Mullite-Fibers-Reinforced Bagasse Cellulose Aerogels with Excellent Mechanical, Flame Retardant, and Thermal Insulation Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of BCE Aerogels and Mubce Aerogels
2.3. Characterization
3. Results
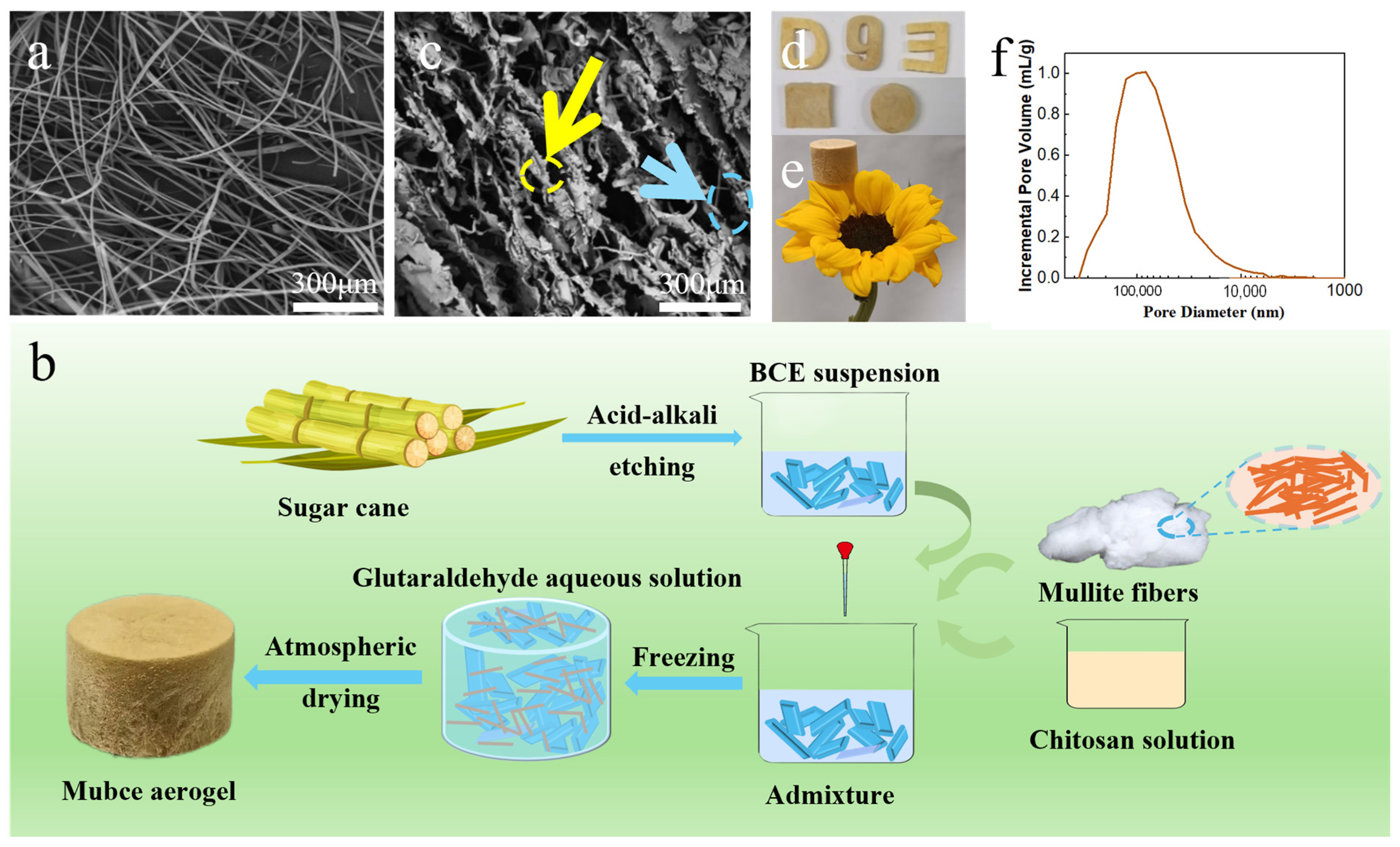
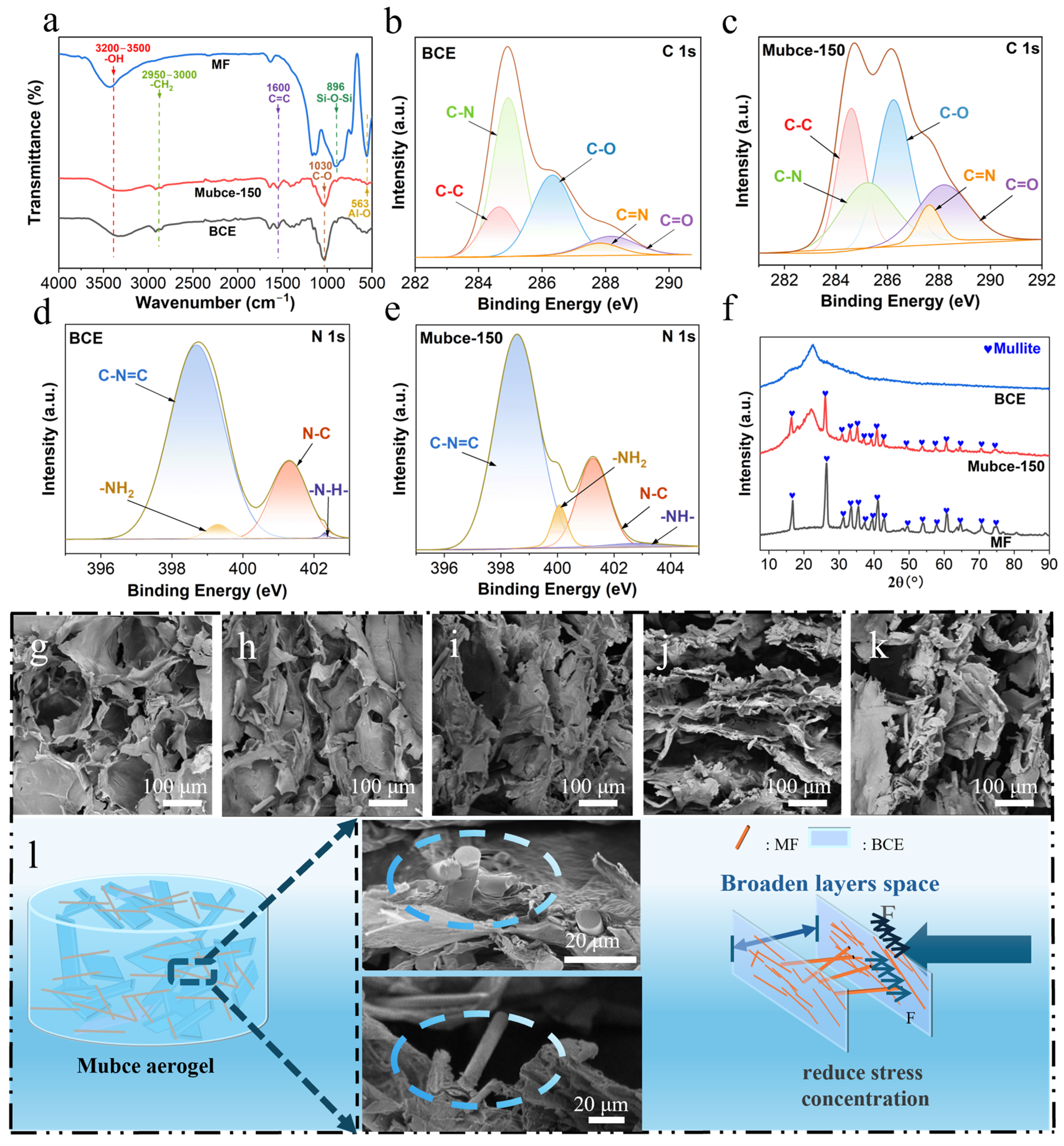
3.1. Preparation and Characterization of Mubce Aerogels
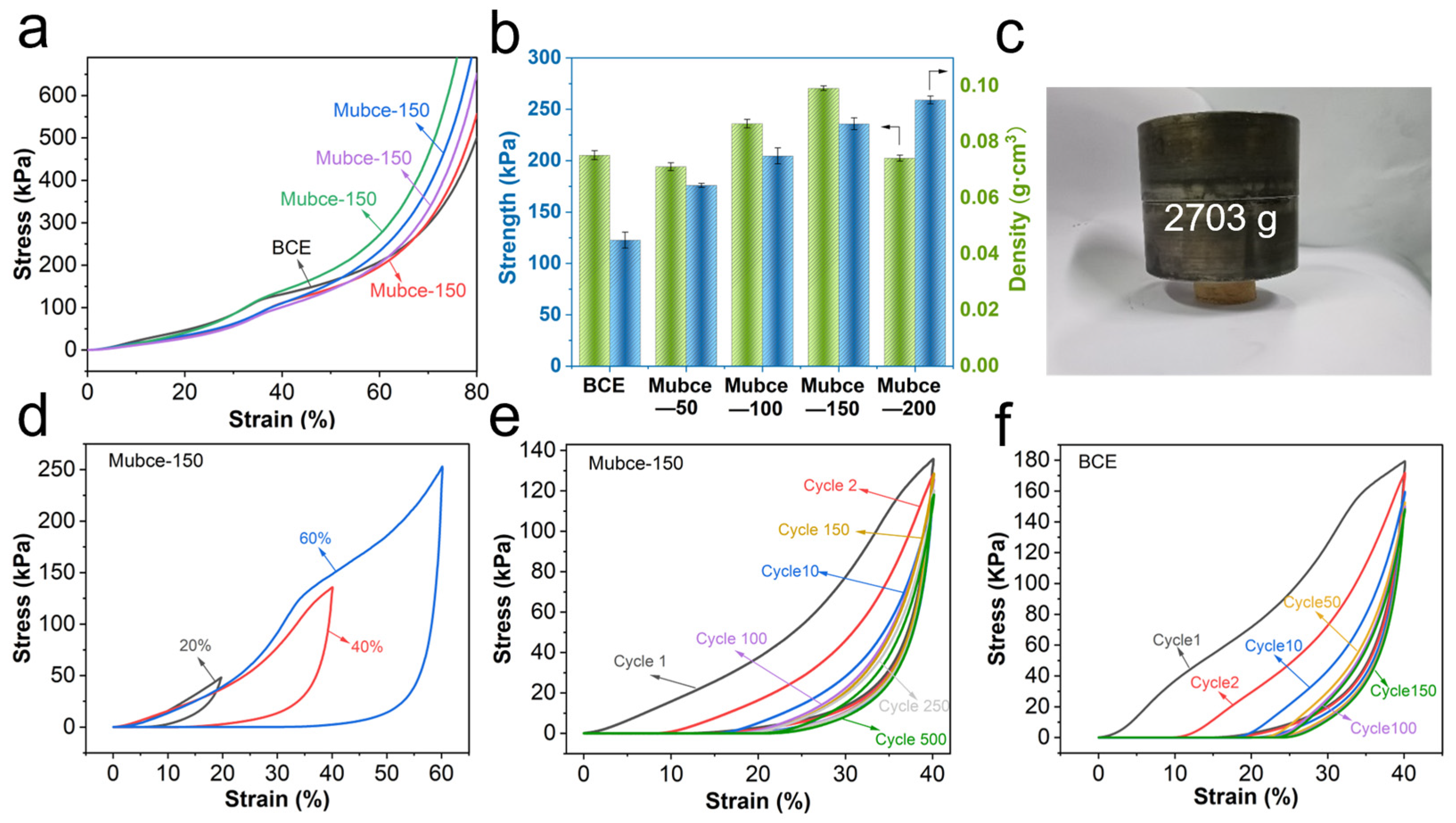
3.2. Mechanical Properties of Mubce Aerogels
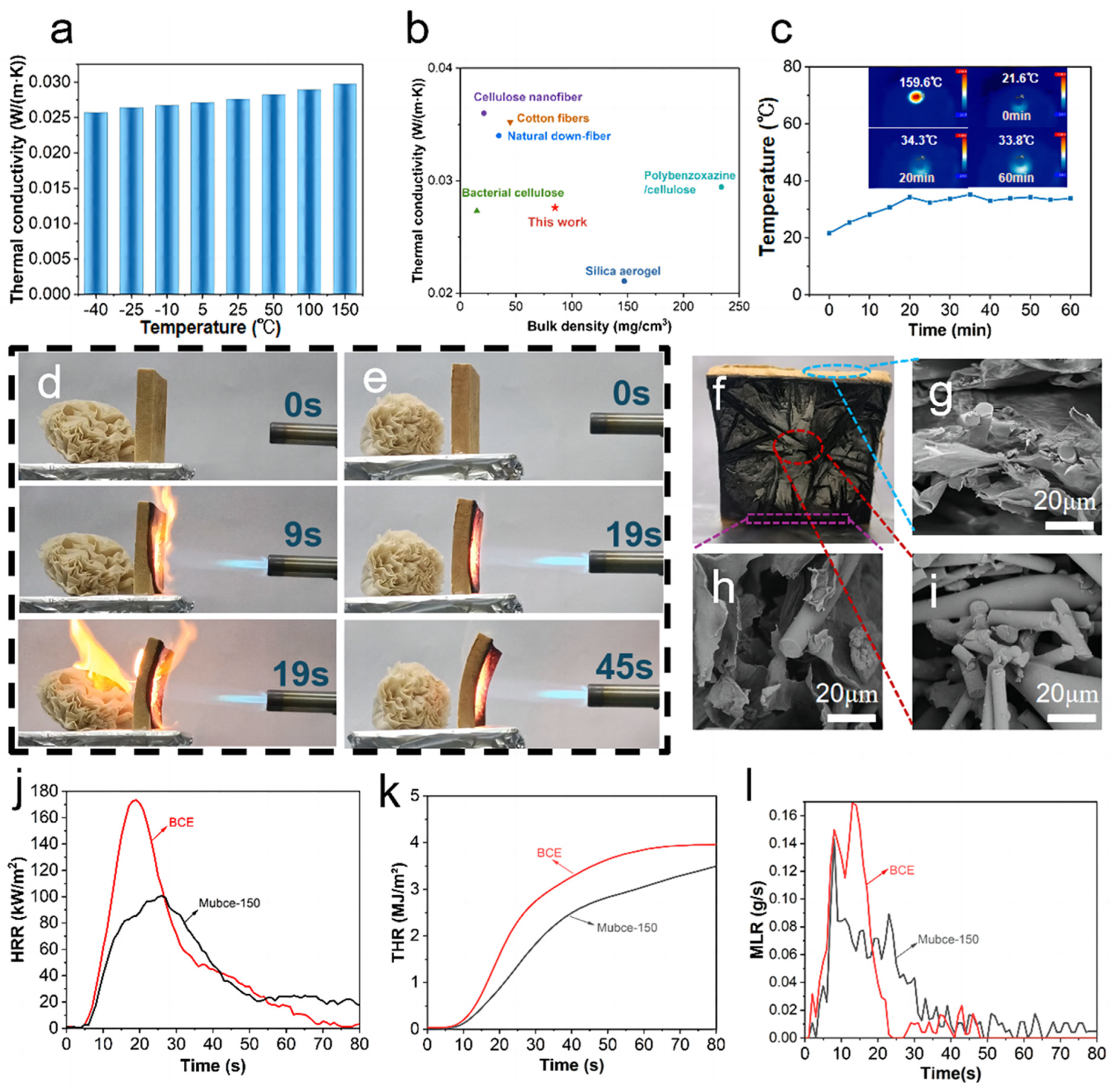
3.3. Thermal Insulation and Fire Resistance Properties of Mubce Aerogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosah, C.P.; Li, S.; Ampofo, G.K.M.; Sangare, I. A continental and global assessment of the role of energy consumption, total natural resource rent, and economic growth as determinants of carbon emissions. Sci. Total Environ. 2023, 892, 164592. [Google Scholar] [PubMed]
- Yuan, J. Impact of Insulation Type and Thickness on the Dynamic Thermal Characteristics of an External Wall Structure. Sustainability 2018, 10, 2835. [Google Scholar] [CrossRef]
- Han, L.; Dong, L.; Zhang, H.; Li, F.; Tian, L.; Li, G.; Jia, Q.; Zhang, S. Thermal insulation TiN aerogels prepared by a combined freeze-casting and carbothermal reduction-nitridation technique. J. Am. Ceram. Soc. 2021, 41, 5127–5137. [Google Scholar]
- Tian, L.; Liang, F.; Dong, L.; Li, J.; Jia, Q.; Zhang, H.; Li, J.; Jia, Q.; Zhang, H.; Yan, S.; et al. Preparation and enhanced adsorption properties for CO2 and dyes of amino-decorated hierarchical porous BCN aerogels. J. Am. Ceram. Soc. 2021, 104, 1110–1119. [Google Scholar] [CrossRef]
- Han, L.; Dong, L.; Li, F.; Duan, H.; Zhang, H.; Li, G.; Jia, Q.; Zhang, S. Preparation of Si3N4-BCxN-TiN composite ceramic aerogels via foam-gelcasting. J. Eur. Ceram. Soc. 2022, 42, 2699–2706. [Google Scholar]
- Li, K.; Yuan, G.; Dong, L.; Deng, G.; Duan, H.; Jia, Q.; Zhang, H.; Zhang, S. Boehmite aerogel with ultrahigh adsorption capacity for Congo Red removal: Preparation and adsorption mechanism. Sep. Purif. Technol. 2022, 302, 122065. [Google Scholar] [CrossRef]
- Huang, Z.; Zheng, Y.; Zhang, H.; Li, F.; Zeng, Y.; Jia, Q.; Zhang, J.; Li, J.; Zhang, S. High-yield production of carbon nanotubes from waste polyethylene and fabrication of graphene-carbon nanotube aerogels with excellent adsorption capacity. J. Mater. Sci. Technol. 2021, 94, 90–98. [Google Scholar]
- Fan, B.; Chen, S.; Yao, Q.; Sun, Q.; Jin, C. Fabrication of Cellulose Nanofiber/AlOOH Aerogel for Flame Retardant and Thermal Insulation. Materials 2017, 10, 311. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Zhao, X.; Li, N.; Guo, X.; Zhao, L.; Yin, Y. Anisotropic composite aerogel with thermal insulation and flame retardancy from cellulose nanofibers, calcium alginate and boric acid. Int. J. Biol. Macromol. 2024, 267, 131450. [Google Scholar]
- Ahmad, H.; Anguilano, L.; Fan, M. Microstructural architecture and mechanical properties of empowered cellulose-based aerogel composites via TEMPO-free oxidation. Carbohydr. Polym. 2022, 298, 120117. [Google Scholar]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic force microscopy reveals how relative humidity impacts the Young’s modulus of lignocellulosic polymers and their adhesion with cellulose nanocrystals at the nanoscale. Int. J. Biol. Macromol. 2020, 147, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Muharja, M.; Hasanah, S.; Sari, D.A.D.; Khoirunnafiuddin, M.; Fadilah, S.N.; Darmayanti, R.F.; Satrio, D.; Ismayati, M. Enhanced oil removal by graphene oxide/N,N′-methylene bisacrylamide modified magnetite-cellulose aerogel derived from ramie stem waste: Adsorption performance, kinetics, and economical analysis. Bioresour. Technol. Rep. 2023, 24, 101645. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, S.; Yang, B.; Hao, M.; Chen, Z.; Liu, Y.; Wang, X.; Yao, J. Preparation of ambient-dried multifunctional cellulose aerogel by freeze-linking technique. Chem. Eng. J. 2023, 477, 147044. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, S.; Li, M.; Wang, N.; Liu, L.; Ge, A. Superior stable, hydrophobic and multifunctional nanocellulose hybrid aerogel via rapid UV induced in-situ polymerization. Carbohydr. Polym. 2022, 288, 119370. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zhang, S.; Qi, F.; Huang, A. Highly stable cellulose nanofiber/polyacrylamide aerogel via in-situ physical/chemical double crosslinking for highly efficient Cu(II) ions removal. Int. J. Biol. Macromol. 2022, 209, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Preparation, and Mechanical and Thermal Properties of Bagasse Cellulose Aerogel by Atmospheric Pressure Drying. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2023. [Google Scholar]
- Mi, H.; Li, H.; Jing, X.; Zhang, Q.; Feng, P.; He, P.; Liu, Y. Superhydrophobic cellulose nanofibril/silica fiber/Fe3O4 nanocomposite aerogel for magnetically driven selective oil absorption. Cellulose 2020, 27, 8909–8922. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, X.; Yan, L.; Huyan, X.; Zhang, T.; Liu, S.; Guo, A.; Liu, J.; Hou, F. Super-insulated, flexible, and high resilient mullite fiber reinforced silica aerogel composites by interfacial modification with nanoscale mullite whisker. Compos. Part B 2022, 230, 109549. [Google Scholar]
- Huang, Y.; Zhou, T.; He, S.; Xiao, H.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Flame-retardant polyvinyl alcohol/cellulose nanofibers hybrid carbon aerogel by freeze drying with ultra-low phosphorus. Appl. Surf. Sci. 2019, 497, 143775. [Google Scholar] [CrossRef]
- Luo, X.; Shen, J.; Ma, Y.; Liu, L.; Meng, R.; Yao, J. Robust, sustainable cellulose composite aerogels with outstanding flame retardancy and thermal insulation. Carbohydr. Polym. 2020, 230, 115623. [Google Scholar]
- Ma, B.; Hou, X.; Li, Y. Mechanically strong and thermal insulating polyimide/SiO2 aerogel prepared by ambient drying. Mater. Lett. 2022, 327, 133039. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, L.; Yang, M.; Chen, Z.; Song, K.; Wang, Y.; Erisen, D.E.; Xie, J.; Wu, Q.; Kou, Z. Electrospinning of SiO2-based composites embedded TiO2 nanoparticles with ultra-strong suppression of radiative heat transfer. J. Alloys Compd. 2023, 957, 170331. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.; Wang, X.; Huan, S.; Yang, H.; Wang, C. Low-cost, superhydrophobic, flame-retardant sunflower straw-based xerogel as thermal insulation materials for energy-efficient buildings. Sustain. Mater. Technol. 2023, 38, e00748. [Google Scholar] [CrossRef]
- Wei, L.; Sun, L.; Zhao, H.; Lu, J.; Liu, L.; Yao, J. Aggregation-induced microfilaments enabled cotton cellulose aerogels with highly compressive and fatigue resistance, greatly thermal insulation and fireproofing. Ind. Crop. Prod. 2023, 206, 117666. [Google Scholar] [CrossRef]
- Naseem, S.; Durrani, A.I.; Rizwan, M.; Yasmeen, F.; Siddiqui, S.; Habib, F. Sono-Microwave Assisted Chlorine free and Ionic Liquid (SMACIL) extraction of cellulose from Urtica dioica: A benign to green approach. Int. J. Biol. Macromol. 2024, 259, 129059. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zuo, K.; Wu, W.; Xu, Z.; Yi, Y.; Jing, Y.; Dai, H.; Fang, G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydr. Polym. 2018, 196, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Z.; Jiang, W.; Fang, X.; Xia, Z.; Niu, H.; Zhou, H. Multifunctional amphibious superhydrophilic-oleophobic cellulose nanofiber aerogels for oil and water purification. Carbohydr. Polym. 2024, 230, 121774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ouyang, G.; Sun, S.; Wang, J.; Wang, C.; Qu, C.; Lu, K.; Zhe, Q. Low-cost preparation and characterization of high-purity mullite nanofibrous membranes. Ceram. Int. 2023, 50, 9105–9113. [Google Scholar]
- Munier, P.; Gordeyeva, K.; Bergström, L.; Fall, A.B. Directional Freezing of Nanocellulose Dispersions Aligns the Rod-Like Particles and Produces Low-Density and Robust Particle Networks. Biomacromolecules 2016, 17, 1875–1881. [Google Scholar] [CrossRef]
- Gowtham, S.; Jeevanantham, T.; Emelda, J.; Edric, J. Investigation on effect of fibre orientation on mechanical behaviour of polymer matrix natural fibre reinforced composite material. Mater. Today Proc. 2024, 50, 9105–9113. [Google Scholar]
- Zhang, M.; Jiang, S.; Han, F.; Li, M.; Wang, N.; Liu, L. Anisotropic cellulose nanofiber/chitosan aerogel with thermal management and oil absorption properties. Carbohydr. Polym. 2021, 264, 118033. [Google Scholar] [CrossRef]
- Shi, Y.; Miao, Y.; Li, L.; Li, W.; Zheng, X.; Zhao, J.; Liu, Z. Efficient thermal insulation through all-biomass silk fibroin composite aerogel 3D-reinforced by natural down-fiber. Mater. Today Phys. 2024, 40, 101323. [Google Scholar] [CrossRef]
- Ke, W.; Ge, F.; Shi, X.; Zhang, Y.; Wu, T.; Zhu, X.; Cheng, Y.; Shi, Y.; Wang, Z.; Yuan, L.; et al. Superelastic and super flexible cellulose aerogels for thermal insulation and oil/water separation. Int. J. Biol. Macromol. 2024, 260, 129245. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Huang, P.; Wei, X.; Yu, J.; Wang, S.; Liao, J. Ultra-high strength, highly deformable and superhydrophobic polybenzoxazine@cellulose nanofiber composite aerogel for thermal insulation. Compos. Part A Appl. Sci. Manuf. 2023, 175, 107771. [Google Scholar]
- Yu, D.; Liu, M.; Xu, F.; Kong, Y.; Shen, X. Structure tailoring and thermal performances of water glass-derived silica aerogel composite with high specific surface area and enhanced thermal stability. J. Non-Cryst. Solids 2024, 630, 122889. [Google Scholar] [CrossRef]
- Guo, W.; Hu, Y.; Wang, X.; Zhang, P.; Song, L.; Xing, W. Exceptional flame-retardant cellulosic foams modified with phosphorus-hybridized graphene nanosheets. Cellulose 2018, 26, 1247–1260. [Google Scholar]
- Guo, W.; Chen, S.; Liang, F.; Jin, L.; Ji, C.; Zhang, P.; Fei, B. Ultra-light-weight, anti-flammable and water-proof cellulosic aerogels for thermal insulation applications. Int. J. Biol. Macromol. 2023, 246, 125343. [Google Scholar]
- Wang, X.; Zhou, S.; Guo, W.; Wang, P.; Xing, W.; Song, L.; Hu, Y. Renewable Cardanol-Based Phosphate as a Flame Retardant Toughening Agent for Epoxy Resins. ACS Sustain. Chem. Eng. 2017, 5, 3409–3416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Sun, M.; Lv, J.; Gu, J.; Xu, Q.; Li, Y.; Zhang, X.; Duan, H.; Li, S. Mullite-Fibers-Reinforced Bagasse Cellulose Aerogels with Excellent Mechanical, Flame Retardant, and Thermal Insulation Properties. Materials 2024, 17, 3737. https://doi.org/10.3390/ma17153737
Wang S, Sun M, Lv J, Gu J, Xu Q, Li Y, Zhang X, Duan H, Li S. Mullite-Fibers-Reinforced Bagasse Cellulose Aerogels with Excellent Mechanical, Flame Retardant, and Thermal Insulation Properties. Materials. 2024; 17(15):3737. https://doi.org/10.3390/ma17153737
Chicago/Turabian StyleWang, Shuang, Miao Sun, Junyi Lv, Jianming Gu, Qing Xu, Yage Li, Xin Zhang, Hongjuan Duan, and Shaoping Li. 2024. "Mullite-Fibers-Reinforced Bagasse Cellulose Aerogels with Excellent Mechanical, Flame Retardant, and Thermal Insulation Properties" Materials 17, no. 15: 3737. https://doi.org/10.3390/ma17153737





