Increased Cytotoxicity of Bimetallic Ultrasmall Silver–Platinum Nanoparticles (2 nm) on Cells and Bacteria in Comparison to Silver Nanoparticles of the Same Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Electron Microscopy
2.3. X-ray Powder Diffraction (XRD)
2.4. Small-Angle X-ray Scattering (SAXS)
2.5. NMR Spectroscopy
2.6. DOSY-NMR Spectroscopy
2.7. X-ray Photoelectron Spectroscopy (XPS)
2.8. Elemental Analysis (AAS, ICP-MS)
2.9. Differential Centrifugal Sedimentation (DCS)
2.10. UV-Vis Spectroscopy
2.11. Fluorescence Spectroscopy
2.12. Synthesis of Glutathione-Coated Nanoparticles
2.13. Fluorescent Labeling of GSH-Coated Nanoparticles
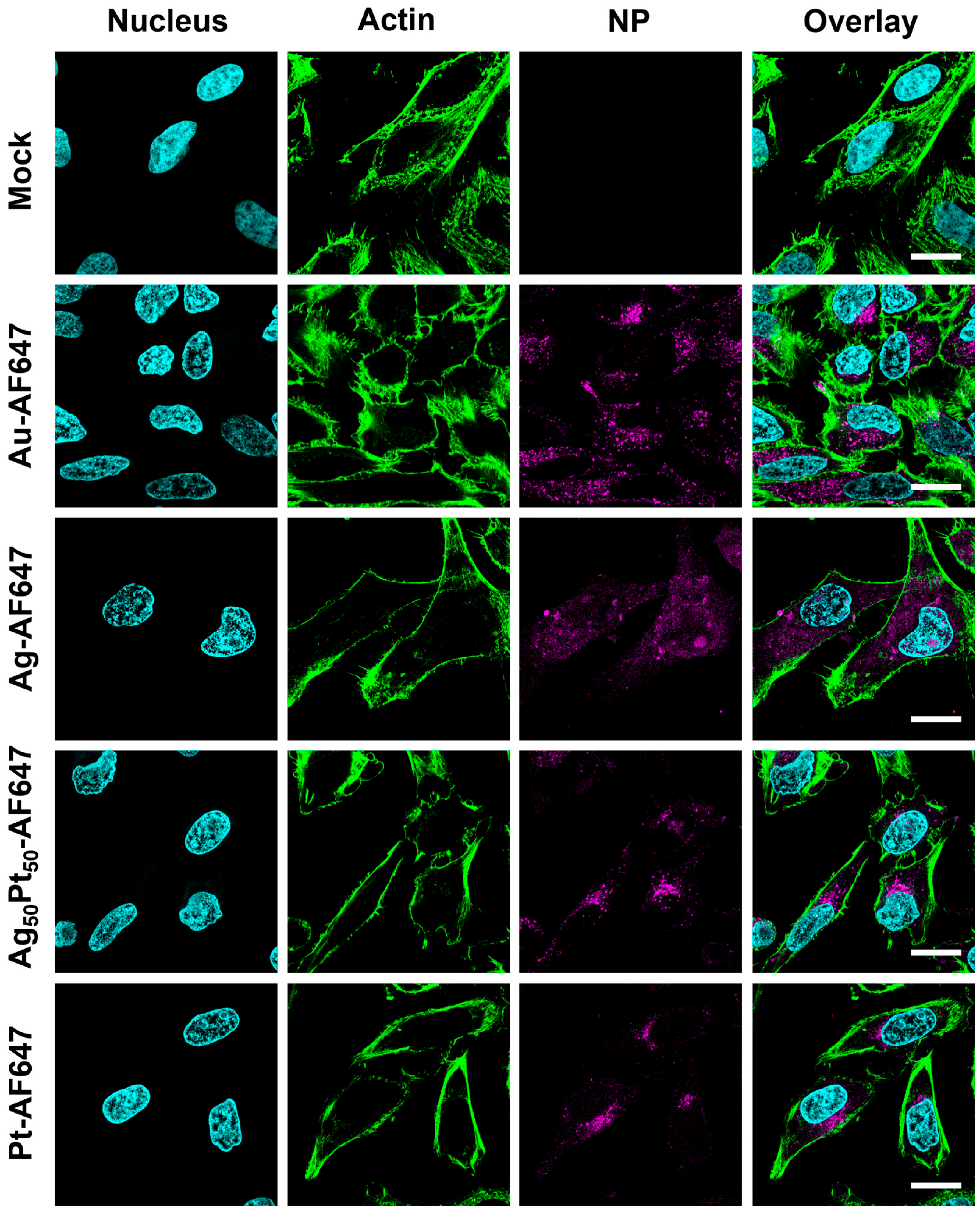
2.14. Uptake of M-AF647 Nanoparticles by HeLa Cells
2.15. MTT Tests of M-GSH Nanoparticles with HeLa Cells
2.16. Antibacterial Tests of M-GSH Nanoparticles with Staphylococcus xylosus and Escherichia coli
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.N.; Chen, X.; Dobrovolskaia, M.A.; Lammers, T. Cancer nanomedicine. Nat. Rev. Cancer 2022, 22, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Agatea, L.; Crotti, S.; Ragazzi, E.; Bedin, C.; Urso, E.; Mammi, I.; Traldi, P.; Pucciarelli, S.; Nitti, D.; Agostini, M. Peptide patterns as discriminating biomarkers in plasma of patients with familial adenomatous polyposis. Clin. Color. Cancer 2016, 15, e75–e92. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Guo, L.H.; Li, J.Q.; Thu, H.E.; Hussain, Z. Nanomedicines guided nanoimaging probes and nanotherapeutics for early detection of lung cancer and abolishing pulmonary metastasis: Critical appraisal of newer developments and challenges to clinical transition. J. Control. Release 2018, 292, 29–57. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Rice, K.M.; Ginjupalli, G.K.; Manne, N.; Jones, C.B.; Blough, E.R. A review of the antimicrobial potential of precious metal derived nanoparticle constructs. Nanotechnology 2019, 30, 372001. [Google Scholar] [CrossRef]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Koller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Streich, C.; Stein, F.; Jakobi, J.; Ingendoh-Tsakmakidis, A.; Heine, N.; Rehbock, C.; Winkel, A.; Grade, S.; Kühnel, M.; Migunov, V.; et al. The origin of the intracellular silver in bacteria: A comprehensive study using targeting gold-silver alloy nanoparticles. Adv. Healthc. Mater. 2023, 12, 2302084. [Google Scholar] [CrossRef]
- Guisbiers, G.; Mendoza-Cruz, R.; Bazan-Diaz, L.; Velazquez-Salazar, J.J.; Mendoza-Perez, R.; Robledo-Torres, J.A.; Rodriguez-Lopez, J.L.; Montejano-Carrizales, J.M.; Whetten, R.L.; Jose-Yacaman, M. Electrum, the gold–silver alloy, from the bulk scale to the nanoscale: Synthesis, properties, and segregation rules. ACS Nano 2016, 10, 188–198. [Google Scholar] [CrossRef]
- Al-Zubeidi, A.; Stein, F.; Flatebo, C.; Rehbock, C.; Hosseini Jebeli, S.A.; Landes, C.F.; Barcikowski, S.; Link, S. Single-particle hyperspectral imaging reveals kinetics of silver ion leaching from alloy nanoparticles. ACS Nano 2021, 15, 8363–8375. [Google Scholar] [CrossRef]
- Grasmik, V.; Rurainsky, C.; Loza, K.; Evers, M.V.; Prymak, O.; Heggen, M.; Tschulik, K.; Epple, M. Deciphering the surface composition and the internal structure of alloyed silver–gold nanoparticles. Chem. Eur. J. 2018, 24, 9051–9060. [Google Scholar] [CrossRef] [PubMed]
- Ristig, S.; Chernousova, S.; Meyer-Zaika, W.; Epple, M. Synthesis, characterization and in-vitro effects of 7 nm alloyed silver-gold nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Etterlin, G.D.; Spyrogianni, A.; Krumeich, F.; Leroux, J.C.; Pratsinis, S.E. Plasmonic biocompatible silver-gold alloyed nanoparticles. Chem. Commun. 2014, 50, 13559–13562. [Google Scholar] [CrossRef]
- Breisch, M.; Grasmik, V.; Loza, K.; Pappert, K.; Rostek, A.; Ziegler, N.; Ludwig, A.; Heggen, M.; Epple, M.; Tiller, J.C.; et al. Bimetallic silver platinum nanoparticles with combined osteo-promotive and antimicrobial activity. Nanotechnology 2019, 30, 305101. [Google Scholar] [CrossRef]
- Grasmik, V.; Breisch, M.; Loza, K.; Heggen, M.; Köller, M.; Sengstock, C.; Epple, M. Synthesis and biological characterization of alloyed silver–platinum nanoparticles: From compact core–shell nanoparticles to hollow nanoalloys. RSC Adv. 2018, 8, 38582–38590. [Google Scholar] [CrossRef]
- Ryu, H.S.; Bae, I.H.; Lee, K.G.; Hwang, H.S.; Lee, K.H.; Koh, J.T.; Cho, J.H. Antibacterial effect of silver-platinum coating for orthodontic appliances. Angle Orthod. 2012, 82, 151–157. [Google Scholar] [CrossRef]
- Epple, M.; Rotello, V.M.; Dawson, K. The why and how of ultrasmall nanoparticles. Acc. Chem. Res. 2023, 56, 3369–3378. [Google Scholar] [CrossRef]
- Klein, K.; Loza, K.; Heggen, M.; Epple, M. An efficient method for covalent surface functionalization of ultrasmall metallic nanoparticles by surface azidation, followed by copper-catalyzed azide-alkyne cycloaddition. ChemNanoMat 2021, 7, 1330–1339. [Google Scholar] [CrossRef]
- Thust, A.; Barthel, J.; Tillmann, K. FEI Titan 80–300 TEM. J. Large-Scale Res. Facil. 2016, 2, A41. [Google Scholar] [CrossRef]
- Hammersley, A.P. FIT2D: A multi-purpose data reduction, analysis and visualization program. J. Appl. Crystallogr. 2016, 49, 646–652. [Google Scholar] [CrossRef]
- Oliveira, C.L.P.; Vorup-Jensen, T.; Andersen, C.B.F.; Andersen, G.R.; Pedersen, J.S. Discovering new features of protein complexes structures by small-angle X-ray scattering. In Applications of Synchrotron Light to Scattering and Diffraction in Materials and Life Sciences; Gomez, M., Nogales, A., Garcia-Gutierrez, M.C., Ezquerra, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 231–244. [Google Scholar]
- Garcia, P.R.A.F.; Prymak, O.; Grasmik, V.; Pappert, K.; Wlysses, W.; Otubo, L.; Epple, M.; Oliveira, C.L.P. An in situ SAXS investigation of the formation of silver nanoparticles and bimetallic silver–gold nanoparticles in controlled wet-chemical reduction synthesis. Nanoscale Adv. 2019, 2, 225–238. [Google Scholar] [CrossRef]
- Wolff, N.; Loza, K.; Heggen, M.; Schaller, T.; Niemeyer, F.; Bayer, P.; Beuck, C.; Oliveira, C.L.P.; Prymak, O.; Weidenthaler, C.; et al. Ultrastructure and surface composition of glutathione-terminated ultrasmall silver, gold, platinum, and alloyed silver–platinum nanoparticles (2 nm). Inorg. Chem. 2023, 62, 17470–17485. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Altieri, A.S.; Hinton, D.P.; Byrd, R.A. Association of biomolecular systems via pulsed-field gradient NMR self-diffusion measurements. J. Am. Chem. Soc. 1995, 117, 7566–7567. [Google Scholar] [CrossRef]
- Einstein, A. Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen. Ann. Phys. 1905, 322, 549–560. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Van der Meer, S.B.; Loza, K.; Wey, K.; Heggen, M.; Beuck, C.; Bayer, P.; Epple, M. Click chemistry on the surface of ultrasmall gold nanoparticles (2 nm) for covalent ligand attachment followed by NMR spectroscopy. Langmuir 2019, 35, 7191–7204. [Google Scholar] [CrossRef]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 1655–1656. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M. Gold nanoparticle research before and after the Brust–Schiffrin method. Chem. Comm. 2013, 49, 16–18. [Google Scholar] [CrossRef]
- Ferreira, R.S.; Lira, A.L.; Torquato, R.J.S.; Schuck, P.; Sousa, A.A. Mechanistic insights into ultrasmall gold nanoparticle-protein interactions through measurement of binding kinetics. J. Phys. Chem. C 2019, 123, 28450–28459. [Google Scholar] [CrossRef]
- Calborean, A.; Martin, F.; Marconi, D.; Turcu, R.; Kacso, I.E.; Buimaga-Iarinca, L.; Graur, F.; Turcu, I. Adsorption mechanisms of L-glutathione on Au and controlled nano-patterning through Dip Pen Nanolithography. Mater. Sci. Eng. C 2015, 57, 171–180. [Google Scholar] [CrossRef]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef]
- Wolff, N.; Beuck, C.; Schaller, T.; Epple, M. Possibilities and limitations of solution-state NMR spectroscopy to analyze the ligand shell of ultrasmall metal nanoparticles. Nanoscale Adv. 2024, 6, 3285–3298. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Klein, K.; Hayduk, M.; Kollenda, S.; Schmiedtchen, M.; Voskuhl, J.; Epple, M. Covalent attachment of aggregation-induced emission molecules to the surface of ultrasmall gold nanoparticles to enhance cell penetration. Molecules 2022, 27, 1788. [Google Scholar] [CrossRef]
- Yu, R.; Liz-Marzan, L.M.; Garcia de Abajo, F.J. Universal analytical modeling of plasmonic nanoparticles. Chem. Soc. Rev. 2017, 46, 6710–6724. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Cui, Z.H.; He, H.L.; Wu, S.B.; Dong, C.L.; Lu, S.Y.; Shan, T.J.; Fang, L.X.; Liao, X.P.; Liu, Y.H.; Sun, J. Rapid screening of essential oils as substances which enhance antibiotic activity using a modified well diffusion method. Antibiotics 2021, 10, 463. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, Y.; Qu, Q.; Cui, W.; God’spower, B.O.; Liu, Y.; Chen, X.; Chen, M.; Yang, Y.; Li, Y. Azithromycin inhibits biofilm formation by Staphylococcus xylosus and affects histidine biosynthesis pathway. Front. Pharmacol. 2018, 9, 740. [Google Scholar] [CrossRef]
- Tashiro, Y.; Eida, H.; Ishii, S.; Futamata, H.; Okabe, S. Generation of small colony variants in biofilms by Escherichia coli harboring a conjugative F plasmid. Microbes Environ. 2017, 32, 40–46. [Google Scholar] [CrossRef]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV-Vis. Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef]
- Loza, K.; Epple, M. Silver nanoparticles in complex media: An easy procedure to discriminate between metallic silver nanoparticles, reprecipitated silver chloride, and dissolved silver species. RSC Adv. 2018, 8, 24386–24391. [Google Scholar] [CrossRef]
- Graf, C.; Nordmeyer, D.; Sengstock, C.; Ahlberg, S.; Diendorf, J.; Raabe, J.; Epple, M.; Köller, M.; Lademann, J.; Vogt, A.; et al. Shape-dependent dissolution and cellular uptake of silver nanoparticles. Langmuir 2018, 34, 1506–1519. [Google Scholar] [CrossRef]
- Merrifield, R.C.; Stephan, C.; Lead, J. Determining the concentration dependent transformations of Ag nanoparticles in complex media: Using SP-ICP-MS and Au@Ag core shell nanoparticles as tracers. Environ. Sci. Technol. 2017, 51, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.P.; Roesslein, M.; Diener, L.; Wichser, A.; Nowack, B.; Wick, P. Cytotoxic effects of nanosilver are highly dependent on the chloride concentration and the presence of organic compounds in the cell culture media. J. Nanobiotechnol. 2017, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Ocwieja, M.; Mrowiec, H.; Walas, S.; Lupa, D. Oxidative dissolution of silver nanoparticles: A new theoretical approach. J. Colloid Interface Sci. 2016, 469, 355–364. [Google Scholar] [CrossRef]
- Loza, K.; Diendorf, J.; Greulich, C.; Ruiz-Gonzales, L.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Koeller, M.; Epple, M. The dissolution and biological effect of silver nanoparticles in biological media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Wong, C.K.; Yau, S.K.W.; Lok, C.N.; Che, C.M. Oxidative dissolution of silver nanoparticles by dioxygen: A kinetic and mechanistic study. Chem. Asian J. 2011, 6, 2506–2511. [Google Scholar] [CrossRef]
- Liu, L.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Ho, C.M.; Yau, S.K.W.; Lok, C.N.; So, M.H.; Che, C.M. Oxidative dissolution of silver nanoparticles by biologically relevant oxidants: A kinetic and mechanistic study. Chem. Asian J. 2010, 5, 285–293. [Google Scholar] [CrossRef]
- Nazarenus, M.; Zhang, Q.; Soliman, M.G.; del Pino, P.; Pelaz, B.; Carregal-Romero, S.; Rejman, J.; Rothen-Rutishauser, B.; Clift, M.J.D.; Zellner, R.; et al. In vitro interaction of colloidal nanoparticles with mammalian cells: What have we learned thus far? Beilstein J. Nanotechnol. 2014, 5, 1477–1490. [Google Scholar] [CrossRef]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Jin, S.; Ma, X.; Xue, X.; Yang, K.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef]
- Yang, L.; Shang, L.; Nienhaus, G.U. Mechanistic aspects of fluorescent gold nanocluster internalization by live HeLa cells. Nanoscale 2013, 5, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrion, C.; Bocanegra, A.I.; Arnaiz, B.; Feliu, N.; Zhu, D.C.; Parak, W.J. Triple-labeling of polymer-coated quantum dots and adsorbed proteins for tracing their fate in cell cultures. ACS Nano 2019, 13, 4631–4639. [Google Scholar] [CrossRef]
- Sokolova, V.; Ebel, J.F.; Kollenda, S.; Klein, K.; Kruse, B.; Veltkamp, C.; Lange, C.M.; Westendorf, A.M.; Epple, M. Uptake of functional ultrasmall gold nanoparticles in 3D gut cell models. Small 2022, 18, 2201167. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Wetzel, O.; Kostka, K.; Heggen, M.; Loza, K.; Epple, M. Pathways for oral and rectal delivery of gold nanoparticles (1.7 nm) and gold nanoclusters into the colon: Enteric-coated capsules and suppositories. Molecules 2021, 26, 5069. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Kreyling, W.G.; Simon, U. Toxic effects and biodistribution of ultrasmall gold nanoparticles. Arch. Toxicol. 2017, 91, 3011–3037. [Google Scholar] [CrossRef]
- Rostek, A.; Breisch, M.; Pappert, K.; Loza, K.; Heggen, M.; Köller, M.; Sengstock, C.; Epple, M. Comparative biological effects of spherical noble metal nanoparticles (Rh, Pd, Ag, Pt, Au) with 4–8 nm diameter. Beilstein J. Nanotechnol. 2018, 9, 2763–2774. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Gross-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Loza, K.; Sengstock, C.; Chernousova, S.; Koeller, M.; Epple, M. The predominant species of ionic silver in biological media is colloidally dispersed nanoparticulate silver chloride. RSC Adv. 2014, 4, 35290–35297. [Google Scholar] [CrossRef]
- Ahlberg, S.; Meinke, M.C.; Werner, L.; Epple, M.; Diendorf, J.; Blume-Peytavi, U.; Lademann, J.; Vogt, A.; Rancan, F. Comparison of silver nanoparticles stored under air or argon with respect to the induction of intracellular free radicals and toxic effects toward keratinocytes. Eur. J. Pharm. Biopharm. 2014, 88, 651–657. [Google Scholar] [CrossRef]
- Wetzel, O.; Hosseini, S.; Loza, K.; Heggen, M.; Prymak, O.; Bayer, P.; Beuck, C.; Schaller, T.; Niemeyer, F.; Weidenthaler, C.; et al. Metal–ligand interface and internal structure of ultrasmall silver nanoparticles (2 nm). J. Phys. Chem. B 2021, 125, 5645–5659. [Google Scholar] [CrossRef]
- Breisch, M.; Loza, K.; Pappert, K.; Rostek, A.; Rurainsky, C.; Tschulik, K.; Heggen, M.; Epple, M.; Tiller, J.C.; Schildhauer, T.A.; et al. Enhanced dissolution of silver nanoparticles in a physical mixture with platinum nanoparticles based on the sacrificial anode effect. Nanotechnology 2020, 31, 055703. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Mehata, A.K.; Priya, V.; Malik, A.K.; Setia, A.; Suseela, M.N.L.; Vikas; Gokul, P.; Samridhi; Singh, S.K.; et al. Bimetallic Au-Ag nanoparticles: Advanced nanotechnology for tackling antimicrobial resistance. Molecules 2022, 27, 7059. [Google Scholar] [CrossRef]
- Cipriano, L.A.; Kristoffersen, H.H.; Munhos, R.L.; Pittkowski, R.; Arenz, M.; Rossmeisl, J. Tuning the chemical composition of binary alloy nanoparticles to prevent their dissolution. Nanoscale 2023, 15, 16697–16705. [Google Scholar] [CrossRef]
- Chang, Y.; Cheng, Y.; Feng, Y.L.; Li, K.; Jian, H.; Zhang, H.Y. Upshift of the d band center toward the Fermi level for promoting silver ion release, bacteria inactivation, and wound healing of alloy silver nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 12224–12231. [Google Scholar] [CrossRef] [PubMed]
- Abuayyash, A.; Ziegler, N.; Gessmann, J.; Sengstock, C.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Antibacterial efficacy of sacrifical anode thin films combining silver with platinum group elements within a bacteria-containing human plasma clot. Adv. Eng. Mater. 2018, 20, 1700493. [Google Scholar] [CrossRef]
- Koeller, M.; Bellova, P.; Javid, S.M.; Motemani, Y.; Khare, C.; Sengstock, C.; Tschulik, K.; Schildhauer, T.A.; Ludwig, A. Antibacterial activity of microstructured sacrificial anode thin films by combination of silver with platinum group elements (platinum, palladium, iridium). Mater. Sci. Eng. C 2017, 74, 536–541. [Google Scholar] [CrossRef]
- El Arrassi, A.; Bellova, P.; Memar Javid, S.; Motemani, Y.; Khare, C.; Sengstock, C.; Köller, M.; Ludwig, A.; Tschulik, K. A unified interdisciplinary approach to design superior antibacterial coatings for fast silver release. ChemElectroChem 2017, 4, 1975–1983. [Google Scholar] [CrossRef]
- Köller, M.; Sengstock, C.; Motemani, Y.; Khare, C.; Buenconsejo, P.J.S.; Geukes, J.; Schildhauer, T.A.; Ludwig, A. Antibacterial activity of microstructured Ag/Au sacrificial anode thin films. Mater. Sci. Eng. C 2015, 46, 276–280. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Chen, Y.C.; Kang, L.; Yu, J.; Wang, Y.; Lu, C.; Mashimo, T.; Yoshiasa, A.; Lin, C.H. Homogeneously alloyed nanoparticles of immiscible Ag–Cu with ultrahigh antibacterial activity. Colloids Surf. B Biointerfaces 2019, 180, 466–472. [Google Scholar] [CrossRef]
- Singh, A.V.; Patil, R.; Kasture, M.B.; Gade, W.N.; Prasad, B.L.V. Synthesis of Ag-Pt alloy nanoparticles in aqueous bovine serum albumin foam and their cytocompatibility against human gingival fibroblasts. Colloids Surf. B Biointerfaces 2009, 69, 239–245. [Google Scholar] [CrossRef]
- Utembe, W.; Potgieter, K.; Stefaniak, A.B.; Gulumian, M. Dissolution and biodurability: Important parameters needed for risk assessment of nanomaterials. Part. Fibre Toxicol. 2015, 12, 11. [Google Scholar] [CrossRef]
- Ahlberg, S.; Antonopulos, A.; Diendorf, J.; Dringen, R.; Epple, M.; Flöck, R.; Goedecke, W.; Graf, C.; Haberl, N.; Helmlinger, J.; et al. PVP-coated, negatively charged silver nanoparticles: A multi-center study of their physicochemical characteristics, cell culture and in vivo experiments. Beilstein J. Nanotechnol. 2014, 5, 1944–1965. [Google Scholar] [CrossRef]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, Z.Y.; Liu, F.D.; Kane, A.B.; Hurt, R.H. Chemical transformations of nanosilver in biological environments. ACS Nano 2012, 6, 9887–9899. [Google Scholar] [CrossRef]
- Levard, C.; Reinsch, B.C.; Michel, F.M.; Oumahi, C.; Lowry, G.V.; Brown, G.E. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: Impact on dissolution rate. Environ. Sci. Technol. 2011, 45, 5260–5266. [Google Scholar] [CrossRef]
- Pakiari, A.H.; Jamshidi, Z. Nature and strength of M-S bonds (M = Au, Ag, and Cu) in binary alloy gold clusters. J. Phys. Chem. A 2010, 114, 9212–9221. [Google Scholar] [CrossRef]
- Marchioni, M.; Battocchio, C.; Joly, Y.; Gateau, C.; Nappini, S.; Pis, I.; Delangle, P.; Michaud-Soret, I.; Deniaud, A.; Veronesi, G. Thiolate-capped silver nanoparticles: Discerning direct grafting from sulfidation at the metal-ligand interface by interrogating the sulfur atom. J. Phys. Chem. C 2020, 124, 13467–13478. [Google Scholar] [CrossRef]



| Au | Ag | Ag50Pt50 | Pt | |
|---|---|---|---|---|
| GSH-terminated nanoparticles used for the synthesis | 15 mg Au, 0.31 µmol nanoparticles | 15 mg Ag, 0.57 µmol nanoparticles | 6.47 mg Ag, 11.7 mg Pt, 0.48 µmol nanoparticles | 18.8 mg Pt, 0.35 µmol nanoparticles |
| round-bottomed flask | 250 mL | 500 mL | 250 mL | 250 mL |
| H2O | 18 mL | 36 mL | 20 mL | 24 mL |
| methanol | 54 mL | 108 mL | 60 mL | 72 mL |
| ISA | 184 mg, 676 µmol | 394 mg, 1.45 mmol | 221 mg, 812 µmol | 357 mg, 1.31 mmol |
| K2CO3 | 94 mg, 676 µmol | 201 mg, 1.45 mmol | 113 mg, 812 µmol | 183 mg, 1.31 mmol |
| 5 mM CuSO4 solution | 1.04 mL, 5.2 µmol | 2.52 mL, 12.6 µmol | 3.67 mL, 18.4 µmol | 3.91 mL, 19.6 µmol |
| 1 M NaOH solution | 1 mL | 2 mL | 2 mL | 4 mL |
| Reaction time | 72 h | 48 h | 48 h | 72 h |
| Au | Ag | Ag50Pt50 | Pt | |
|---|---|---|---|---|
| N3-terminated nanoparticles used for the synthesis | 3 mg Au, 62 nmol nanoparticles | 2.54 mg Ag, 96 nmol nanoparticles | 1.85 mg Pt, 0.61 mg Ag, 61 nmol nanoparticles | 3.7 mg Pt, 68 nmol nanoparticles |
| 11.2 mM AF647-alkyne solution | 100 µL, 1.12 µmol, 1 mg | 155 µL, 1.73 µmol, 1.54 mg | 100 µL, 1.12 µmol, 1 mg | 61 µL, 0.68 µmol, 0.61 mg |
| Cu-THPTA solution | 583 µL | 853 µL | 459 µL | 1.5 mL |
| 10 mM sodium ascorbate solution | 301 µL, 3.6 µmol, 0.6 mg | 246 µL, 2.46 µmol, 0.49 mg | 164 µL, 1.6 µmol, 0.33 mg | 535 µL, 5.4 µmol, 1.06 mg |
| Reaction time | 17 h | 6 h | 17 h | 17 h |
| Ag | Ag70Pt30 | Ag50Pt50 | Ag20Pt80 | Pt | Au | |
|---|---|---|---|---|---|---|
| particle core volume/nm3 | 4.19 * | 4.19 * | 4.19 * | 4.19 * | 4.19 * | 4.19 * |
| particle core weight/g·1023 | 4.39 * | 5.54 * | 6.69 * | 7.84 * | 8.98 * | 8.09 * |
| particle density/g cm−3 | 10.49 | 13.78 ** | 15.97 ** | 19.26 ** | 21.45 | 19.32 |
| particle core surface area/nm2 | 12.57 * | 12.57 * | 12.57 * | 12.57 * | 12.57 * | 12.57 * |
| hydrodynamic diameter (DCS)/nm | 1.7 ± 0.5 *** | 1.5 ± 0.3 | 1.6 ± 0.3 *** | 1.6 ± 0.4 | 1.6 ± 0.4 *** | 1.5 ± 0.3 *** |
| diffusion coefficient (1H-DOSY)/10−10 m2 s−1 | 1.47 *** | 1.53 | 1.60 *** | 1.69 | 1.56 *** | 1.28 *** |
| hydrodynamic diameter (1H-DOSY)/nm | 3.32 *** | 3.19 | 3.05 *** | 2.88 | 3.14 *** | 3.81 *** |
| particle core diameter (HRTEM)/nm | 2.2 ± 0.5 *** | 1.9 ± 0.6 | 1.8 ± 0.4 *** | 1.8 ± 0.3 | 2.0 ± 0.4 *** | 2.0 ± 0.4 *** |
| particle core diameter (SAXS)/nm | 1.0 ± 0.1 *** | 1.0 ± 0.1 | 1.6 ± 0.1 *** | 1.0 ± 0.1 | 0.9 ± 0.1 *** | 0.8 ± 0.2 |
| crystallinity by TEM | crystalline *** | amorphous | amorphous *** | crystalline | crystalline *** | crystalline *** |
| oxidation state of metals by XPS | Ag+ *** | Ag+, Pt, Pt2+ | Ag+, Pt, Pt2+ *** | Ag+, Pt, Pt2+ | Pt, Pt2+ *** | Au *** |
| normalized molar ratio metal(M):sulfur(S) by ICP-MS | 1.00 (Ag):1.28 (S) *** | 0.71 (Ag):0.29 (Pt):0.65 (S) | 0.59 (Ag):0.41 (Pt):0.68 (S) *** | 0.16 (Ag):0.84 (Pt):1.35 (S) | 1.00 (Pt):0.73 (S) *** | 1.00 (Au):0.82 (S) *** |
| overall nominal composition of one nanoparticle | Ag245GSH315 *** | Ag184Pt74GSH170 | Ag156Pt110GSH180 *** | Ag43Pt230GSH370 | Pt277GSH206 *** | Au247GSH203 *** |
| GSH molecular footprint/nm2 | 0.040 *** | 0.074 | 0.070 *** | 0.034 | 0.062 *** | 0.062 *** |
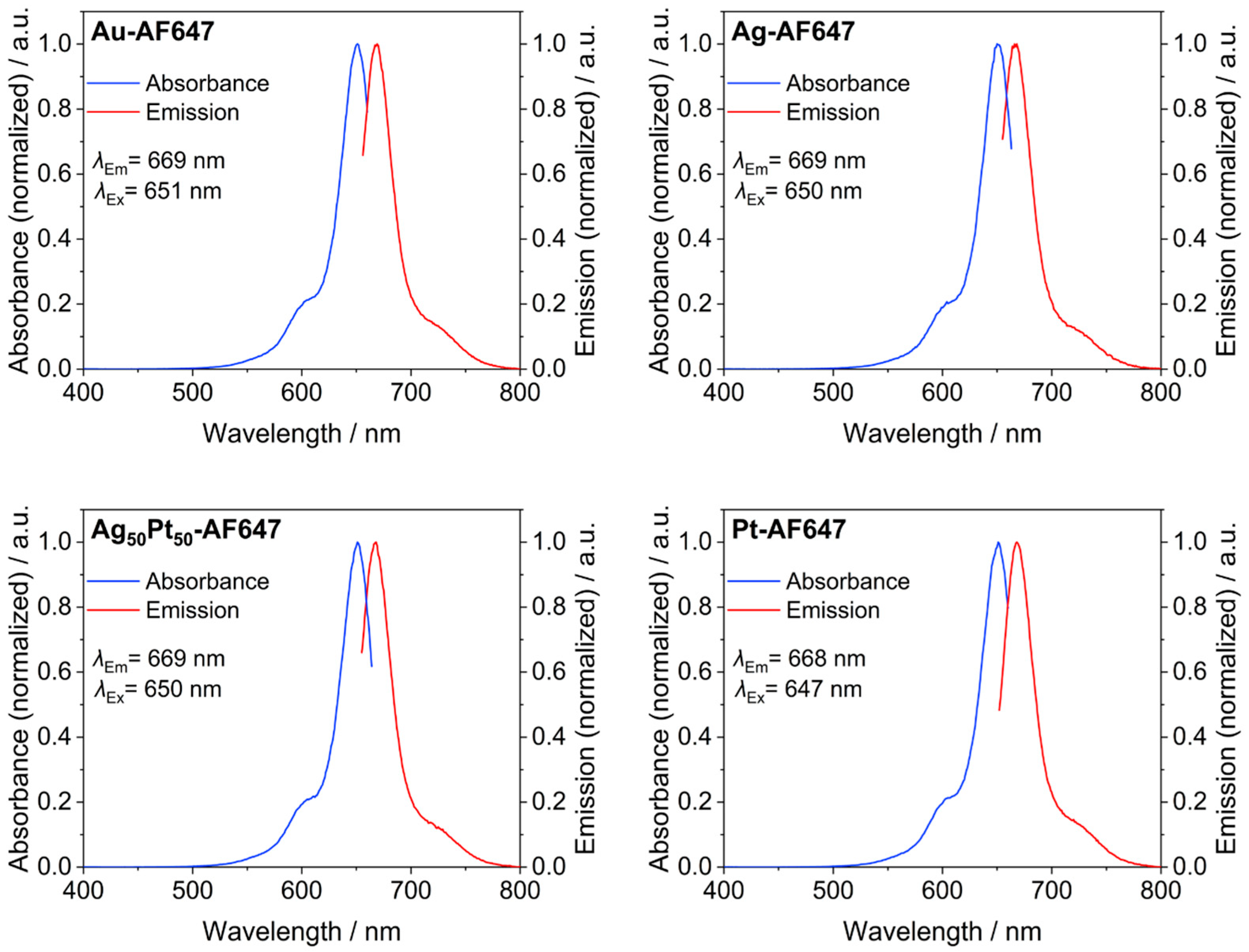
| number of conjugated AlexaFluor-647 molecules on each M-AF647 nanoparticle by AAS and UV-VIS | 13 | - | 8 | - | 6 | 12 |
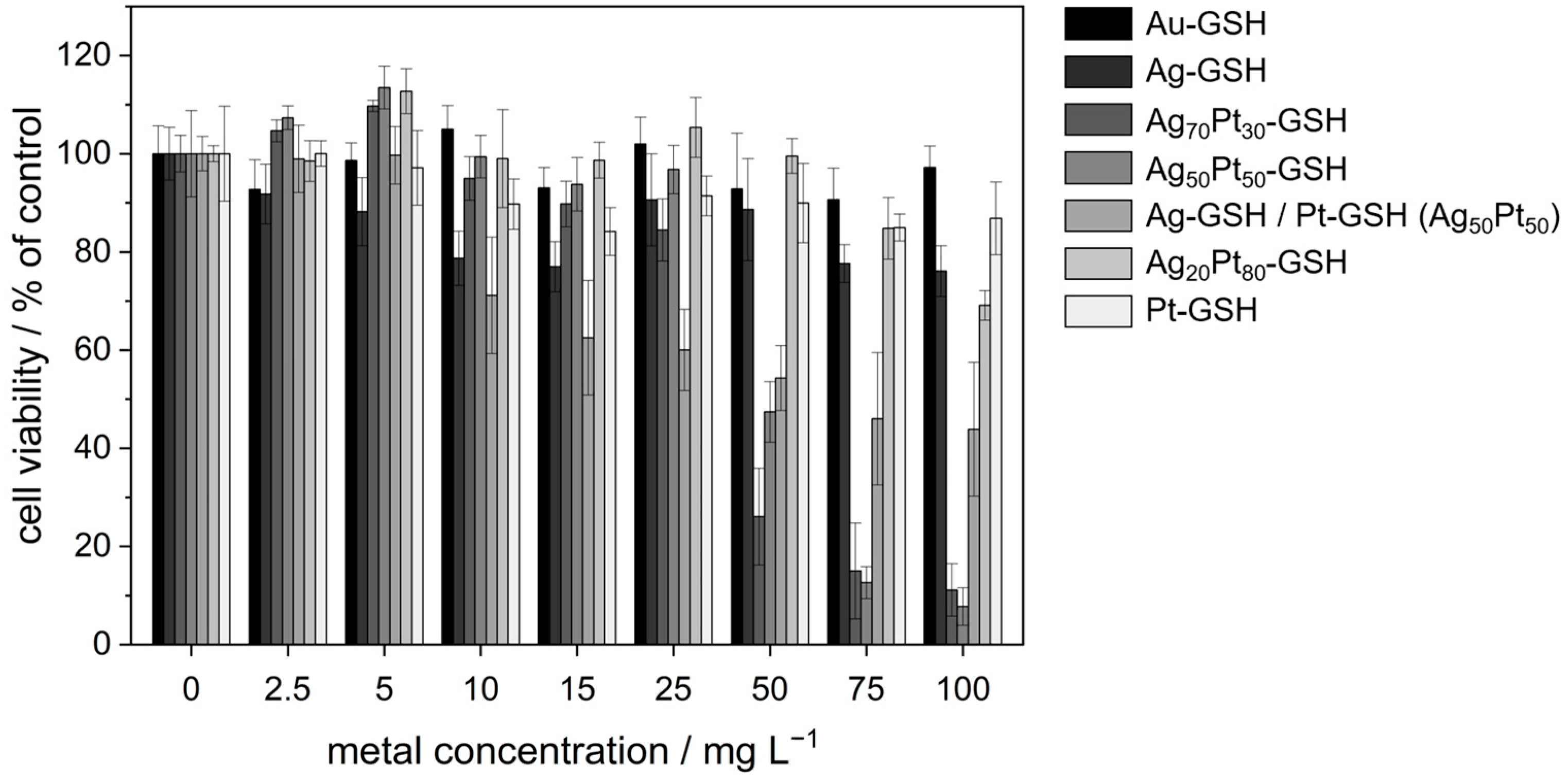
| c(Metal)/in µg mL−1 | Au | Ag | Ag70Pt30 | Ag50Pt50 | Ag/Pt (50:50) | Ag20Pt80 | Pt |
|---|---|---|---|---|---|---|---|
| 0 | 100 ± 6 | 100 ± 5 (Ag: 0) | 100 ± 4 (Ag: 0) | 100 ± 9 (Ag: 0) | 100 ± 4 (Ag: 0) | 100 ± 2 (Ag: 0) | 100 ± 10 |
| 2.5 | 93 ± 6 | 92 ± 6 (Ag: 2.5) | 105 ± 2 (Ag: 1) | 107 ± 2 (Ag: 0.9) | 99 ± 7 (Ag: 0.9) | 98 ± 4 (Ag: 0) | 100 ± 3 |
| 5 | 99 ± 4 | 88 ± 7 (Ag: 5) | 110 ± 1 (Ag: 2.8) | 114 ± 4 (Ag: 1.8) | 100 ± 6 (Ag: 1.8) | 113 ± 5 (Ag: 0.6) | 97 ± 8 |
| 10 | 105 ± 5 | 79 ± 6 (Ag: 10) | 95 ± 4 (Ag: 5.6) | 99 ± 4 (Ag: 3.6) | 71 ± 12 (Ag: 3.6) | 99 ± 10 (Ag: 1) | 90 ± 5 |
| 15 | 93 ± 4 | 77 ± 5 (Ag: 15) | 90 ± 5 (Ag: 8.5) | 94 ± 5 (Ag: 5) | 62 ± 12 (Ag: 5) | 99 ± 4 (Ag: 1.8) | 84 ± 5 |
| 25 | 102 ± 6 | 91 ± 9 (Ag: 25) | 84 ± 6 (Ag: 14) | 97 ± 5 (Ag: 8.9) | 60 ± 8 (Ag: 8.9) | 105 ± 6 (Ag: 3) | 91 ± 4 |
| 50 | 93 ± 11 | 89 ± 10 (Ag: 50) | 26 ± 10 (Ag: 28) | 47 ± 6 (Ag: 18) | 54 ± 7 (Ag: 18) | 100 ± 4 (Ag: 6) | 90 ± 8 |
| 75 | 91 ± 6 | 78 ± 4 (Ag: 75) | 15 ± 10 (Ag: 42) | 13 ± 3 (Ag: 27) | 46 ± 14 (Ag: 27) | 85 ± 6 (Ag: 9) | 85 ± 3 |
| 100 | 97 ± 4 | 76 ± 5 (Ag: 100) | 11 ± 5 (Ag: 56) | 8 ± 4 (Ag: 36) | 44 ± 14 (Ag: 36) | 69 ± 3 (Ag: 12) | 87 ± 7 |
| Sample | E. coli (Gram-Negative) | S. xylosus (Gram-Positive) |
|---|---|---|
| AgNO3 | 6–10 (6–10) | 15–25 (15–25) |
| Ag | >100 (>100) | >100 (>100) |
| Au | >100 (−) | >100 (−) |
| Pt | >100 (−) | >100 (−) |
| Ag50Pt50 | 11–15 (4–5.4) | 1–5 (0.4–1.8) |
| Ag/Pt (50:50) | 76–100 (27–36) | >100 (>36) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolff, N.; Białas, N.; Loza, K.; Heggen, M.; Schaller, T.; Niemeyer, F.; Weidenthaler, C.; Beuck, C.; Bayer, P.; Prymak, O.; et al. Increased Cytotoxicity of Bimetallic Ultrasmall Silver–Platinum Nanoparticles (2 nm) on Cells and Bacteria in Comparison to Silver Nanoparticles of the Same Size. Materials 2024, 17, 3702. https://doi.org/10.3390/ma17153702
Wolff N, Białas N, Loza K, Heggen M, Schaller T, Niemeyer F, Weidenthaler C, Beuck C, Bayer P, Prymak O, et al. Increased Cytotoxicity of Bimetallic Ultrasmall Silver–Platinum Nanoparticles (2 nm) on Cells and Bacteria in Comparison to Silver Nanoparticles of the Same Size. Materials. 2024; 17(15):3702. https://doi.org/10.3390/ma17153702
Chicago/Turabian StyleWolff, Natalie, Nataniel Białas, Kateryna Loza, Marc Heggen, Torsten Schaller, Felix Niemeyer, Claudia Weidenthaler, Christine Beuck, Peter Bayer, Oleg Prymak, and et al. 2024. "Increased Cytotoxicity of Bimetallic Ultrasmall Silver–Platinum Nanoparticles (2 nm) on Cells and Bacteria in Comparison to Silver Nanoparticles of the Same Size" Materials 17, no. 15: 3702. https://doi.org/10.3390/ma17153702





