Comparative Study of the Foaming Behavior of Ethylene–Vinyl Acetate Copolymer Foams Fabricated Using Chemical and Physical Foaming Processes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Chemical and Physical Foaming of EVA Foams
2.3. Characterization
3. Results and Discussion
3.1. Basic Properties of EVA
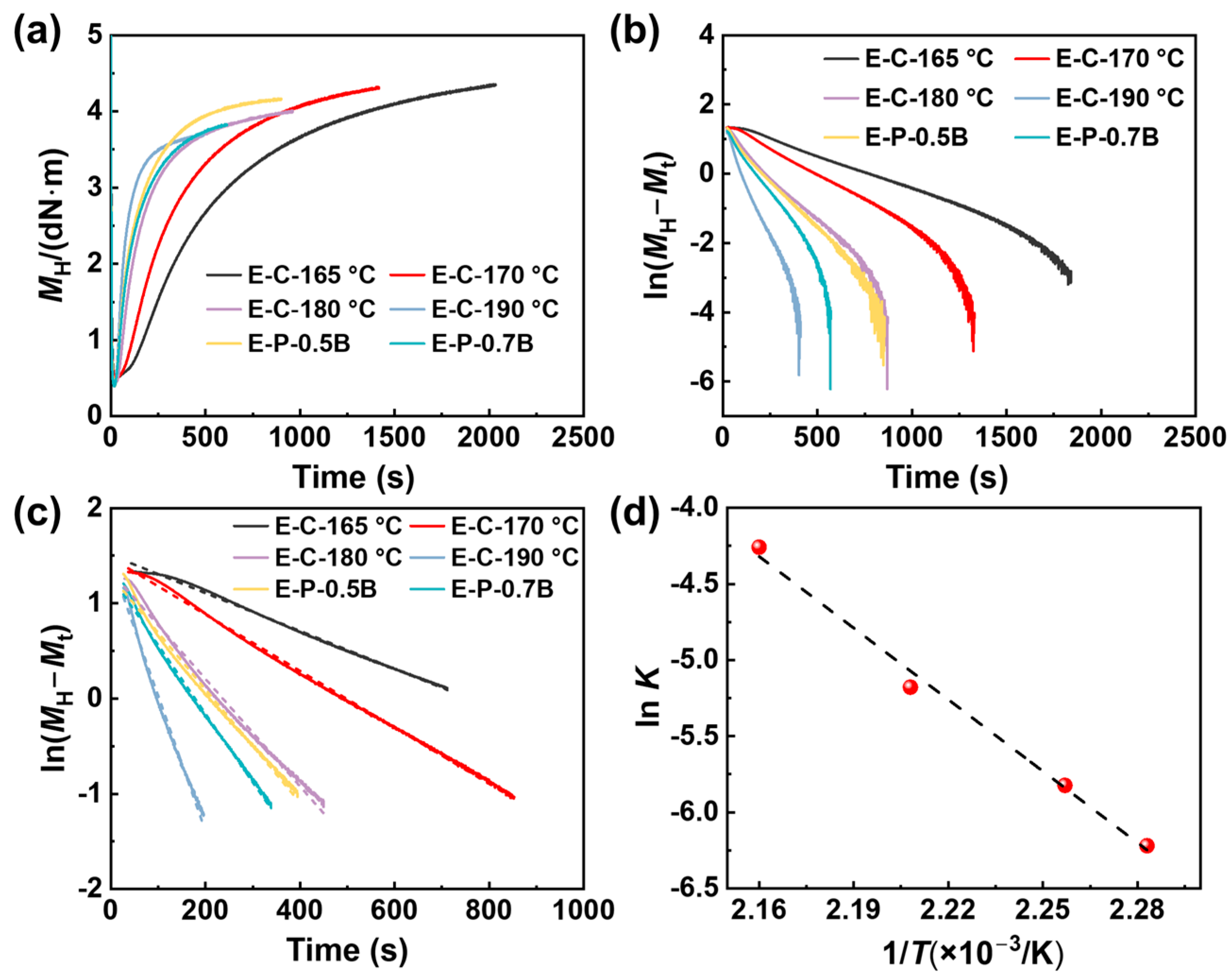
3.1.1. Vulcanization Characteristic
3.1.2. Thermal Properties
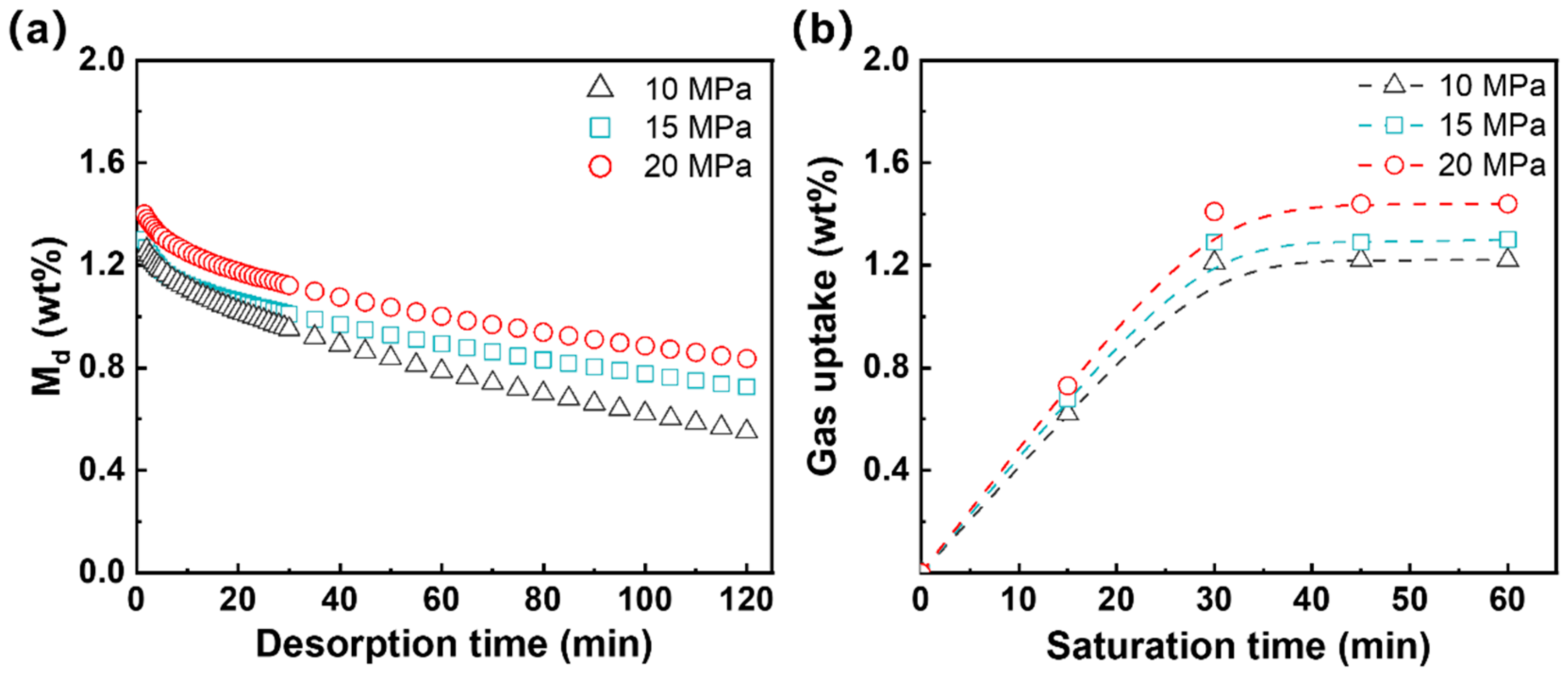
3.1.3. Gas Solubility and Diffusivity
3.2. The Foaming Behaviors of EVA during the Chemical and Physical Processes
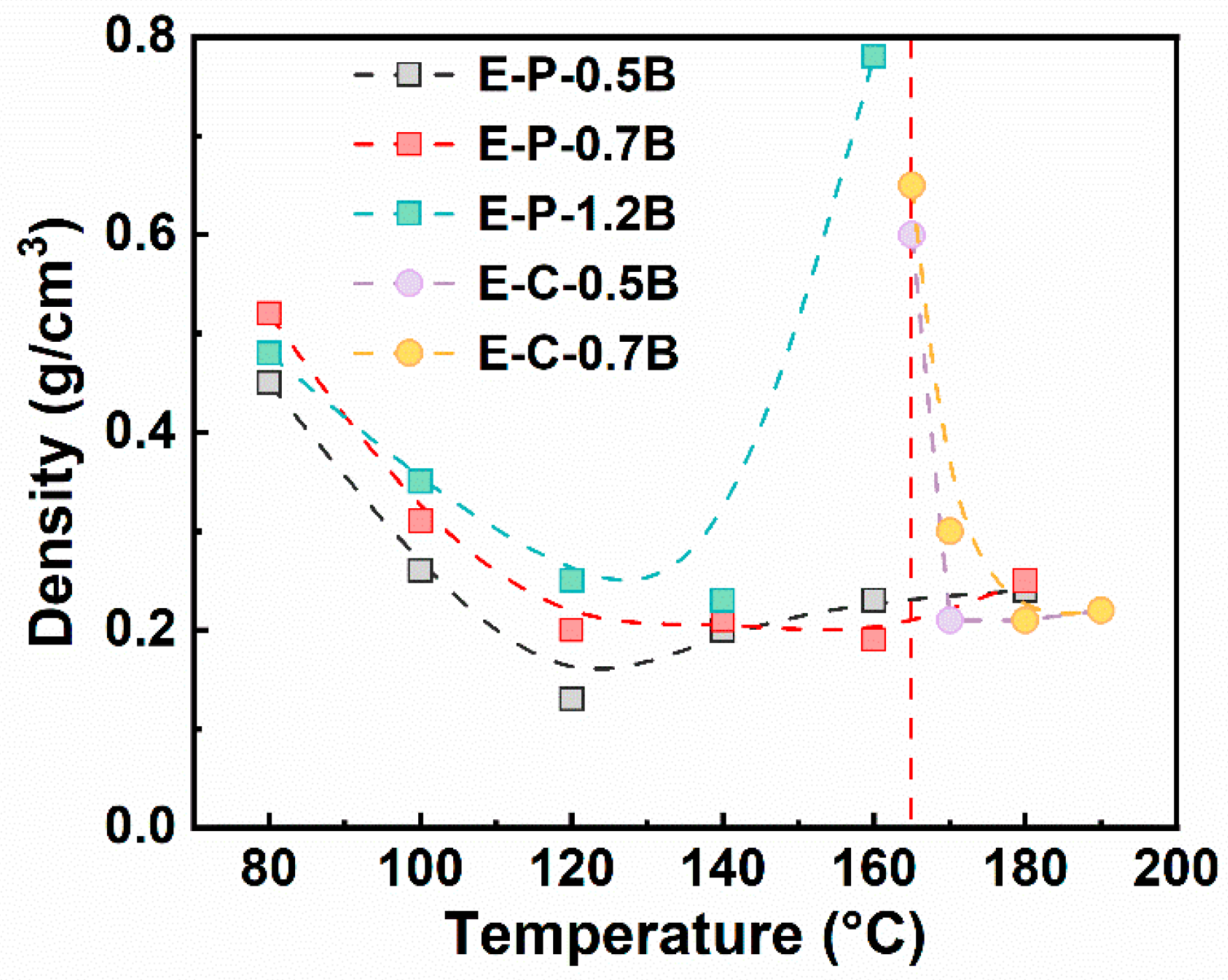
3.2.1. The Foaming Temperature Window of EVA
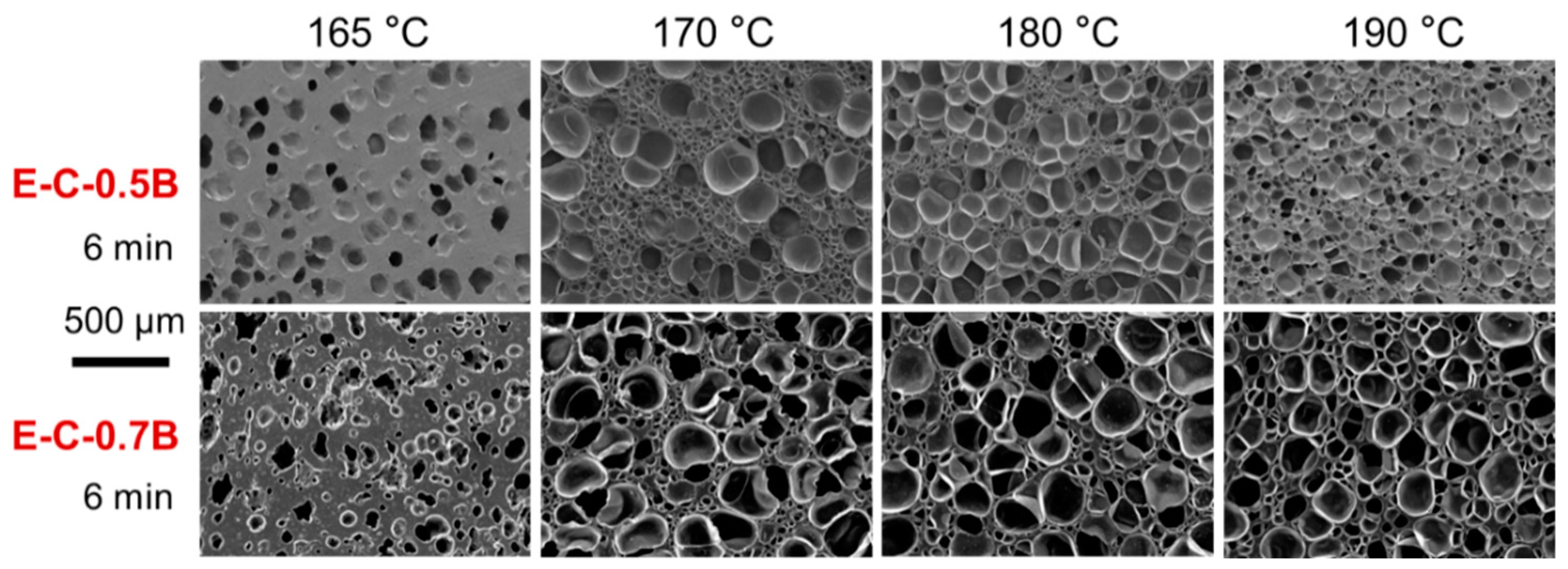
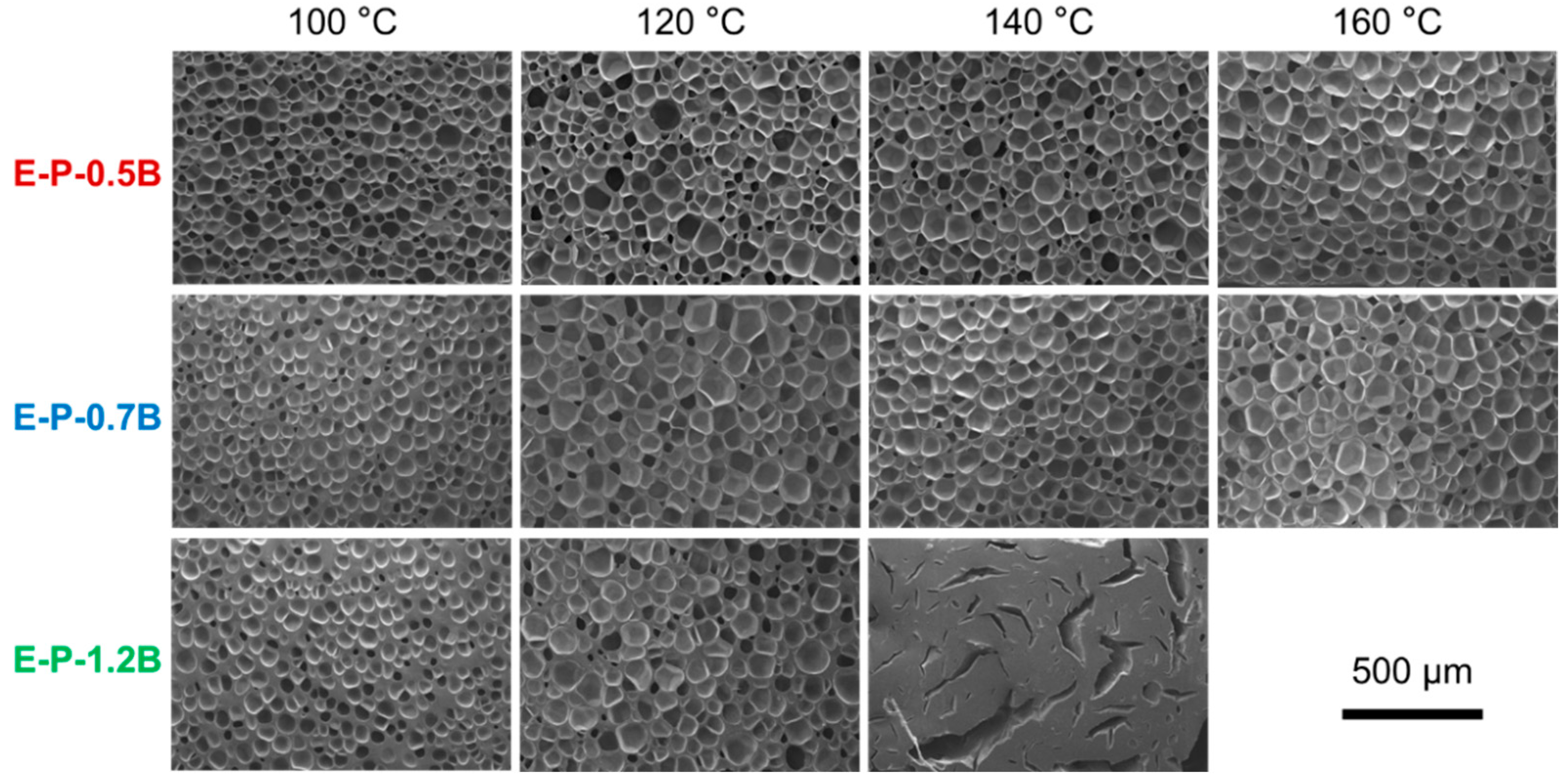
3.2.2. Influence of Foaming Temperature on the Cell Morphology
3.2.3. Influence of Gas Content on the Cell Morphology
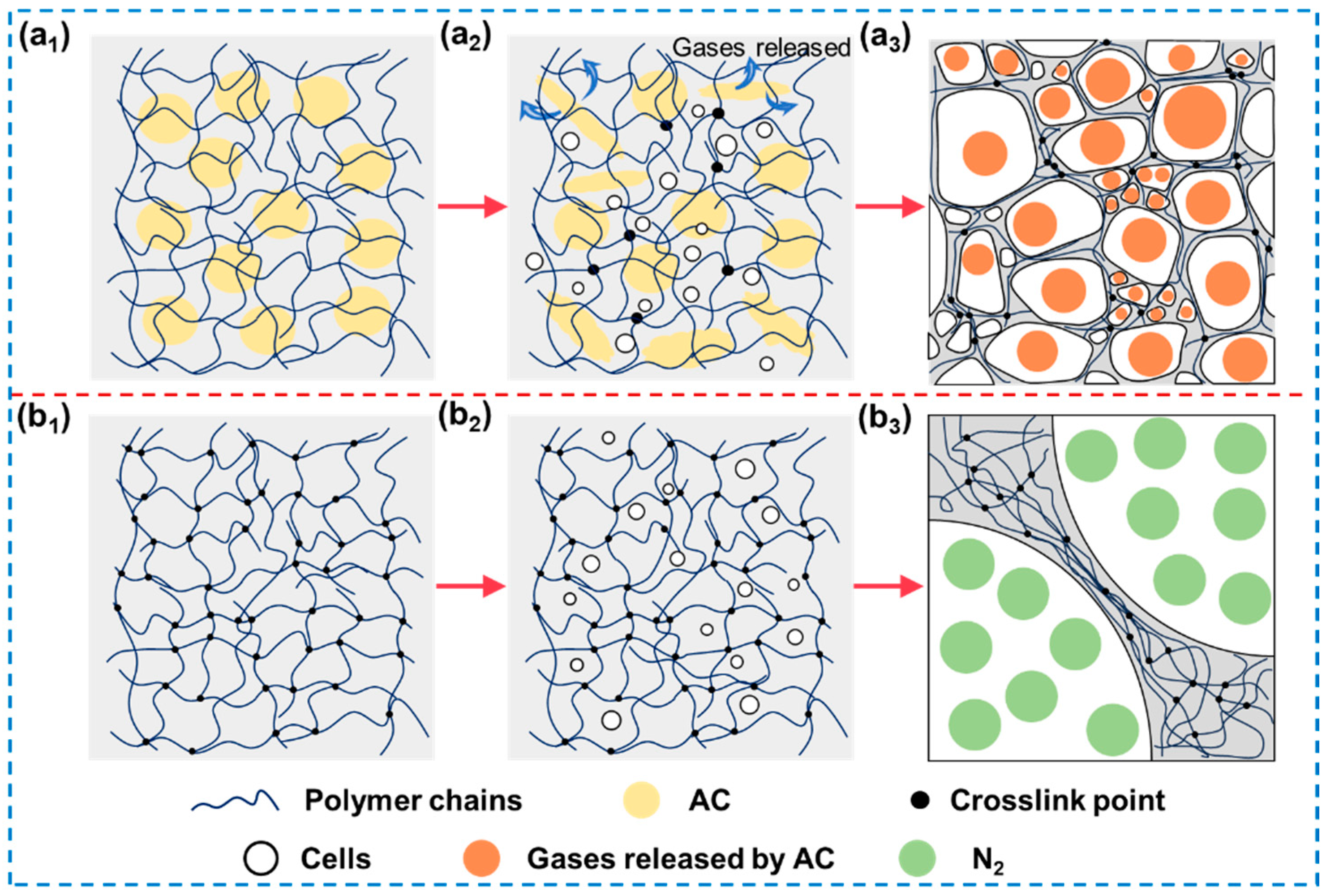
3.2.4. Mechanisms of Cell Structure Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stark, W.; Jaunich, M. Investigation of Ethylene/Vinyl Acetate Copolymer (EVA) by Thermal Analysis DSC and DMA. Polym. Test. 2011, 30, 236–242. [Google Scholar] [CrossRef]
- Shao, L.; Ji, Z.Y.; Ma, J.Z.; Xue, C.H.; Deng, F.Q. Morphology and Interaction of Nanocomposite Foams Formed with Organo-palygorskite and Ethylene-vinyl Acetate Copolymers. Polym. Bull. 2017, 74, 413–429. [Google Scholar] [CrossRef]
- Ma, J.Z.; Shao, L.; Xue, C.H.; Deng, F.Q.; Duan, Z.Y. Compatibilization and Properties of Ethylene Vinyl Acetate Copolymer (EVA) and Thermoplastic Polyurethane (TPU) Blend Based Foam. Polym. Bull. 2014, 71, 2219–2234. [Google Scholar] [CrossRef]
- Zhai, W.T.; Jiang, J.J.; Park, C.B. A Review on Physical Foaming of Thermoplastic and Vulcanized Elastomers. Polym. Rev. 2021, 62, 95–141. [Google Scholar] [CrossRef]
- Jiang, J.J.; Liu, F.; Yang, X.; Xiong, Z.J.; Liu, H.W.; Xu, D.H.; Zhai, W.T. Evolution of Ordered Structure of TPU in High-elastic State and Their Influences on the Autoclave Foaming of TPU and Inter-bead Bonding of Expanded TPU Beads. Polymer 2021, 228, 123872. [Google Scholar] [CrossRef]
- Zhong, W.; Hu, D.; Jia, X.; Huang, Y.; Wang, Y.; Lei, K.; Jiang, X.; Yu, J.; Chen, Y.; Zhao, L. A Novel Semi-Continuous Preparation Mode of Ultra-Low Density Thermoplastic Polyurethane Foam. Chem. Eng. J. 2024, 481, 148402. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, G.; Zhao, J.; Zhang, A.; Zhao, G. Super-elastic and Structure-tunable Poly(ether-block-amide) Foams Achieved by Microcellular Foaming. J. CO2 Util. 2022, 55, 101807. [Google Scholar] [CrossRef]
- Yu, L.; Yu, Z.; Yang, L.; Wen, S.; Zhang, Z.X. Development of Thermoplastic Polyether Ester Elastomer Microcellular Foam with High Resilience: Effect of Chain Extension on Foaming Behavior and Mechanical Properties. J. Appl. Polym. Sci. 2023, 140, e53912. [Google Scholar] [CrossRef]
- Rodrigo-Carranza, V.; Hoogkamer, W.; González-Ravé, J.M.; Horta-Muñoz, S.; Serna-Moreno, M.d.C.; Romero-Gutierrez, A.; González-Mohíno, F. Influence of Different Midsole Foam in Advanced Footwear Technology Use on Running Economy and Biomechanics in Trained Runners. Scand. J. Med. Sci. Sports 2024, 34, e14526. [Google Scholar] [CrossRef]
- Muniz-Pardos, B.; Sutehall, S.; Angeloudis, K.; Guppy, F.M.; Bosch, A.; Pitsiladis, Y. Recent Improvements in Marathon Run Times Are Likely Technological, Not Physiological. Sports Med. 2021, 51, 371–378. [Google Scholar] [CrossRef]
- Rodriguez-Perez, M.A.; Simoes, R.D.; Constantino, C.J.L.; de Saja, J.A. Structure and Physical Properties of EVA/Starch Precursor Materials for Foaming Applications. J. Appl. Polym. Sci. 2011, 121, 2324–2330. [Google Scholar] [CrossRef]
- Ji, Z.Y.; Ma, J.Z.; Qin, X.; Wu, Y.K.; Xu, R.; Ma, Z.L.; Xue, C.H.; Qin, J.B.; Shao, L. Improved Dimensional Stability of Styrene Butadiene Rubber/Ethylene Vinyl Acetate Composite Foams with Skeleton Support Structure Based on Alternately Cross-Linking Process. Polymer 2018, 157, 103–110. [Google Scholar] [CrossRef]
- Park, K.-W.; Kim, G.-H. Ethylene Vinyl Acetate Copolymer (EVA)/Multiwalled Carbon Nanotube (MWCNT) Nanocomposite Foams. J. Appl. Polym. Sci. 2009, 112, 1845–1849. [Google Scholar] [CrossRef]
- Sun, T.S.; Shi, K. Reinforcing the Tensile Strength of Lightweight and Soft BBIR/EVA Foam with Organic Modified Potassium Titanate Whisker and its Thermal Insulation Performance. Polym. Sci. Ser. A 2019, 61, 931–939. [Google Scholar]
- Reyes-Labarta, J.A.; Marcilla, A. Thermal Treatment and Degradation of Cross-Linked Ethylene Vinyl Acetate–Polyethylene–Azodicarbonamide–ZnO Foams. Complete Kinetic Modeling and Analysis. Ind. Eng. Chem. Res. 2012, 51, 9515–9530. [Google Scholar] [CrossRef]
- Huang, G.; Li, S.; Li, Y.; Wu, X.; Feng, X.; Gui, Y.; Deng, J.; Wang, C.; Pan, K. Preparation and Characterization of Microcellular Foamed Thermoplastic Polyamide Elastomer Composite Consisting of EVA/TPAE1012. J. Appl. Polym. Sci. 2021, 138, 50952. [Google Scholar] [CrossRef]
- Kwon, M.J.; Lim, D.H.; Choi, I.C.; Kwon, H.-W.; Ha, C.-S. Preparation and Properties of Ethylene-Vinyl Acetate Copolymer-Based Blend Foams. J. Elastom. Plast. 2021, 53, 68–82. [Google Scholar] [CrossRef]
- Chang, B.P.; Kurkin, A.; Kashcheev, A.; Leong, K.F.; Tok, A.l.Y.; Lipik, V. Enhancing Dynamic Impact Performance and Cushioning of EVA Copolymer Foams with Thermoplastic Elastomers. Mater. Today Commun. 2024, 38, 107888. [Google Scholar] [CrossRef]
- Ma, J.Z.; Duan, Z.Y.; Xue, C.H.; Deng, F.Q. Morphology and Mechanical Properties of EVA/OMMT Nanocomposite Foams. J. Thermoplast. Compos. Mater. 2012, 26, 555–569. [Google Scholar] [CrossRef]
- Duan, Z.; Ma, J.; Xue, C.; Deng, F. Effect of Stearic Acid/Organic Montmorillonite on EVA/SA/OMMT Nanocomposite Foams by Melting Blending. J. Cell. Plast. 2014, 50, 263–277. [Google Scholar] [CrossRef]
- Lunchev, A.V.; Kashcheev, A.; Tok, A.l.Y.; Lipik, V. Mechanical Characteristics of Poly(ethylene vinyl acetate) Foams with Graphene for the Applications in Sport Footwear. Polym. Test. 2022, 113, 107688. [Google Scholar] [CrossRef]
- Chang, B.P.; Kashcheev, A.; Veksha, A.; Lisak, G.; Goei, R.; Leong, K.F.; Tok, A.L.; Lipik, V. Nanocomposite Foams with Balanced Mechanical Properties and Energy Return from EVA and CNT for the Midsole of Sports Footwear Application. Polymers 2023, 15, 948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Zhang, T.; Wang, D.; Zhang, X.; Xin, Z.X.; Prakashan, K. Physicomechanical, Friction, and Abrasion Properties of EVA/PU Blend Foams Foamed by Supercritical Nitrogen. Polym. Eng. Sci. 2018, 58, 673–682. [Google Scholar] [CrossRef]
- Rangappa, R.; Yeh, S.-K. Investigating the Role and Impact of N2 as a Blowing Agent in the Fabrication of Thermoplastic Polyurethane Foams. Cell. Polym. 2023, 42, 187–203. [Google Scholar] [CrossRef]
- Li, R.S.; Lee, J.H.; Wang, C.D.; Mark, L.H.; Park, C.B. Solubility and Diffusivity of CO2 and N2 in TPU and Their Effects on Cell Nucleation in Batch Foaming. J. Supercrit. Fluids 2019, 154, 104623. [Google Scholar] [CrossRef]
- Kiran, E.; Sarver, J.A.; Hassler, J.C. Solubility and Diffusivity of CO2 and N2 in Polymers and Polymer Swelling, Glass Transition, Melting, and Crystallization at High Pressure: A Critical Review and Perspectives on Experimental Methods, Data, and Modeling. J. Supercrit. Fluids 2022, 185, 105378. [Google Scholar] [CrossRef]
- Michaeli, W.; Westermann, K.; Sitz, S. Extrusion of Physically Foamed Rubber Profiles. J. Cell. Plast. 2011, 47, 483–495. [Google Scholar] [CrossRef]
- Yu, Z.W.; Ma, L.Y.; Zhu, B.J.; Phule, A.D.; Wen, S.B.; Zhao, Y.X.; Zhang, Z.X. Development of EVA/POE/SEBS Microcellular Foam: Network Structure, Mechanics Performance and Midsole Application. Express Polym. Lett. 2022, 16, 1322–1330. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, C.; Zhang, X.; Zhao, Y.; Wen, S.; Zhang, Z.X. Controlled Damping Ethylene-Vinyl Acetate Copolymer/Styrene Ethylene Butylene Styrene Foam in Wide Temperature Range Prepared by Supercritical Nitrogen. Polym. Eng. Sci. 2023, 63, 3429–3432. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids-Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Pang, Y.Y.; Cao, Y.Y.; Zheng, W.G.; Park, C.B. A Comprehensive Review of Cell Structure Variation and General Rules for Polymer Microcellular Foams. Chem. Eng. J. 2022, 430, 132662. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Zhao, J.; Li, S.; Zhao, G.; Park, C.B. Structure-Gradient Thermoplastic Polyurethane Foams with Enhanced Resilience Derived by Microcellular Foaming. J. Supercrit. Fluids 2022, 188, 105667. [Google Scholar] [CrossRef]
- Wang, G.; Ren, T.; Zhang, W.; Liu, J.; Xu, Z.; Zhao, J.; Li, X.; Li, S.; Zhao, G. Research on the Cyclic Compression Performance of Polycarbonate-Based Thermoplastic Polyurethane Foams Prepared by Microcellular Foaming. J. CO2 Util. 2022, 65, 102218. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, G.; Pei, X.; Huang, P.; Liu, X.; Zheng, W. Customized Multimodal Cellular Elastomer Foams through Presetting Cells before CO2 Foaming. ACS Appl. Polym. Mater. 2024, 6, 3003–3014. [Google Scholar] [CrossRef]
- Li, Y.; Gong, P.; Liu, Y.; Niu, Y.; Park, C.B.; Li, G. Environmentally Friendly and Zero-Formamide EVA/LDPE Microcellular Foams via Supercritical Carbon Dioxide Solid Foaming. ACS Appl. Polym. Mater. 2021, 3, 4213–4222. [Google Scholar] [CrossRef]
- Choi, S.-S.; Kim, E. A Novel System for Measurement of Types and Densities of Sulfur Crosslinks of a Filled Rubber Vulcanizate. Polym. Test. 2015, 42, 62–68. [Google Scholar] [CrossRef]
- Thitithammawong, A.; Uthaipan, N.; Junhasavasdikul, B.; Nakason, C.; Kalkornsurapranee, E. Curing Characteristics and Kinetics of EPDM and EOC Compounds in Co-vulcanization as Blend. J. Appl. Polym. Sci. 2019, 136, 47613. [Google Scholar] [CrossRef]
- Bianchi, O.; Martins, J.D.N.; Fiorio, R.; Oliveira, R.V.B.; Canto, L.B. Changes in Activation Energy and Kinetic Mechanism During EVA Crosslinking. Polym. Test. 2011, 30, 616–624. [Google Scholar] [CrossRef]
- Bianchi, O.; Oliveira, R.V.B.; Fiorio, R.; Martins, J.D.N.; Zattera, A.J.; Canto, L.B. Assessment of Avrami, Ozawa and Avrami–Ozawa Equations for Determination of EVA Crosslinking Kinetics from DSC Measurements. Polym. Test. 2008, 27, 722–729. [Google Scholar] [CrossRef]
- Valentín, J.L.; Rodríguez, A.; Marcos-Fernández, A.; González, L. Dicumyl Peroxide Cross-Linking of Nitrile Rubbers with Different Content in Acrylonitrile. J. Appl. Polym. Sci. 2005, 96, 1–5. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Malatesta, V.; Ceraulo, M.; Mistretta, M.C.; Koci, P. Photooxidation and Photostabilization of EVA and Cross-Linked EVA. Polym. Test. 2016, 51, 6–12. [Google Scholar] [CrossRef]
- Luna, C.B.B.; da Silva Barbosa Ferreira, E.; Siqueira, D.D.; dos Santos Filho, E.A.; Araújo, E.M. Additivation of The Ethylene–Vinyl Acetate Copolymer (EVA) with Maleic Anhydride (MA) and Dicumyl Peroxide (DCP): The Impact of Styrene Monomer on Cross-Linking and Functionalization. Polym. Bull. 2022, 79, 7323–7346. [Google Scholar] [CrossRef]
- Hoang, T.; Chinh, N.T.; Trang, N.T.T.; Hang, T.T.X.; Thanh, D.T.M.; Hung, D.V.; Ha, C.-S.; Aufray, M. Effects of Maleic Anhydride Grafted Ethylene/Vinyl Acetate Copolymer (Eva) on The Properties of Eva/Silica Nanocomposites. Macromol. Res. 2013, 21, 1210–1217. [Google Scholar] [CrossRef]
- Yang, C.; Wang, G.; Zhang, A.; Zhao, J.; Xu, Z.; Li, S.; Zhao, G. High-elastic and Strong Hexamethylene Diisocyanate (HDI)-Based Thermoplastic Polyurethane Foams Derived by Microcellular Foaming with Co-blowing Agents. J. CO2 Util. 2023, 74, 102543. [Google Scholar] [CrossRef]
- Zhong, W.P.; Yu, Z.; Zhu, T.Y.; Zhao, Y.X.; Phule, A.D.; Zhang, Z.X. Influence of Different Ratio of CO2/N2 and Foaming Additives on Supercritical Foaming of Expanded Thermoplastic Polyurethane. Express Polym. Lett. 2022, 16, 318–336. [Google Scholar] [CrossRef]
- Jiang, J.J.; Zheng, H.; Liu, H.W.; Zhai, W.T. Tunable Cell Structure and Mechanism in Porous Thermoplastic Polyurethane Micro-Film Fabricated by a Diffusion-Restricted Physical Foaming Process. J. Supercrit. Fluids 2021, 171, 105205. [Google Scholar] [CrossRef]
- Zhai, W.T.; Jiang, J.J. Fundamental Issues for Batch Foaming of Thermoplastic Elastomers with Supercritical Fluids. Acta Polym. Sin. 2024, 55, 369–395. [Google Scholar]
- Zheng, H.; Pan, G.; Huang, P.K.; Xu, D.H.; Zhai, W.T. Fundamental Influences of Crosslinking Structure on the Cell Morphology, Creep Property, Thermal Property, and Recycling Behavior of Microcellular EPDM Foams Blown with Compressed CO2. Ind. Eng. Chem. Res. 2020, 59, 1534–1548. [Google Scholar] [CrossRef]
- Shafeeq, V.H.; Unnikrishnan, G. Experimental and theoretical evaluation of mechanical, thermal and morphological features of EVA-millable polyurethane blends. J. Polym. Res. 2020, 27, 53. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, J.; Li, Y.; Zhou, M.; Wang, Z.; Wang, L.; Zhai, W. Supercritical Fluid Microcellular Foaming of High-Hardness TPU via a Pressure-Quenching Process: Restricted Foam Expansion Controlled by Matrix Modulus and Thermal Degradation. Molecules 2022, 27, 8911. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, S.; Yu, W. Recyclable ethylene-vinyl acetate copolymer vitrimer foams. Polymer 2021, 222, 123662. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, P.; Lee, P.C.; Chang, N.R.S.; Zhao, Y.; Su, Y.; Wu, F.; Liu, X.; Zheng, W. Lightweight olefin block copolymers foams with shrinkable and recoverable performance via supercritical CO2 foaming. J. Supercrit. Fluids 2023, 200, 105981. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Dai, X.R.; Zou, L.; Wen, S.B.; Sinha, T.K.; Li, H. A Developed, Eco-Friendly, and Flexible Thermoplastic Elastomeric Foam from SEBS for Footwear Application. Express Polym. Lett. 2019, 13, 948–958. [Google Scholar] [CrossRef]

 ) solid crosslinked EVA sheet used for physical foaming; (
) solid crosslinked EVA sheet used for physical foaming; ( ) EVA foams prepared using chemical foaming.
) EVA foams prepared using chemical foaming.
 ) solid crosslinked EVA sheet used for physical foaming; (
) solid crosslinked EVA sheet used for physical foaming; ( ) EVA foams prepared using chemical foaming.
) EVA foams prepared using chemical foaming.


| Chemical Foaming | Physical Foaming | ||||
|---|---|---|---|---|---|
| Samples | E-C-0.5B | E-C-0.7B | E-P-0.5B | E-P-0.7B | E-P-1.2B |
| EVA (phr) | 100 | 100 | |||
| BIPB (phr) | 0.5 | 0.7 | 0.5 | 0.7 | 1.2 |
| TiO2 (phr) | 3 | 0 | |||
| CaCO3 (phr) | 15 | 0 | |||
| ZnO (phr) | 2 | 0 | |||
| SA (phr) | 1 | 0 | |||
| AC (phr) | 1.35 | 0 | |||
| Sample | t90 (s) | MH (dN·m) | ML (dN·m) | Crosslink Density (mol/m3) | Elastic Torque (dN·m) |
|---|---|---|---|---|---|
| E-P-0.5B-180 °C | 394.4 | 4.16 | 0.30 | 45 | 3.85 |
| E-P-0.7B-180 °C | 338.7 | 4.81 | 0.30 | 66 | 4.50 |
| E-P-1.2B-180 °C | 303.5 | 4.62 | 0.28 | 217 | 4.39 |
| E-C-0.5B-165 °C | 1645.6 | 4.18 | 0.34 | / | 3.92 |
| E-C-0.5B-170 °C | 853.1 | 4.28 | 0.32 | / | 3.86 |
| E-C-0.5B-180 °C | 449.6 | 3.97 | 0.28 | / | 3.69 |
| E-C-0.5B-190 °C | 196.0 | 3.66 | 0.27 | / | 3.39 |
| Sample | T (°C) | K (s−1) | R-Square |
|---|---|---|---|
| E-C-0.5B | 165 | 0.00199 | 0.998 |
| 170 | 0.00296 | 0.999 | |
| 180 | 0.00564 | 0.992 | |
| 190 | 0.01420 | 0.992 | |
| E-P-0.5B | 180 | 0.00594 | 0.992 |
| E-P-0.7B | 180 | 0.00720 | 0.996 |
| E-P-1.2B | 180 | 0.00660 | 0.998 |
| Pressure (MPa) | Solubility (%) | Dd (m2/s) |
|---|---|---|
| 10 | 1.35 ± 0.11 | (2.55 ± 0.07) × 10−12 |
| 15 | 1.56 ± 0.14 | (3.08 ± 0.12) × 10−12 |
| 20 | 1.80 ± 0.15 | (3.45 ± 0.09) × 10−12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Jiang, J.; Huang, H.; Wang, Z.; Wang, L.; Chen, B.; Zhai, W. Comparative Study of the Foaming Behavior of Ethylene–Vinyl Acetate Copolymer Foams Fabricated Using Chemical and Physical Foaming Processes. Materials 2024, 17, 3719. https://doi.org/10.3390/ma17153719
Li Y, Jiang J, Huang H, Wang Z, Wang L, Chen B, Zhai W. Comparative Study of the Foaming Behavior of Ethylene–Vinyl Acetate Copolymer Foams Fabricated Using Chemical and Physical Foaming Processes. Materials. 2024; 17(15):3719. https://doi.org/10.3390/ma17153719
Chicago/Turabian StyleLi, Yaozong, Junjie Jiang, Hanyi Huang, Zelin Wang, Liang Wang, Bichi Chen, and Wentao Zhai. 2024. "Comparative Study of the Foaming Behavior of Ethylene–Vinyl Acetate Copolymer Foams Fabricated Using Chemical and Physical Foaming Processes" Materials 17, no. 15: 3719. https://doi.org/10.3390/ma17153719





