Numerical Investigation of the Performance of a Proton Exchange Membrane Water Electrolyzer under Various Outlet Manifold Structure Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Liquid Water and Oxygen Flow Model
2.1.1. Governing Equations
2.1.2. Boundary Conditions
2.2. 3D Electrolyzer Model
2.2.1. Governing Equations
2.2.2. Boundary Conditions
2.3. Numerical Implementation
3. Results
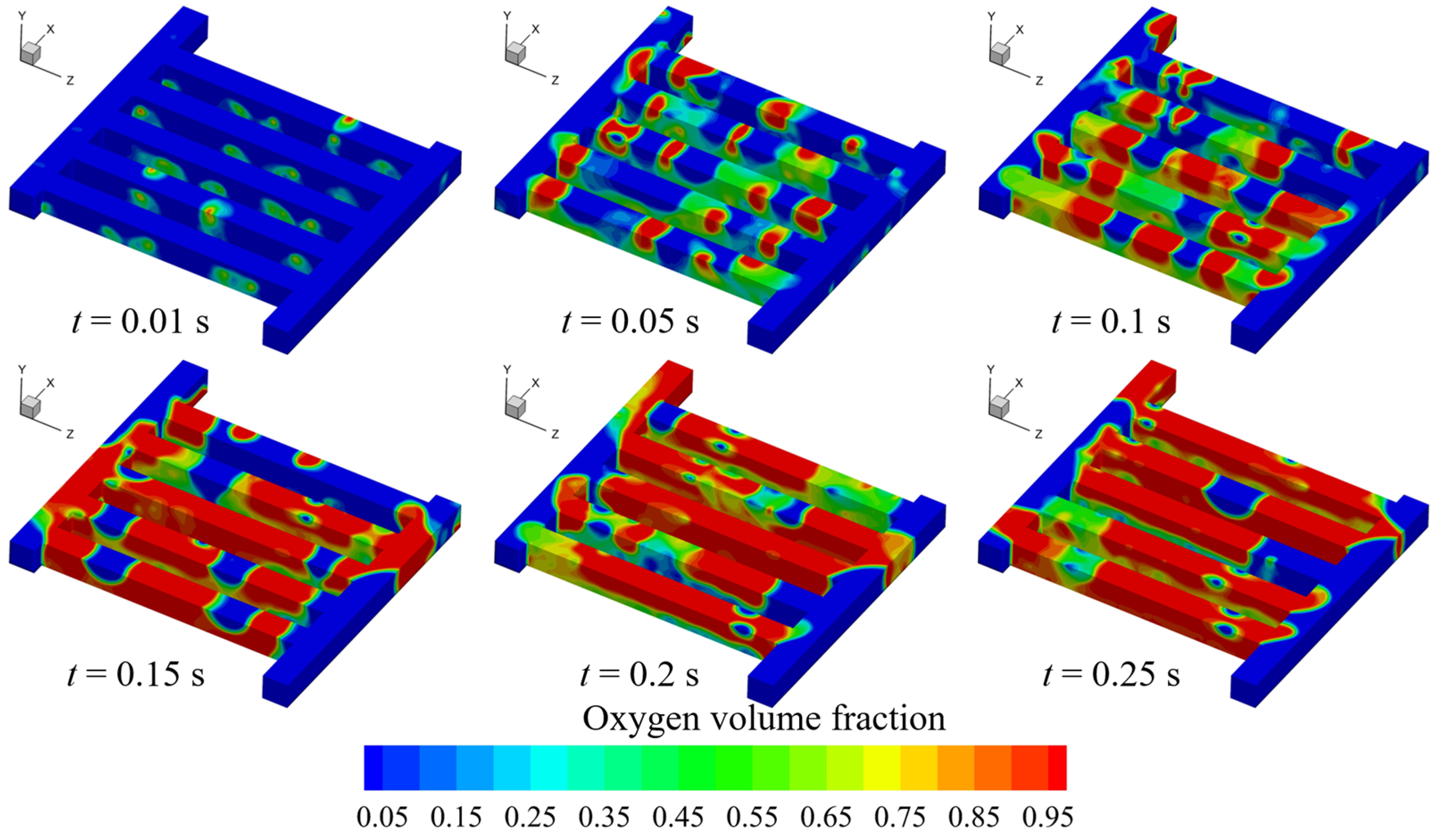
3.1. Oxygen Discharge Characteristics in Anode Flow Field
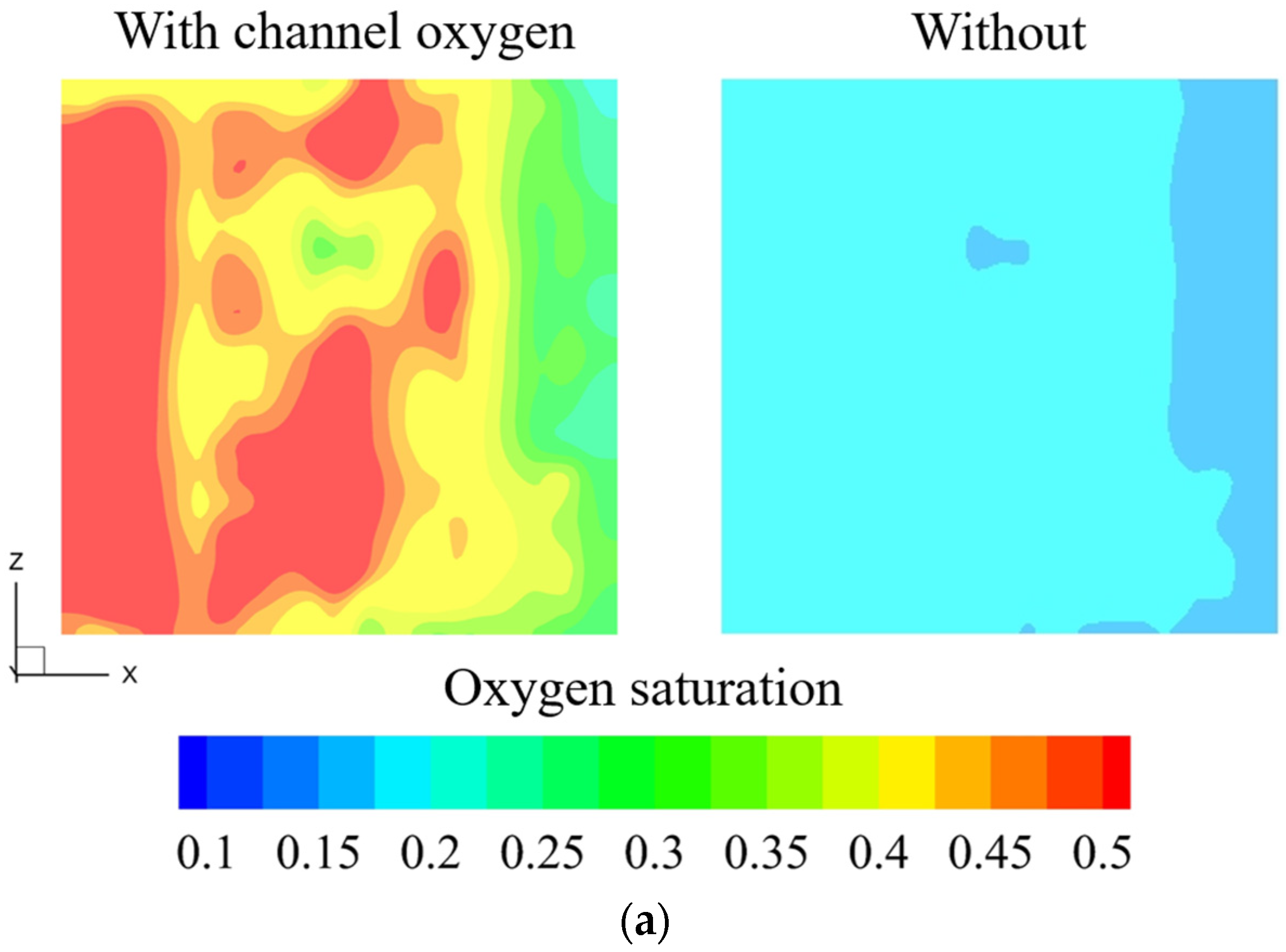
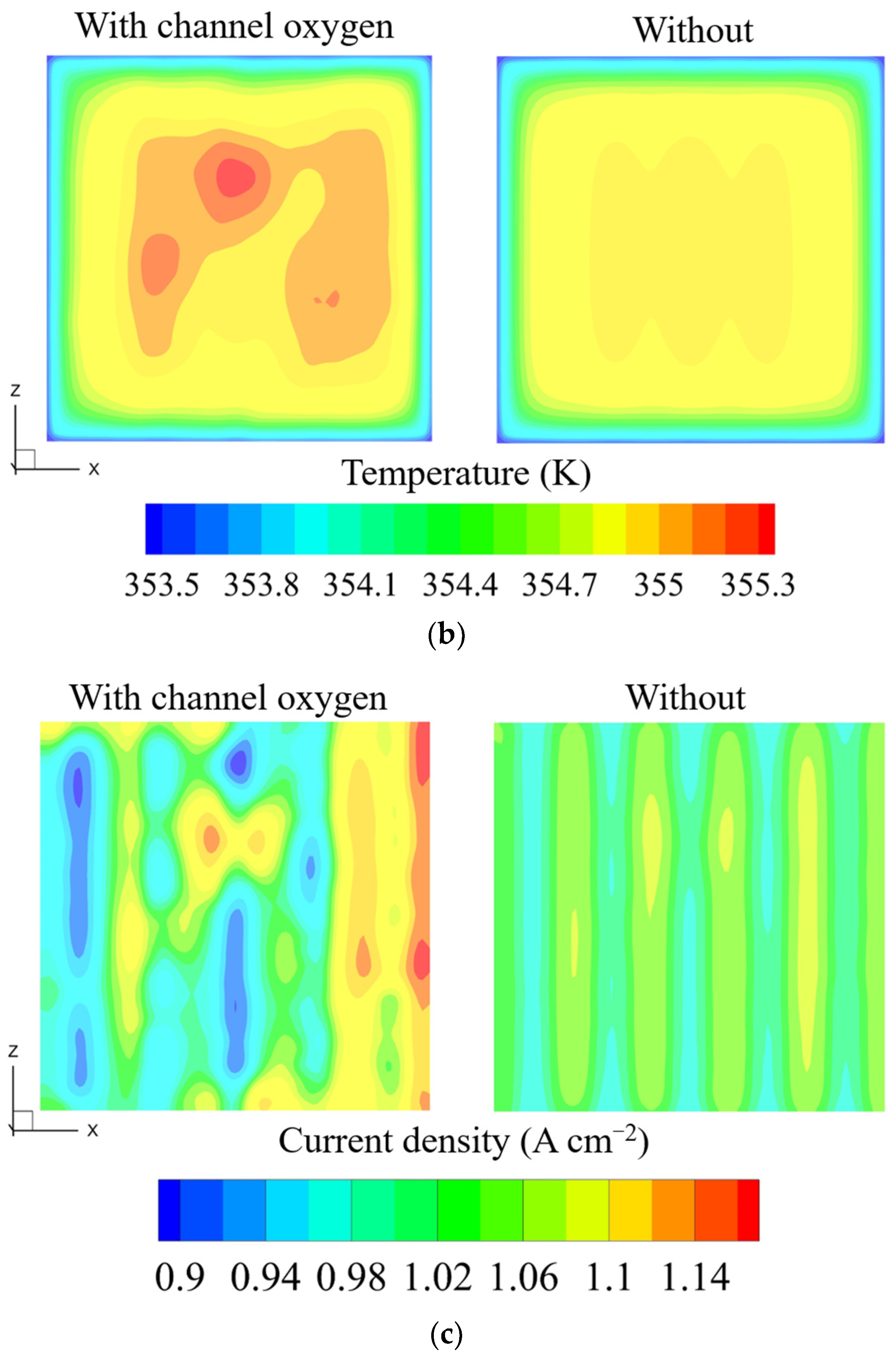
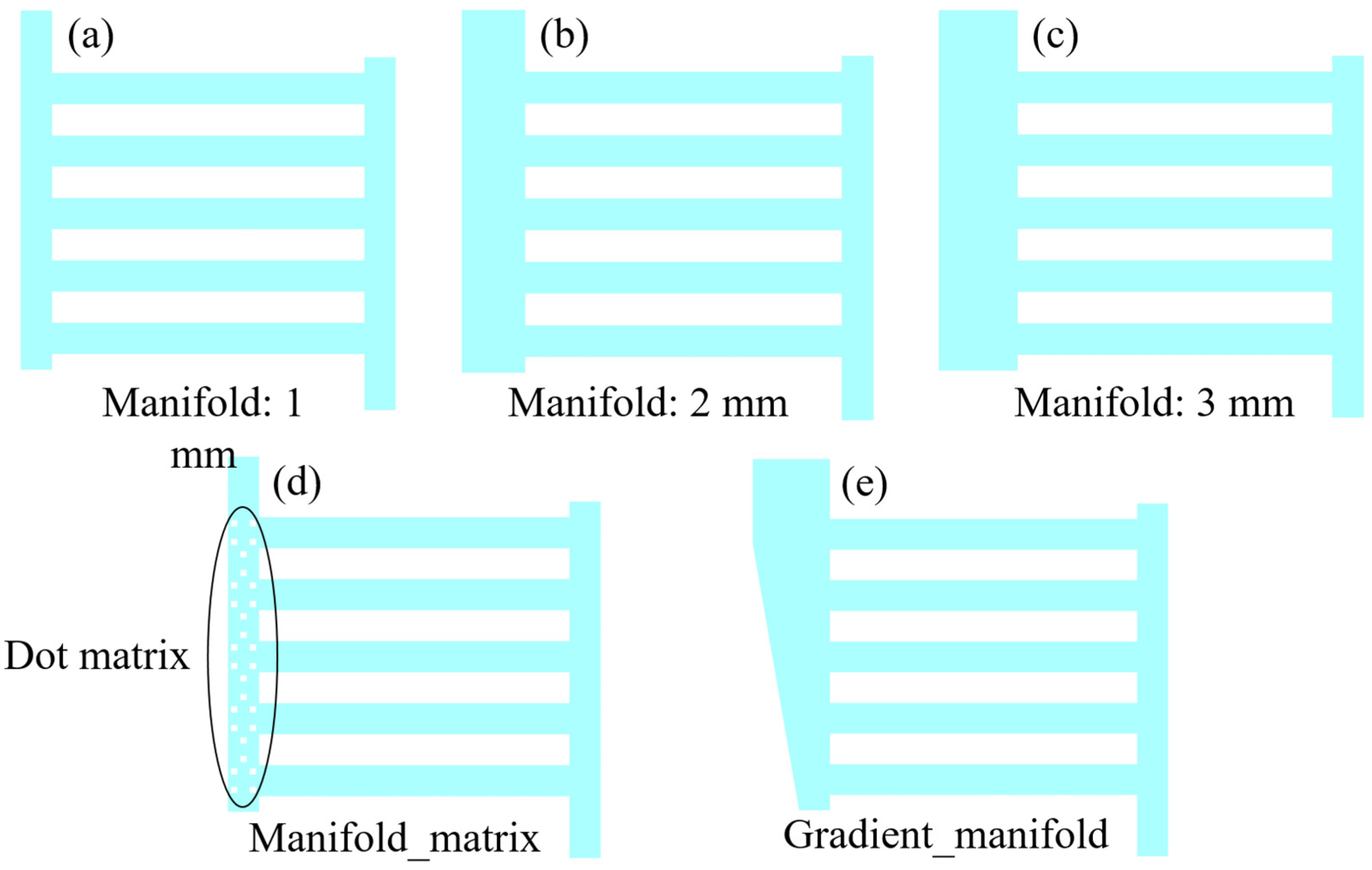
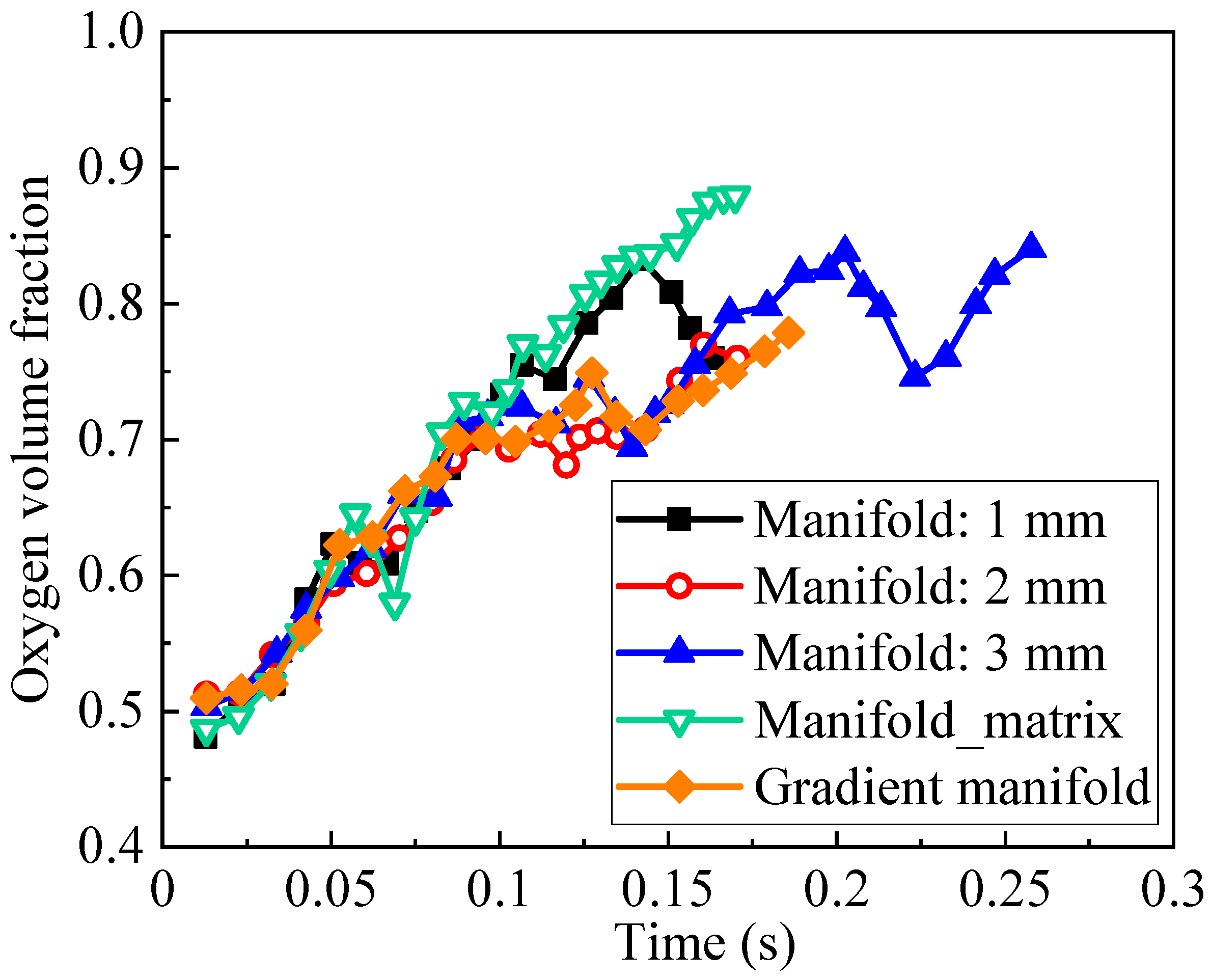
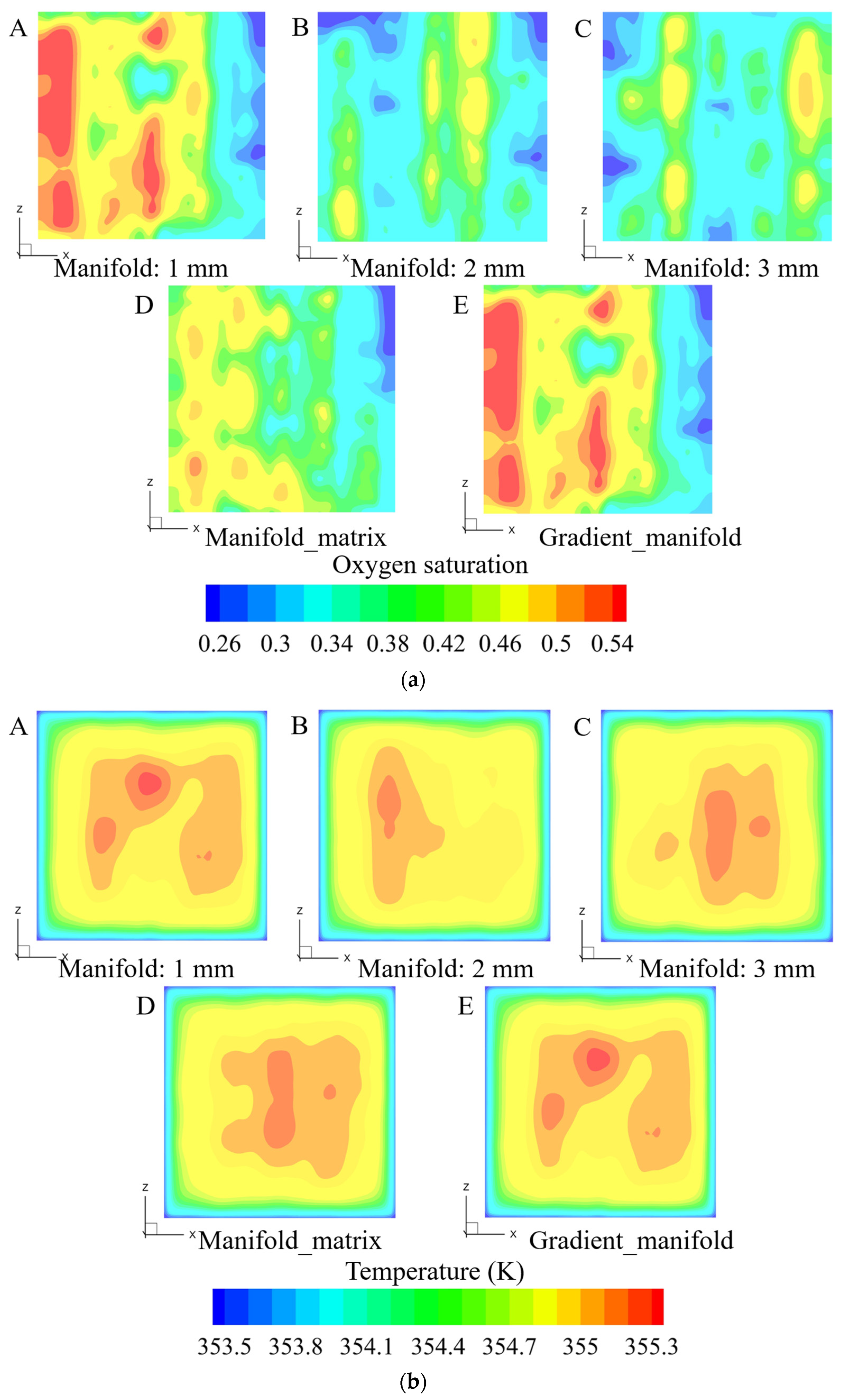
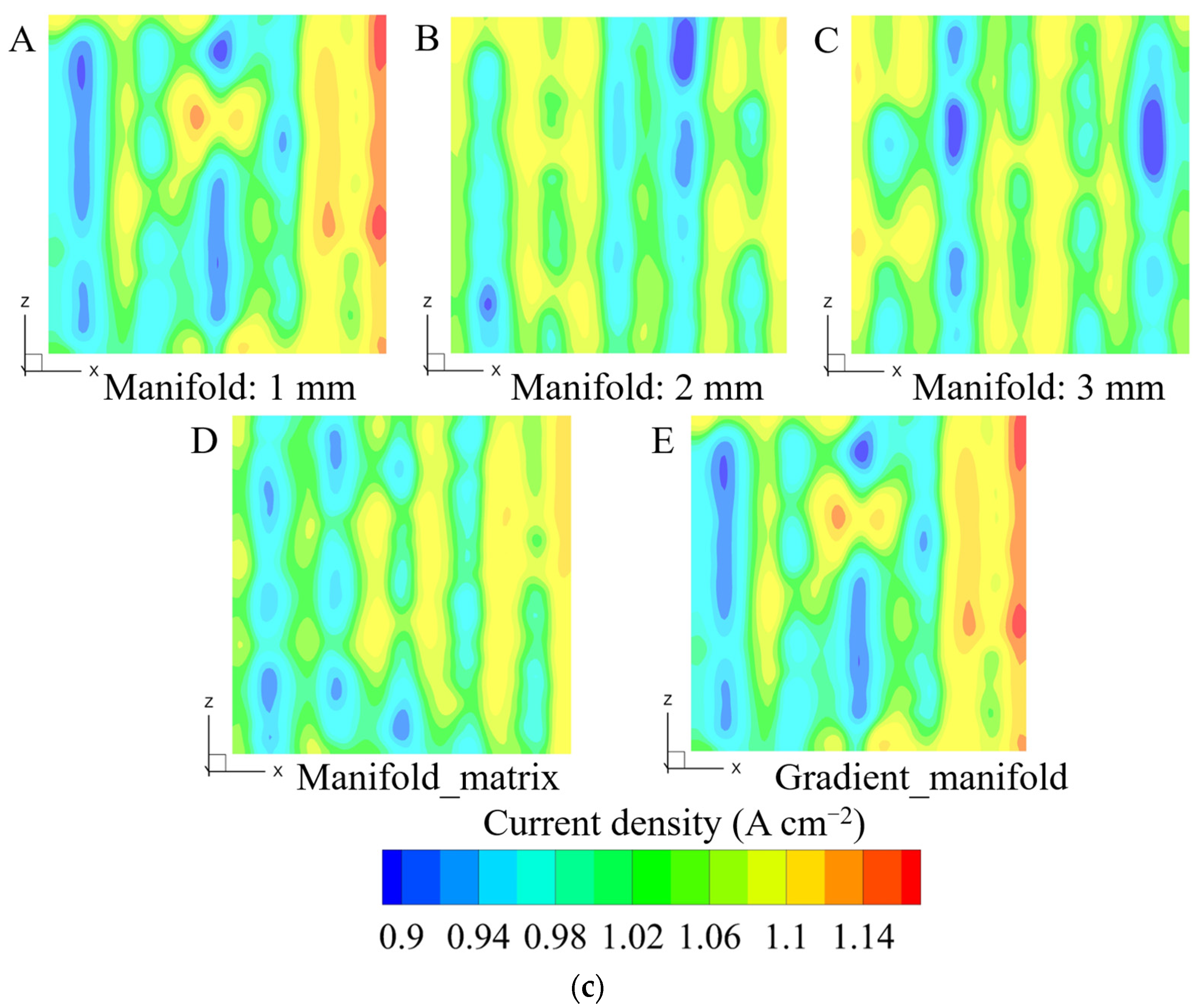
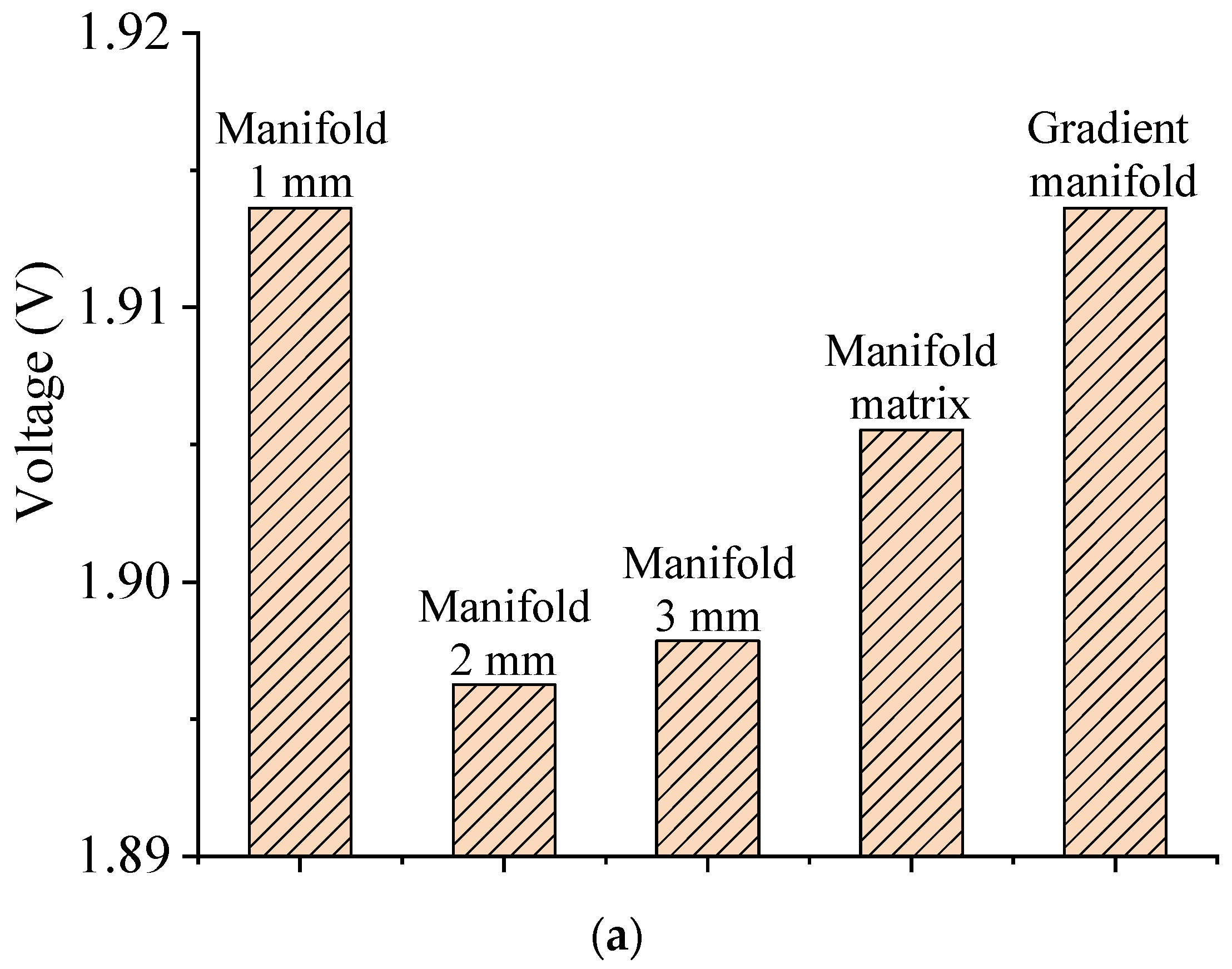
3.2. Influence of Outlet Manifold Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, G.; Qu, Z.; Tao, W.-Q.; Mu, Y.; Jiao, K.; Xu, H.; Wang, Y. Advancing next-generation proton-exchange membrane fuel cell development in multi-physics transfer. Joule 2024, 8, 45–63. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel Cell and Electrolysis Cell Technologies and Hydrogen Infrastructure Development—A Review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Advanced membrane-based electrode engineering toward efficient and durable water electrolysis and cost-effective seawater electrolysis in membrane electrolyzers. Exploration 2024, 4, 20220112. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Cebola, M.-J.; Santos, D.M.F. Towards the Hydrogen Economy—A Review of the Parameters That Influence the Efficiency of Alkaline Water Electrolyzers. Energies 2021, 14, 3193. [Google Scholar] [CrossRef]
- Fu, J.L.; Qu, Z.G.; Zhang, J.F.; Zhang, G.B. Performance analysis of PEMEC with non-uniform deformation based on a comprehensive numerical framework coupling image recognition and CFD. Appl. Energy 2023, 350, 121772. [Google Scholar] [CrossRef]
- Rakousky, C.; Keeley, G.P.; Wippermann, K.; Carmo, M.; Stolten, D. The stability challenge on the pathway to high-current-density polymer electrolyte membrane water electrolyzers. Electrochim. Acta 2018, 278, 324–331. [Google Scholar] [CrossRef]
- Villagra, A.; Millet, P. An analysis of PEM water electrolysis cells operating at elevated current densities. Int. J. Hydrogen Energy 2019, 44, 9708–9717. [Google Scholar] [CrossRef]
- Sayed-Ahmed, H.; Toldy, Á.I.; Santasalo-Aarnio, A. Dynamic operation of proton exchange membrane electrolyzers—Critical review. Renew. Sustain. Energy Rev. 2024, 189, 113883. [Google Scholar] [CrossRef]
- Lee, H.-O.; Yesuraj, J.; Kim, K. Parametric study to optimize proton exchange membrane electrolyzer cells. Appl. Energy 2022, 314, 118928. [Google Scholar] [CrossRef]
- Lin, R.; Lu, Y.; Xu, J.; Huo, J.; Cai, X. Investigation on performance of proton exchange membrane electrolyzer with different flow field structures. Appl. Energy 2022, 326, 120011. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, Y.; Zhang, Y.; Wang, K.; Xu, C.; Ye, F. Relationship of local current and two-phase flow in proton exchange membrane electrolyzer cells. J. Power Sources 2022, 542, 231742. [Google Scholar] [CrossRef]
- Su, X.; Xu, L.; Zhu, D.; Hu, B.; Mi, L. Electrochemical performance study of proton exchange membrane electrolyzer considering the effect of bubble coverage. Int. J. Hydrogen Energy 2023, 48, 27079–27094. [Google Scholar]
- Majasan, J.O.; Cho, J.I.S.; Dedigama, I.; Tsaoulidis, D.; Shearing, P.; Brett, D.J.L. Two-phase flow behaviour and performance of polymer electrolyte membrane electrolysers: Electrochemical and optical characterisation. Int. J. Hydrogen Energy 2018, 43, 15659–15672. [Google Scholar] [CrossRef]
- Li, Y.; Kang, Z.; Mo, J.; Yang, G.; Yu, S.; Talley, D.A.; Han, B.; Zhan, F.-Y. In-situ investigation of bubble dynamics and two-phase flow in proton exchange membrane electrolyzer cells. Int. J. Hydrogen Energy 2018, 43, 11223–11233. [Google Scholar] [CrossRef]
- Panchenko, O.; Giesenberg, L.; Borgardt, E.; Zwaygardt, W.; Kardjilov, N.; Markötter, H.; Arlt, T.; Manke, I.; Müller, M.; Stolten, D.; et al. Influence of Stoichiometry on the Two-Phase Flow Behavior of Proton Exchange Membrane Electrolyzers. Energies 2019, 12, 350. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, C.; Cai, X.; An, L.; Shen, S.; Yan, X.; Zhang, J. Bubble evolution and transport in PEM water electrolysis: Mechanism, impact, and management. Prog. Energy Combust. Sci. 2023, 96, 101075. [Google Scholar] [CrossRef]
- Toghyani, S.; Afshari, E.; Baniasadi, E. Metal foams as flow distributors in comparison with serpentine and parallel flow fields in proton exchange membrane electrolyzer cells. Electrochim. Acta 2018, 290, 506–519. [Google Scholar] [CrossRef]
- Wu, L.; Pan, Z.; Yuan, S.; Shi, X.; Liu, Y.; Liu, F.; Yan, X.; An, L. A dual-layer flow field design capable of enhancing bubble self-pumping and its application in water electrolyzer. Chem. Eng. J. 2024, 488, 151000. [Google Scholar] [CrossRef]
- Su, X.; Zhang, Q.; Xu, L.; Hu, B.; Wu, X.; Qin, T. A novel combined flow field design and performance analysis of proton exchange membrane electrolysis cell. Int. J. Hydrogen Energy 2024, 61, 444–459. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, G.; Xie, B.; Tongsh, C.; Jiao, K. Integration of the detailed channel two-phase flow into three-dimensional multi-phase simulation of proton exchange membrane electrolyzer cell. Int. J. Green Energy 2021, 18, 541–555. [Google Scholar] [CrossRef]
- Wu, L.; An, L.; Jiao, D.; Xu, Y.; Zhang, G.; Jiao, K. Enhanced oxygen discharge with structured mesh channel in proton exchange membrane electrolysis cell. Appl. Energy 2022, 323, 119651. [Google Scholar] [CrossRef]
- Leverett, M. Capillary Behavior in Porous Solids. Trans. AIME 1941, 142, 152–169. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X. Water transport in polymer electrolyte membrane fuel cells. Prog. Energy Combust. Sci. 2011, 37, 221–291. [Google Scholar] [CrossRef]



| Parameters | Values |
|---|---|
| Working pressure, current density, temperature | 1.0 atm, 1.0 A cm−2, 353.15 K |
| MEA area | 1.0 cm2 |
| Membrane, CL, L/GDL thickness | 127, 10, 300 μm |
| Channel/BP width, height | 1.0, 1.0 mm |
| Porosity: CL, anode L/GDL, cathode L/GDL | 0.3, 0.75, 0.6 |
| Reference hydrogen and oxygen concentrations | 33.5, 33.5 mol m−3 |
| Anode and cathode reference exchange current densities | 1.0 × 109, 8.0 × 104 A m−3 |
| Transfer coefficient | 0.5 |
| Electrical conductivity: anode L/GDL, cathode L/GDL, anode CL, cathode CL, BP | 20,000, 2000, 1000, 5000, 20,000 |
| Membrane’s equivalent weight and density | 1.1 kg mol−1, 1980 kg m−3 |
| Electrolyte volume fraction in CLs | Anode: 0.2, cathode: 0.3 |
| Contact angles: anode L/GDL, cathode L/GDL, anode CL, cathode CL, L/GDL surface, BP surface | 70°, 120°, 80°, 100°, 120°, 90° |
| Permeabilities: anode L/GDL, cathode L/GDL, anode CL, cathode CL, membrane | 4.9 × 10−11, 2.0 × 10−12, 2.0 × 10−12, 1.0 × 10−13, 2.0 × 10−20 |
| Water phase change coefficient | 100 s−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Qu, Z. Numerical Investigation of the Performance of a Proton Exchange Membrane Water Electrolyzer under Various Outlet Manifold Structure Conditions. Materials 2024, 17, 3694. https://doi.org/10.3390/ma17153694
Zhang G, Qu Z. Numerical Investigation of the Performance of a Proton Exchange Membrane Water Electrolyzer under Various Outlet Manifold Structure Conditions. Materials. 2024; 17(15):3694. https://doi.org/10.3390/ma17153694
Chicago/Turabian StyleZhang, Guobin, and Zhiguo Qu. 2024. "Numerical Investigation of the Performance of a Proton Exchange Membrane Water Electrolyzer under Various Outlet Manifold Structure Conditions" Materials 17, no. 15: 3694. https://doi.org/10.3390/ma17153694
APA StyleZhang, G., & Qu, Z. (2024). Numerical Investigation of the Performance of a Proton Exchange Membrane Water Electrolyzer under Various Outlet Manifold Structure Conditions. Materials, 17(15), 3694. https://doi.org/10.3390/ma17153694







