Study on the Preparation and PEC-Type Photodetection Performance of β-Bi2O3 Thin Films
Abstract
:1. Introduction
2. Experimental Details
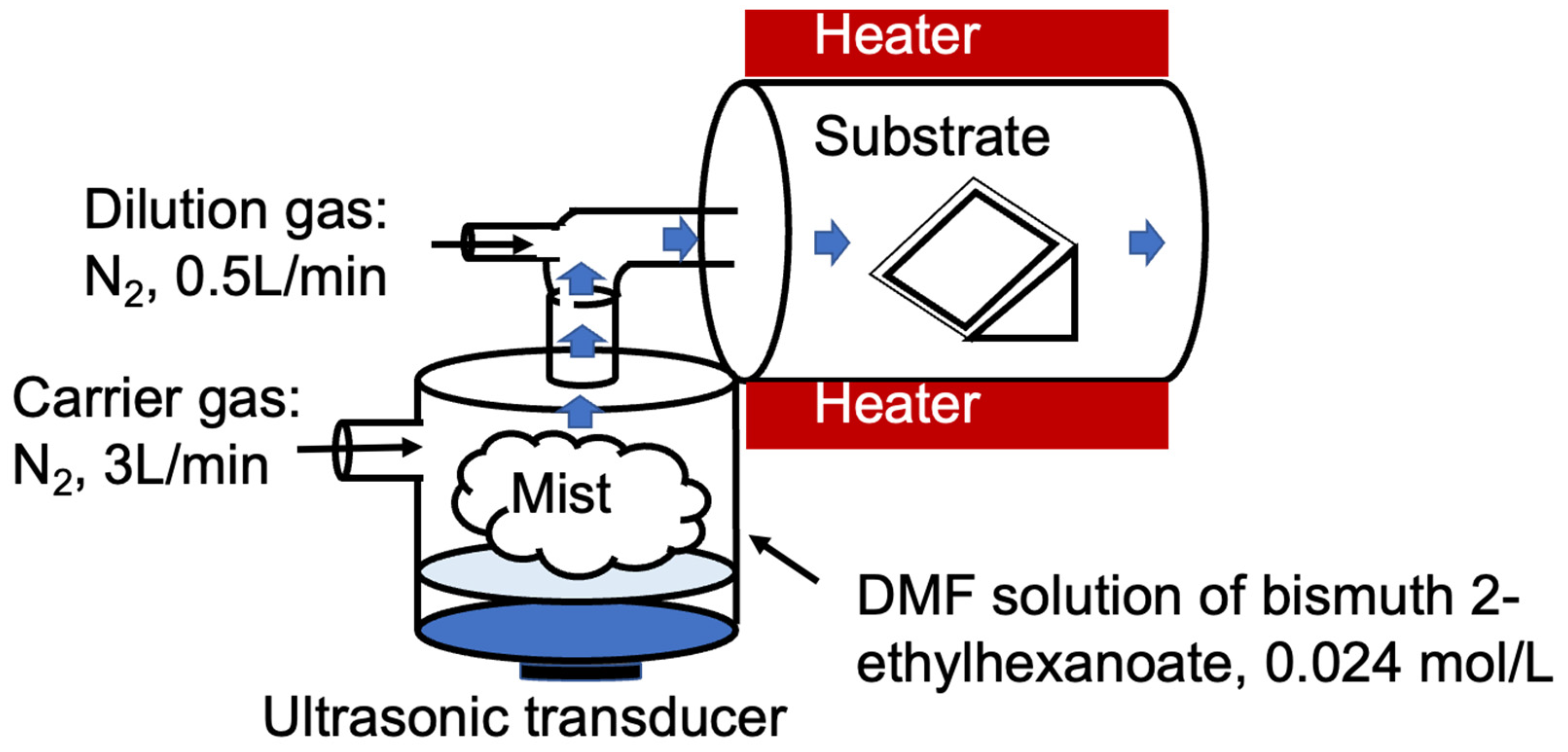
2.1. Thin Film Deposition
2.2. Characterization
3. Results and Discussions
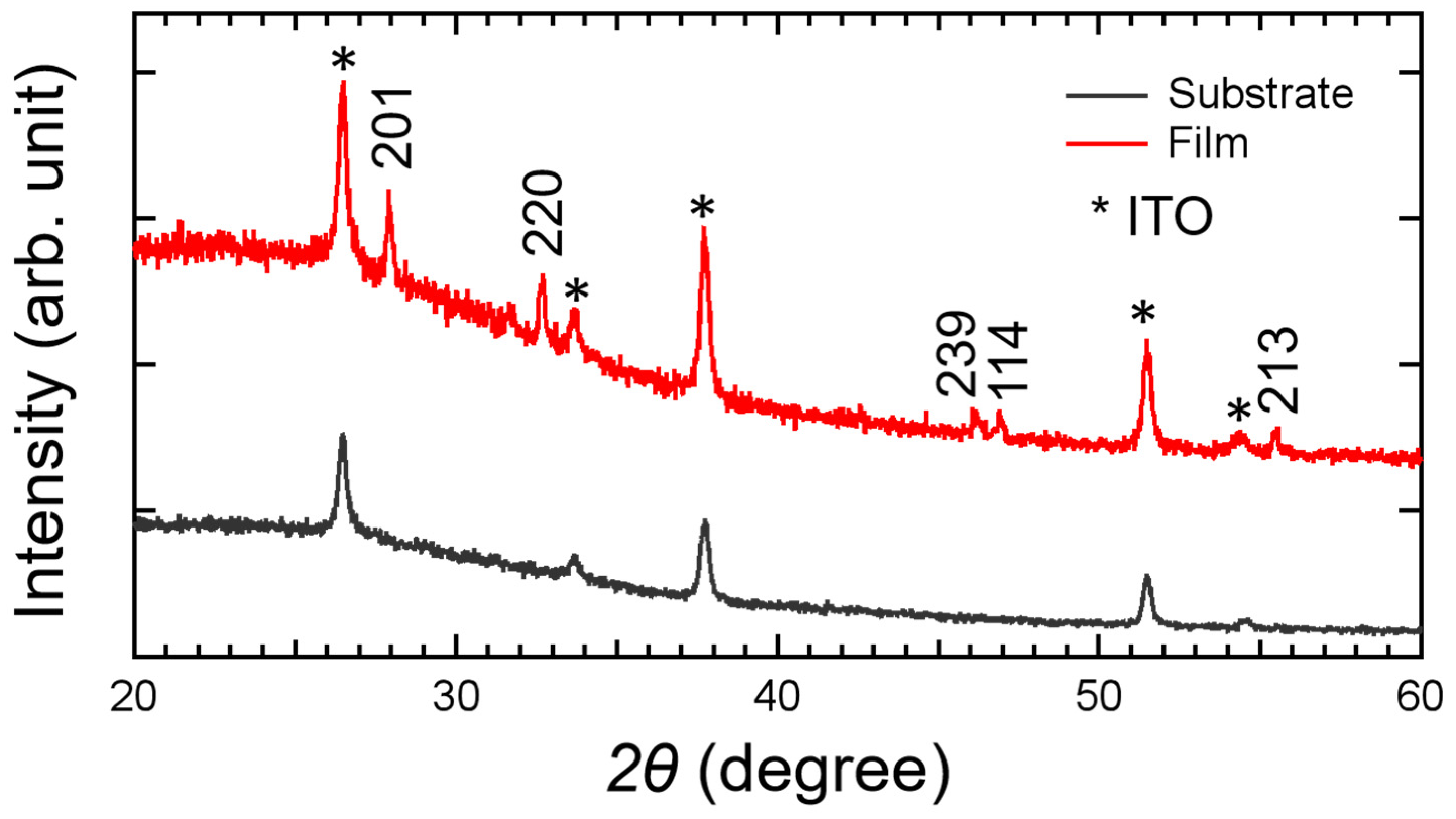
3.1. Phase Structure Analysis
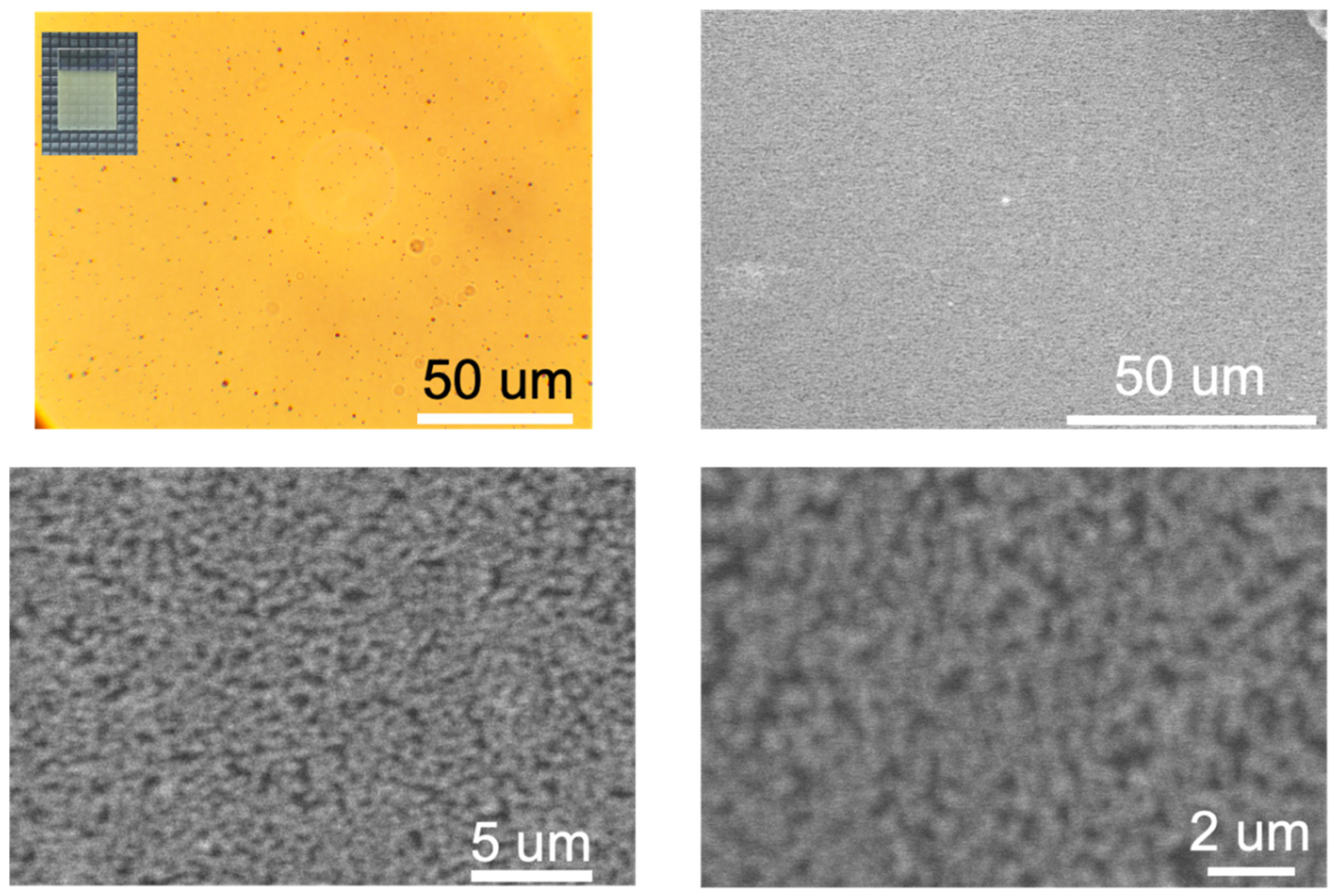
3.2. Surface Morphology
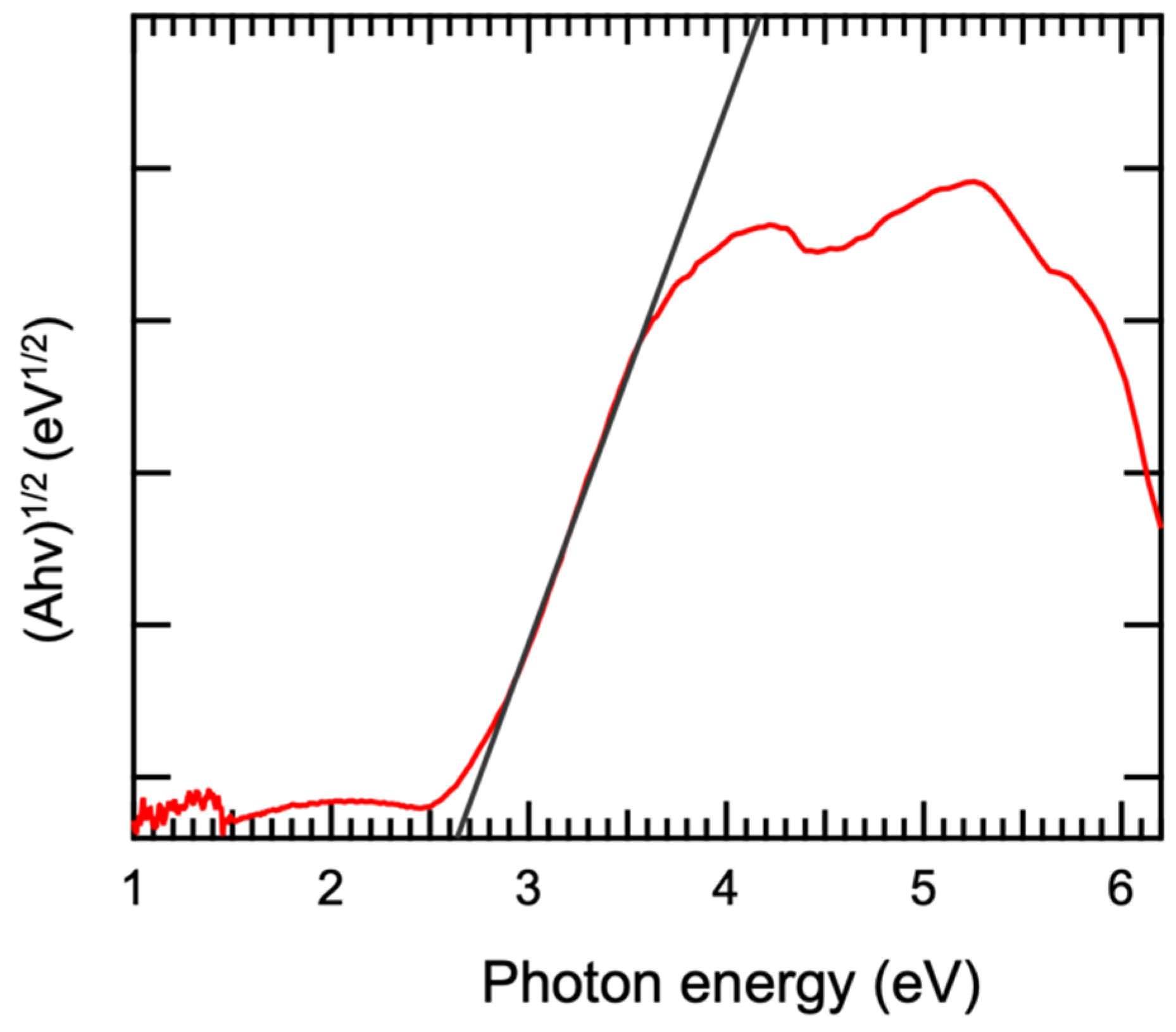
3.3. Optical Performance Analysis
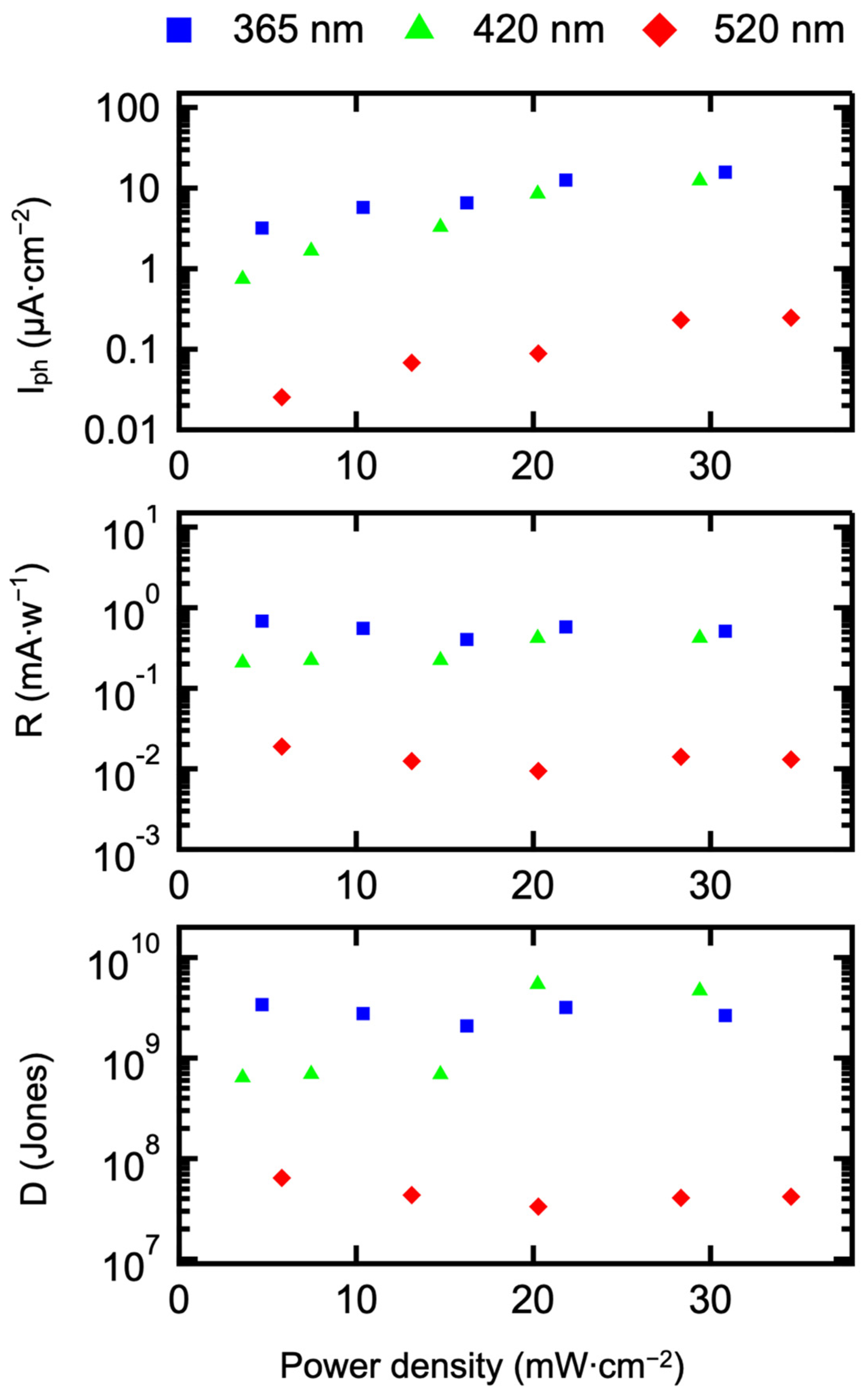
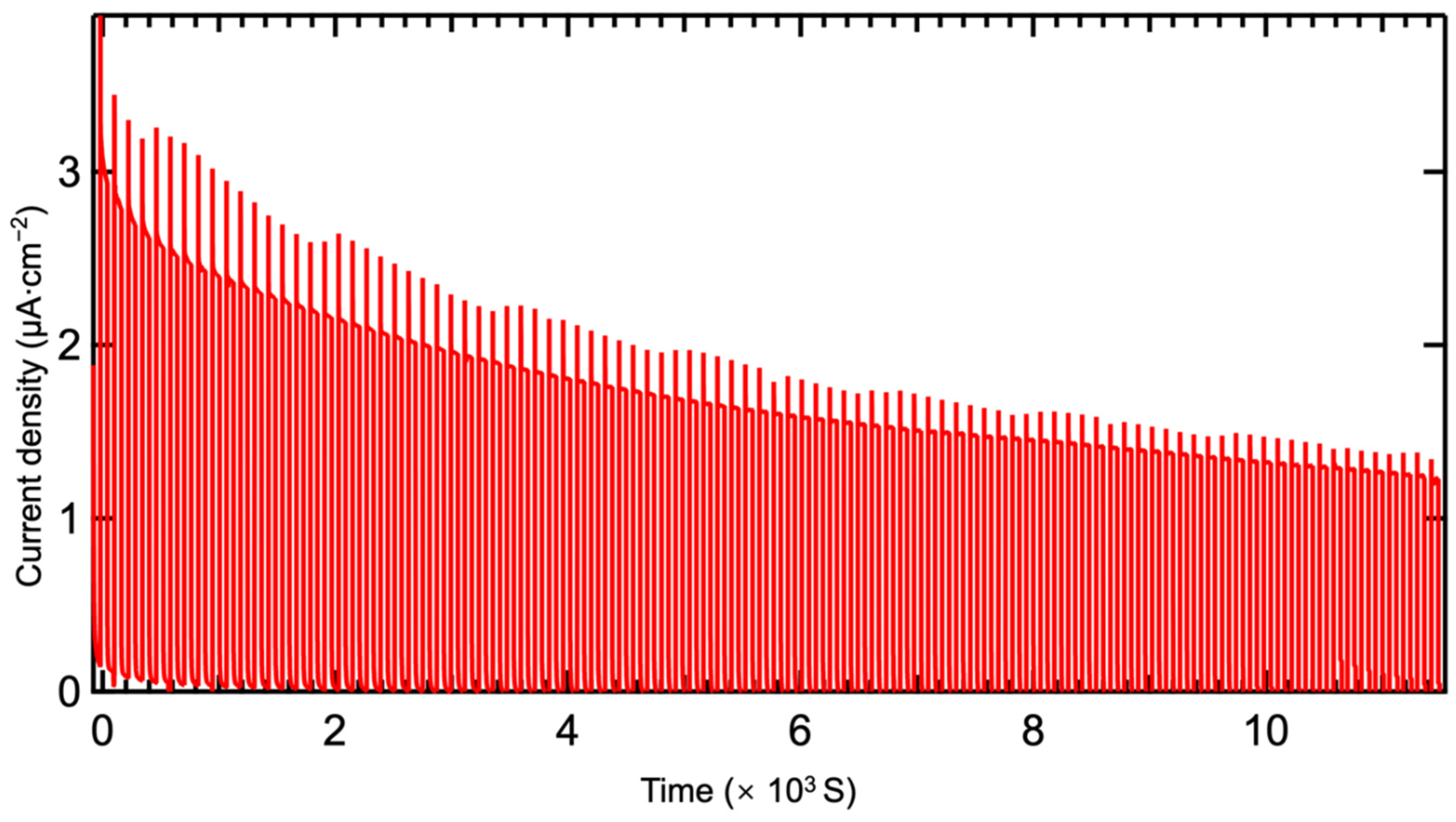
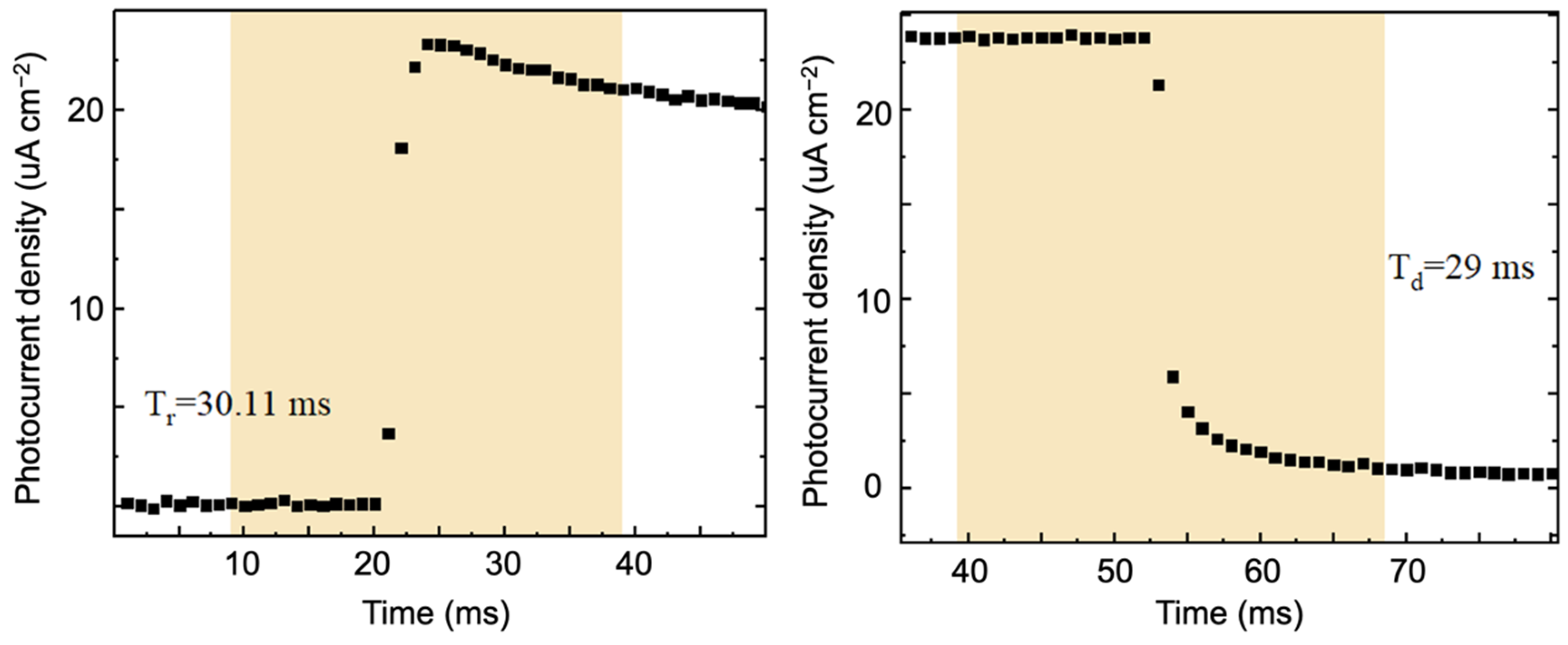
3.4. Construction and Performance Research of Photodetectors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, R.; Zhou, S.; Zhang, Z.; Zheng, R.; Huang, J. Engineering nanostructure–interface of photoanode materials toward photoelectrochemical water oxidation. Adv. Mater. 2021, 33, 2005389. [Google Scholar] [CrossRef]
- Gopinath, C.S.; Nalajala, N. A scalable and thin film approach for solar hydrogen generation: A review on enhanced photocatalytic water splitting. J. Mater. Chem. A 2021, 9, 1353–1371. [Google Scholar] [CrossRef]
- Chen, Q.; Cheng, X.; Long, H.; Rao, Y. A short review on recent progress of Bi/semiconductor photocatalysts: The role of Bi metal. Chin. Chem. Lett. 2020, 31, 2583–2590. [Google Scholar] [CrossRef]
- Xu, M.; Yang, J.; Sun, C.; Liu, L.; Cui, Y.; Liang, B. Performance enhancement strategies of bi-based photocatalysts: A review on recent progress. Chem. Eng. J. 2020, 389, 124402. [Google Scholar] [CrossRef]
- Zahid, A.H.; Han, Q. A review on the preparation, microstructure, and photocatalytic performance of Bi2O3 in polymorphs. Nanoscale 2021, 13, 17687–17724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mei, B.; Chen, H.; Sun, Z. Review on synthetic approaches and PEC activity performance of bismuth binary and mixed-anion compounds for potential applications in marine engineering. Dalton Trans. 2024, 53, 10376–10402. [Google Scholar] [CrossRef]
- Utami, B.A.; Sutanto, H.; Hidayanto, E.; Alkian, I. Recent advances in doped, Bi2O3; its photocatalytic activity: A review. Int. J. Res. Rev. 2022, 9, 216–230. [Google Scholar] [CrossRef]
- Díaz-Guerra Viejo, C.; Almodóvar, P.; Camacho López, M.; Camacho López, S.; Piqueras de Noriega, J. Formation of beta-Bi2O3 and delta-Bi2O3 during laser irradiation of Bi films studied in-situ by spatially resolved Raman spectroscopy. J. Alloys Compd. 2017, 723, 520–526. [Google Scholar] [CrossRef]
- Kim, M.W.; Joshi, B.; Samuel, E.; Kim, K.; Kim, Y.I.; Kim, T.G.; Swihart, M.T.; Yoon, S.S. Highly nanotextured β-Bi2O3 pillars by electrostatic spray deposition as photoanodes for solar water splitting. J. Alloys Compd. 2018, 764, 881–889. [Google Scholar] [CrossRef]
- Li, Y.; Song, J.; Ji, Y.; Lu, X.; Tian, Q.; Chen, J.; Sui, Z. Bismuth/bismuth trioxide with a dual-carbon support for high and long life lithium storage. J. Phys. Chem. Solids 2022, 163, 110562. [Google Scholar] [CrossRef]
- Wu, Z.; Chong, M.C.; Zhang, S.; Li, J.; Zhu, Y. Regulating the crystal phase of bismuth-based semiconductors for promoted photocatalytic performance. Sci. China Chem. 2024, 67, 1839–1864. [Google Scholar] [CrossRef]
- Ilves, V.G.; Gaviko, V.S.; Murzakaev, A.M.; Sokovnin, S.Y.; Uimin, M.A.; Zuev, M.G. Phase transformation in air of Bi2O3 nanopowder produced by pulsed electron beam evaporation. Ceram. Int. 2023, 49, 21848–21854. [Google Scholar] [CrossRef]
- Guo, P.; Yin, F.; Zhang, J.; Chen, B.; Ni, Z.; Shi, L.; Han, M.; Wu, Z.; Li, G. Crystal-Phase and Surface-Structure Engineering of Bi2O3 for Enhanced Electrochemical N2 Fixation to NH3. ACS Appl. Mater. Interfaces 2024, 16, 17540–17552. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, P.H.E.; Soares, F.C.; Rodrigues, G.N.; Del Duque, D.M.; de Oliveira, W.R.; Gianelli, B.F.; de Mendonca, V.R. Synthesis and photocatalytic performance of Bi2O3 thin films obtained in a homemade spin coater. Mater. Today Commun. 2021, 27, 102214. [Google Scholar] [CrossRef]
- Hofmann, M.; Rößner, L.; Armbrüster, M.; Mehring, M. Thin Coatings of α-and β-Bi2O3 by Ultrasonic Spray Coating of a Molecular Bismuth Oxido Cluster and their Application for Photocatalytic Water Purification Under Visible Light. ChemistryOpen 2020, 9, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Cosham, S.D.; Hill, M.S.; Horley, G.A.; Johnson, A.L.; Jordan, L.; Molloy, K.C.; Stanton, D.C. Synthesis and materials chemistry of bismuth Tris-(di-i-propylcarbamate): Deposition of photoactive Bi2O3 thin films. Inorg. Chem. 2014, 53, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ibanez, P.; McMichael, S.; Cabanillas, A.R.; Alkharabsheh, S.; Moranchel, A.T.; Byrne, J.A. New trends on photoelectrocatalysis (PEC): Nanomaterials, wastewater treatment and hydrogen generation. Curr. Opin. Chem. Eng. 2021, 34, 100725. [Google Scholar] [CrossRef]
- Alulema-Pullupaxi, P.; Espinoza-Montero, P.J.; Sigcha-Pallo, C.; Vargas, R.; Fernández, L.; Peralta-Hernandez, J.M.; Paz, J.L. Fundamentals and applications of photoelectrocatalysis as an efficient process to remove pollutants from water: A review. Chemosphere 2021, 281, 130821. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Gu, M.; Kim, K.H. Effect of annealing of β-Bi2O3 over enhanced photoelectrochemical performance. Mater. Sci. Semicond. Process. 2022, 141, 106439. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Y.; Zhao, J.; Song, Y.; Huang, Z.; Gao, F.; Li, N.; Li, Y. Induced aqueous synthesis of metastable β-Bi2O3 microcrystals for visible-light photocatalyst study. Cryst. Growth Des. 2015, 15, 1031–1042. [Google Scholar] [CrossRef]
- Selvamani, T.; Anandan, S.; Granone, L.; Bahnemann, D.W.; Ashokkumar, M. Phase-controlled synthesis of bismuth oxide polymorphs for photocatalytic applications. Mater. Chem. Front. 2018, 2, 1664–1673. [Google Scholar] [CrossRef]
- Schlesinger, M.; Schulze, S.; Hietschold, M.; Mehring, M. Metastable β-Bi2O3 nanoparticles with high photocatalytic activity from polynuclear bismuth oxido clusters. Dalton Trans. 2013, 42, 1047–1056. [Google Scholar] [CrossRef]
- Tang, X.; Ma, C.; Liu, N.; Liu, C.; Liu, S. Visible light β-Bi2O3/BiOCl heterojunction photocatalyst with highly enhanced photocatalytic activity. Chem. Phys. Lett. 2018, 709, 82–87. [Google Scholar] [CrossRef]
- Trentelman, K. A note on the characterization of bismuth black by Raman microspectroscopy. J. Raman Spectrosc. 2009, 40, 585–589. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E. The molecular structure of bismuth oxide by Raman spectroscopy. J. Solid State Chem. 1992, 97, 319–331. [Google Scholar] [CrossRef]
- In, J.; Yoon, I.; Seo, K.; Park, J.; Choo, J.; Lee, Y.; Kim, B. Polymorph-Tuned synthesis of α-and β-Bi2O3 nanowires and determination of their growth direction from polarized raman single nanowire microscopy. Chem.–Eur. J. 2011, 17, 1304–1309. [Google Scholar] [CrossRef]
- Wang, K.; Qiao, H.; Li, J.; Qi, X. A robust photoelectrochemical photodetectors based on the self-healing properties of Bi2O2S nanoplates. Appl. Surf. Sci. 2021, 565, 150444. [Google Scholar] [CrossRef]
- Xu, L.; Yang, M.; Lu, Q.; Ren, Y.; Chen, Y.; Guo, J.; Cai, M.; Miao, X.; Teng, F.; Fan, H.; et al. 2D BiSeI Nanosheets for Broadband Self-Powered Photoelectrochemical Photodetector. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2024, 18, 2300183. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, D.; Zeng, Z.; Zhao, C.; Liu, Y.; Zhang, B.; Pan, J.; Liu, D.; Wang, J. PVP-Assisted Hydrothermal Synthesis of Bi2O2Se Nanosheets for Self-Powered Photodetector. J. Sol. Energy Res. Updates 2022, 9, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, W.; Sun, Z.; Li, C. Highly Sensitive and Stable Visible-Light Self-Power Photodetectors Based on Solution-Processed Bi4O5I2. Cryst. Growth Des. 2023, 23, 2852–2859. [Google Scholar] [CrossRef]
- Yan, X.; Shi, B.; Cao, H.; Tian, Z.; Dai, C.; Liu, W.; Yang, Q.; Wang, Y. Flexible self-powered photoelectrochemical-type photodetectors based on Bi2O2S/GO composites. J. Mater. Chem. C 2023, 11, 6928–6934. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, Z.; Mei, B. Oxygen Vacancies Modified Bi4O5I2 for Sensitive and Stable Photoelectrochemical-Type Photodetection. Adv. Opt. Mater. 2024, 12, 2302029. [Google Scholar] [CrossRef]
- Zhao, G.; Mei, B.; Chen, Y.; Sun, Z. Mist Chemical Vapor Deposition of Bi13S18I2 for Photoelectrochemical-type Photodetection. Inorg. Chem. 2024, 63, 3460–3466. [Google Scholar] [CrossRef] [PubMed]






| Method | Ref. | Temperature | Precursor | Time |
|---|---|---|---|---|
| Solid-state reaction | [14] | 450–600 °C | Bi2SiO5 | 2 h |
| Solution methods | [15] | 300–500 °C | Bi38O45(OMc)24(DMSO)9·2DMSO·7H2O | 75 min |
| CVD | [16] | 500–800 °C | Bismuth carbamate | 10–40 min |
| PVD | [12] | 300–600 °C | Bi (001) film, BiOx, amorphous film | 30–35 min |
| Chemical Reagents | Chemical Formula | Purity | Manufacturer |
|---|---|---|---|
| 25 wt% bismuth(III) 2-ethylhexanoate (C24H45BiO6) | ≥99.5% | Aladdin (Shanghai, China) | |
| N. N-Dimethylformamide | C3H7NO | ≥99.50% | National Pharmaceutical Group (Beijing, China) |
| High purity nitrogen gas | N2 | ≥99.90% | Wuhan Xiangyun Company (Wuhan, China) |
| Anhydrous ethanol | C2H5OH | ≥99.00% | National Pharmaceutical Group (Beijing, China) |
| Deionized water | H2O | ≥99.99% | Self-prepared |
| Acetone | C3H6O | ≥99.00% | National Pharmaceutical Group (Beijing, China) |
| Parameter | Value |
|---|---|
| a = b | 0.770 nm |
| c | 0.568 nm |
| Crystallite size | 18.90 nm |
| Wavelengths | I | II | III | IV | V |
|---|---|---|---|---|---|
| 365 nm | 4.46 | 6.55 | 10.12 | 15.32 | 21.64 |
| 420 nm | 3.58 | 7.45 | 14.75 | 20.34 | 29.40 |
| 520 nm | 5.79 | 10.76 | 13.13 | 20.28 | 28.33 |
| Materials | Ref. | Electrolyte | Light (nm) | Voltage (V) | R (mA/W) | τr/τd (ms) | Stability (per Cycle Decrease) |
|---|---|---|---|---|---|---|---|
| Bi2O2S | [27] | 0.5 M KOH | Sun | 0.6 | 0.23 | 80/70 | – |
| BiSeI | [28] | 0.2 M Na2SO4 | 600 | 0.5 | 2.88 | 7/16 | 0.09% |
| Bi2O2Se | [29] | Na2SO3 | 365 | 0 | 14.24 | 9/12 | – |
| Bi4O5I2 | [30] | 0.5 M Na2SO3 | 420 | 0 | 19.4 | 1.9/3.0 | 0.12% |
| Bi2O2S/GO | [31] | KOH/PVA | Sun | 0 | 0.035 | 420/2300 | 0.05% |
| OV-Bi4O5I2 | [32] | 0.5 M H2SO4 | 365 | 0 | 18.2 | 1.4/1.0 | 0.082% |
| Bi13S18I2 | [33] | 0.5 M Na2SO4 + 0.7M Na2SO3 | 0 | 20.2 | 2.0/1.0 | 0.05% | – |
| β-Bi2O3 | This work | 0.5 M KOH | 365 | 0 | 2.84 | 30.1/29.1 | 0.09% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Xiong, Z.; Wang, Z.; Sun, J. Study on the Preparation and PEC-Type Photodetection Performance of β-Bi2O3 Thin Films. Materials 2024, 17, 3779. https://doi.org/10.3390/ma17153779
Zhang J, Xiong Z, Wang Z, Sun J. Study on the Preparation and PEC-Type Photodetection Performance of β-Bi2O3 Thin Films. Materials. 2024; 17(15):3779. https://doi.org/10.3390/ma17153779
Chicago/Turabian StyleZhang, Jiaji, Zhihua Xiong, Zi Wang, and Jinlong Sun. 2024. "Study on the Preparation and PEC-Type Photodetection Performance of β-Bi2O3 Thin Films" Materials 17, no. 15: 3779. https://doi.org/10.3390/ma17153779





