Summary of the Research Progress on Advanced Engineering, Processes, and Process Parameters of Rare Earth Green Metallurgy
Abstract
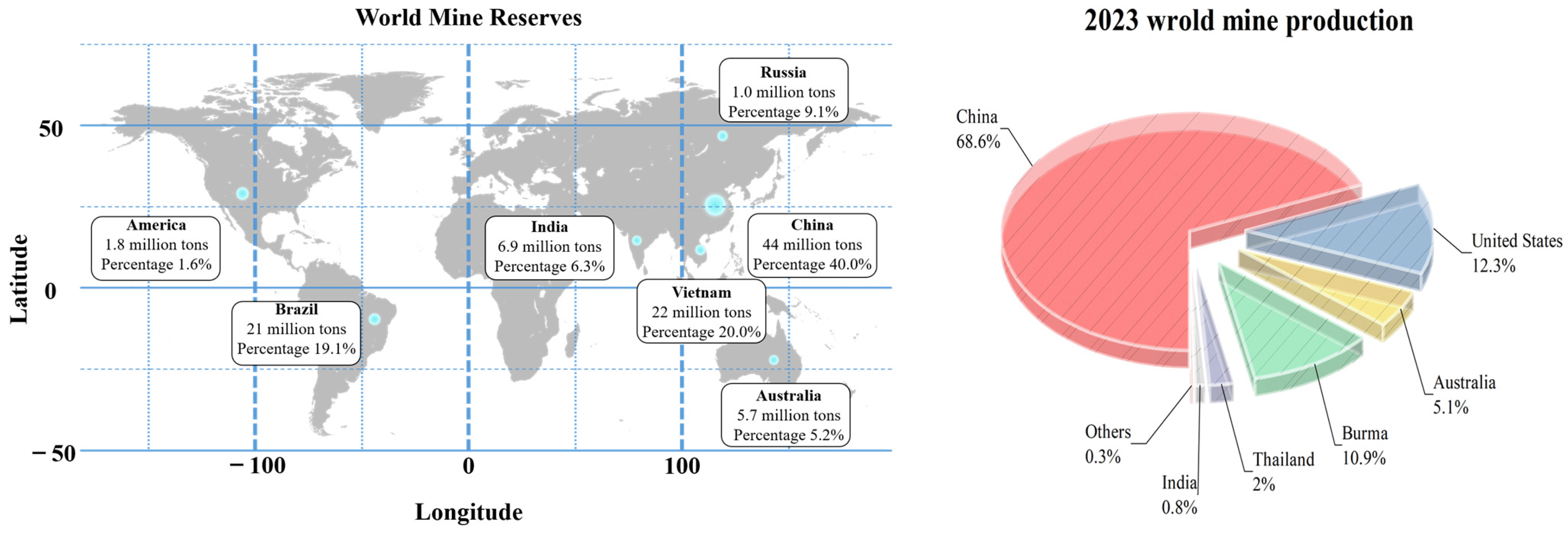
:1. Introduction
2. Engineering Progress in the Preparation of Rare Earth Compounds
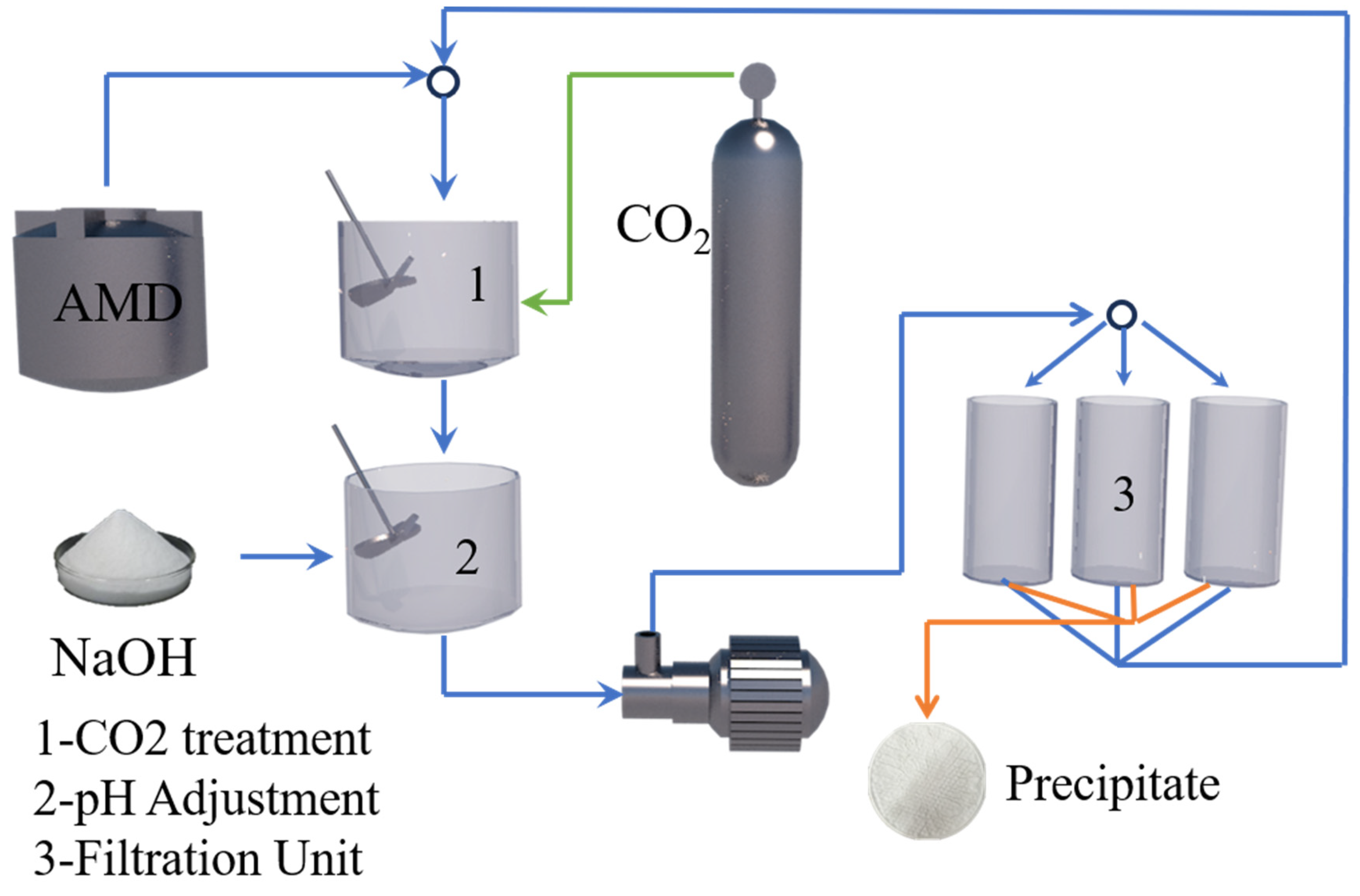
2.1. Precipitation Method
2.2. Microemulsion Method
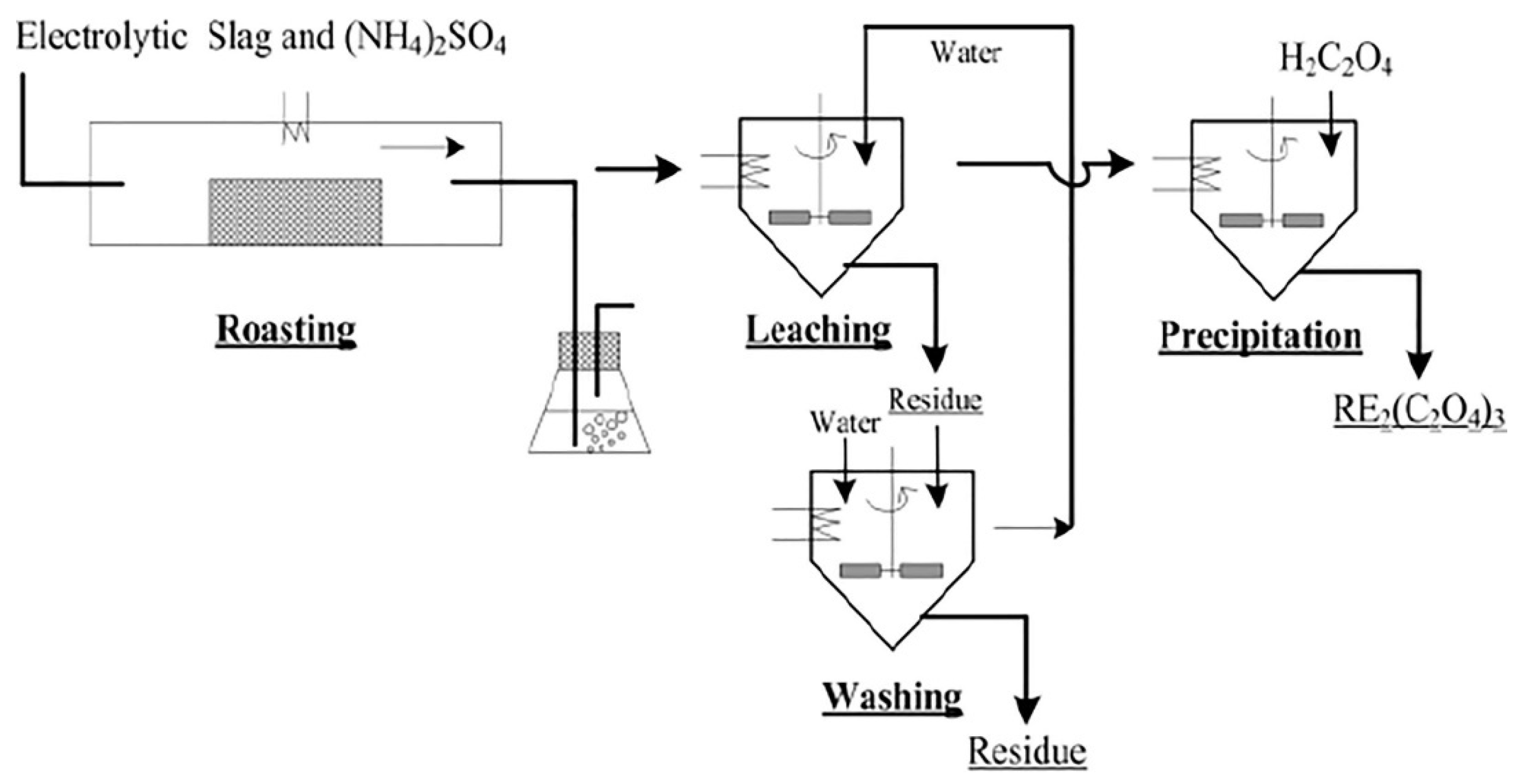
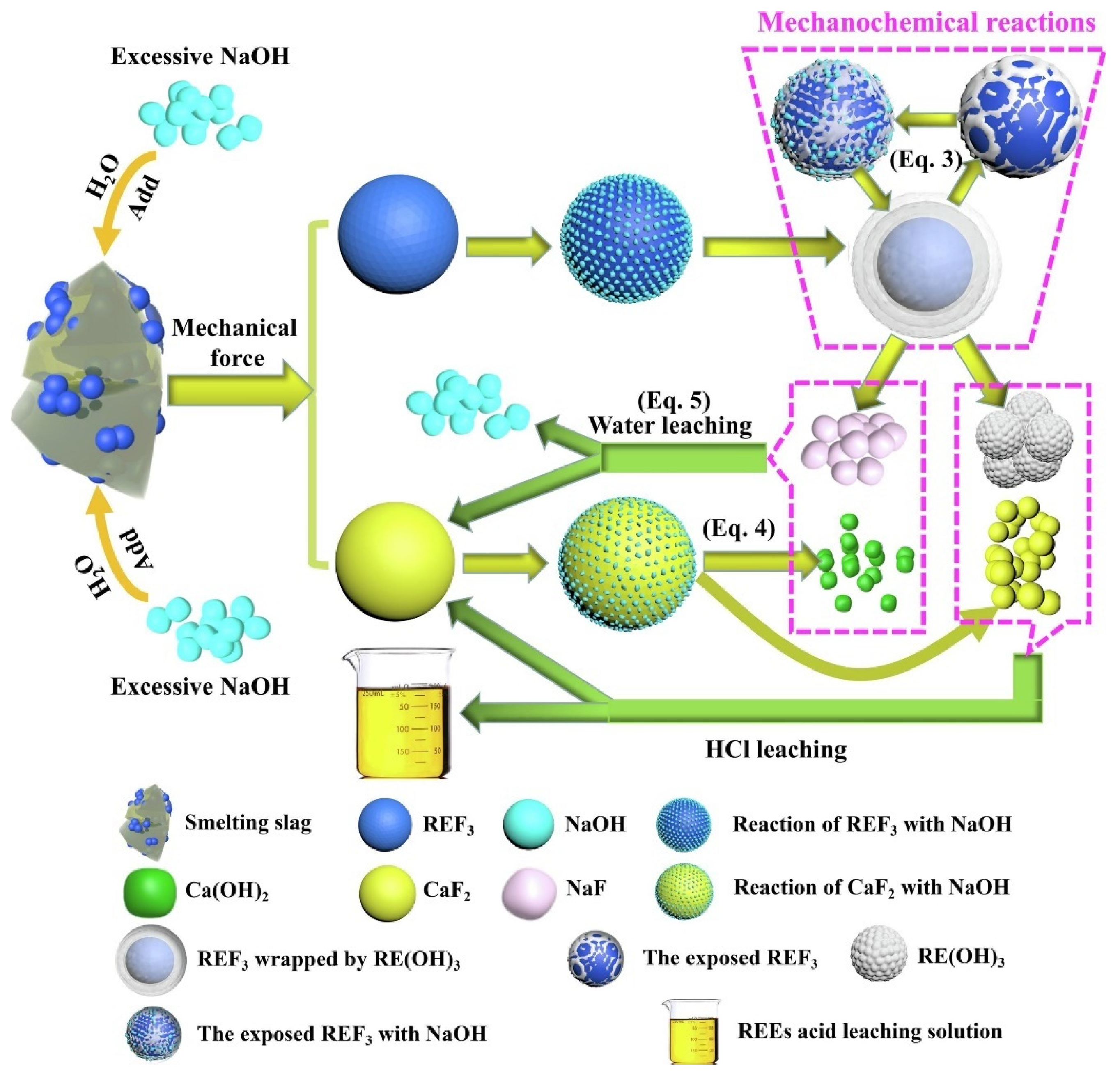
2.3. Roasting-Sulfuric Acid Leaching Method
2.4. Electrolysis and Solvent Extraction
3. Process Comparison of the Preparation of Rare Earth Compounds by Engineering Methods
4. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Liu, X.; Huang, W.; Bettinelli, M.; Liu, X. Lanthanide-Activated Phosphors Based on 4f-5d Optical Transitions: Theoretical and Experimental Aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef] [PubMed]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Soller, B.S.; Salzinger, S.; Rieger, B. Rare Earth Metal-Mediated Precision Polymerization of Vinylphosphonates and Conjugated Nitrogen-Containing Vinyl Monomers. Chem. Rev. 2016, 116, 1993–2022. [Google Scholar] [CrossRef] [PubMed]
- Ortu, F. Rare Earth Starting Materials and Methodologies for Synthetic Chemistry. Chem. Rev. 2022, 122, 6040–6116. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, J.; Yue, J.; Wei, Y.; Gao, C.; Xie, X.; Huang, L. Recent Development in Sensitizers for Lanthanide-Doped Upconversion Luminescence. Chem. Rev. 2022, 122, 15998–16050. [Google Scholar] [CrossRef]
- Matulionyte, M.; Skripka, A.; Ramos-Guerra, A.; Benayas, A.; Vetrone, F. The Coming of Age of Neodymium: Redefining Its Role in Rare Earth Doped Nanoparticles. Chem. Rev. 2023, 123, 515–554. [Google Scholar] [CrossRef] [PubMed]
- Berends, A.C.; Van De Haar, M.A.; Krames, M.R. YAG:Ce3+ Phosphor: From Micron-Sized Workhorse for General Lighting to a Bright Future on the Nanoscale. Chem. Rev. 2020, 120, 13461–13479. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Van Hecke, K.; Van Deun, R. Nano- and Micro-Sized Rare-Earth Carbonates and Their Use as Precursors and Sacrificial Templates for the Synthesis of New Innovative Materials. Chem. Soc. Rev. 2015, 44, 2032–2059. [Google Scholar] [CrossRef]
- Wirth, S.; Steglich, F. Exploring Heavy Fermions from Macroscopic to Microscopic Length Scales. Nat. Rev. Mater. 2016, 1, 16051. [Google Scholar] [CrossRef]
- Azimi, G.; Dhiman, R.; Kwon, H.-M.; Paxson, A.T.; Varanasi, K.K. Hydrophobicity of Rare-Earth Oxide Ceramics. Nat. Mater 2013, 12, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, P.; Chen, X.-Q.; Fu, P.; Luan, Y.; Hu, X.; Liu, H.; Sun, M.; Chen, Y.; Cao, Y.; et al. Low-Oxygen Rare Earth Steels. Nat. Mater. 2022, 21, 1137–1143. [Google Scholar] [CrossRef]
- Arai, Y.; Kuroda, K.; Nomoto, T.; Tin, Z.H.; Sakuragi, S.; Bareille, C.; Akebi, S.; Kurokawa, K.; Kinoshita, Y.; Zhang, W.-L.; et al. Multipole Polaron in the Devil’s Staircase of CeSb. Nat. Mater. 2022, 21, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.W.; Felker, D.A.; Bark, C.W.; Wang, Y.; Niranjan, M.K.; Nelson, C.T.; Zhang, Y.; Su, D.; Folkman, C.M.; Baek, S.H.; et al. Metallic and Insulating Oxide Interfaces Controlled by Electronic Correlations. Science 2011, 331, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Perlepe, P.; Oyarzabal, I.; Mailman, A.; Yquel, M.; Platunov, M.; Dovgaliuk, I.; Rouzières, M.; Négrier, P.; Mondieig, D.; Suturina, E.A.; et al. Metal-Organic Magnets with Large Coercivity and Ordering Temperatures up to 242 °C. Science 2020, 370, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Van Loy, S.; Binnemans, K.; Van Gerven, T. Mechanochemical-Assisted Leaching of Lamp Phosphors: A Green Engineering Approach for Rare-Earth Recovery. Engineering 2018, 4, 398–405. [Google Scholar] [CrossRef]
- Wei, P.; Zhuang, L.; Yu, H.; Qin, Y.; Chu, Y. Low-temperature Molten Salt Synthesis of Ultrafine High-entropy Rare-earth Silicate Powders. J. Am. Ceram. Soc. 2024, 107, 1372–1382. [Google Scholar] [CrossRef]
- Takano, M.; Asano, S.; Goto, M. Recovery of Nickel, Cobalt and Rare-Earth Elements from Spent Nickel–Metal-Hydride Battery: Laboratory Tests and Pilot Trials. Hydrometallurgy 2022, 209, 105826. [Google Scholar] [CrossRef]
- Wei, P.; Zhao, S.; Zhuang, L.; Yu, H.; Qin, Y.; Chu, Y. Chemical Co-precipitation Synthesis of High-entropy Rare-earth Silicate Nanopowders. J. Am. Ceram. Soc. 2024, 107, 3577–3586. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.; Qiu, T.; Zhao, G.; Fang, X. Optimization of Rare Earth Carbonate Reactive-Crystallization Process Based on Response Surface Method. J. Rare Earths 2021, 39, 98–104. [Google Scholar] [CrossRef]
- Meng, X.; Zhao, H.; Zhao, Y.; Shen, L.; Gu, G.; Qiu, G. Heap Leaching of Ion Adsorption Rare Earth Ores and REEs Recovery from Leachate with Lixiviant Regeneration. Sci. Total Environ. 2023, 898, 165417. [Google Scholar] [CrossRef] [PubMed]
- Onoda, H.; Iinuma, A. Selective Preparation of Neodymium Phosphates from Iron Mixed Solution by Two-Step Precipitation. J. Environ. Chem. Eng. 2020, 8, 104083. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Luo, B.; Li, L.; Sun, X. A Quantitative Recovery Process of Rare Earth in Bastnaesite Leachate for Energy Saving and Emission Reduction. Miner. Eng. 2022, 190, 107920. [Google Scholar] [CrossRef]
- He, L.; Xu, Q.; Li, W.; Dong, Q.; Sun, W. One-Step Separation and Recovery of Rare Earth and Iron from NdFeB Slurry via Phosphoric Acid Leaching. J. Rare Earths 2022, 40, 338–344. [Google Scholar] [CrossRef]
- Shi, Q.; Natarajan, A.R.; Van Der Ven, A.; Allison, J. Partitioning of Ca to Metastable Precipitates in a Mg-Rare Earth Alloy. Mater. Res. Lett. 2023, 11, 222–230. [Google Scholar] [CrossRef]
- Meng, X.; Zhao, H.; Zhao, Y.; Shen, L.; Gu, G.; Qiu, G. Effective Recovery of Rare Earth from (Bio)Leaching Solution through Precipitation of Rare Earth-Citrate Complex. Water Res. 2023, 233, 119752. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Rezaee, M.; Pisupati, S.V. Precipitation of Rare Earth Elements from Acid Mine Drainage by CO2 Mineralization Process. Chem. Eng. J. 2020, 399, 125716. [Google Scholar] [CrossRef]
- Li, R.; Bai, G.; Zhang, H.; Song, E.; Zhang, J.; Li, D.; Zhang, J.; Xu, S. Promoting Thermal Stability and Acid Resistance of Rare Earth Sulfide Powders by Coating ZnO and SiO2 Double Shells. J. Am. Ceram. Soc. 2023, 107, 3415–3423. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Tang, L.; Zhong, C.; Liu, Y.; Lu, L.; Qiu, T.; Liu, H. Behavior and Mechanism of Low-Concentration Rare Earth Ions Precipitated by the Microbial Humic-like Acids. Environ. Sci. Pollut. Res. 2020, 27, 21965–21976. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Li, K.; Wang, L.; Niu, F.; Liu, D.; Meng, Y. Maintenance of the Metastable State and Induced Precipitation of Dissolved Neodymium (III) in an Na2CO3 Solution. Minerals 2021, 11, 952. [Google Scholar] [CrossRef]
- Yin, J.; Zou, Z.; Tian, J. Preparation of Crystalline Rare Earth Carbonates with Large Particle Size from the Lixivium of Weathered Crust Elution-Deposited Rare Earth Ores. Int. J. Miner. Metall. Mater. 2020, 27, 1482–1488. [Google Scholar] [CrossRef]
- Zhi, H.; Feng, L.; Ni, S.; Cui, J.; Sun, X. The Transformation and Enrichment of Rare Earth Sulfate in Bayan Obo Ore Water Leaching Solution by Dibenzyl Phosphate Based Extraction-Precipitation Method. Sep. Purif. Technol. 2021, 277, 119464. [Google Scholar] [CrossRef]
- Chen, F.; Liu, F.; Wang, L.; Wang, J. Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes 2022, 10, 2310. [Google Scholar] [CrossRef]
- He, Q.; Lai, F.; Lai, A.; Qiu, J.; Xiao, Y. Removal of Sulfate Ions from Calcium Oxide Precipitation Enrichment of a Rare Earth Leaching Liquor by Stirring Washing with Sodium Hydroxide. ACS Omega 2021, 6, 5209–5220. [Google Scholar] [CrossRef]
- Trisnawati, I.; Yulandra, A.; Prameswara, G.; Pusparini, W.R.; Mulyono, P.; Prasetya, A.; Petrus, H.T.B.M. Optimization of Multistage Precipitation Processes for Rare Earth Element Purification from Indonesian Zircon Tailings. J. Sustain. Metall. 2021, 7, 537–546. [Google Scholar] [CrossRef]
- Barve, R.A.; Panigrahi, B.S.; Suriyamurthy, N.; Venkatraman, B. Luminescent Properties of CaSiO3: Ce3+: A Preliminary Study. Phys. B Condens. Matter 2021, 612, 412925. [Google Scholar] [CrossRef]
- Aslam, S.; Shahzad Shifa, M.; Abbas Gilani, Z.; Noor Ul Huda Khan Asghar, H.M.; Nauman Usmani, M.; Ur Rehman, J.; Azhar Khan, M.; Perveen, A.; Khalid, M. Structural, Optical and Magnetic Elucidation of Co-Doping of Nd3+ and Pr3+ on Lithium Nanoferrite and Its Technological Application. Results Phys. 2019, 12, 1334–1339. [Google Scholar] [CrossRef]
- Ali, I.; Islam, M.U.; Ashiq, M.N.; Asif Iqbal, M.; Karamat, N.; Awan, M.S.; Naseem, S. Role of Tb–Mn Substitution on the Magnetic Properties of Y-Type Hexaferrites. J. Alloys Compd. 2014, 599, 131–138. [Google Scholar] [CrossRef]
- Xiong, L.-Q.; Chen, Z.-G.; Yu, M.-X.; Li, F.-Y.; Liu, C.; Huang, C.-H. Synthesis, Characterization, and in Vivo Targeted Imaging of Amine-Functionalized Rare-Earth up-Converting Nanophosphors. Biomaterials 2009, 30, 5592–5600. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Li, J.; Qi, X.; Guo, Z.; Wei, H.; Chu, H. Gold Nanoparticles on Nanosheets Derived from Layered Rare-Earth Hydroxides for Catalytic Glycerol-to-Lactic Acid Conversion. ACS Appl. Mater. Interfaces 2021, 13, 522–530. [Google Scholar] [CrossRef]
- Kumar, D.; Verma, S.; Verma, K.; Som, S.; Kumar, V.; Swart, H.C. Enhanced Upconversion Study of Er3+-Yb3+ Codoped NaYF4 Phosphors Synthesized by the Reverse Microemulsion Method. Ceram. Int. 2018, 44, 13649–13653. [Google Scholar] [CrossRef]
- Predeanu, G.; Slăvescu, V.; Bălănescu, M.; Dorina Mihalache, R.; Mihaly, M.; Marin, A.C.; Meghea, A.; Valentim, B.; Guedes, A.; Abagiu, A.T.; et al. Coal Bottom Ash Processing for Capitalization According to Circular Economy Concept. Miner. Eng. 2021, 170, 107055. [Google Scholar] [CrossRef]
- Karamat, N.; Ali, I.; Aziz, A.; Sher, M.; Ashiq, M.N. Electrical and Dielectric Studies of Substituted Holmium Based Pyrochlore Zirconates Nanomaterials. J. Alloys Compd. 2015, 652, 83–90. [Google Scholar] [CrossRef]
- Zhang, J.; Wulantuya; Di, X.; Liu, Z.; Xu, G.; Xu, S. Preparation of YVO4:RE (RE = Yb3+/Er3+, Yb3+/Tm3+) Nanoparticles via Microemulsion-Mediated Hydrothermal Method. Trans. Nonferrous Met. Soc. China 2010, 20, s231–s235. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, L.; Ma, J.; Yang, R.; Chen, Y. Effect of the Thermodynamic Properties of W/O Microemulsions on Samarium Oxide Nanoparticle Size. J. Colloid Interface Sci. 2009, 340, 119–125. [Google Scholar] [CrossRef]
- Xu, Y.; Suthar, J.; Egbu, R.; Weston, A.J.; Fogg, A.M.; Williams, G.R. Reverse Microemulsion Synthesis of Layered Gadolinium Hydroxide Nanoparticles. J. Solid State Chem. 2018, 258, 320–327. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Huang, C.-H.; Cheng, B.-M.; Lu, C.-H. Influence of Microemulsion Conditions on the VUV-Excited Luminescence and Microstructures of Y3Al5O12: Eu3+ Phosphors. Mater. Chem. Phys. 2010, 124, 632–638. [Google Scholar] [CrossRef]
- Mortier, M.; Bensalah, A.; Dantelle, G.; Patriarche, G.; Vivien, D. Rare-Earth Doped Oxyfluoride Glass-Ceramics and Fluoride Ceramics: Synthesis and Optical Properties. Opt. Mater. 2007, 29, 1263–1270. [Google Scholar] [CrossRef]
- Janssens, S.; Williams, G.V.M.; Clarke, D. Synthesis and Characterization of Rare Earth and Transition Metal Doped BaMgF4 Nanoparticles. J. Lumin. 2013, 134, 277–283. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X.; Tang, Q.; Li, Y. A New Type of Silica-Coated Gd2(CO3)3:Tb Nanoparticle as a Bifunctional Agent for Magnetic Resonance Imaging and Fluorescent Imaging. Nanotechnology 2012, 23, 205103. [Google Scholar] [CrossRef]
- Wang, G.; Qin, W.; Zhang, J.; Zhang, J.; Wang, Y.; Cao, C.; Wang, L.; Wei, G.; Zhu, P.; Kim, R. Synthesis and Spectral Properties of Eu3+-Doped YF3 Nanobundles. J. Fluor. Chem. 2008, 129, 621–624. [Google Scholar] [CrossRef]
- Wang, G.; Qin, W.; Zhang, D.; Wei, G.; Zheng, K.; Wang, L.; Ding, F. Synthesis and Upconversion Luminescence Properties of Yb3+/Tm3+-Codoped BaSiF6 Nanorods. J. Fluor. Chem. 2009, 130, 755–758. [Google Scholar] [CrossRef]
- Wang, G.; Qin, W.; Wei, G.; Wang, L.; Zhu, P.; Kim, R.; Zhang, D.; Ding, F.; Zheng, K. Synthesis and Upconversion Luminescence Properties of YF3:Yb3+/Tm3+ Octahedral Nanocrystals. J. Fluor. Chem. 2009, 130, 158–161. [Google Scholar] [CrossRef]
- Wu, S.; Fang, J.; Xu, X.; Liu, Z.; Zhu, X.; Xu, W. Microemulsion Synthesis, Characterization of Highly Visible Light Responsive Rare Earth-Doped Bi2O3. Photochem. Photobiol. 2012, 88, 1205–1210. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, W.; Zhang, J.; Cao, C.; Zhang, J.; Jin, Y.; Ren, X.; Zheng, Z.; Lü, S. Synthesis, Photoluminescence and Bioconjugation of Rare-Earth (Eu) Complexes-Embedded Silica Nanoparticles. Solid State Commun. 2007, 142, 689–693. [Google Scholar] [CrossRef]
- Jian, Z.; Pu, Y.; Fang, J.; Ye, Z. Microemulsion Synthesis of Nanosized TiO2 Particles Doping with Rare-Earth and Their Photocatalytic Activity. Photochem. Photobiol. 2010, 86, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cao, B.; Ma, R.; Song, H.; Song, H. Polyvinylpyrrolidone-Stabilized Pt Colloidal Catalysts in Chloronitrobenzene Hydrogenation and Modification with Rare Earth Ions. React. Kinet. Mech. Catal. 2015, 116, 479–489. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, W.; Zhang, J.; Cao, C.; Zhang, J.; Jin, Y.; Ren, X.; Zhu, P.; Wei, G.; Wang, G.; et al. Europium(III) Complexes/Silica Hybrid Nanospheres Synthesized in Microemulsion. J. Nanosci. Nanotechnol. 2008, 8, 1218–1220. [Google Scholar] [CrossRef]
- Gao, L.-L.; Song, S.-Y.; Ma, J.-F.; Yang, J. Microemulsion-Mediated Solvothermal Synthesis and Photoluminescent Properties of Europium Tungstate Nanostructures. J. Nanosci. Nanotechnol. 2013, 13, 4228–4234. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H. Selective Extraction of Rare Earths and Lithium from Rare Earth Fluoride Molten-Salt Electrolytic Slag by Sulfation. Miner. Eng. 2021, 160, 106711. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, F.; Chen, T.; Wang, C.; Hu, K.; Zhang, C.; Huang, Z.; Li, X.; Wan, Y. Effective Recovery of Rare Earth and Fluorine from REF3 Smelting Slag by a Mechanochemical Process. Sep. Purif. Technol. 2023, 316, 123832. [Google Scholar] [CrossRef]
- Chen, S.; Feng, Z.; Wang, M.; Zhao, L.; Yu, Z.; Xia, C.; Huang, X. Leaching Kinetic Study of Sulfuric Acid Roasted Mixed-Type Rare Earth Concentrate for Reducing the Solid-Waste Production and Chemical Consumption. J. Clean. Prod. 2020, 260, 120989. [Google Scholar] [CrossRef]
- Zhao, J.; Li, B.; Wei, X. Dry Processing Technology of Exhaust Gas Emitted by Roasting of Rare Earth Concentrates with Concentrated Sulfuric Acid. J. Clean. Prod. 2021, 327, 129489. [Google Scholar] [CrossRef]
- Nawab, A.; Yang, X.; Honaker, R. An Acid Baking Approach to Enhance Heavy Rare Earth Recovery from Bituminous Coal-Based Sources. Miner. Eng. 2022, 184, 107610. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Liu, Z.; Zhang, D.; Gao, K.; Li, M. A Novel, Clean, Closed-Loop Process for Directional Recovery of Rare Earth Elements, Fluorine, and Phosphorus from Mixed Rare Earth Concentrate. J. Clean. Prod. 2021, 321, 128784. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, L.; Wang, M.; Feng, Z.; Xia, C.; Xu, Y.; Huang, X. Effects of Iron and Temperature on Solubility of Light Rare Earth Sulfates in Multicomponent System of Fe2(SO4)3-H3PO4-H2SO4 Synthetic Solution. J. Rare Earths 2020, 38, 1243–1250. [Google Scholar] [CrossRef]
- Wang, M.; Huang, X.; Feng, Z.; Xia, C.; Meng, D.; Yu, Z. Behavior of Sulfate in Preparation of Single Light Rare Earth Carbonate by Mg(HCO3)2 Precipitation Method. J. Rare Earths 2021, 39, 850–857. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, X.; Meng, D.; Liu, X.; Zhong, Q.; Feng, Z. Effect of Phase Transition during Roasting of Mountain Pass Rare Earth Concentrate on Leaching Efficiency of Rare Earths. J. Rare Earths 2024, 42, 409–414. [Google Scholar] [CrossRef]
- Zinoveev, D.; Grudinsky, P.; Zhiltsova, E.; Grigoreva, D.; Volkov, A.; Dyubanov, V.; Petelin, A. Research on High-Pressure Hydrochloric Acid Leaching of Scandium, Aluminum and Other Valuable Components from the Non-Magnetic Tailings Obtained from Red Mud after Iron Removal. Metals 2021, 11, 469. [Google Scholar] [CrossRef]
- Chadirji-Martinez, K.; Grosvenor, A.P.; Crawford, A.; Chernikov, R.; Heredia, E.; Feng, R.; Pan, Y. Thorium Speciation in Synthetic Anhydrite: Implications for Remediation and Recovery of Thorium from Rare-Earth Mine Tailings. Hydrometallurgy 2022, 214, 105965. [Google Scholar] [CrossRef]
- Gupta, T.; Nawab, A.; Honaker, R. Pretreatment of Bituminous Coal By-Products for the Hydrometallurgical Extraction of Rare Earth Elements. Minerals 2023, 13, 614. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Wang, N.; Gu, H. Selective Extraction of Rare Earth Elements from Red Mud Using Oxalic and Sulfuric Acids. J. Environ. Chem. Eng. 2022, 10, 108650. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, Z.; Luo, T.; Wu, X.; Xie, Z.; Lan, S.; Xie, S.; Zhou, D. Conversion of Cerium and Lanthanum from Rare Earth Polishing Powder Wastes to CeO2 and La0.6Ca0.4CoO3. Hydrometallurgy 2020, 193, 105317. [Google Scholar] [CrossRef]
- Shakiba, G.; Saneie, R.; Abdollahi, H.; Ebrahimi, E.; Rezaei, A.; Mohammadkhani, M. Application of Deep Eutectic Solvents (DESs) as a Green Lixiviant for Extraction of Rare Earth Elements from Caustic-Treated Monazite Concentrate. J. Environ. Chem. Eng. 2023, 11, 110777. [Google Scholar] [CrossRef]
- Kang, C.; Yang, S.; Qiao, J.; Zhao, Y.; Dong, S.; Wang, Y.; Duan, C.; Liu, J. Extraction of Valuable Critical Metals from Coal Gangue by Roasting Activation-Sulfuric Acid Leaching. Int. J. Coal Prep. Util. 2024, 1–18. [Google Scholar] [CrossRef]
- Jürjo, S.; Oll, O.; Paiste, P.; Külaviir, M.; Zhao, J.; Lust, E. Electrochemical Co-Reduction of Praseodymium and Bismuth from 1-Butyl-1-Methylpyrrolidinium Bis(Fluorosulfonyl)Imide Ionic Liquid. Electrochem. Commun. 2022, 138, 107285. [Google Scholar] [CrossRef]
- Zhou, Y.; Schulz, S.; Haberstroh, J.; Wenzel, M.; Du, H.; Weigand, J.J. CeRES Process−Separation of Ce Rium from Lanthanum by R Edox E Xtraction and S Tripping. ACS Sustain. Chem. Eng. 2022, 10, 16290–16298. [Google Scholar] [CrossRef]
- Hirota, K.; Okabe, T.H.; Saito, F.; Waseda, Y.; Jacob, K.T. Electrochemical Deoxidation of RE–O (RE = Gd, Tb, Dy, Er) Solid Solutions. J. Alloys Compd. 1999, 282, 101–108. [Google Scholar] [CrossRef]
- Kamimoto, Y.; Itoh, T.; Kuroda, K.; Ichino, R. Recovery of Rare-Earth Elements from Neodymium Magnets Using Molten Salt Electrolysis. J. Mater. Cycles Waste Manag. 2017, 19, 1017–1021. [Google Scholar] [CrossRef]
- Venkatesan, P.; Vander Hoogerstraete, T.; Binnemans, K.; Sun, Z.; Sietsma, J.; Yang, Y. Selective Extraction of Rare-Earth Elements from NdFeB Magnets by a Room-Temperature Electrolysis Pretreatment Step. ACS Sustain. Chem. Eng. 2018, 6, 9375–9382. [Google Scholar] [CrossRef]
- Kumari, A.; Dipali; Randhawa, N.S.; Sahu, S.K. Electrochemical Treatment of Spent NdFeB Magnet in Organic Acid for Recovery of Rare Earths and Other Metal Values. J. Clean. Prod. 2021, 309, 127393. [Google Scholar] [CrossRef]
- Chung, H.; Prasakti, L.; Stopic, S.R.; Feldhaus, D.; Cvetković, V.S.; Friedrich, B. Recovery of Rare Earth Elements from Spent NdFeB Magnets: Metal Extraction by Molten Salt Electrolysis (Third Part). Metals 2023, 13, 559. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X. Characteristics of Electrical Resistance Alteration during In Situ Leaching of Ion-Adsorption-Type Rare Earth Ore. Minerals 2024, 14, 92. [Google Scholar] [CrossRef]
- Bourbos, E.; Giannopoulou, I.; Karantonis, A.; Paspaliaris, I.; Panias, D. Reduction of Light Rare Earths and a Proposed Process for Nd Electrorecovery Based on Ionic Liquids. J. Sustain. Metall. 2018, 4, 395–406. [Google Scholar] [CrossRef]
- Nakanishi, B.R.; Allanore, A. Electrochemical Investigation of Molten Lanthanum-Yttrium Oxide for Selective Liquid Rare-Earth Metal Extraction. J. Electrochem. Soc. 2019, 166, E420–E428. [Google Scholar] [CrossRef]
- Pérez-Cardona, J.R.; Huang, T.-Y.; Zhao, F.; Sutherland, J.W.; Atifi, A.; Fox, R.V.; Baek, D.L. Molten Salt Electrolysis and Room Temperature Ionic Liquid Electrochemical Processes for Refining Rare Earth Metals: Environmental and Economic Performance Comparison. Sustain. Energy Technol. Assess. 2022, 54, 102840. [Google Scholar] [CrossRef]
- Sronsri, C.; Sittipol, W.; Panitantum, N.; U-yen, K. Optimization of Elemental Recovery from Electronic Wastes Using a Mild Oxidizer. Waste Manag. 2021, 135, 420–427. [Google Scholar] [CrossRef]
- Abbasalizadeh, A.; Seetharaman, S.; Venkatesan, P.; Sietsma, J.; Yang, Y. Use of Iron Reactive Anode in Electrowinning of Neodymium from Neodymium Oxide. Electrochim. Acta 2019, 310, 146–152. [Google Scholar] [CrossRef]
- Zhou, Y.; Schulz, S.; Lindoy, L.F.; Du, H.; Zheng, S.; Wenzel, M.; Weigand, J.J. Separation and Recovery of Rare Earths by in Situ Selective Electrochemical Oxidation and Extraction from Spent Fluid Catalytic Cracking (FCC) Catalysts. Hydrometallurgy 2020, 194, 105300. [Google Scholar] [CrossRef]
- Xu, X.; Sturm, S.; Samardzija, Z.; Scancar, J.; Markovic, K.; Zuzek Rozman, K. A Facile Method for the Simultaneous Recovery of Rare-Earth Elements and Transition Metals from Nd–Fe–B Magnets. Green Chem. 2020, 22, 1105–1112. [Google Scholar] [CrossRef]
- Vasudevan, S.; Sozhan, G.; Mohan, S.; Pushpavanam, S. An Electrochemical Process for the Separation of Cerium from Rare Earths. Hydrometallurgy 2005, 76, 115–121. [Google Scholar] [CrossRef]
- Venkatesan, P.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. An Environmentally Friendly Electro-Oxidative Approach to Recover Valuable Elements from NdFeB Magnet Waste. Sep. Purif. Technol. 2018, 191, 384–391. [Google Scholar] [CrossRef]
- Ji, X.; Wu, C.; Jan, S.; Wang, Z.; Jin, X. Using Rare Earth Oxychlorides as Precursors to Prepare Rare Earth Alloys through Solid Cathode Electrolysis in Molten CaCl2. Electrochem. Commun. 2019, 103, 27–30. [Google Scholar] [CrossRef]
- Peng, X.; Feng, S.; Lai, S.; Liu, Z.; Gao, J.; Javanbakht, M.; Gao, B. Structural Engineering of Rare-Earth-Based Perovskite Electrocatalysts for Advanced Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2022, 47, 39470–39485. [Google Scholar] [CrossRef]
- Yasuda, K.; Kondo, K.; Kobayashi, S.; Nohira, T.; Hagiwara, R. Selective Formation of Rare Earth-Nickel Alloys via Electrochemical Reactions in NaCl-KCl Molten Salt. ECS Trans. 2014, 64, 601–607. [Google Scholar] [CrossRef]
- Junaid, M.; Jacob, J.; Nadeem, M.; Jabbar, N.; Khan, M.A.; Manzoor, A.; Chughtai, A.H.; Ali, A.; Mahmood, K.; Hussain, S. Structural Elucidation and Dielectric Behavior Evaluation of Dy–Ni Substituted Manganese Ferrites. Phys. B Condens. Matter 2021, 602, 412494. [Google Scholar] [CrossRef]



| Preparation Methods | Prepared Products | Reaction Temperature | Raw Material | Reaction Conditions | Product Characteristics | Yield/ Recovery |
|---|---|---|---|---|---|---|
| Molten salt precipitation method [18] | HEREDs | 1073 K | RE2O3:SiO2 = 1:2.4 | 2 h | Grain size: 1.32 μm | |
| Molten salt precipitation method [18] | HEREMs | 1173 K | RE2O3:SiO2 = 1:0.9 | 3 h | Grain size; 1.64 μm | |
| Bisulfate coprecipitation method [19] | RE2O3 | 50–90 °C | Solid waste | 650 rpm, 1 h | RE2O3 | La > 99%, Ce > 99% |
| Chemical precipitation method [20] | HEREs | 1173 K | RE/Si = 1:0.4 | pH = 7~8 | Type X2 monosilicic acid | - |
| Chemical precipitation method [21] | RE2(CO3)3 | Room temperature | Heavy rare earth | Concentration: 1.75 g/L, seed crystal: 13.56 wt%, maturing: 8 h | 28.23 μm, Water rhombic structure | 97.82% |
| Organic precipitation method [22] | REEs | Room temperature | Citrate:50 mmol/L, L:S = 1:2 | Ph5 | RE2O3 | 96% |
| Two-step precipitation method [23] | NdPO4 | Room temperature | 0.002 mol/LNdCl3·6H2O, NaOH, 0.2 mol/LH3PO4 | pH 4 | NdPO4 | 99.97% |
| Phosphoric acid one-step precipitation method [25] | REEs | 80 °C | 4 mol/LH3PO4, S:L = 30:1 | Extraction: 1.5 h | RE2O3 | 99.49% |
| CO2 mineralization and precipitation method [28] | REEs | Room temperature | CO2/NaOH | Step 1 pH 5 Step 2 pH 7 | RE2(CO3)3 | 95% |
| Heterogeneous precipitation method [29] | γ-Ce2S3 | Room temperature | γ-Ce2S3, CTAB, Zn(NO3)2 | 50 °C, agitation: 5 h, calcination: 200°C | γ-Ce2S3 powder coated with ZnO | - |
| Bioleaching three-step precipitation method [27] | RE2(CO3)3 | Room temperature | CaCO3, Na2CO3 | pH > 2 | RE2(CO3)3 | 96% |
| Sludge-based humic acid precipitation method [30] | RE2(CO3)3 | 25 °C | Low concentration rare earth solution | L:S = 1:1, 100 rpm, pH 8.6 | Amorphous particle, No crystal characteristic peaks | 89% |
| Extraction-precipitation method [24] | RE (III) | 60 °C | NLSA, rare earth solution | 6 mol/L HCl | 61.5 μm | 96% |
| Chemical precipitation method [31] | Nd | Room temperature | NaCl, CO | 0.5 mol/L NaCl, 450 min | RE2(CO3)3 | 93% |
| Chemical precipitation method [32] | RE2(CO3)3 | 40–70 °C | NH4HCO3 | pH 5–8, 500 rpm | 50~200 μm | 95% |
| Chemical precipitation method [33] | RECl3 | Room temperature | DBP, DPP, TPP, DPPO | pH 4.5 | 300 μm | 99.99% |
| Enrichment and precipitation method [35] | RE2O3 | 35 °C | n(NaOH)/n(RE3+) = 2.85, L:S = 6.5 mL/g | Agitation: 20 min | 5 μm | 94.389% |
| Multistage precipitation method [36] | LREEs, HREEs | 50 °C | RE3+, oxalic acid, NaOH | 15%Na2CO3, pH 4.5, 200 rpm | 10 μm | 88%/74% |
| Coprecipitation method [37] | γ-Ca2SiO4, β-Ca2SiO4 | 1200 °C | RE, Ca, Si | n(Ce) = 1, 1.43, 2 | Fluorescence lifetime: 33.5, 34.3, 36.5 ns | - |
| Prepared Products | Process Means | Means of Characterization | Product Characteristics | Product Performance |
|---|---|---|---|---|
| LiNi0.35−yCo0.15PryNdxFe2−xO4 [38] | Doping method | XRD, FTIR, VSM | 28~70 nm | Magnetic(al) matrix performance: −10,000~10,000 Oe |
| Sr2Co2xMnxTbyFe12yO22 [39] | Doping method | SEM, VDXS, VSM | Y type hexagonal ferrite | Magnetic(al) matrix performance > 3200 Oe |
| UCNPs [40] | Hydrothermal method | LSUCLM, XRD, EDXA, FTIR | 20~40 nm | Amino amount: (9.5 ± 0.8) × 10−5 mol/g |
| LREH nanosheets [41] | Inversion method | XRD, SEM, FTIR | Homogeneous nanosheets | Dimension < 3 nm) |
| NaYF4 fluorescent nanocrystalline powder [42] | Inversion method | XRD, TEM, SEM | Hexagonal nanopowder | a = b = 5.9168 Å, c = 3.331 Å |
| Enrichment and concentration of REE [43] | Concentrated nitric acid enrichment and water washing | XRD, SEM | Extract | - |
| Ho2Zr2−xGexO7 [44] | Conventional law | XRD, SEM | Single-phase cubic structure | Dielectric loss < 1.3 GHz |
| YVO4: RE (RE = Yb3+/Er3+, Yb3+/Tm3+) [45] | Hydrothermal method | XRD, TEM | Nanoparticles | Controllable particle size, narrow particle size distribution, and less agglomeration |
| Sm(OH)3 [46] | W/O type | DSC-TGA, XRD, TEM, UV–VIS | Nanoparticles | Nanoparticles |
| Layered gadolinium hydroxide (LGdH) [47] | Inversion method | XRD, TEM, FTIR | 200 nm | Size-controlled nanoparticles |
| YAG: Eu3+ phosphor powder [48] | W/O type | XRD, TEM | 100 nm | Good particle size and good luminosity |
| Glass-ceramic [49] | Doping method | XRD, TEM, DTA | 20 nm | Strong optical performance |
| BaMgF4:Mn2+:Ce3+ nanoparticles [50] | Doping method | XRD, TEM, EDS | 50~80 nm | Internal electronic efficiency = 45% |
| Silica-coated Gd2(CO3)3:Tb nanoparticles [51] | Inversion method | HRTEM, EDS, FTIR | 16 nm | This nanoprobe was successfully delivered to gastric cancer SGC7901 cells in a short period of time and delivered to NCl-H460 lung cancer cells |
| YF3:Eu3+ nanobeams [52] | Doping method | XRD, SEM, TEM | Length = 500 nm, diameter = 2 nm | High luminous intensity |
| BaSiF6:Yb3+ (20%)/Tm3+ (1.2%) [53] | Doping method | XED, TEM | Length = 1 mm | High luminous intensity |
| YF3:Yb3+ (20%)/Tm3+ (2%) [54] | Doping method | XRD, SEM | 100 nm | Good luminosity |
| Bi2O3 doped RE catalyst [55] | Doping method | XRD, TEM, BET | Nano monoclinic crystal | High catalytic ability |
| Eu(DBM)3 [56] | W/O type | TEM, Olympus optical camera | 40 nm | Regular shape, high photobleaching resistance |
| Nano-La-TiO2 and Ce-TiO2 particles [57] | Inversion method | XRD, TEM, FTIR, TG | 20~50 nm | Good photocatalytic activity |
| Polyvinylpyrrolidone (PVP) stabilized Pt colloidal catalyst [58] | Catalytic reflux method | TEM, XPS | Nanoparticles | High catalytic activity |
| Eu(DBM)3Phen/SiO2 nanosphere [59] | Doping method | SEM, TEM | 40 nm | good spherical surface |
| Eu2(WO4)3 nanocrystalline [60] | Solvothermal method | XRD, TEM | Nanoparticles | Controllable morphology |
| Mn1−xNixFe2−yDyyO4 soft magnetic oxide [97] | Doping method | XRD, FTIR, VSM | 20~30 nm | Increase in saturation magnetization |
| Process Means | Means of Characterization | Process Conditions | Reaction Material | Yield/Recovery/Conclusion |
|---|---|---|---|---|
| Selective sulfation method [61] | TGA-DSC, XRD, SEM | (NH4)2SO4/slag = 3:1, 750 °C, 60 min | REF3 1.0MLiOH | REEs > 96.5% |
| Echanized method [62] | XRD, SEM | NaOH/RSS = 0.4:1, 400 rpm, 40 min | REF3 | REEs > 96% |
| Sulfuric acid leaching method [63] | ICP–AES, XRD, SEM | 15 min, 40 °C, 250 rpm, L/S = 7:1 | REF3 | REEs > 97% |
| Exhaust gas dry process [64] | XRD | Adding cyclone separator and heat exchange-condensing acid system | REEs | H2SO4 > 91.17% fluoride > 93.44% |
| Acid-roasting method [65] | ICP–OES, XRD, TGA-DSC | 600 °C, 50 g/L, 2 h | Coal | REEs > 80% |
| Oxidation roasting-acid leaching [66] | ICP–AES, TGA-DSC, SEM | Calcination: 550 °C, 2 h acid pickling 8 MHCl, 20 min, 70 °C | Rare earth concentrate | REO > 280 g/L |
| Acid leaching [67] | ICP–AES | 25~65 °C, L:S = 2:1 Fe2(SO4)3 (0~50.13 g/L), H3PO4 (20.34 g/L) 0.5 M H2SO4 | Rare earth sulfate | Independent system is higher than hybrid system |
| Forward addition method [68] | TGA-DSC, XRD, SEM | n(RE3+): n(HCO3+) = 1:3, 30°C, 300 rpm | Rare earth sulfate | Forward feeding improves efficiency |
| Oxidation roasting-acid leaching [69] | TGA-DSC, XRD, SEM | 4 M HCl, 25 °C, 300 rpm, 1 h, L:S = 6:1 | Astnaesite | REEs > 70.32% |
| Carbonate thermal reduction method [70] | XRD, SEM | 150 °C, acid concentration: 10%, L:S = 1:11, 60 min | Red mud | REEs > 98% |
| Sulfuric acid-roasting method [71] | XRD, SEM, ICP-MS | 353~473 K, pH = 0.3~3 | Monazite mine | Th4+ > 1780 mg/L |
| LTP oxidation method [72] | XRD, SEM | 600 °C, 5 h, L:S = 1.6:1 | Coal | HREE > 53% |
| Multistage extraction acid leaching [73] | TGA-DSC, XRD, SEM | 1 M H2SO4, 3 h, 95 °C, L:S = 5:1 | Red mud | REEs > 80% |
| Roasting-acid leaching [74] | TGA-DSC, XRD, SEM | Six-stage treatment | Rare earth solid waste | REEs > 91.73% |
| Acid leaching [75] | XRD, SEM | 500 °C, 2 h, S:L = 10:1 | Monazite | HREE > 94% |
| Step-by-step chemical extraction method [76] | TGA-DSC, XRD, SEM | Calcination conditions: 650 °C, 2 h; leaching conditions: 120 °C, acid concentration 6 M, L/S = 10:1, 1 h | Coal gangue | Rees = 344 μg/g |
| Purpose | Process Means | Means of Characterization | Experimental Conditions | Conclusion |
|---|---|---|---|---|
| Study on the electrochemical deposition of Pr [77] | Addition of bismuth ions | XRD | Cyclic voltammetry curve | The addition of bismuth ions is more conducive to the precipitation of Pr |
| Separation of La/Ce [78] | In situ electrochemical redox for extraction and back-extraction | Autolab PGSTAT 100 N potentiostatic/galvan-ostatic | Electrolysis 60 min, −1.0 mA/cm2 | La3+ > 99.7% |
| Removing oxygen from RE [79] | Electrochemical oxidation method | Electrochemical workstation | 1189 k, 10 h | Reduction of rare earth metals with oxygen content greater than 2000 mass ppm to 10~50 mass ppm |
| Recycled neodymium magnet [80] | Potential electrolysis | XRD, SEM | −1.8~0.8 V | Recyclable |
| Recovery of REEs from NdFeB magnet scrap [81] | Electrolysis | XRD | 500 rpm, 8 h, 25 °C, INdFeB = 0.5 A, ITi/Pt = 0.2 A | REEs > 97% |
| Recovery of REEs from waste NdFeB magnet [82] | Acid-dissolved electrolysis | XRD, SEM, TG-DTA | 1 M D2EHPA, oxalic acid, 1073 K | REO > 99.9% |
| MRDO extraction of REEs [83] | Low potential potentiostatic deposition | XRD, SEM, ICP-OES | 1050 °C | REEs > 98% |
| Electrodeposition behavior [85] | Potentiostatic deposition | SEM, EDS | −3.1 V, 25 °C, 5 h | REEs > 98% |
| Electrochemical separation of La/Y [86] | Potentiostatic deposition | SEM | 2.5 V, 2573 K | Successful isolation |
| E-waste recovery of REEs [88] | Potentiostatic deposition | Langmuir model | 160 A/m2, 7 mL/min, n(Fe3+) = 0.8 mol/L | Recovery rate =1.135 mg/min |
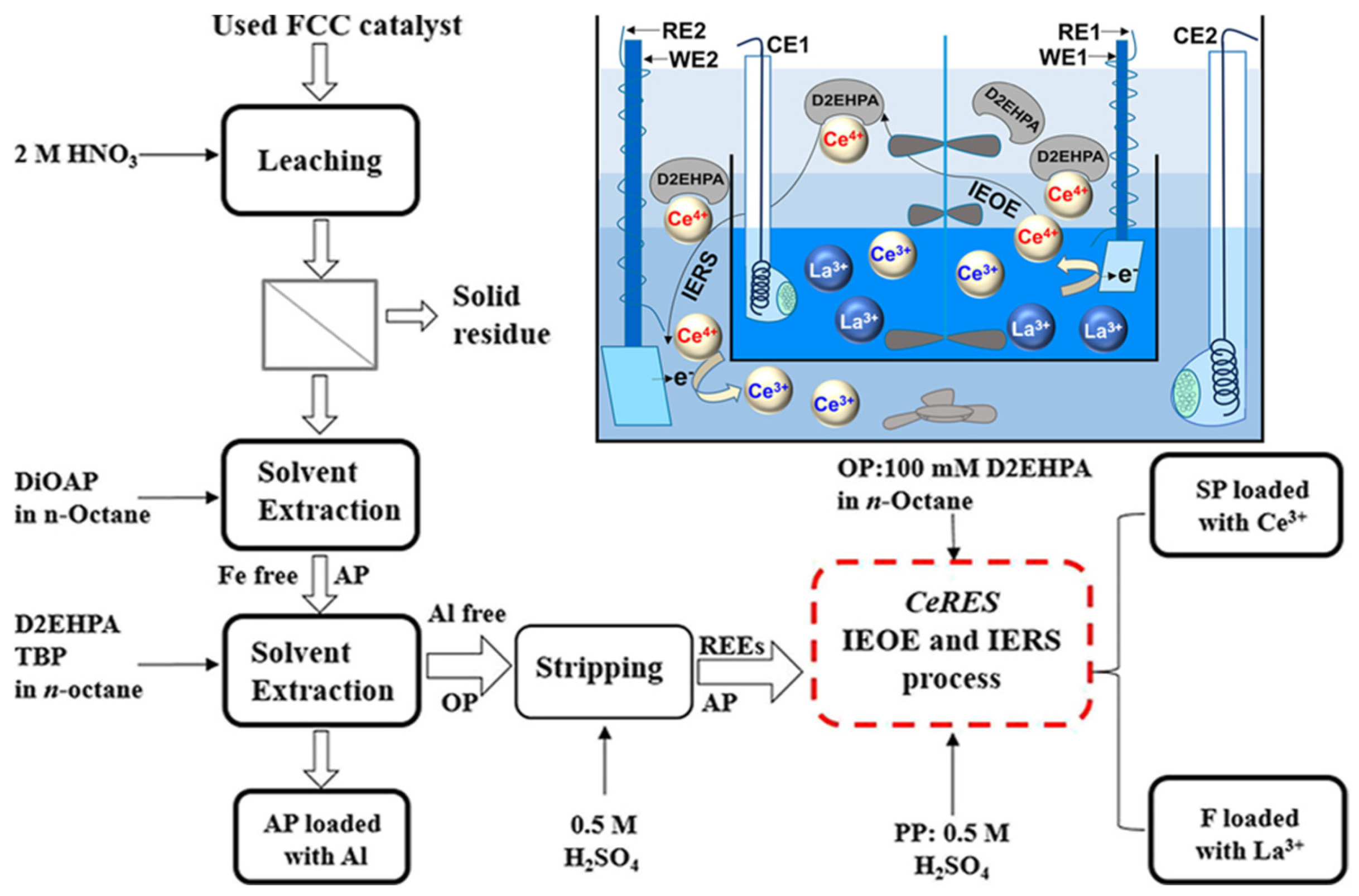
| FCC recovery La3+/Ce3+ [90] | In situ electrochemical oxidation | LSV, CV, SWV | 25% D2EHPA, 25% TBP | La3+ > 99.5%, Ce4+ = 100% |
| Electrodeposition behavior [89] | Potentiostatic deposition | CV | RECl3 | REO > 95% |
| Recovery of REEs from NdFeB magnet scrap [91] | In situ electrochemical oxidation | XRD, CV | Oxalic acid precipitation | REO > 99.2% |
| Recovery of REEs from waste magnets [93] | Potentiostatic deposition | SEM | 973 k, 0.42 V | Effective separation of Nd and Pr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, T.; Dou, Z.; Xie, W.; Lan, C.; Li, G. Summary of the Research Progress on Advanced Engineering, Processes, and Process Parameters of Rare Earth Green Metallurgy. Materials 2024, 17, 3686. https://doi.org/10.3390/ma17153686
Li Y, Zhang T, Dou Z, Xie W, Lan C, Li G. Summary of the Research Progress on Advanced Engineering, Processes, and Process Parameters of Rare Earth Green Metallurgy. Materials. 2024; 17(15):3686. https://doi.org/10.3390/ma17153686
Chicago/Turabian StyleLi, Yingqi, Tingan Zhang, Zhihe Dou, Wei Xie, Chuidai Lan, and Guangtao Li. 2024. "Summary of the Research Progress on Advanced Engineering, Processes, and Process Parameters of Rare Earth Green Metallurgy" Materials 17, no. 15: 3686. https://doi.org/10.3390/ma17153686





