage cells and PHOSPHO1-positive osteoblasts were found on the surface of new bone and on β-TCP granules. Thereafter, ALP-positive osteoblast lineage cells were more widespread on the surface of new bone than PHOSPHO1-positive osteoblasts. In the group implanted with a mixture of phosphorylated pullulan and β-TCP, ALP-positive osteoblast lineage cells and PHOSPHO1-positive osteoblasts were located not only on the surfaces of β-TCP and new bone but also on phosphorylated pullulan. Interestingly, osteopontin, a bone matrix protein able to bind to crystalline calcium, was found on the surfaces of β-TCP and phosphorylated pullulan, suggesting that phosphorylated pullulan may function as a scaffold material for osteoblast fixation. In addition, an analysis using strain-derived osteoblasts (MC3T3-E1 cells) suggested that phosphorylated pullulan did not have a direct effect on osteoblast differentiation or function.
On the other hand, bone resorption was evaluated by osteoclast distribution using tartrate-resistant acid phosphatase (TRAP) staining. Osteoclasts were most abundant 2 weeks after implantation in all groups and then decreased. In the control group, TRAP-positive osteoclasts were located on the surface of the new bone, whereas in the β-TCP implantation group, they were located not only on the new bone but also on β-TCP. In the group implanted with a mixture of phosphorylated pullulan and β-TCP, the TRAP-positive osteoclasts were mainly located on the surface of the new bone, but some osteoclasts were also localized on the surface of phosphorylated pullulan.
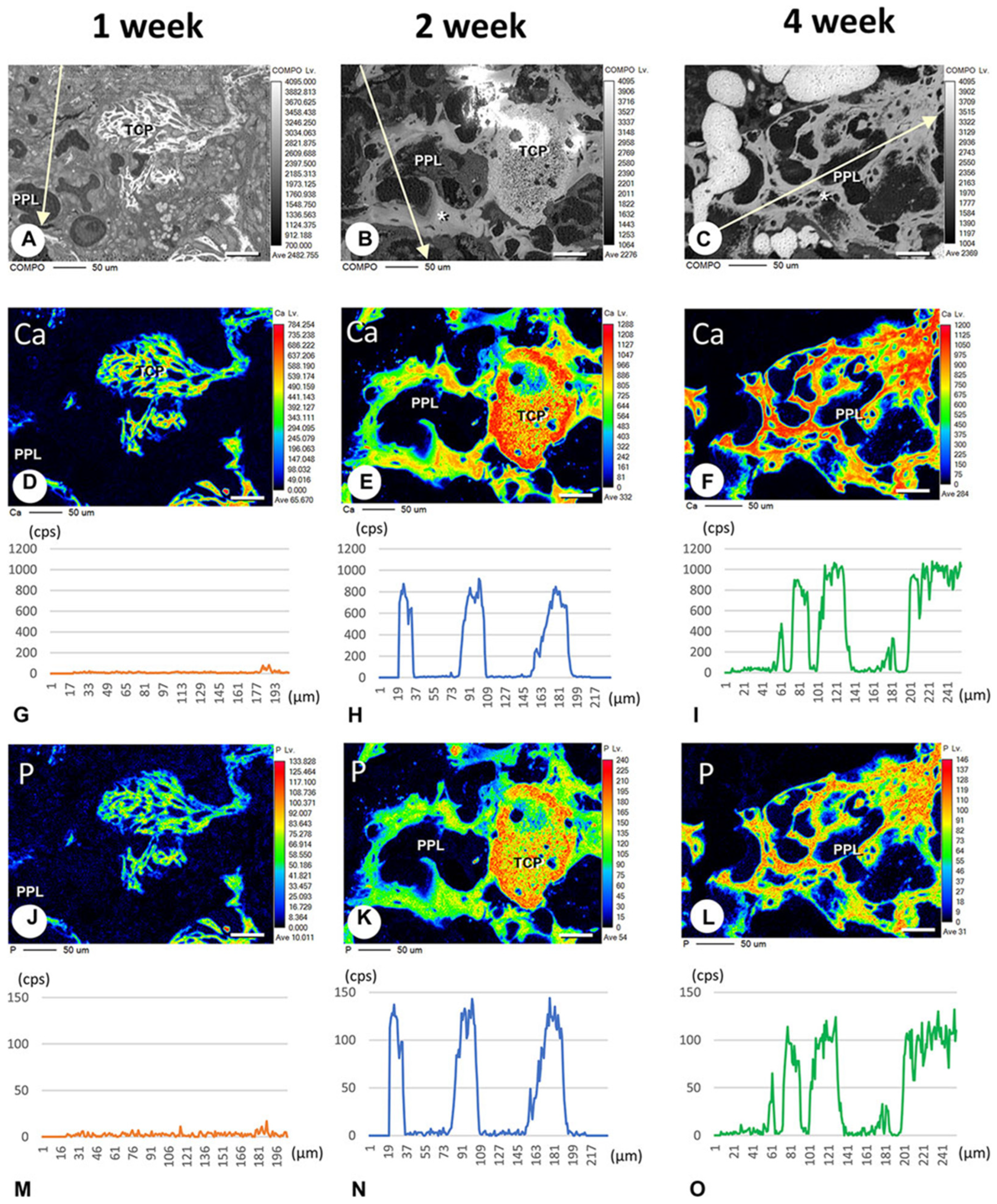
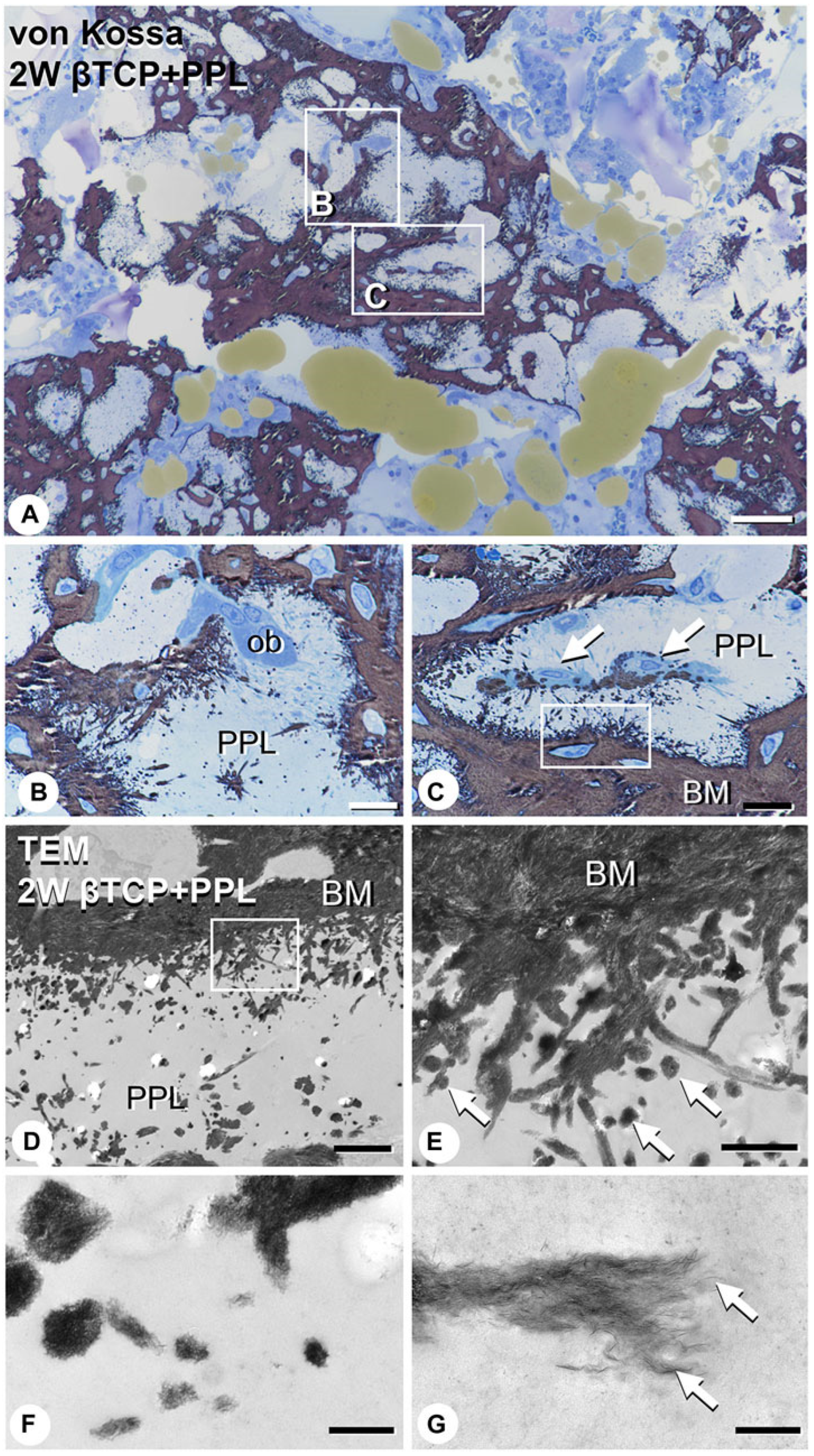
In addition, Morimoto et al. evaluated calcification related to phosphorylated pullulan using the elemental mapping of calcium and phosphorus using an electron probe micro-analyzer. Calcium and phosphorus were not detected in the phosphorylated pullulan 1 week after implantation, but 2 to 4 weeks after implantation, calcium and phosphorus deposits were observed on the phosphorylated pullulan surface and the surrounding new bone. Although the X-ray fluorescence intensity of phosphorus on phosphorylated pullulan 2 and 4 weeks after implantation did not differ, the X-ray fluorescence intensity of calcium increased over time (
Figure 6). These results suggested that calcium might accumulate in phosphorylated pullulan and the surrounding new bone over time. Microstructural analysis revealed the formation of numerous calcite spheres and needle-like calcified crystal masses on the surface of pullulan phosphate (
Figure 7), suggesting that phosphorylated pullulan may have an affinity for crystalline calcium and may promote calcification.
Based on these results, Morimoto et al. speculated that phosphorylated pullulan mixed with β-TCP exhibited osteoblast anchorage and osteoconductive ability as a scaffold material and might induce calcification by retaining calcium.
9. Application of Phosphorylated Pullulan to Areas Other than Hard Tissue
Phosphorylated pullulan is promoted for practical use in fields other than hard tissue. The submucosal injection material used for the endoscopic resection of gastric and other cancers is one of them. Satomi et al. confirmed that submucosal injection material-based phosphorylated pullulan could demonstrate a tissue elevation volume and duration comparable to existing submucosal injection materials using pigs and has high operability [
62]. From these characteristics, the submucosal injection material containing phosphorylated pullulan as a key component has been approved under the Pharmaceutical Affairs Law in Japan and is marketed as “enRise”.
10. Conclusions
Phosphorylated pullulan is an unprecedented bioabsorbable material with bone adhesion properties developed based on experience in basic research on dental adhesive materials. It also can assist with bone formation. From these characteristics, phosphorylated pullulan is expected to be a novel bone replacement material. Currently, clinical trials of this material will begin in 2024 as a Class IV medical device, which is defined by the Japanese Act on Securing Quality, Efficacy, and Safety of Products Including Pharmaceuticals and Medical Devices. In addition to bone, phosphorylated pullulan is also expected to be used as a scaffold for the regeneration of various organs, as a carrier for drug delivery systems, and in a wide range of applications as a bioabsorbable material able to replace collagen and hyaluronic acid.
Author Contributions
Conceptualization, K.N. and Y.Y.; methodology, K.N. and Y.Y.; formal analysis, K.N., T.A. and Y.Y.; investigation, K.N., H.H., K.Y., T.N. and M.N.; data curation, K.N.; writing—original draft preparation, K.N., T.A. and Y.Y.; writing—review and editing, K.N., H.H., B.V.M. and Y.Y.; visualization, K.N. and T.A.; supervision, B.V.M. and Y.Y.; project administration, Y.Y.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Parts of these researches were supported by AMED under Grant Numbers JP18hk0102055 and JP22hk0102087 and by the Suzuken Memorial Foundation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The corresponding author, Yasuhiro Yoshida, owns shares in Japan MDB Solutions Co., Inc., the manufacturer and distributor of the endoscopic submucosal injection material “enRize”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Noelken, R.; Pausch, T.; Wagner, W.; Al-Nawas, B. Peri-implant defect grafting with autogenous bone or bone graft material in immediate implant placement in molar extraction sites—1- to 3-year results of a prospective randomized study. Clin. Oral Implant. Res. 2020, 31, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Lin, T.; Khalaf, A.T.; Zhang, Y.; He, H.; Yang, L.; Zhu, J.; Shi, Z. The preparation and application of calcium phosphate biomedical composites in filling of weight-bearing bone defects. Sci. Rep. 2021, 11, 4283. [Google Scholar] [CrossRef]
- Graham, S.M.; Leonidou, A.; Aslam-Pervez, N.; Hamza, A.; Panteliadis, P.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Biological therapy of bone defects: The immunology of bone allo-transplantation. Expert Opin. Biol. Ther. 2010, 10, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, W.; Zhu, K.; Liu, W.; Luo, Y.; Yuan, X.; Wang, J.; Cheng, T.; Zhang, X. Lithium chloride with immuno-modulatory function for regulating titanium nanoparticle-stimulated inflammatory response and accelerating osteogenesis through suppression of MAPK signaling pathway. Int. J. Nanomed. 2019, 14, 7475–748823. [Google Scholar] [CrossRef]
- Ishikawa, K. Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions. Materials 2010, 3, 1138–1155. [Google Scholar] [CrossRef]
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Octacalcium Phosphate and Hydroxyapatite: Crystallographic and Chemical Relations between Octacalcium Phosphate and Hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenecity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Shepherd, D.V.; Best, S.M. Substituted hydroxyapatites for bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 2335–2347. [Google Scholar] [CrossRef]
- Bracci, B.; Torricelli, P.; Panzavolta, S.; Boanini, E.; Giardino, R.; Bigi, A. Effect of Mg2+, Sr2+, and Mn2+ on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J. Inorg. Biochem. 2009, 103, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-substituted hydroxyapatite: From synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Zhao, R.; Tang, W.; Yang, F.; Tian, H.; Peng, S.; Pan, H.; Shuai, C. Structural and Functional Adaptive Artificial Bone: Materials, Fabrications, and Properties. Adv. Funct. Mater. 2023, 33, 2214726. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Gui, Z.; Zheng, J.; Yang, P.; Liu, A.; Wei, S.; Yang, Z. Carbon fiber reinforced hydroxyapatite composites with excellent mechanical properties and biological activities prepared by spark plasma sintering. Ceram. Int. 2020, 46, 27446–27456. [Google Scholar] [CrossRef]
- Zeng, Y.; Yan, Y.; Yan, H.; Liu, C.; Li, P.; Dong, P.; Zhao, Y.; Chen, J. 3D printing of hydroxyapatite scaffolds with good mechanical and biocompatible properties by digital light processing. J. Mater. Sci. 2018, 53, 6291–6301. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Galois, L.; Mainard, D.; Delagoutte, J.P. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int. Orthop. 2002, 26, 109. [Google Scholar] [PubMed]
- Frayssinet, P.; Mathon, D.; Lerch, A.; Autefage, A.; Collard, P.; Rouquet, N. Osseointegration of composite calcium phosphate bioceramics. J. Biomed. Mater. Res. 2000, 50, 125–130. [Google Scholar] [CrossRef]
- Nakajima, Y.; Fiorellini, J.; Kim, D.; Weber, H. Regeneration of standardized mandibular bone defects using expanded polytetrafluoroethylene membrane and various bone fillers. Int. J. Periodontics Restor. Dent. 2007, 27, 151–159. [Google Scholar]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224. [Google Scholar] [CrossRef]
- Nery, E.B.; LeGeros, R.Z.; Lynch, K.L.; Lee, K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J. Periodontol. 1992, 63, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.A.; Rozental, T.D. Bone Graft Substitutes. Hand Clin. 2012, 28, 457–468. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Dong, L.; Shen, Y.; Hu, H.; Gao, H.; Zhao, S.; Li, X. Microstructural, mechanical properties and strengthening mechanism of DLP produced β-tricalcium phosphate scaffolds by incorporation of MgO/ZnO/58S bioglass. Ceram. Int. 2021, 47, 25863–25874. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharjee, A.; Banerjee, D.; Boccaccini, A.R.; Bandyopadhyay, A. Influence of random and designed porosities on 3D printed tricalcium phosphate-bioactive glass scaffolds. Addit. Manuf. 2021, 40, 101895. [Google Scholar] [CrossRef]
- Ishikawa, K.; Hayashi, K. Carbonate apatite artificial bone. Sci. Technol. Adv. Mater. 2021, 22, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, K.; Fukuda, N.; Kasugai, S.; Tachikawa, N.; Koyano, K.; Matsushita, Y.; Ogino, Y.; Ishikawa, K.; Miyamoto, Y. Maxillary Sinus Floor Augmentation Using Low-Crystalline Carbonate Apatite Granules With Simultaneous Implant Installation: First-in-Human Clinical Trial. J. Oral Maxillofac. Surg. 2019, 77, e1–e985. [Google Scholar] [CrossRef]
- Ishikawa, K. Carbonate apatite bone replacement: Learn from the bone. J. Ceram. Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Tsuchiya, A.; Hayashi, K.; Tsuru, K.; Ohe, G. Physical and Histological Comparison of Hydroxyapatite, Carbonate Apatite, and β-Tricalcium Phosphate Bone Substitutes. Materials 2018, 11, 1993. [Google Scholar] [CrossRef]
- Nagai, H.; Kobayashi-Fujioka, M.; Fujisawa, K.; Ohe, G.; Takamaru, N.; Hara, K.; Uchida, D.; Tamatani, T.; Ishikawa, K.; Miyamoto, Y. Effects of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. J. Mater. Sci. Mater. Med. 2015, 26, 99. [Google Scholar] [CrossRef]
- Elsheikh, M.; Kishida, R.; Hayashi, K.; Tsuchiya, A.; Shimabukuro, M.; Ishikawa, K. Effects of pore interconnectivity on bone regeneration in carbonate apatite blocks. Regen. Biomater. 2022, 9, rbac010. [Google Scholar] [CrossRef]
- Suzuki, O.; Miyasaka, Y.; Sakurai, M.; Nakamura, M.; Kagayama, M. Bone Formation on Synthetic Precursors of Hydroxyapatite. Tohoku J. Exp. Med. 1991, 164, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.; Müller, K.H.; Wong, W.C.; Pickard, C.J.; Reid, D.G.; Skepper, J.N.; Duer, M.J. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. USA 2014, 111, E1354–E1363. [Google Scholar] [CrossRef] [PubMed]
- Takami, M.; Mochizuki, A.; Yamada, A.; Tachi, K.; Zhao, B.; Miyamoto, Y.; Anada, T.; Honda, Y.; Inoue, T.; Nakamura, M.; et al. Osteoclast differentiation induced by synthetic octacalcium phosphate through receptor activator of NF-kappaB ligand expression in osteoblasts. Tissue Eng. Part A 2009, 15, 3991–4000. [Google Scholar] [CrossRef]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Kamakura, S.; Matsui, K.; Fukuda, M.; Takano, H.; Iino, M.; Ishikawa, S.; Kawana, H.; Soma, T.; Imamura, E.; et al. Clinical study of octacalcium phosphate and collagen composite in oral and maxillofacial surgery. J. Tissue Eng. 2020, 11, 2041731419896449. [Google Scholar] [CrossRef]
- Komlev, V.S.; Barinov, S.M.; Bozo, I.I.; Deev, R.V.; Eremin, I.I.; Fedotov, A.Y.; Gurin, A.N.; Khromova, N.V.; Kopnin, P.B.; Kuvshinova, E.A.; et al. Bioceramics composed of octacalcium phosphate demonstrate enhanced biological behavior. ACS Appl. Mater. Interfaces 2014, 6, 16610–16620. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Ishikawa, K. Fabrication of pure octacalcium phosphate blocks from dicalcium hydrogen phosphate dihydrate blocks via a dissolution–precipitation reaction in a basic solution. Mater. Lett. 2019, 239, 143–146. [Google Scholar] [CrossRef]
- Hamada, S.; Mori, Y.; Shiwaku, Y.; Hamai, R.; Tsuchiya, K.; Baba, K.; Oizumi, I.; Kanabuchi, R.; Miyatake, N.; Aizawa, T.; et al. Octacalcium Phosphate/Gelatin Composite (OCP/Gel) Enhances Bone Repair in a Critical-sized Transcortical Femoral Defect Rat Model. Clin. Orthop. Relat. Res. 2022, 480, 2043–2055. [Google Scholar] [CrossRef]
- Oizumi, I.; Hamai, R.; Shiwaku, Y.; Mori, Y.; Anada, T.; Baba, K.; Miyatake, N.; Hamada, S.; Tsuchiya, K.; Nishimura, S.; et al. Impact of simultaneous hydrolysis of OCP and PLGA on bone induction of a PLGA-OCP composite scaffold in a rat femoral defect. Acta Biomater. 2021, 124, 358–373. [Google Scholar] [CrossRef]
- Shi, H.; Ye, X.; Wu, T.; Zhang, J.; Ye, J. Regulating the physicochemical and biological properties in vitro of octacalcium phosphate by substitution with strontium in a large doping range. Mater. Today Chem. 2017, 5, 81–91. [Google Scholar] [CrossRef]
- Inoue, S.; Koshiro, K.; Yoshida, Y.; De Munck, J.; Nagakane, K.; Suzuki, K.; Sano, H.; Van Meerbeek, B. Hydrolytic stability of self-etch adhesives bonded to dentin. J. Dent. Res. 2005, 84, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Hamai, R.; Shiwaku, Y.; Kurobane, T.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Angio-osteogenic capacity of octacalcium phosphate co-precipitated with copper gluconate in rat calvaria critical-sized defect. Sci. Technol. Adv. Mater. 2022, 23, 120–139. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Jayr, N.; Singh, D.; Purewal, S.S.; Kennedy, J.F. Pullulan in pharmaceutical and cosmeceutical formulations: A review. Int. J. Biol. Macromol. 2023, 231, 123353. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Snauwaert, J.; Hellemans, L.; Lambrechts, P.; Vanherle, G.; Wakasa, K. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J. Dent. Res. 2000, 79, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Yoshida, Y.; Nagaoka, N.; Fukegawa, D.; Hayakawa, S.; Mine, A.; Nakamura, M.; Minagi, S.; Osaka, A.; Suzuki, K.; et al. Nano-controlled molecular interaction at adhesive interfaces for hard tissue reconstruction. Acta Biomater. 2010, 6, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Irie, M.; Ogawa, T.; Van Landuyt, K.; Osaka, A.; Suzuki, K.; Minagi, S.; et al. Nanolayering of phosphoric acd ester monomer on enamel and dentin. Acta Biomater. 2011, 7, 3187–3195. [Google Scholar] [CrossRef]
- Yoshida, Y.; Inoue, S. Chemical analyses in dental adhesive technology. Jpn. Dent. Sci. Rev. 2012, 48, 141–152. [Google Scholar] [CrossRef]
- Yoshida, Y.; Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Torii, Y.; Ogawa, T.; Osaka, A.; Van Meerbeek, B. Self-assembled nano-layering at the adhesive interface. J. Dent. Res. 2012, 91, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Yoshida, Y.; Van Meerbeek, B.; Hayakawa, S. Atomic level observation and structural analysis of phosphoric-acid ester interaction at dentin. Acta Biomater. 2019, 97, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; Chaudhari, A.; Yoshida, Y.; Van Meerbeek, B.; Naert, I.; Duyck, J. Bone tissue response to implant surfaces functionalized with phosphate-containing polymers. Clin. Oral Implants Res. 2014, 25, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; Chaudhari, A.; Yoshihara, K.; Mesquita, M.F.; Yoshida, Y.; Van Meerbeek, B.; Vandamme, K.; Duyck, J. Phosphorylated Pullulan Coating Enhances Titanium Implant Osseointegration in a Pig Model. Int. J. Oral Maxillofac. Implants 2017, 32, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; de Rycker, J.; Chaudhari, A.; Coutinho, E.; Yoshida, Y.; Van Meerbeek, B.; Mesquita, M.F.; da Silva, W.J.; Yoshihara, K.; Vandamme, K.; et al. Titanium implant functionalization with phosphate-containing polymers may favour in vivo osseointegration. J. Clin. Periodontol. 2017, 44, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Nagamoto, K.; Nakanishi, K.; Akasaka, T.; Abe, S.; Yoshihara, K.; Nakamura, M.; Hayashi, H.; Takemoto, S.; Tamura, M.; Kitagawa, Y.; et al. Investigation of a new implant surface modification using phosphorylated pullulan. Front. Bioeng. Biotechnol. 2024, 12, 1378039. [Google Scholar] [CrossRef] [PubMed]
- Pedano, M.S.; Li, X.; Li, S.; Sun, Z.; Cokic, S.M.; Putzeys, E.; Yoshihara, K.; Yoshida, Y.; Chen, Z.; Van Landuyt, K.; et al. Freshly-mixed and setting calcium-silicate cements stimulate human dental pulp cells. Dent. Mater. 2018, 34, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Pedano, M.S.; Li, X.; Camargo, B.; Hauben, E.; De Vleeschauwer, S.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Van Meerbeek, B. Injectable phosphopullulan-functionalized calcium-silicate cement for pulp-tissue engineering: An in-vivo and ex-vivo study. Dent. Mater. 2020, 36, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.R.; Islam, R.; Liu, Y.; Toida, Y.; Yoshida, Y.; Sano, H.; Ahmed, H.M.A.; Tomokiyo, A. Biological evaluation of novel phosphorylated pullulan-based calcium hydroxide formulations as direct pulp capping materials: An in-vivo study on rat molars. Int. Endod. J. 2024. in printing. [Google Scholar]
- Takahata, T.; Okihara, T.; Yoshida, Y.; Yoshihara, K.; Shiozaki, Y.; Yoshida, A.; Yamane, K.; Watanabe, N.; Yoshimura, M.; Nakamura, M.; et al. Bone engineering by phosphorylated-pullulan and β-TCP composite. Biomed. Mater. 2015, 10, 065009. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hasegawa, T.; Hongo, H.; Yamamoto, T.; Maruoka, H.; Haraguchi-Kitakamae, M.; Nakanishi, K.; Yamamoto, T.; Ishizu, H.; Shimizu, T.; et al. Phosphorylated pullulan promotes calcification during bone regeneration in the bone defects of rat tibiae. Front. Bioeng. Biotechnol. 2023, 11, 1243951. [Google Scholar] [CrossRef]
- Satomi, T.; Ochi, Y.; Okihara, T.; Fujii, H.; Yoshida, Y.; Mominoki, K.; Hirayama, H.; Toyosawa, J.; Yamasaki, Y.; Kawano, S.; et al. Innovative submucosal injection solution for endoscopic resection with phosphorylated pullulan: A preclinical study. Gastrointest. Endosc. 2024, 99, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).