Physicochemical Properties of (La,Sr)CoO3 Thick Films on Fe-25Cr Steel under Exposure to SOFC Cathode Operating Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of (La,Sr)CoO3 Powders by EDTA Gel Processes
2.2. Sintering of (La,Sr)CoO3 Bulk Sample
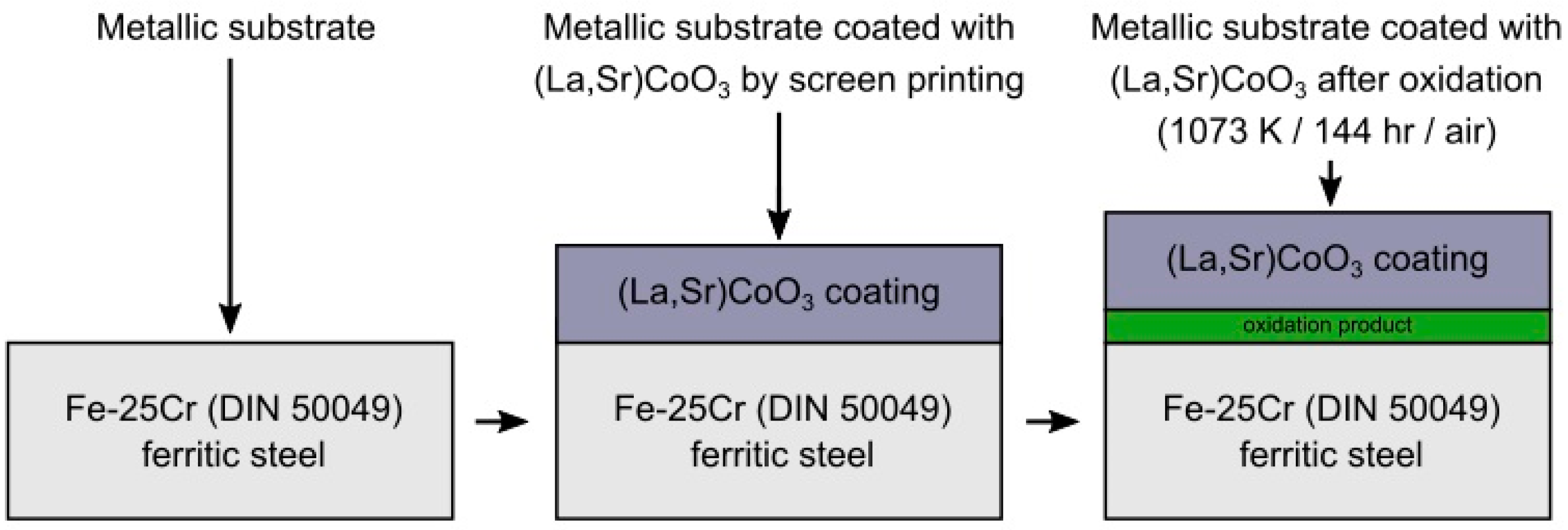
2.3. Composition of (La,Sr)CoO3 Paste and Its Deposition on Steel Substrate by Screen Printing Method and Oxidation Process Procedure
2.4. Analysis Method
3. Results
3.1. Physicochemical Properties of (La,Sr)CoO3 Powder
3.2. Physicochemical Properties of (La,Sr)CoO3 Sinter
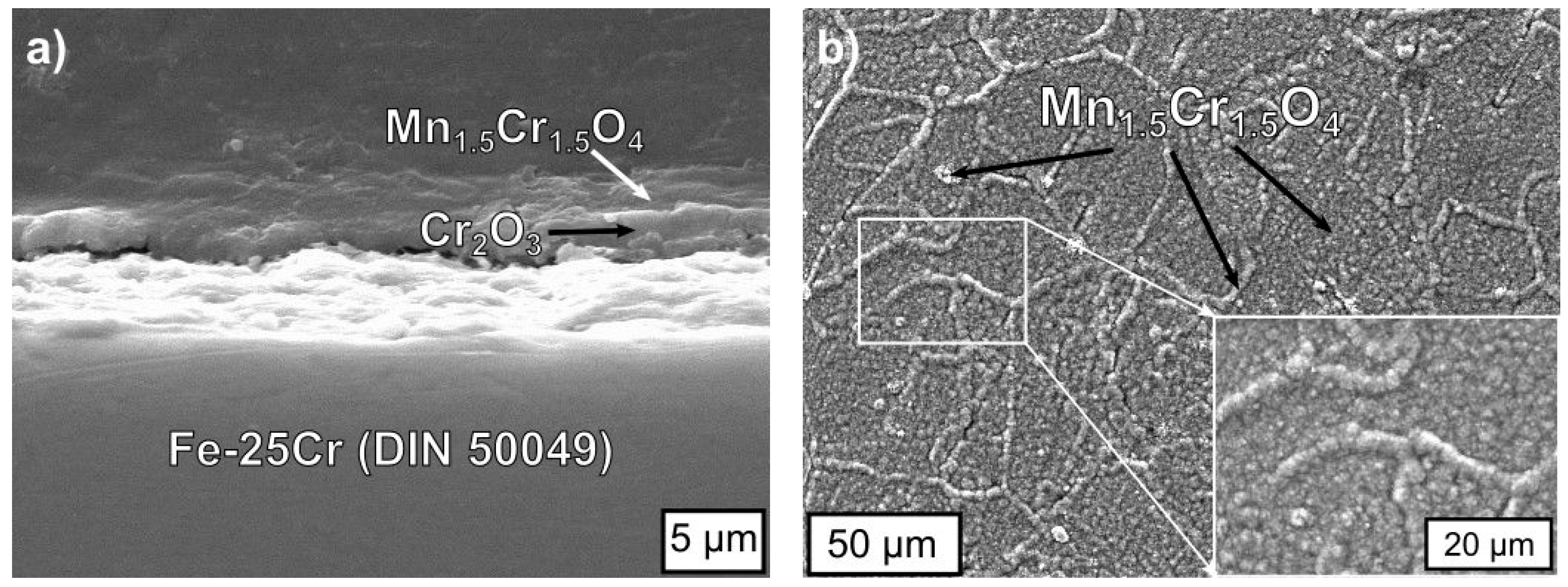
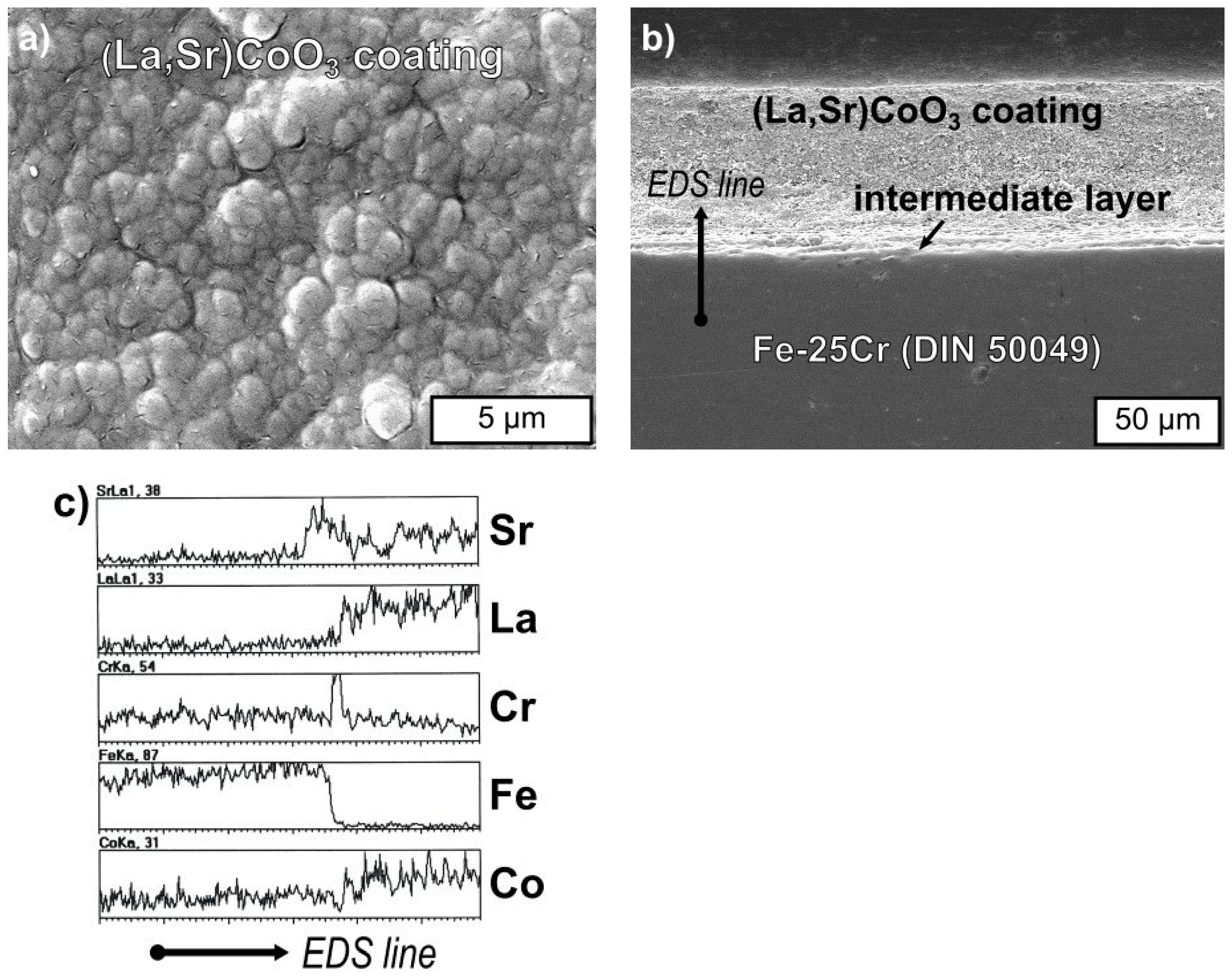
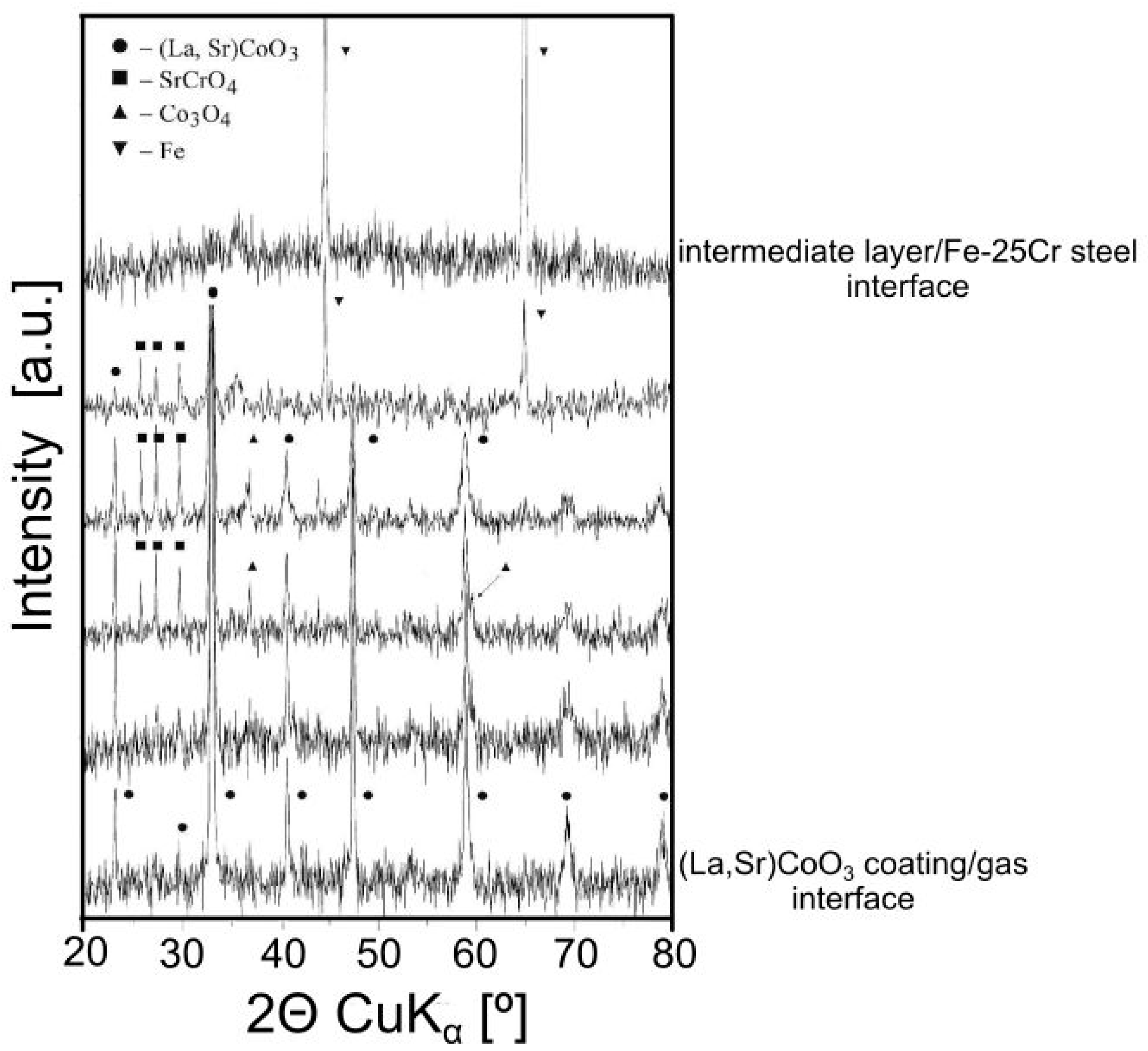
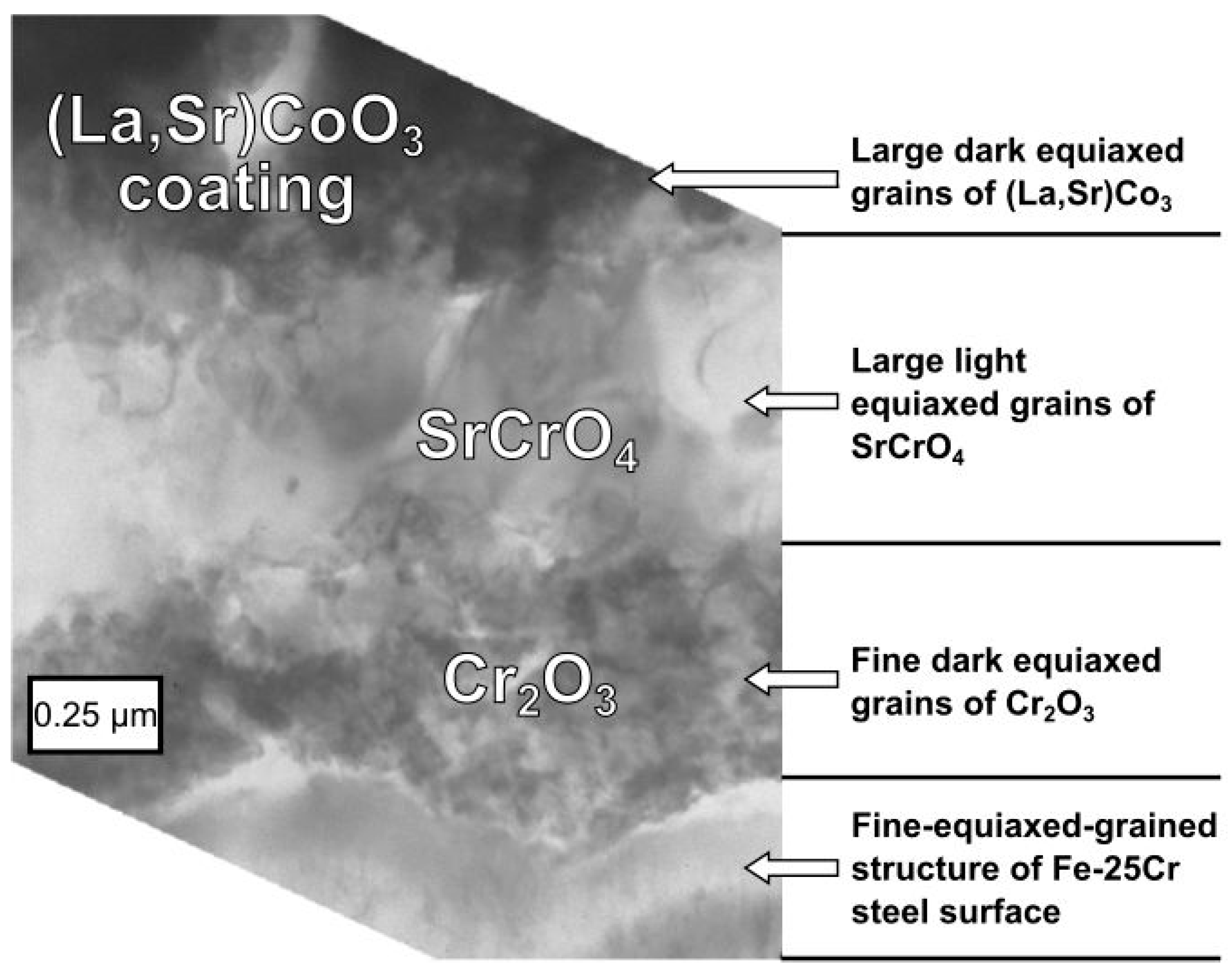
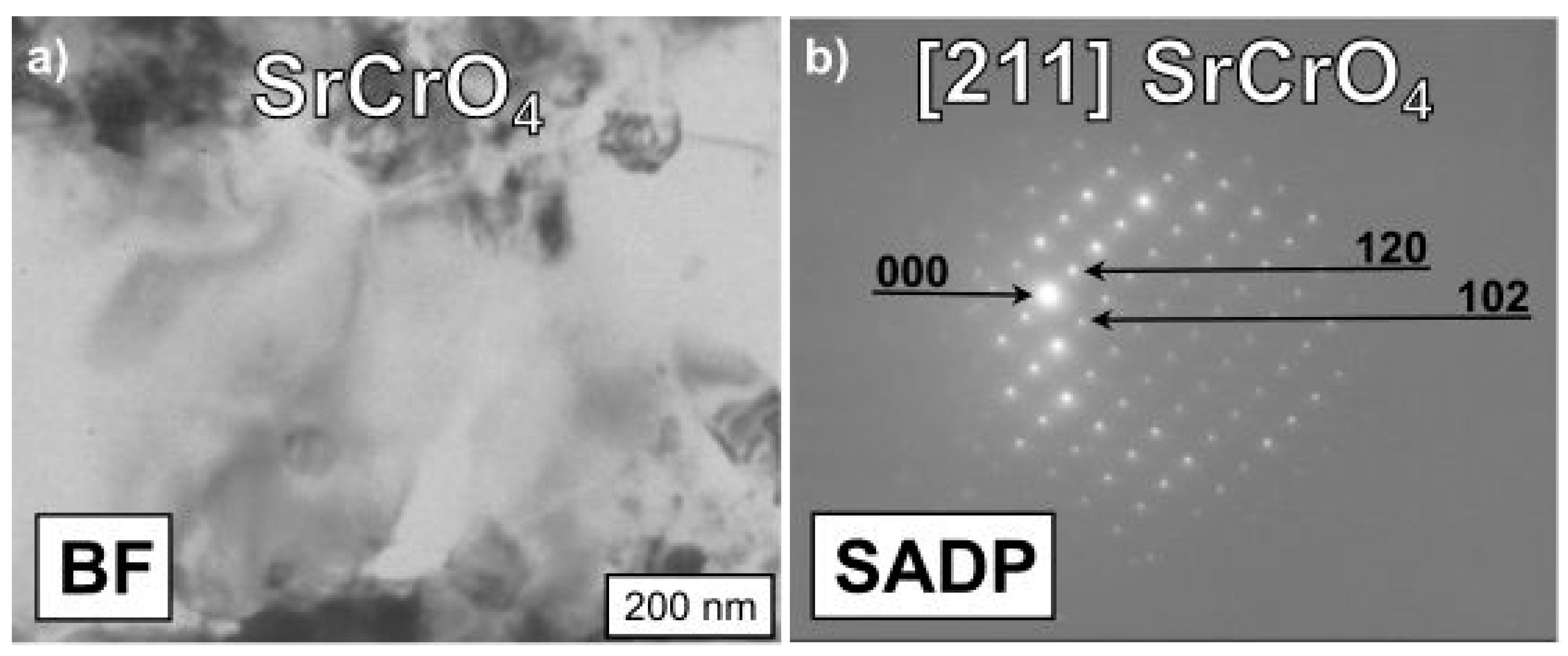
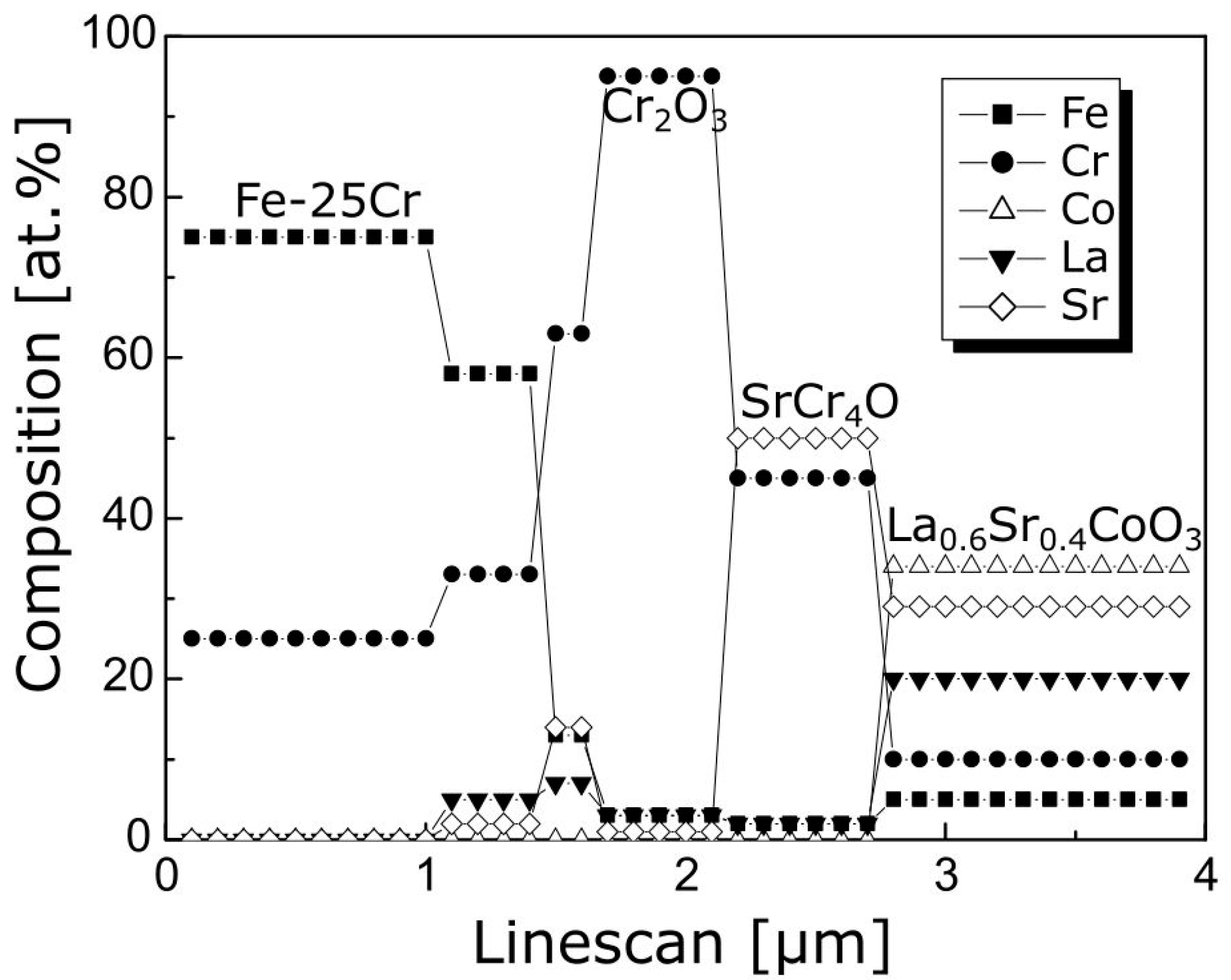
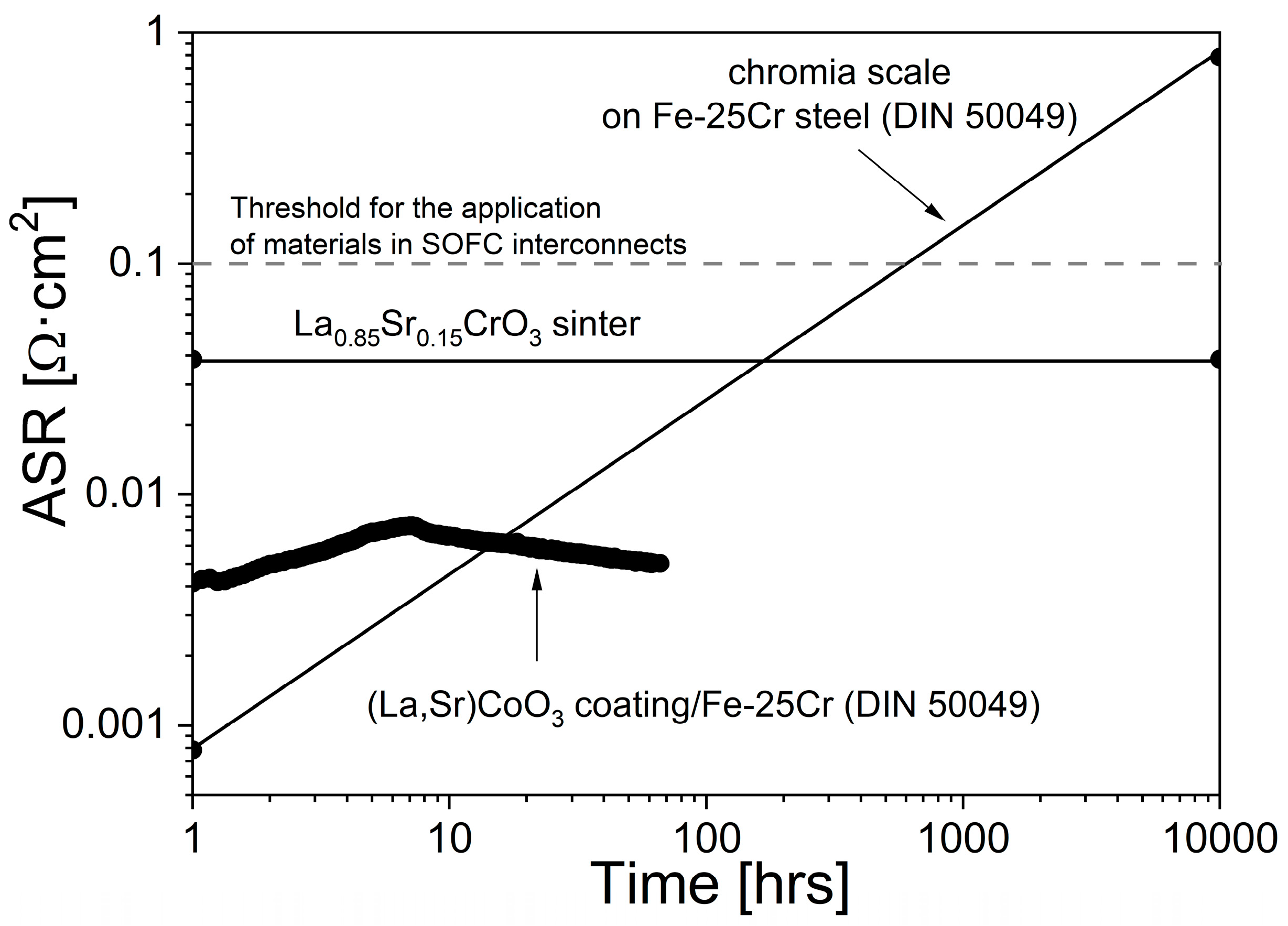
3.3. Physicochemical Properties of the Coating (La,Sr)CoO3/Fe-25Cr Steel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.S.; de Souza, T.M. Novel materials for solid oxide fuel cell technologies: A literature review. Int. J. Hydrogen Energy 2017, 42, 26020–26036. [Google Scholar] [CrossRef]
- Huang, J.; Xie, F.; Wang, C.; Mao, Z. Development of solid oxide fuel cell materials for intermediate-to-low temperature operation. Int. J. Hydrogen Energy 2012, 37, 877–883. [Google Scholar] [CrossRef]
- Kendal, K.; Kendal, M. High-Temperature Solid Oxide Fuel Cells for the 21st Century. Fundamentals, Design and Applications; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Fergus, J.W. Metallic interconnects for solid oxide fuel cells. Mater. Sci. Eng. A 2005, 397, 271–283. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Piron-Abellan, J.; Shemet, V.; Singheiser, L. Metallic interconnectors for solid oxide fuel cells—A review. Mater. High Temp. 2003, 20, 115–127. [Google Scholar]
- Yang, Z.; Weil, K.S.; Paxton, D.M.; Stevenson, J.W. Selection and evaluation of heat-resistant alloys for SOFC interconnect applications. J. Electrochem. Soc. 2003, 150, A1188–A1201. [Google Scholar] [CrossRef]
- Blum, L.; (Bert) de Haart, L.G.J.; Malzbender, J.; Menzler, N.H.; Remmel, J.; Steinberger-Wilckens, R. Recent results in Jülichsolid oxide fuel cell technology development. J. Power Sources 2013, 241, 477–485. [Google Scholar] [CrossRef]
- Brylewski, T.; Nanko, M.; Maruyama, T.; Przybylski, K. Application of Fe-16Cr ferritic alloy to interconnector for a solid oxide fuel cell. Solid State Ion. 2001, 143, 131–140. [Google Scholar] [CrossRef]
- Molin, S.; Chen, M.; Bentzen, J.J.; Hendriksen, P.V. High Temperature Oxidation of Ferritic Steels for Solid Oxide Electrolysis Stacks. ECS Trans. 2013, 50, 11–20. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Deevi, S.C. Development of interconnect materials for solid oxide fuel cells. Mater. Sci. Eng. A 2003, 348, 227–243. [Google Scholar] [CrossRef]
- Bian, L.; Chen, Z.; Wang, L.; Li, F.; Chou, K. Oxidation resistance, thermal expansion and area specific resistance of Fe-Cr alloy interconnector for solid oxide fuel cell. J. Iron Steel Res. Int. 2017, 24, 77–83. [Google Scholar] [CrossRef]
- Hilpert, K.; Das, D.; Miller, M.; Peck, D.H.; Weiβ, R. Chromium vapor species over solid oxide fuel cell interconnect materials and their potential for degradation processes. J. Electrochem. Soc. 1996, 143, 3642–3647. [Google Scholar] [CrossRef]
- Paulson, S.C.; Birss, V.I. Chromium poisoning of LSM-YSZ SOFC cathodes. J. Electrochem. Soc. 2004, 151, A1961–A1968. [Google Scholar] [CrossRef]
- Sachitan, R.; Sattari, M.; Svensson, J.-E.; Froitzheim, J. Evaluation of the oxidation and Cr evaporation properties of selected FeCr alloys used as SOFC interconnects. Int. J. Hydrogen Energy 2013, 38, 15328–15334. [Google Scholar] [CrossRef]
- Grolig, J.G.; Froitzheim, J.; Svensson, J.-E. Coated stainless stee l441 as interconnect material for solid oxide fuel cells: Oxidation performance and chromium evaporation. J. Power Sources 2014, 248, 1007–1013. [Google Scholar] [CrossRef]
- Kadowaki, T.; Shiomitsu, T.; Matsuda, E.; Nakagawa, H.; Tsuneizumi, H. Maruyama, Applicability of heat resisting alloys to the separator of planar type solid oxide fuel cell. Solid State Ion. 1993, 67, 65–69. [Google Scholar] [CrossRef]
- Maruyama, T.; Inoue, T.; Nagata, K. Electrical Conductivity of SrCrO4 and Sr3Cr2O8 at Elevated Temperatures in Relation to the Highly Conductive Chromia Scale Formed on an Alloy Separator in SOFC. In Proceedings of the 4th International Symposium on Solid Oxide Fuel Cells (SOFC-IV), Yokohama, Japan, 18–23 June 1995; Dokiya, M., Yamamoto, O., Tagawa, H., Singhal, S.C., Eds.; The Electrochemical Society Proceedings Series. The Electrochemical Society, Inc.: Pennington, NJ, USA, 1995; p. 850. [Google Scholar]
- Przybylski, K.; Prażuch, J.; Brylewski, T.; Maruyama, T. Oxidation properties of Fe-Cr ferritic alloys coated with (La,Sr)CoO3 for SOFC application. In Proceedings of the 14th International Corrosion Congress, Cape Town, South Africa, 26 September–1 October 1999; Volume 2, pp. 1–9. [Google Scholar]
- Prażuch, J.; Brylewski, T.; Przybylski, K. Interaction of (La,Sr)CoO3 thick films with Fe-25Cr steel in SOFC cathode operatingconditions at 1073 K in air at 1073 K. Pol. Ceram. Bull. 2000, 61, 167–171. [Google Scholar]
- Przybylski, K.; Brylewski, T.; Prażuch, J. High temperature oxidation of Fe-Cr steels with regard to their application as interconnectors for solid oxide fuel cells. In Proceedings of the 10th International IUPAC Conference on High-Temperature Materials Chemistry, Jülich, Germany, 10–14 April 2000; Schriften des Forschungszentrum Jülich, Series: Energy Technology. Forschungszentrum: Jülich, Germany, 2000; Volume 15, Part II. pp. 741–744. [Google Scholar]
- Przybylski, K.; Brylewski, T.; Prażuch, J. Synthesis and physicochemical properties of (La,Sr)CoO3 for SOFC application. In Proceedings of the 10th International IUPAC Conference on High-Temperature Materials Chemistry, Jülich, Germany, 10–14 April 2000; Schriften des Forschungszentrum Jülich, Series: Energy Technology. Forschungszentrum: Jülich, Germany, 2000; Volume 15, Part II. pp. 745–748. [Google Scholar]
- Ohno, Y.; Nagata, S.; Sato, H. Properties of oxides for high temperature solid electrolyte fuel cell. Solid State Ion. 1983, 9–10, 1001–1007. [Google Scholar] [CrossRef]
- Petrov, A.N.; Kofstad, P. Solid Oxide Fuel Cells (SOFC VI)—Electrochemistry Society Proceedings Vol. 95-1; Dokiya, M., Yamamoto, O., Tagawa, H., Singhal, S.C., Eds.; The Electrochemical Society: Pennington, NJ, USA, 1995; p. 220. [Google Scholar]
- Mizusaki, J. Nonstoichiometry, diffusion, and electrical properties of perovskite-type oxide electrode materials. Solid State Ion. 1992, 52, 79–91. [Google Scholar] [CrossRef]
- Chen, X.; Wu, N.J.; Ignatiev, A. Structure and conducting properties of La1-xSrxCoO3-δ films. J. Eur. Ceram. Soc. 1999, 19, 819–822. [Google Scholar] [CrossRef]
- Mizusaki, J.; Tabuchi, J.; Matsuura, T.; Yamauchi, S.; Fueki, K. Electrical Conductivity and Seebeck Coefficient of Nonstoichiometric La1-xSrxCoO3-δ. J. Electrochem. Soc. 1989, 136, 2082–2088. [Google Scholar] [CrossRef]
- Mizusaki, J.; Mima, Y.; Yamauchi, S.; Fueki, K.; Tagawa, H. Nonstoichiometry of the perovskite-type oxides La1-xSrxCoO3-δ. J. Solid Chem. 1989, 80, 102–111. [Google Scholar] [CrossRef]
- Søgaard, M.; Hendriksen, P.V.; Mogensen, M.; Poulsen, F.W.; Skou, E. Oxygen nonstoichiometry and transport properties of strontium substituted lanthanum cobaltite. Solid State Ion. 2006, 177, 37–38. [Google Scholar] [CrossRef]
- Kim, Y.T.; Shikazono, N. Evaluation of electrochemical reaction mechanisms of La0.6Sr0.4CoO3-δ-Gd0.1Ce0.9O2-δ composite cathodes by 3D numerical simulation. Solid State Ion. 2018, 319, 162–169. [Google Scholar] [CrossRef]
- Samat, A.A.; Somalu, M.R.; Muchtar, A.; Hassan, O.H.; Osman, N.L.S. Cathode prepared by polymeric complexation method for proton-conducting SOFC application. J. Sol-Gel Sci. Technol. 2016, 78, 382–393. [Google Scholar] [CrossRef]
- Lemieszek, B.; Ilickas, M.; Jamroz, J.; Tamuleviciene, A.; Karczewski, J.; Błaszczak, P.; Maximenko, A.; Abakeviciene, B.; Małys, M.; Tamulevicius, S.; et al. Enhanced electrochemical performance of partially amorphous La0.6Sr0.4CoO3-δ oxygen electrode materials for low-temperature solid oxide cells operating at 400 °C. Appl. Surf. Sci. 2024, 670, 160620. [Google Scholar] [CrossRef]
- Barsoum, M.W. Fundamentals of Ceramics, 2nd ed.; McGraw-Hill: Singapore, 1997. [Google Scholar]
- Azaroff, L.V. Elements of X-Ray Crystallography; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed.; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Kurokawa, H.; Jacobson, C.P.; De Jonghe, L.C.; Visco, S. Chromium vaporization of bare and of coated iron-chromium alloys at 1073 K. Solid State Ion. 2007, 178, 287–296. [Google Scholar] [CrossRef]
- Brylewski, T. Metaliczne Interkonektory w Układzie Metal/Ceramika do Zastosowania w Ogniwach Paliwowych SOFC/Metallic Interconnects in a Metal/Ceramics System for Application in Solid Oxide Fuel Cells; Wyd. Naukowe “Akapit”: Krakow, Poland, 2008. (In Polish) [Google Scholar]
- Brylewski, T.; Przybylski, K. Oxidation properties of coated Fe-25Cr steel with regard to the chromium vaporization effects under SOFC cathode operation conditions. Ann. Chim. Sci. Mat. 2008, 33, 75–82. [Google Scholar]
- Chevalier, S.; Caboche, G.; Przybylski, K.; Brylewski, T. Effect of nano-layered coatings on the electrical conductivity of oxide scale grown on ferritic steels. J. Appl. Electrochem. 2009, 39, 529–534. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Semiconducting Materials, Butterworths; Scientific Publications: London, UK, 1951. [Google Scholar]
- Landauer, R. Electrical conductivity in inhomogeneous media. AIP Conf. Proc. 1978, 40, 2–45. [Google Scholar]
- Pohl, A.; Westin, G. Alkoxide route to La0.5Sr0.5CoO3 films and powders. J. Am. Ceram. 2005, 88, 2099–2105. [Google Scholar] [CrossRef]
- Rao, C.N.R. Transition Metal Oxides; VCH Publishers, Inc.: Nowy York, NY, USA, 1995. [Google Scholar]
- Cox, P.A. Transition Metal Oxides; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Brylewski, T.; Gil, A.; Rakowska, A.; Chevalier, S.; Adamczyk, A.; Dąbek, J.; Kruk, A.; Stygar, M.; Przybylski, K. Improving the physicochemical properties of Fe-25Cr ferritic steel for SOFC interconnects via Y-implantation and Y2O3-deposition. Oxid. Met. 2013, 80, 83–111. [Google Scholar] [CrossRef]
- Cox, M.G.E.; Mc Enanay, B.; Scott, V.D. A chemical diffusion model for partitioning of transition elements in oxide scales on 587 alloys. Philos. Mag. 1972, 26, 839–851. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Footner, P.K.; Holmes, D.R.; Mortimer, D. Oxidation of iron-chromium binary alloys. Nature 1967, 216, 54–56. [Google Scholar] [CrossRef]
- Hay, K.A.; Hicks, F.G.; Holmes, D.R. The transport properties and defect structure of the oxide (Fe,Cr)2O3 formed on Fe-Cr alloys. Werkst. Korros. 1970, 11, 917–924. [Google Scholar] [CrossRef]
- Maruyama, T.; Inoue, T.; Akashi, T. Standard Gibbs Energies of Formation of SrCrO4 and Sr3Cr2O8. Mater. Trans. JIM 1998, 39, 1158–1161. [Google Scholar] [CrossRef]
- Akashi, A.; Inoue, T.; Maruyama, T. Electrical conductivity measurement for estimation of standard Gibss energy change of ternary oxide formation. Mater. Trans. JIM 2000, 12, 1646–1650. [Google Scholar] [CrossRef]
- Negas, T.; Roth, R.S. System SrO-Chromium oxide in air and oxygen. J. Res. Nat. Bur. Stand. A Phys. Chem. 1969, 73, 431–442. [Google Scholar] [CrossRef]
- Schafer, W.; Schmidberger, R. High Tech Ceramics; Vincenzini, P., Ed.; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Konysheva, E.; Penkalla, H.; Wessel, E.; Mertens, J.; Seeling, U.; Singheiser, L.; Hilpert, K. Chromium poisoning of perovskite cathodes by the ODS alloy Cr5Fe1Y2O3 and the High Chromium Ferritic Steel Crofer 22 APU. J. Electrochem. Soc. 2006, 153, A765–A773. [Google Scholar] [CrossRef]
- Kruk, A.; Stygar, M.; Brylewski, T. Mn-Co spinel protective-conductive coatings on AL453 ferritic stainless steel for IT-SOFC interconnect application. J. Solid State Electrochem. 2013, 17, 993–1003. [Google Scholar] [CrossRef]
- Brylewski, T.; Kruk, A.; Bobruk, M.; Adamczyk, A.; Partyka, J.; Rutkowski, P. Structure and electrical properties of Cu-doped Mn-Co-O spinel prepared via soft chemistry and its application in IT-SOFC interconnects. J. Power Sources 2016, 333, 145–155. [Google Scholar] [CrossRef]
- Brylewski, T.; Dąbek, J.; Przybylski, K.; Morgiel, J.; Rękas, M. Screen-printed (La,Sr)CrO3 coatings on ferritic stainless steel interconnects for SOFCs using nanopowders prepared by means of ultrasonic spray pyrolysis. J. Power Sources 2012, 208, 86–95. [Google Scholar] [CrossRef]




| Chemical Composition [mass%] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Cr | Mn | Si | Ni | C | P | S | Ti |
| 73.347 | 24.55 | 0.28 | 0.74 | 0.99 | 0.04 | 0.03 | 0.013 | 0.01 |
| BET Surface Area [m2·g−1] | BET Mean Grain [µm] | Powder Characterising Parameters [µm] |
|---|---|---|
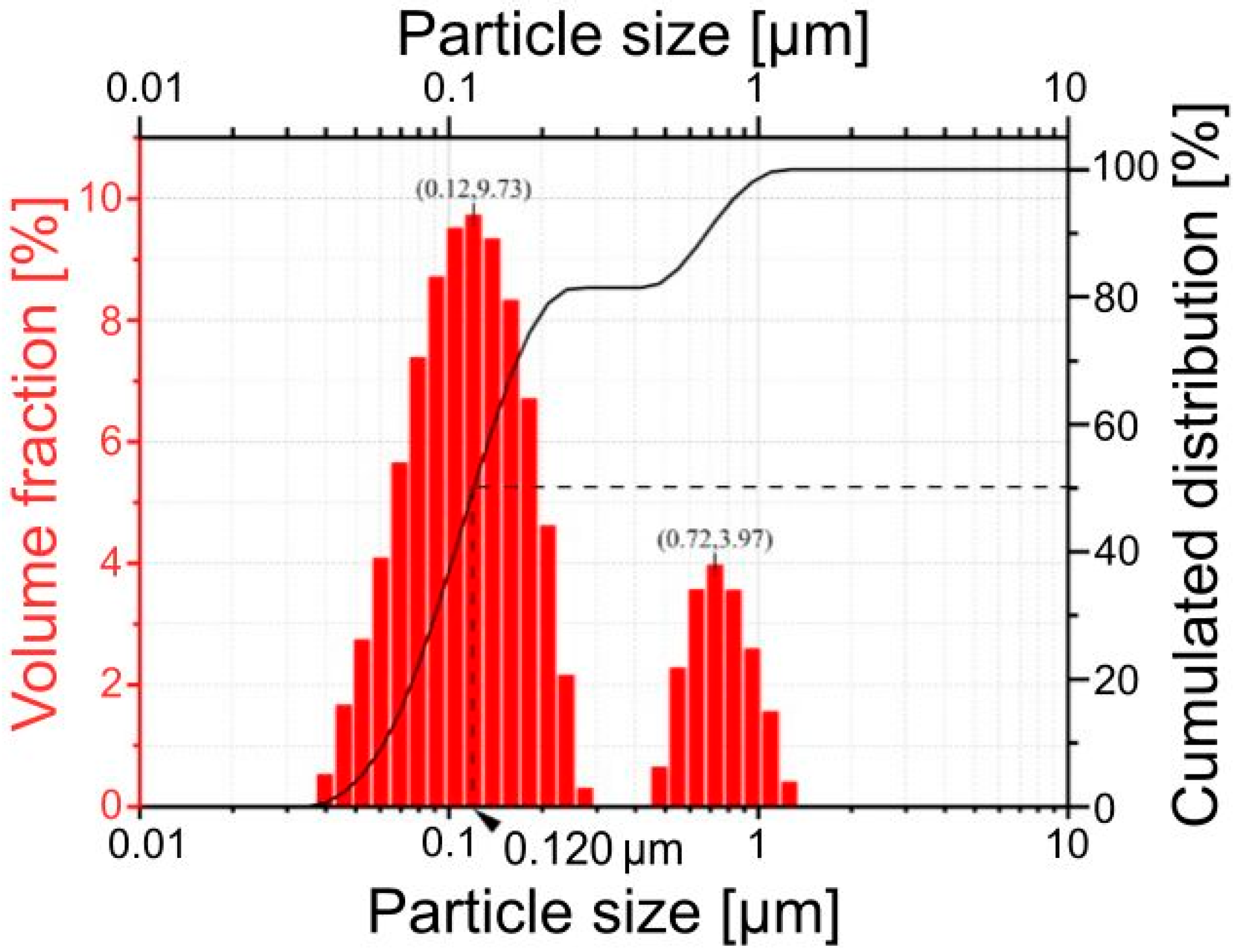
| 14.68 ± 0.13 | 0.06 | Surface weighted mean D[3.2] = 0.109 µm Volume weighted mean D[3.2] = 0.229 µm 1st mode: 9.73% for 0.12 µm 2nd mode: 3.97% for 0.72 µm Dv10 = 0.062 µm Dv50 = 0.120 µm (median) Dv90 = 0.678 µm |
| Apparent Density [g·cm−3] | Relative Density [%] | Total Porosity [%] |
|---|---|---|
| 6.36 ± 0.09 | 96.58 | 3.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prażuch, J.; Pyzalski, M.; Fernández González, D.; Brylewski, T. Physicochemical Properties of (La,Sr)CoO3 Thick Films on Fe-25Cr Steel under Exposure to SOFC Cathode Operating Conditions. Materials 2024, 17, 3791. https://doi.org/10.3390/ma17153791
Prażuch J, Pyzalski M, Fernández González D, Brylewski T. Physicochemical Properties of (La,Sr)CoO3 Thick Films on Fe-25Cr Steel under Exposure to SOFC Cathode Operating Conditions. Materials. 2024; 17(15):3791. https://doi.org/10.3390/ma17153791
Chicago/Turabian StylePrażuch, Janusz, Michał Pyzalski, Daniel Fernández González, and Tomasz Brylewski. 2024. "Physicochemical Properties of (La,Sr)CoO3 Thick Films on Fe-25Cr Steel under Exposure to SOFC Cathode Operating Conditions" Materials 17, no. 15: 3791. https://doi.org/10.3390/ma17153791
APA StylePrażuch, J., Pyzalski, M., Fernández González, D., & Brylewski, T. (2024). Physicochemical Properties of (La,Sr)CoO3 Thick Films on Fe-25Cr Steel under Exposure to SOFC Cathode Operating Conditions. Materials, 17(15), 3791. https://doi.org/10.3390/ma17153791








