Prussian Blue Anchored on Reduced Graphene Oxide Substrate Achieving High Voltage in Symmetric Supercapacitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Analysis
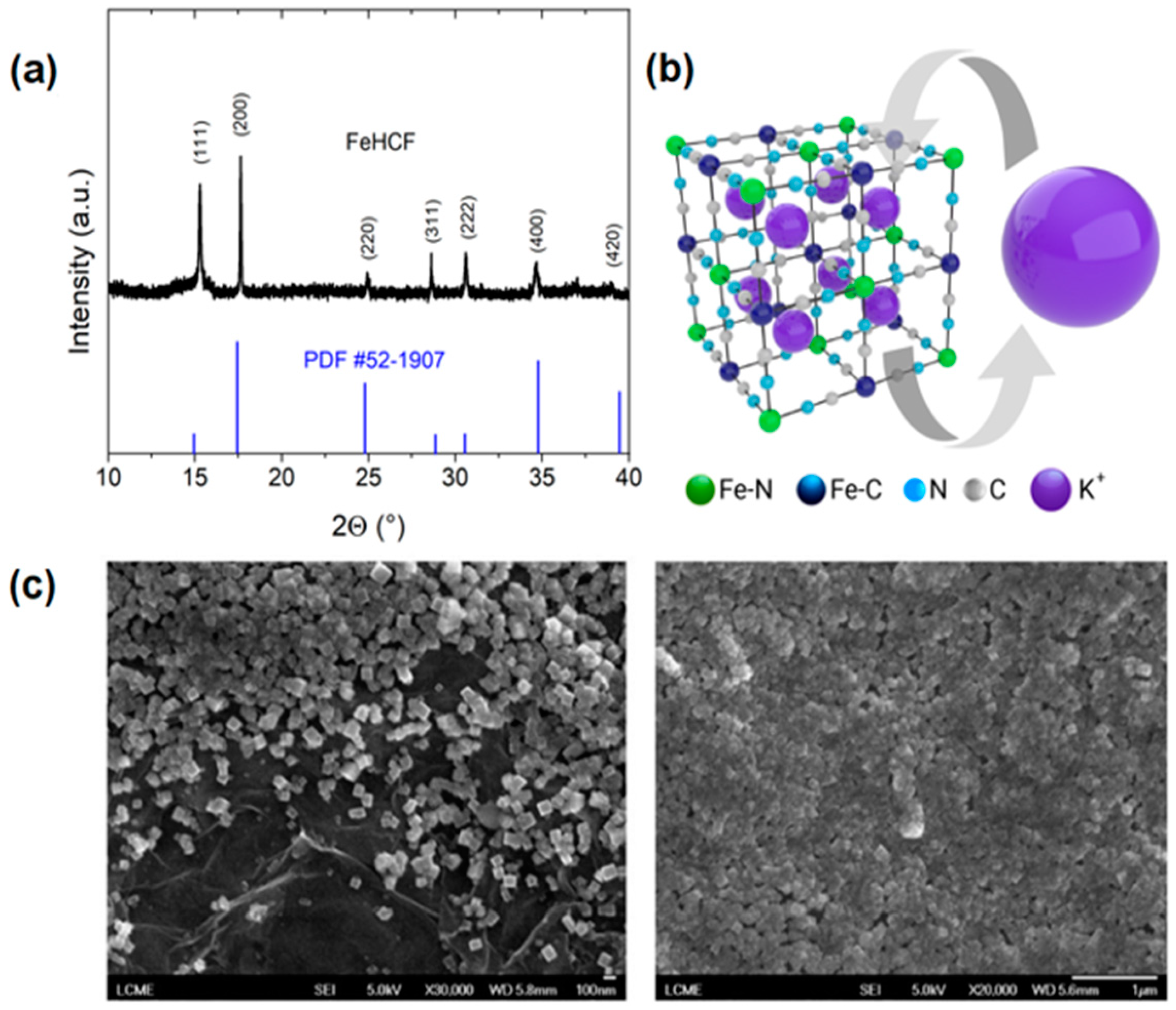
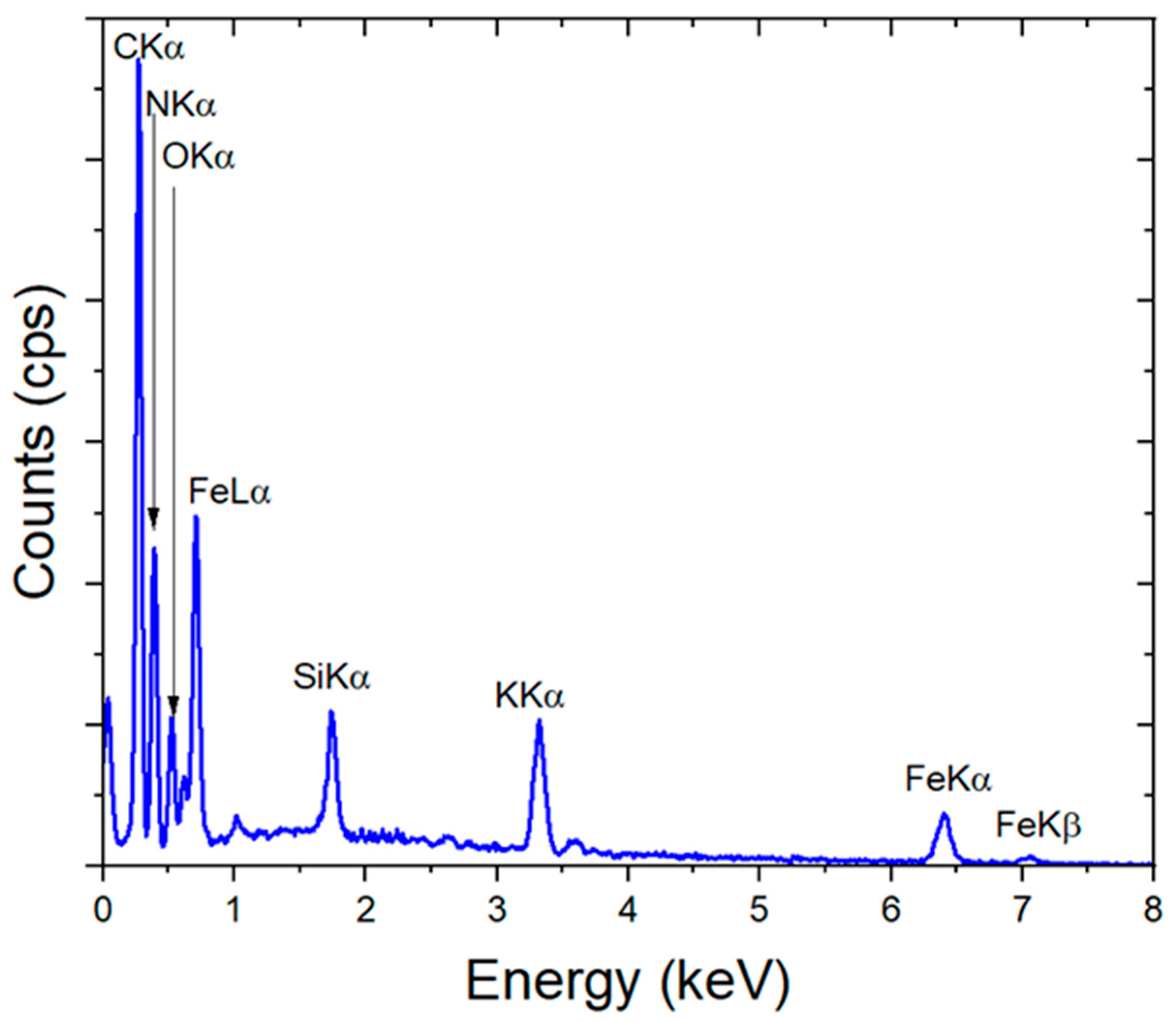
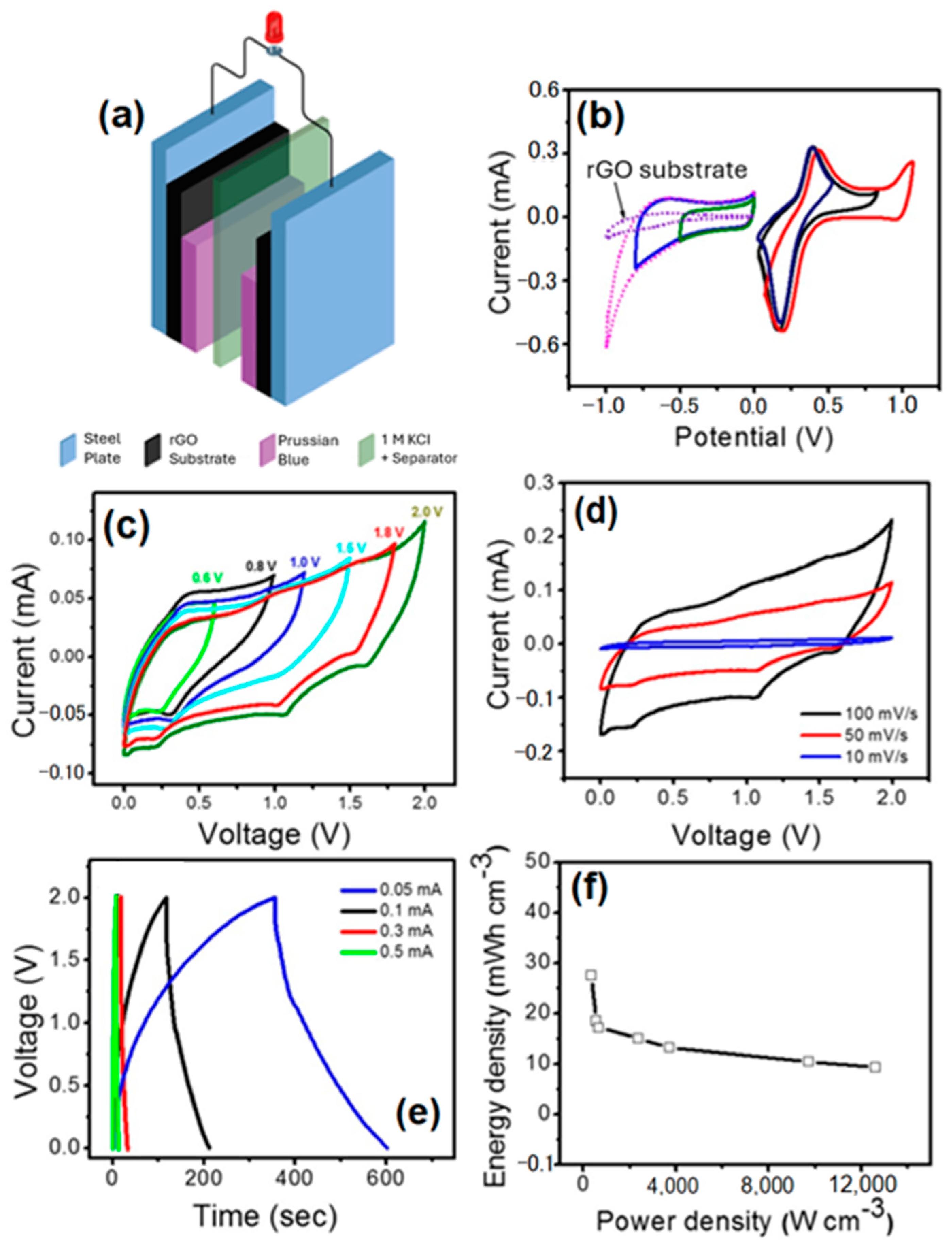
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goda, E.S.; Lee, S.; Sohail, M.; Yoon, K.R. Prussian blue and its analogues as advanced supercapacitor electrodes. J. Energy Chem. 2020, 50, 206–229. [Google Scholar] [CrossRef]
- Tang, Z.; Shang, X.; Hu, B.; Nie, P.; Shi, W.; Yang, J.; Liu, J. Fabrication of various metal hexacyanoferrates@CNF through acid-regulation for high-performance supercapacitor with superior stability. Carbon 2022, 187, 47–55. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Amarnath, C.A.; Sawant, S.N. Tailoring synthesis strategies for polyaniline-prussian blue composite in view of energy storage and H2O2 sensing application. Electrochim. Acta 2019, 295, 294–301. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Madhavi, S.; Hng, H.H.; Lou, X.W. Formation of Fe2O3 Microboxes with Hierarchical Shell Structures from Metal–Organic Frameworks and Their Lithium Storage Properties. J. Am. Chem. Soc. 2012, 134, 17388–17391. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.A.; Idrees, R.; Saeed, S. A critical review on polyimide derived carbon materials for high-performance supercapacitor electrodes. J. Energy Storage 2022, 55, 105667. [Google Scholar] [CrossRef]
- Chen, L.; Sun, W.; Xu, K.; Dong, Q.; Zheng, L.; Wang, J.; Lu, D.; Shen, Y.; Zhang, J.; Fu, F.; et al. How Prussian Blue Analogues Can Be Stable in Concentrated Aqueous Electrolytes. ACS Energy Lett. 2022, 7, 1672–1678. [Google Scholar] [CrossRef]
- Lamprecht, X.; Speck, F.; Marzak, P.; Cherevko, S.; Bandarenka, A. Electrolyte Effects on the Stabilization of Prussian Blue Analogue Electrodes in Aqueous Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 3513–3525. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, L. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 2022, 430, 132745. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Lichchhavi; Kanwade, A.; Shirage, P.M. A review on synergy of transition metal oxide nanostructured materials: Effective and coherent choice for supercapacitor electrodes. J. Energy Storage 2022, 55, 105692. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, C.; Ke, Q.; Yow, Z.F.; Cheng, C.; Wang, J. Hybrid Fe2O3 Nanoparticle Clusters/rGO Paper as an Effective Negative Electrode for Flexible Supercapacitors. Chem. Mater. 2016, 28, 7296–7303. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.-B.; Liu, M.-C.; Dai, Y.-H.; Yan, K.; Hu, B.; Luo, Y.-C.; Kang, L. Design and preparation of MoO2/MoS2 as negative electrode materials for supercapacitors. Mater. Des. 2016, 112, 88–96. [Google Scholar] [CrossRef]
- Galal, A.; Hassan, H.K.; Atta, N.F.; Jacob, T. Effect of Redox Electrolyte on the Specific Capacitance of SrRuO3 –Reduced Graphene Oxide Nanocomposites. J. Phys. Chem. C 2018, 122, 11641–11650. [Google Scholar] [CrossRef]
- Moyseowicz, A.; Gryglewicz, G. High-performance hybrid capacitor based on a porous polypyrrole/reduced graphene oxide composite and a redox-active electrolyte. Electrochim. Acta 2020, 354, 136661. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.S.; Yuan, G.; Cui, C.; Lu, L. A Symmetric RuO2∕RuO2 Supercapacitor Operating at 1.6 V by Using a Neutral Aqueous Electrolyte. Electrochem. Solid-State Lett. 2012, 15, A60. [Google Scholar] [CrossRef]
- Wei, B.; Wu, J.; Hou, Z.; Fang, M.; Zeng, H.; Yan, X.; Guo, Q.; Yong Yang, Y.; Wang, Z.; Qi, Z. Binder-free V-doped CrN thin film electrode enables high performance symmetric supercapacitor. J. Alloys Compd. 2023, 977, 173424. [Google Scholar] [CrossRef]
- Adnan, S.M.; Shoeb, M.; Ansari, M.Z.; Mashkoor, F.; Mobin, M.; Zaidi, S.; Jeong, C. Fabrication of NiO–CuO decorated polyaniline (PANI/NiO–CuO) nanocomposite based symmetric supercapacitor device for high-energy density performance with wide potential window in aqueous electrolyte. Inorg. Chem. Commun. 2023, 157, 111265. [Google Scholar] [CrossRef]
- Ashok Survase, A.; Hari Sutar, S.; Bhaurao Ubale, S.; Shivraj Kanase, S. Symmetric supercapacitor based on biosynthesized nanosheets of reduced graphene oxide (rGO): Characterization and electrochemical behavior. J. Electroanal. Chem. 2023, 953, 118020. [Google Scholar] [CrossRef]
- Arvas, M.B. Hydrothermal synthesis of polypyrrole/dye-functionalized carbon cloth electrode for wide potential window supercapacitor. Synth. Met. 2022, 293, 117275. [Google Scholar] [CrossRef]
- Bhojane, P. Recent advances and fundamentals of Pseudocapacitors: Materials, mechanism, and its understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Fic, K.; Meller, M.; Frackowiak, E. Strategies for enhancing the performance of carbon/carbon supercapacitors in aqueous electrolytes. Electrochim. Acta 2014, 128, 210–217. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef]
- Ye, W.; Wang, H.; Ning, J.; Zhong, Y.; Hu, Y. New types of hybrid electrolytes for supercapacitors. J. Energy Chem. 2021, 57, 219–232. [Google Scholar] [CrossRef]
- Hillier, N.; Yong, S.; Beeby, S. The good, the bad and the porous: A review of carbonaceous materials for flexible supercapacitor applications. Energy Rep. 2020, 6, 148–156. [Google Scholar] [CrossRef]
- Yue, T.; Shen, B.; Gao, P. Carbon material/MnO2 as conductive skeleton for supercapacitor electrode material: A review. Renew. Sustain. Energy Rev. 2022, 158, 112131. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Zan, G.; Li, S.; Chen, P.; Dong, K.; Wu, Q.; Wu, T. Mesoporous Cubic Nanocages Assembled by Coupled Monolayers With 100% Theoretical Capacity and Robust Cycling. ACS Cent. Sci. 2024, 6, 1283–1294. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Li, X.; Zhang, X.; Chen, J. A new electrochemical immunosensor for sensitive detection of prion based on Prussian blue analogue. Talanta 2018, 179, 726–733. [Google Scholar] [CrossRef]
- Jiayi Liu, J.; Li, X.; Rykov, A.I.; Fan, Q.; Xu, W.; Cong, W.; Jin, C.; Tang, H.; Zhu, K.; Ganeshraja, A.S.; et al. Zinc-modulated Fe–Co Prussian blue analogues with well-controlled morphologies for the efficient sorption of cesium. J. Mater. Chem. A 2017, 5, 3284–3292. [Google Scholar] [CrossRef]
- Avila, L.B.; Serrano Arambulo, P.C.; Cuevas-Arizaca, E.E.; Dantas, A.; Müller, C.K. Study on the Electrical Conduction Mechanism of Unipolar Resistive Switching Prussian White Thin Films. Nanomaterials 2022, 12, 2881. [Google Scholar] [CrossRef]
- Avila, L.B.; Müller, C.K.; Hildebrand, D.; Faita, F.L.; Baggio, B.F.; Cid, C.C.P.; Pasa, A.A. Resistive switching in electrodeposited Prussian blue layers. Materials 2020, 13, 5618. [Google Scholar] [CrossRef]
- Piernas Muñoz, M.J.; Castillo Martínez, E. Electrochemical Performance of Prussian Blue and Analogues in Aqueous Rechargeable Batteries. In Prussian Blue Based Batteries; Springer: Cham, Switzerland, 2018; pp. 23–44. [Google Scholar] [CrossRef]
- Nai, J.; Lou, X.W. (David) Hollow Structures Based on Prussian Blue and Its Analogs for Electrochemical Energy Storage and Conversion. Adv. Mater. 2019, 31, 1706825. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Z.; Yan, K.; Guo, X.; Wan, S.; He, F.; Sun, B.; Wang, G. The Rise of Prussian Blue Analogs: Challenges and Opportunities for High-Performance Cathode Materials in Potassium-Ion Batteries. Small Struct. 2021, 2, 2000054. [Google Scholar] [CrossRef]
- Xu, C.; Yang, Z.; Zhang, X.; Xia, M.; Yan, H.; Li, J.; Yu, H.; Zhang, L.; Shu, J. Prussian Blue Analogues in Aqueous Batteries and Desalination Batteries. Nano-Micro Lett. 2021, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Cheng, W.; Wang, W.; Zhao, Q.; Xie, J.; Zhang, W.; Gao, H.; Xue, L.; Li, J. Hexacyanoferrate-Type Prussian Blue Analogs: Principles and Advances Toward High-Performance Sodium and Potassium Ion Batteries. Adv. Energy Mater. 2021, 11, 2000943. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Hu, Q.; Hao, T.; Cao, H.; Huang, X.; Liu, Y.; Zhang, Y.; Lin, D.; Tang, Y.; et al. Prussian blue analogs cathodes for aqueous zinc ion batteries. Mater. Today Energy 2022, 29, 101095. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, L.; Xiong, Y.; Wei, T.; Fan, Z. Toward the Design of High-performance Supercapacitors by Prussian Blue, its Analogues and their Derivatives. Energy Environ. Mater. 2022, 3, 323–345. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Mariappan, V.M.; Kim, S.-J. Copper tungsten sulfide anchored on Ni-foam as a high-performance binder free negative electrode for asymmetric supercapacitor. Chem. Eng. J. 2019, 359, 409–418. [Google Scholar] [CrossRef]
- Buser, H.J.; Schwarzenbach, D.; Petter, W.; Ludi, A. The crystal structure of Prussian Blue: Fe4[Fe(CN)6]3.xH2O. Inorg. Chem. 1977, 16, 2704–2710. [Google Scholar] [CrossRef]
- Khalid, M.; Tumelero, M.A.; Pasa, A.A. Asymmetric and symmetric solid-state supercapacitors based on 3D interconnected polyaniline-carbon nanotube framework. RSC Adv. 2015, 5, 62033–62039. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef]
- Itaya, K.; Ataka, T.; Toshima, S. Spectroelectrochemistry and Electrochemical Preparation Method of Prussian Blue Modified Electrodes. J. Am. Chem. Soc. 1982, 104, 4767–4772. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Dong, H.; Zeng, Y.; Hu, H.; Zheng, M.; Liu, Y.; Xiao, Y.; Liang, Y. Hierarchical porous carbon with network morphology derived from natural leaf for superior aqueous symmetrical supercapacitors. Electrochim. Acta 2017, 258, 504–511. [Google Scholar] [CrossRef]
- Niu, B.; Jiang, W.; Jiang, B.; Lv, M.; Wang, S.; Wang, W. Determining the depth of surface charging layer of single Prussian blue nanoparticles with pseudocapacitive behaviors. Nat. Comm. 2022, 13, 2316. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, P.; Wang, F.; Wang, L.; Su, Y.; Zhang, F.; Zhuang, X.; Feng, X. Vacancy modification of Prussian-blue nano-thin films for high energy-density micro-supercapacitors with ultralow RC time constant. Nano Energy 2019, 60, 8–16. [Google Scholar] [CrossRef]
- Li, H.; Cao, L.; Zhang, H.; Tian, Z.; Zhang, Q.; Yang, F.; Yang, H.; He, S.; Jiang, S. Intertwined carbon networks derived from Polyimide/Cellulose composite as porous electrode for symmetrical supercapacitor. J. Colloid Interface Sci. 2022, 609, 179–187. [Google Scholar] [CrossRef]

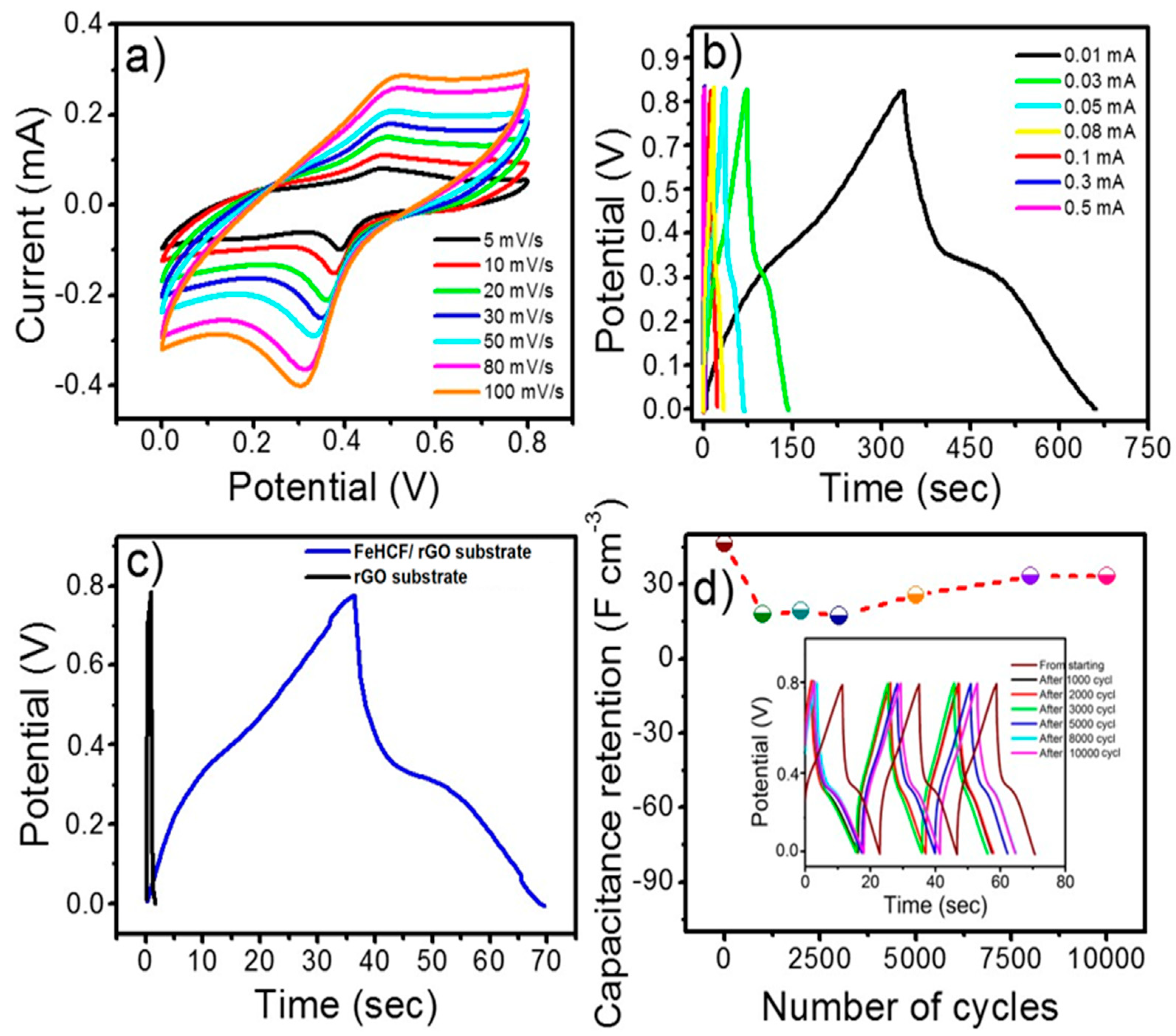
| Current Density, I/A (mA cm−2) | Specific Areal Capacitance, (mF cm−2) | Volumetric Current Density, I/V (A cm−3) | Specific Volumetric Capacitance,
(F cm−3) |
|---|---|---|---|
| 0.01 | 26.6 | 0.06 | 88.0 |
| 0.03 | 17.1 | 0.19 | 63.0 |
| 0.05 | 14.0 | 0.33 | 46.4 |
| 0.08 | 12.8 | 0.52 | 37.2 |
| 0.1 | 8.5 | 0.66 | 23.7 |
| 0.3 | 6.3 | 1.98 | 15.8 |
| 0.5 | 3.9 | 3.30 | 12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila, L.B.; Serrano, P.A.; Quispe, L.T.; Dantas, A.; Costa, D.P.; Arizaca, E.E.C.; Chávez, D.P.P.; Portugal, C.D.V.; Müller, C.K. Prussian Blue Anchored on Reduced Graphene Oxide Substrate Achieving High Voltage in Symmetric Supercapacitor. Materials 2024, 17, 3782. https://doi.org/10.3390/ma17153782
Avila LB, Serrano PA, Quispe LT, Dantas A, Costa DP, Arizaca EEC, Chávez DPP, Portugal CDV, Müller CK. Prussian Blue Anchored on Reduced Graphene Oxide Substrate Achieving High Voltage in Symmetric Supercapacitor. Materials. 2024; 17(15):3782. https://doi.org/10.3390/ma17153782
Chicago/Turabian StyleAvila, Lindiomar Borges, Pablo A. Serrano, Luis Torres Quispe, Adriana Dantas, Diogo Pontes Costa, Edy Elar Cuevas Arizaca, Diana Patricia Paredes Chávez, César Daniel Valdivia Portugal, and Christian Klaus Müller. 2024. "Prussian Blue Anchored on Reduced Graphene Oxide Substrate Achieving High Voltage in Symmetric Supercapacitor" Materials 17, no. 15: 3782. https://doi.org/10.3390/ma17153782
APA StyleAvila, L. B., Serrano, P. A., Quispe, L. T., Dantas, A., Costa, D. P., Arizaca, E. E. C., Chávez, D. P. P., Portugal, C. D. V., & Müller, C. K. (2024). Prussian Blue Anchored on Reduced Graphene Oxide Substrate Achieving High Voltage in Symmetric Supercapacitor. Materials, 17(15), 3782. https://doi.org/10.3390/ma17153782








