A Strategy for Fabricating Ultra-Flexible Thermoelectric Films Using Ag2Se-Based Ink
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Printing Ink and Film
2.3. Materials Characterization
3. Results and Discussion
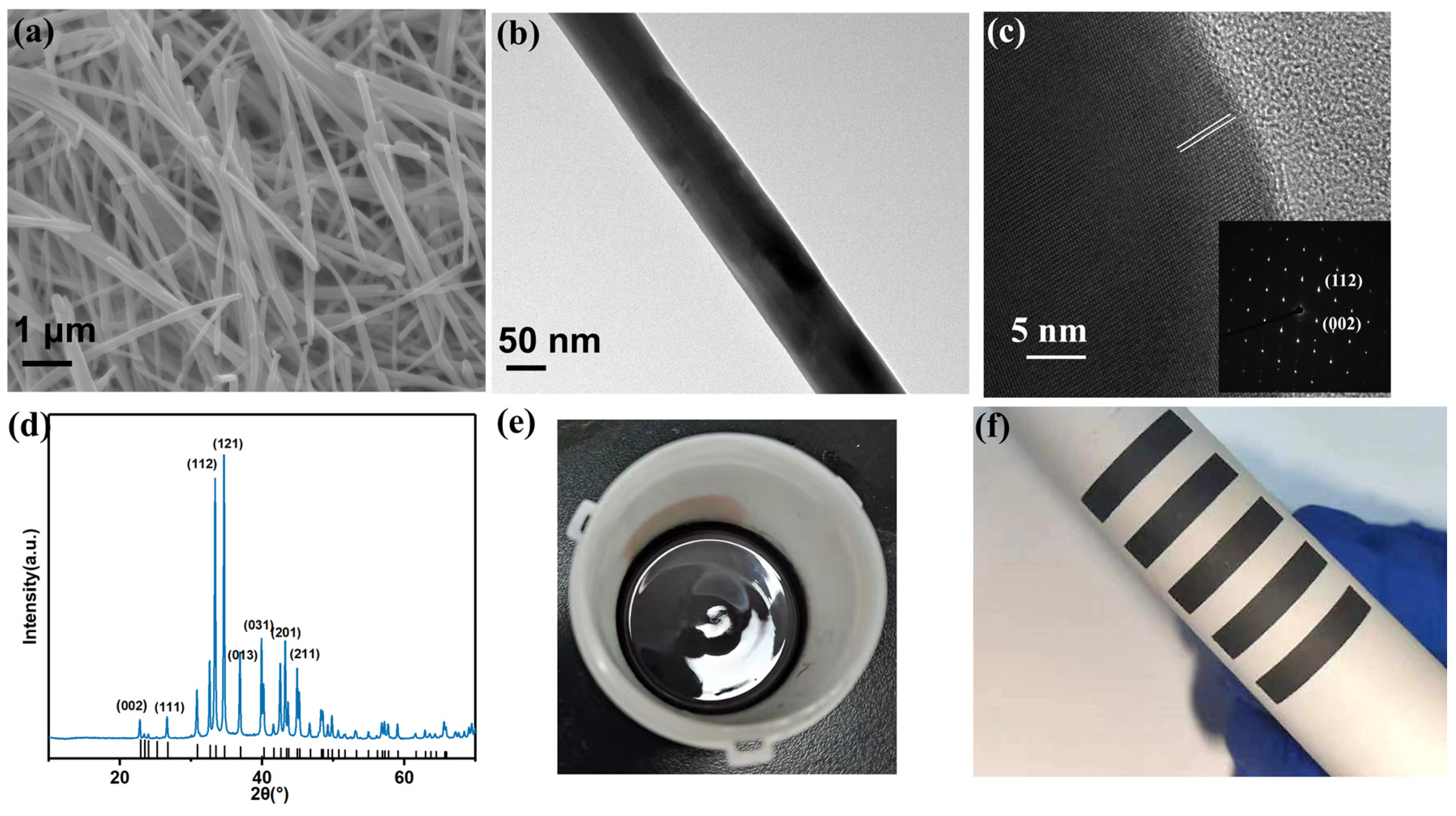
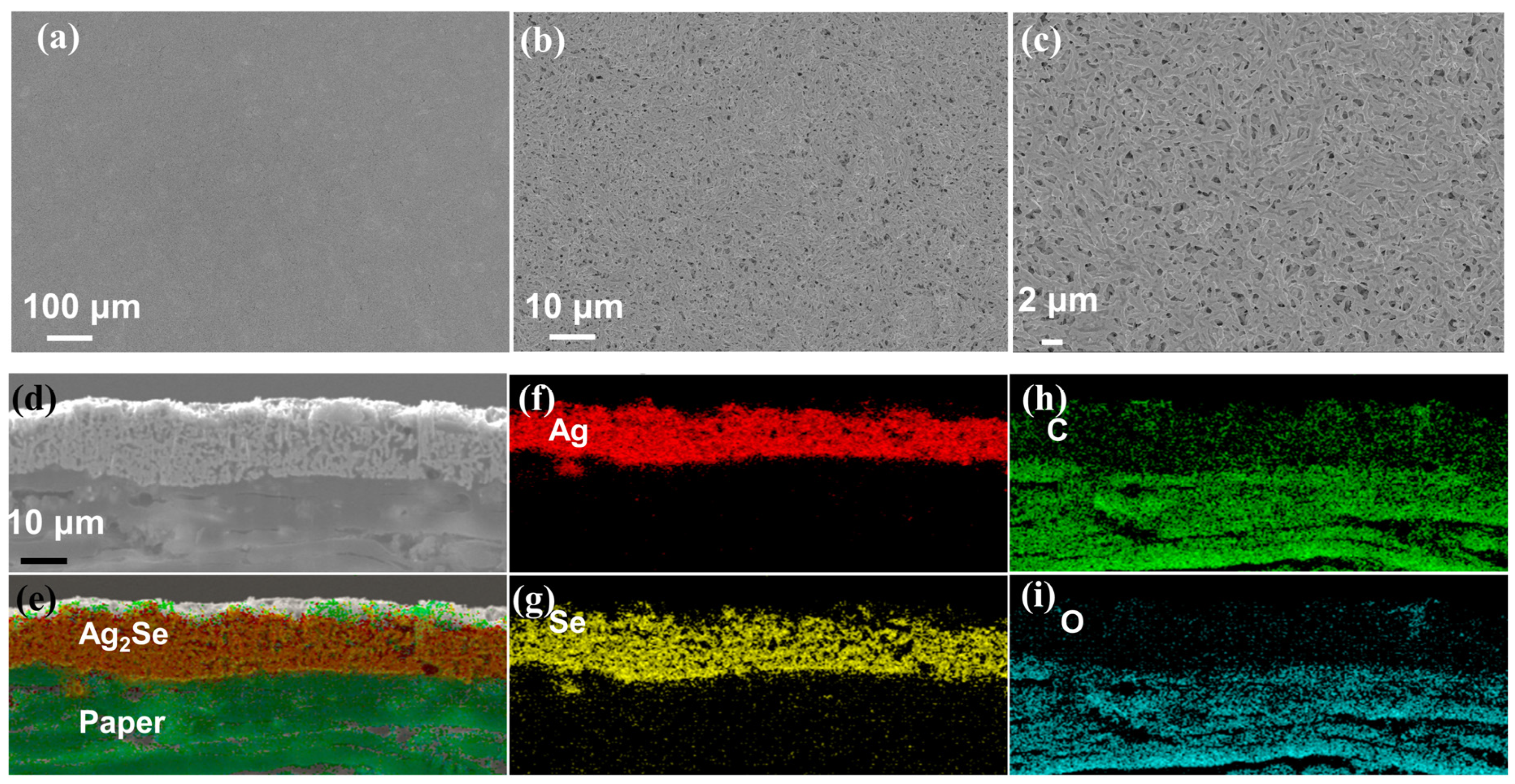
3.1. Characterization of Obtained Powders and Films
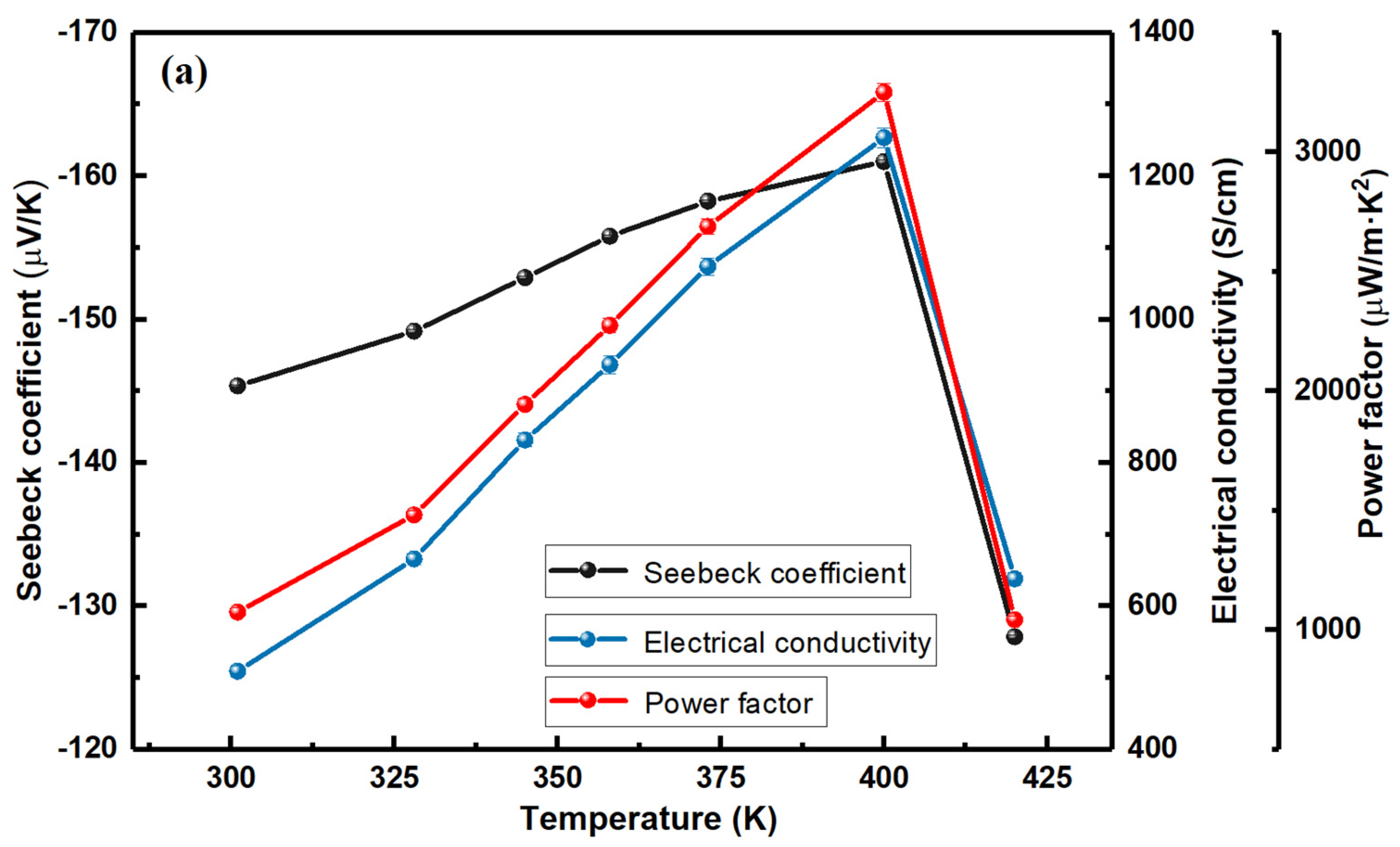
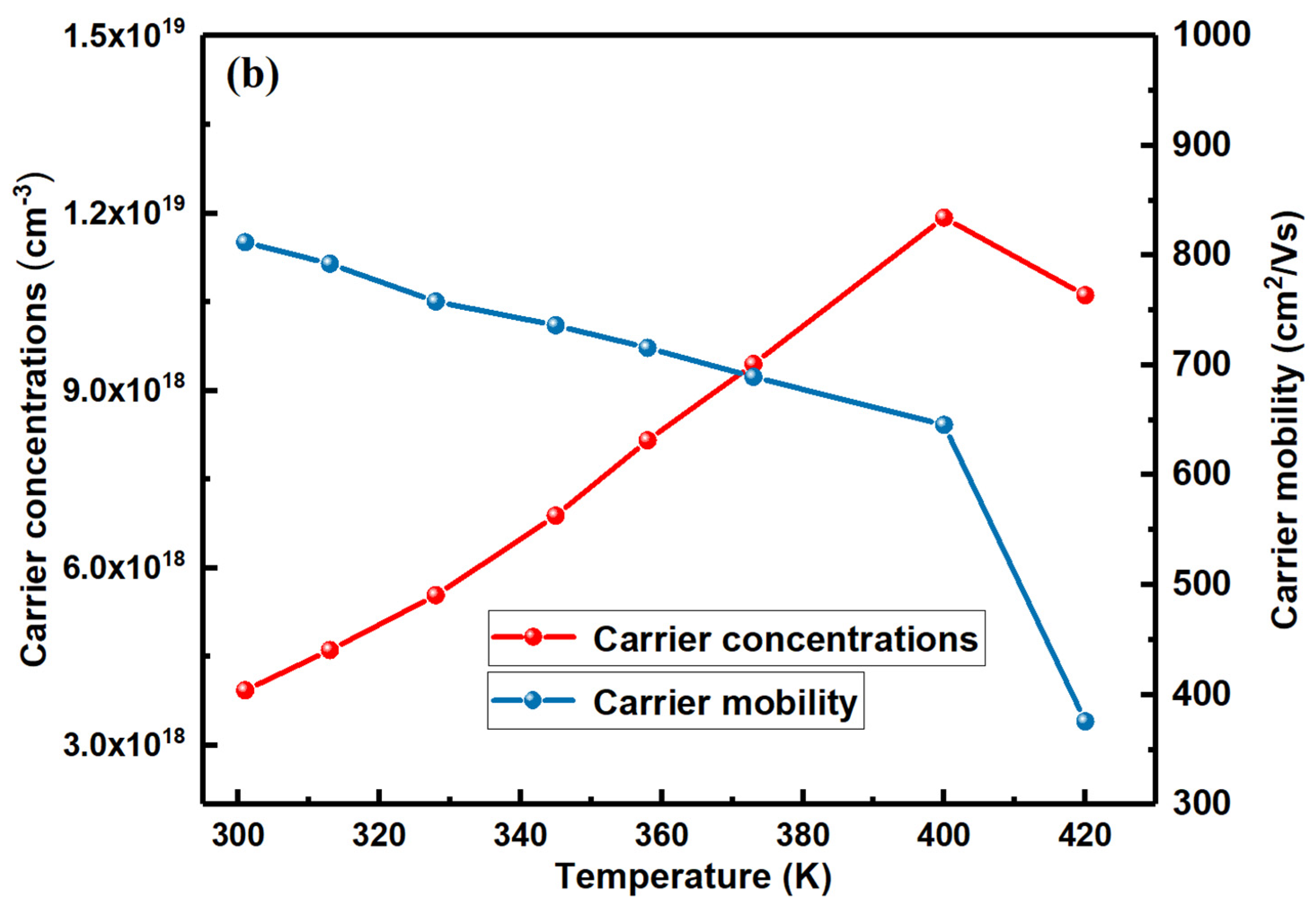
3.2. Electrical Transport Properties
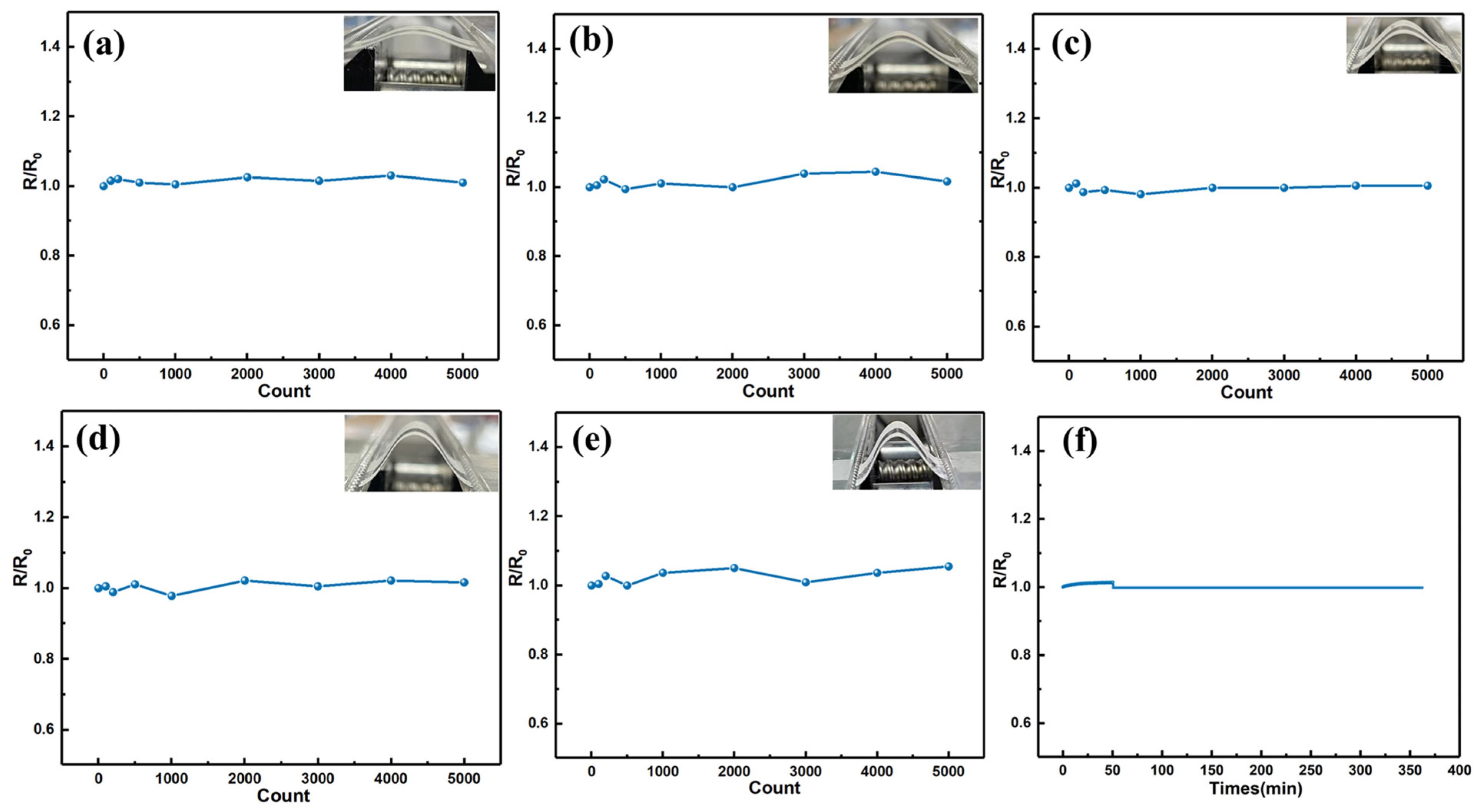
3.3. Highly-Flexible Properties of the Screen-Printed Ag2Se Film
| Materials | (R − R0)/R0 × 100% | Bending Radius (mm) | Bending Times | α (μV/K) | PF (μW/m K2) | Reference |
|---|---|---|---|---|---|---|
| Cu2Se/PI | 8% | 10 | 1000 | 100 | 500 | [43] |
| Ag2Se/nylon | 25% | 4 | 1500 | −140.7 | 1448.1 | [19] |
| Ag2Te/paper | 25% | 5 | 500 | −142 | 192 | [44] |
| Bi2Te3/paper | 140% | / | 400 | / | / | [45] |
| Ag2Te NWs | 30% | 1.5 | 1000 | −154.96 | 359.76 | [46] |
| Bi2Te3/PEDOT | 4% | 3.5 | 100 | 180 | 1350 | [15] |
| Ag2Se/paper | 4% | 5 | 1000 | −161 | 3250.9 | This study |
| Ag2Se/Paper | 6% | 5 | 5000 | / | / | This study |
3.4. Stability of Printed Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Zhang, W.; Gu, B.; Zhao, C.; Ye, B.; Xiao, C.; Xie, Y. Vacancy cluster-induced local disordered structure for the enhancement of thermoelectric property in Cu2ZnSnSe4. J. Mater. Chem. A 2021, 9, 1006–1013. [Google Scholar] [CrossRef]
- d’Angelo, M.; Galassi, C.; Lecis, N. Thermoelectric Materials and Applications: A Review. Energies 2023, 16, 6409. [Google Scholar] [CrossRef]
- Rudysh, M.Y.; Fedorchuk, A.O.; Brik, M.G.; Grechenkov, J.; Bocharov, D.; Piskunov, S.; Popov, A.I.; Piasecki, M. Electronic, Optical, and Vibrational Properties of an AgAlS2 Crystal in a High-Pressure Phase. Materials 2023, 16, 7017. [Google Scholar] [CrossRef] [PubMed]
- Nozariasbmarz, A.; Collins, H.; Dsouza, K.; Polash, M.H.; Hosseini, M.; Hyland, M.; Liu, J.; Malhotra, A.; Ortiz, F.M.; Mohaddes, F.; et al. Review of wearable thermoelectric energy harvesting: From body temperature to electronic systems. Appl. Energy 2020, 258, 114069. [Google Scholar] [CrossRef]
- Kim, F.; Kwon, B.; Eom, Y.; Lee, J.E.; Park, S.; Jo, S.; Park, S.H.; Kim, B.-S.; Im, H.J.; Lee, M.H.; et al. 3D printing of shape-conformable thermoelectric materials using all-inorganic Bi2Te3-based inks. Nat. Energy 2018, 3, 301–309. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Shi, X.L.; Shi, X.; Chen, L.; Dargusch, M.S.; Zou, J.; Chen, Z.G. Flexible Thermoelectric Materials and Generators: Challenges and Innovations. Adv. Mater. 2019, 31, 1807916. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Dong, X.; Sun, L.; Hu, L.; Liu, T.; Zeng, W.; Luo, B.; Jiang, X.; Jiang, Y.; Zhou, Y. Meters-long, sewable, wearable conductive polymer wires for thermoelectric applications. J. Mater. Chem. C 2020, 8, 1571–1576. [Google Scholar] [CrossRef]
- Shang, H.; Li, T.; Luo, D.; Yu, L.; Zou, Q.; Huang, D.; Xiao, L.; Gu, H.; Ren, Z.; Ding, F. High-Performance Ag-Modified Bi0.5Sb1.5Te3 Films for the Flexible Thermoelectric Generator. ACS Appl. Mater. Interfaces 2020, 12, 7358–7365. [Google Scholar] [CrossRef] [PubMed]
- Selvan, K.V.; Mohamed Ali, M.S. Copper–Nickel and Copper–Cobalt Thermoelectric Generators: Power-Generating Optimization through Structural Geometrics. IEEE Trans. Electron Devices 2018, 65, 3394–3400. [Google Scholar] [CrossRef]
- Mehmood, T.; Kim, J.H.; Lee, D.-J.; Dizhur, S.; Hirst, E.S.; Osgood, R.M.; Sayyad, M.H.; Munawar, M.A.; Xu, J. A flexible, printable, thin-film thermoelectric generator based on reduced graphene oxide–carbon nanotubes composites. J. Mater. Sci. Technol. 2020, 55, 10572–10581. [Google Scholar] [CrossRef]
- Hasan, M.N.; Wahid, H.; Nayan, N.; Mohamed Ali, M.S. Inorganic thermoelectric materials: A review. Int. J. Energy Res. 2020, 44, 6170–6222. [Google Scholar] [CrossRef]
- Jiang, C.; Wei, P.; Ding, Y.; Cai, K.; Tong, L.; Gao, Q.; Lu, Y.; Zhao, W.; Chen, S. Ultrahigh performance polyvinylpyrrolidone/Ag2Se composite thermoelectric film for flexible energy harvesting. Nano Energy 2021, 80, 105488. [Google Scholar] [CrossRef]
- Du, Y.; Cai, K.F.; Chen, S.; Cizek, P.; Lin, T. Facile preparation and thermoelectric properties of Bi2Te3 based alloy nanosheet/PEDOT:PSS composite films. ACS Appl. Mater. Interfaces 2014, 6, 5735–5743. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, Y.; Jiang, Q.; Cai, K.; Du, Y.; Song, H.; Gao, M.; Huang, C.; He, J.; Hu, D. Preparation and Characterization of Te/Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate)/Cu(7)Te(4) Ternary Composite Films for Flexible Thermoelectric Power Generator. ACS Appl. Mater. Interfaces 2018, 10, 42310–42319. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Liu, Y.; Wang, B.; Fang, L.; Qiu, J.; Zhang, K.; Wang, S. Exceptional thermoelectric properties of flexible organic-inorganic hybrids with monodispersed and periodic nanophase. Nat. Commun. 2018, 9, 3817. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, M.; Nagao, J. Thermoelectric and transport properties of β-Ag2Se compounds. J. Appl. Phys. 2000, 88, 813–816. [Google Scholar] [CrossRef]
- Gao, J.; Miao, L.; Lai, H.; Zhu, S.; Peng, Y.; Wang, X.; Koumoto, K.; Cai, H. Thermoelectric Flexible Silver Selenide Films: Compositional and Length Optimization. iScience 2020, 23, 100753. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.R.; Qiu, P.; Zhao, K.; Shi, X.; Chen, L. Ag2Q-Based (Q = S, Se, Te) Silver Chalcogenide Thermoelectric Materials. Adv. Mater. 2023, 35, e2110236. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019, 10, 841. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, Y.; Cai, K.; Ding, Y.; Wang, M.; Jiang, C.; Yao, Q.; Huang, C.; Chen, L.; He, J. Ultrahigh power factor and flexible silver selenide-based composite film for thermoelectric devices. Energy Environ. Sci. 2020, 13, 1240–1249. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, W.; Deng, Y.; Song, Q.; Zhang, Q. Enhanced Antioxidation and Thermoelectric Properties of the Flexible Screen-Printed Bi2Te3 Films through Interface Modification. ACS Appl. Energy Mater. 2019, 2, 2828–2836. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, W.; Zhang, Z.; Cao, L.; Yu, Y.; Deng, Y. Enhanced Electrical Transport Properties via Defect Control for Screen-Printed Bi(2)Te(3) Films over a Wide Temperature Range. ACS Appl. Mater. Interfaces 2020, 12, 16630–16638. [Google Scholar] [CrossRef] [PubMed]
- Mallick, M.M.; Rosch, A.G.; Franke, L.; Ahmed, S.; Gall, A.; Gesswein, H.; Aghassi, J.; Lemmer, U. High-Performance Ag-Se-Based n-Type Printed Thermoelectric Materials for High Power Density Folded Generators. ACS Appl. Mater. Interfaces 2020, 12, 19655–19663. [Google Scholar] [CrossRef] [PubMed]
- Mallick, M.M.; Rösch, A.G.; Franke, L.; Gall, A.; Ahmad, S.; Geßwein, H.; Mazilkin, A.; Kübel, C.; Lemmer, U. New frontier in printed thermoelectrics: Formation of β-Ag2Se through thermally stimulated dissociative adsorption leads to high ZT. J. Mater. Chem. A 2020, 8, 16366–16375. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Y.; Yan, Z.; Zhang, Z.; Zhang, Y.; Shi, P.; Xue, C. Screen-Printed Flexible Thermoelectric Device Based on Hybrid Silver Selenide/PVP Composite Films. Nanomaterials 2021, 11, 2042. [Google Scholar] [CrossRef] [PubMed]
- Jeong, U.; Xia, Y.; Yin, Y. Large-scale synthesis of single-crystal CdSe nanowires through a cation-exchange route. Chem. Phys. Lett. 2005, 416, 246–250. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, S.; Ding, C.; Wei, J.; Li, K.; Kong, X.-P.; Xu, J.; Li, K.; Zhao, W. Fabricating different patterns of flexible inorganic semiconductor films via colloidal ink printing on textiles. Mater. Lett. 2022, 307, 131079. [Google Scholar] [CrossRef]
- Ibrahim, N.; Akindoyo, J.O.; Mariatti, M. Recent development in silver-based ink for flexible electronics. J. Sci.-Adv. Mater. Dev. 2022, 7, 100395. [Google Scholar] [CrossRef]
- We, J.H.; Kim, S.J.; Cho, B.J. Hybrid composite of screen-printed inorganic thermoelectric film and organic conducting polymer for flexible thermoelectric power generator. Energy 2014, 73, 506–512. [Google Scholar] [CrossRef]
- Moon, K.S.; Dong, H.; Maric, R.; Pothukuchi, S.; Hunt, A.; Li, Y.I.; Wong, C.P. Thermal Behavior of Silver Nanoparticles for Low-Temperature Interconnect Applications. J. Electron. Mater. 2005, 34, 168–175. [Google Scholar] [CrossRef]
- Mo, L.; Guo, Z.; Yang, L.; Zhang, Q.; Fang, Y.; Xin, Z.; Chen, Z.; Hu, K.; Han, L.; Li, L. Silver Nanoparticles Based Ink with Moderate Sintering in Flexible and Printed Electronics. Int. J. Mol. Sci. 2019, 20, 2124. [Google Scholar] [CrossRef] [PubMed]
- Merilampi, S.; Laine-Ma, T.; Ruuskanen, P. The characterization of electrically conductive silver ink patterns on flexible substrates. Microelectron. Reliab. 2009, 49, 782–790. [Google Scholar] [CrossRef]
- Perez-Taborda, J.A.; Caballero-Calero, O.; Vera-Londono, L.; Briones, F.; Martin-Gonzalez, M. High Thermoelectric zT in n-Type Silver Selenide films at Room Temperature. Adv. Energy Mater. 2018, 8, 1702024. [Google Scholar] [CrossRef]
- Tveryanovich, Y.S.; Razumtcev, A.A.; Fazletdinov, T.R.; Krzhizhanovskaya, M.G.; Borisov, E.N. Stabilization of high-temperature Ag2Se phase at room temperature during the crystallization of an amorphous film. Thin Solid Films 2020, 709, 138187. [Google Scholar] [CrossRef]
- Schwarz, M.; Mews, A.; Dorn, A. Superionic phase transition in individual silver selenide nanowires. Nanoscale 2021, 13, 8017–8023. [Google Scholar] [CrossRef] [PubMed]
- Day, T.; Drymiotis, F.; Zhang, T.; Rhodes, D.; Shi, X.; Chen, L.; Snyder, G.J. Evaluating the potential for high thermoelectric efficiency of silver selenide. J. Mater. Chem. C 2013, 1, 7568–7573. [Google Scholar] [CrossRef]
- Kraemer, D.; Jie, Q.; McEnaney, K.; Cao, F.; Liu, W.; Weinstein, L.A.; Loomis, J.; Ren, Z.; Chen, G. Concentrating solar thermoelectric generators with a peak efficiency of 7.4%. Nat. Energy 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Baca, A.J.; Ahn, J.H.; Sun, Y.; Meitl, M.A.; Menard, E.; Kim, H.S.; Choi, W.M.; Kim, D.H.; Huang, Y.; Rogers, J.A. Semiconductor wires and ribbons for high-performance flexible electronics. Angew. Chem. Int. Edit. 2008, 47, 5524–5542. [Google Scholar] [CrossRef]
- Shi, X.; Chen, H.; Hao, F.; Liu, R.; Wang, T.; Qiu, P.; Burkhardt, U.; Grin, Y.; Chen, L. Room-temperature ductile inorganic semiconductor. Nat. Mater. 2018, 17, 421–426. [Google Scholar] [CrossRef]
- Yu, K.J.; Yan, Z.; Han, M.; Rogers, J.A. Inorganic semiconducting materials for flexible and stretchable electronics. NPJ Flex. Electron. 2017, 1, 4. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, T.; Suo, Z.; Vlassak, J.J. High ductility of a metal film adherent on a polymer substrate. Appl. Phys. Lett. 2005, 87, 161910. [Google Scholar] [CrossRef]
- Yuan, Y.; Xie, S.; Ding, C.; Shi, X.; Xu, J.; Li, K.; Zhao, W. Fabricating flexible wafer-size inorganic semiconductor devices. J. Mater. Chem. C 2020, 8, 915–1922. [Google Scholar] [CrossRef]
- Lin, Z.; Hollar, C.; Kang, J.S.; Yin, A.; Wang, Y.; Shiu, H.Y.; Huang, Y.; Hu, Y.; Zhang, Y.; Duan, X. A Solution Processable High-Performance Thermoelectric Copper Selenide Thin Film. Adv. Mater. 2017, 29, 1606662. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Miao, L.; Liu, C.; Wang, X.; Peng, Y.; Wei, X.; Zhou, J.; Chen, Y.; Hashimoto, R.; Asaka, T.; et al. A novel glass-fiber-aided cold-press method for fabrication of n-type Ag2Te nanowires thermoelectric film on flexible copy-paper substrate. J. Mater. Chem. A 2017, 5, 24740–24748. [Google Scholar] [CrossRef]
- Rojas, J.P.; Conchouso, D.; Arevalo, A.; Singh, D.; Foulds, I.G.; Hussain, M.M. Paper-based origami flexible and foldable thermoelectric nanogenerator. Nano Energy 2017, 31, 296–301. [Google Scholar] [CrossRef]
- Zeng, X.; Ren, L.; Xie, J.; Mao, D.; Wang, M.; Zeng, X.; Du, G.; Sun, R.; Xu, J.B.; Wong, C.P. Room-Temperature Welding of Silver Telluride Nanowires for High-Performance Thermoelectric Film. ACS Appl. Mater. Interfaces 2019, 11, 37892–37900. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Ding, C.; Yin, R.; Lu, S.; Xu, J.; Ren, W.; Li, K.; Zhao, W. A Strategy for Fabricating Ultra-Flexible Thermoelectric Films Using Ag2Se-Based Ink. Materials 2024, 17, 3784. https://doi.org/10.3390/ma17153784
Yuan Y, Ding C, Yin R, Lu S, Xu J, Ren W, Li K, Zhao W. A Strategy for Fabricating Ultra-Flexible Thermoelectric Films Using Ag2Se-Based Ink. Materials. 2024; 17(15):3784. https://doi.org/10.3390/ma17153784
Chicago/Turabian StyleYuan, Yunhuan, Chaogang Ding, Rui Yin, Shun Lu, Jie Xu, Wei Ren, Kang Li, and Weiwei Zhao. 2024. "A Strategy for Fabricating Ultra-Flexible Thermoelectric Films Using Ag2Se-Based Ink" Materials 17, no. 15: 3784. https://doi.org/10.3390/ma17153784
APA StyleYuan, Y., Ding, C., Yin, R., Lu, S., Xu, J., Ren, W., Li, K., & Zhao, W. (2024). A Strategy for Fabricating Ultra-Flexible Thermoelectric Films Using Ag2Se-Based Ink. Materials, 17(15), 3784. https://doi.org/10.3390/ma17153784









