Photo-Thermal Dry Reforming of Methane with PGM-Free and PGM-Based Catalysts: A Review
Abstract
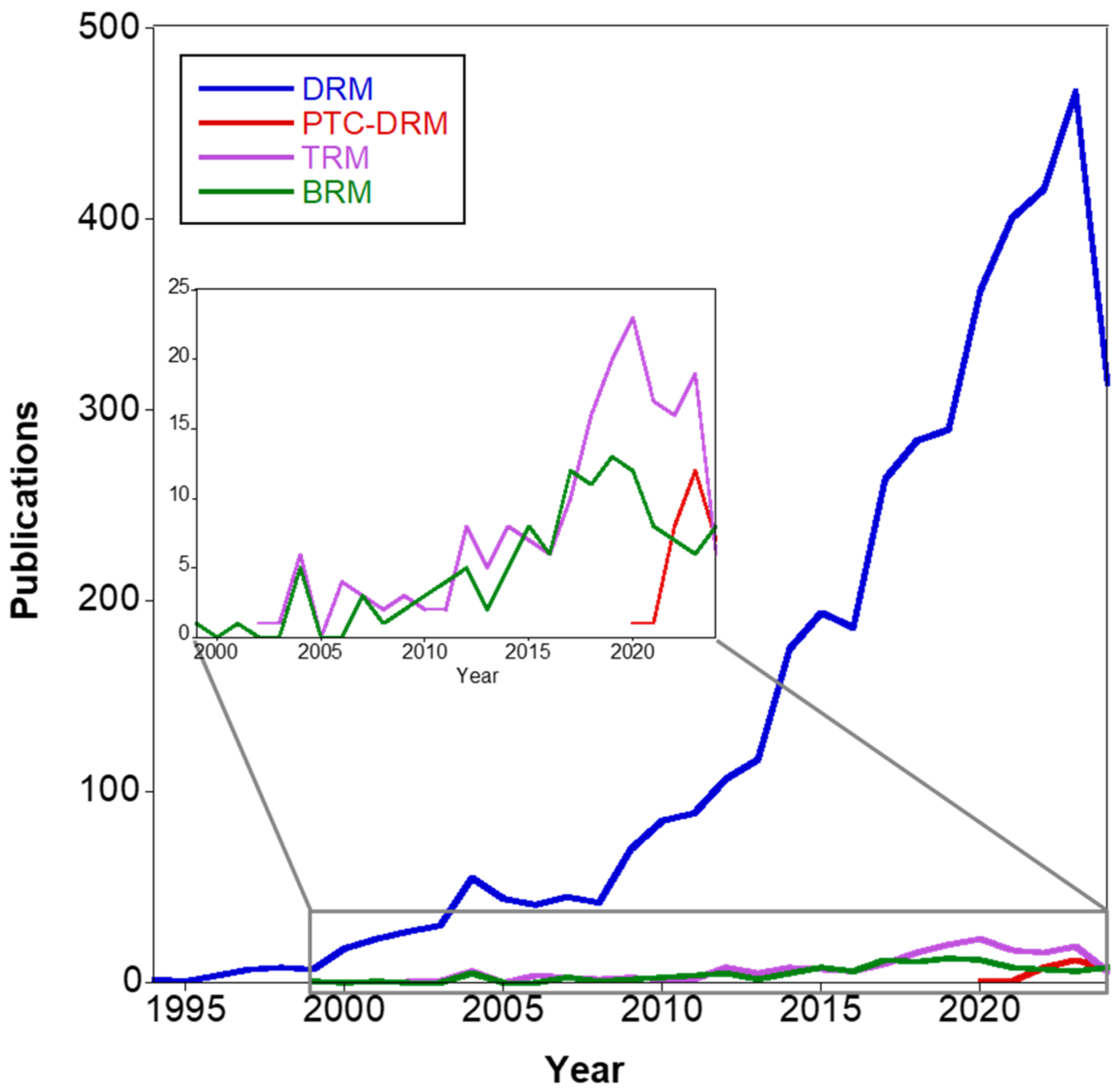
1. Introduction
2. Dry Reforming of Methane (DRM) Reaction
3. Bi- and Tri-Reforming of Methane
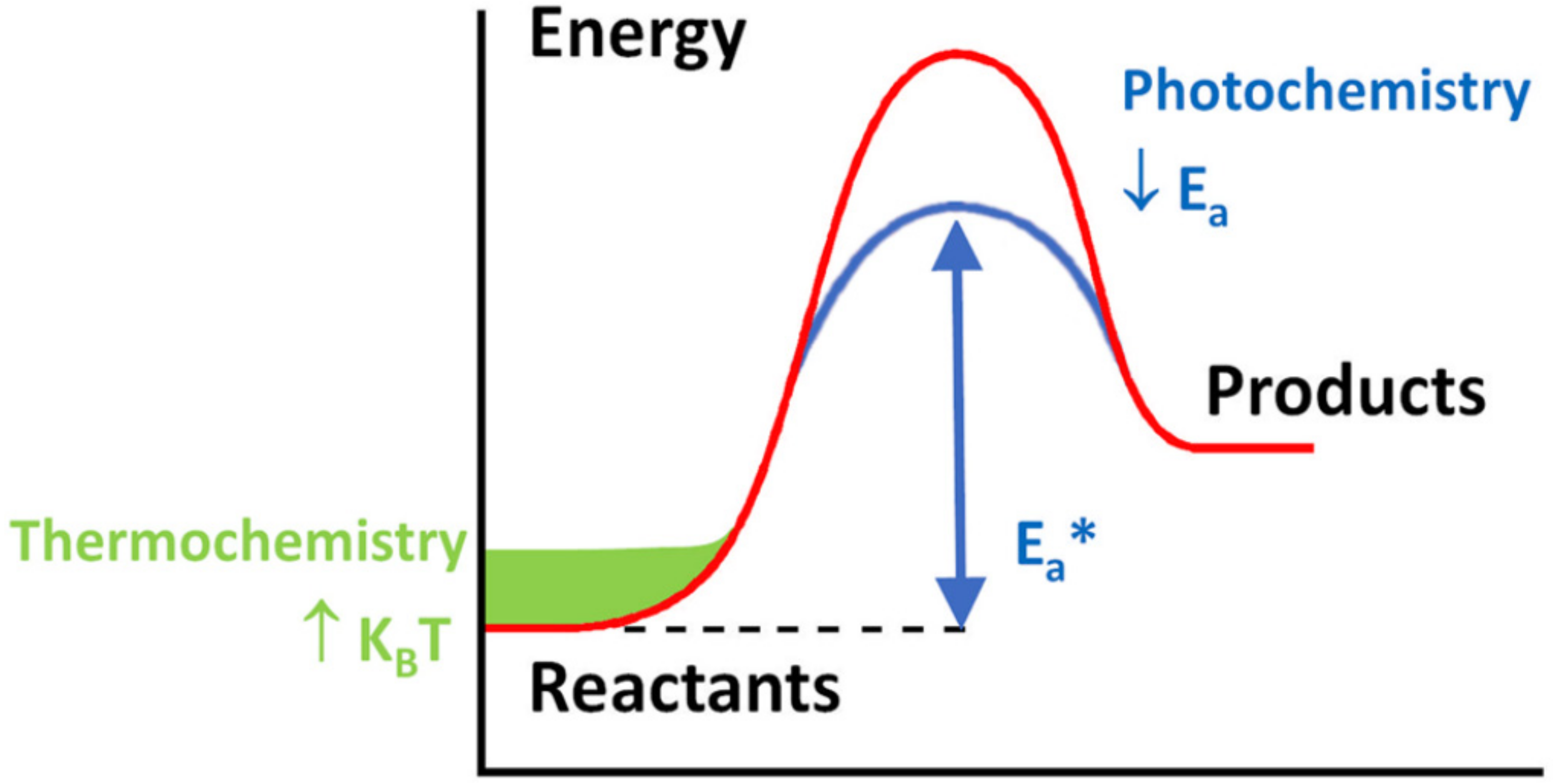
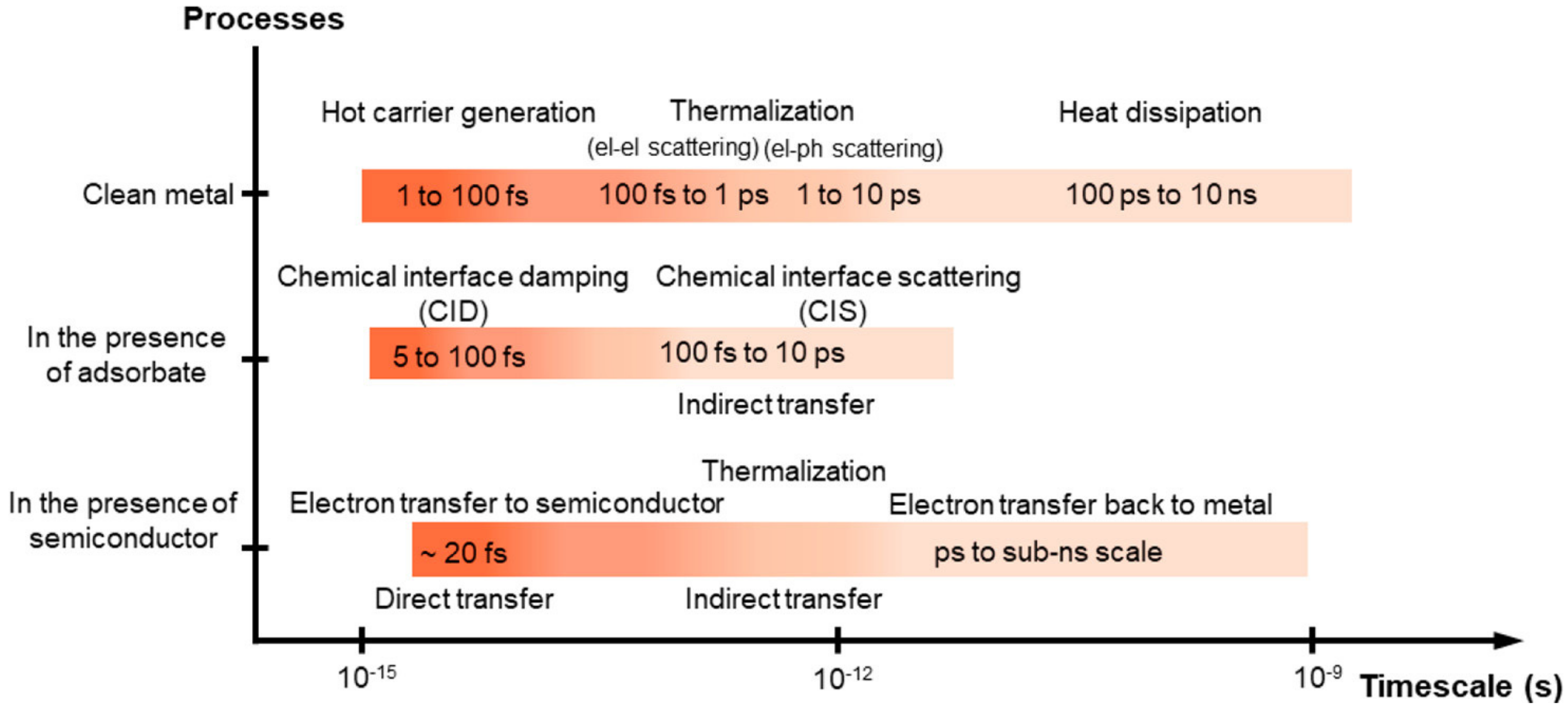
4. Fundamentals of Photo-Thermal Catalysis
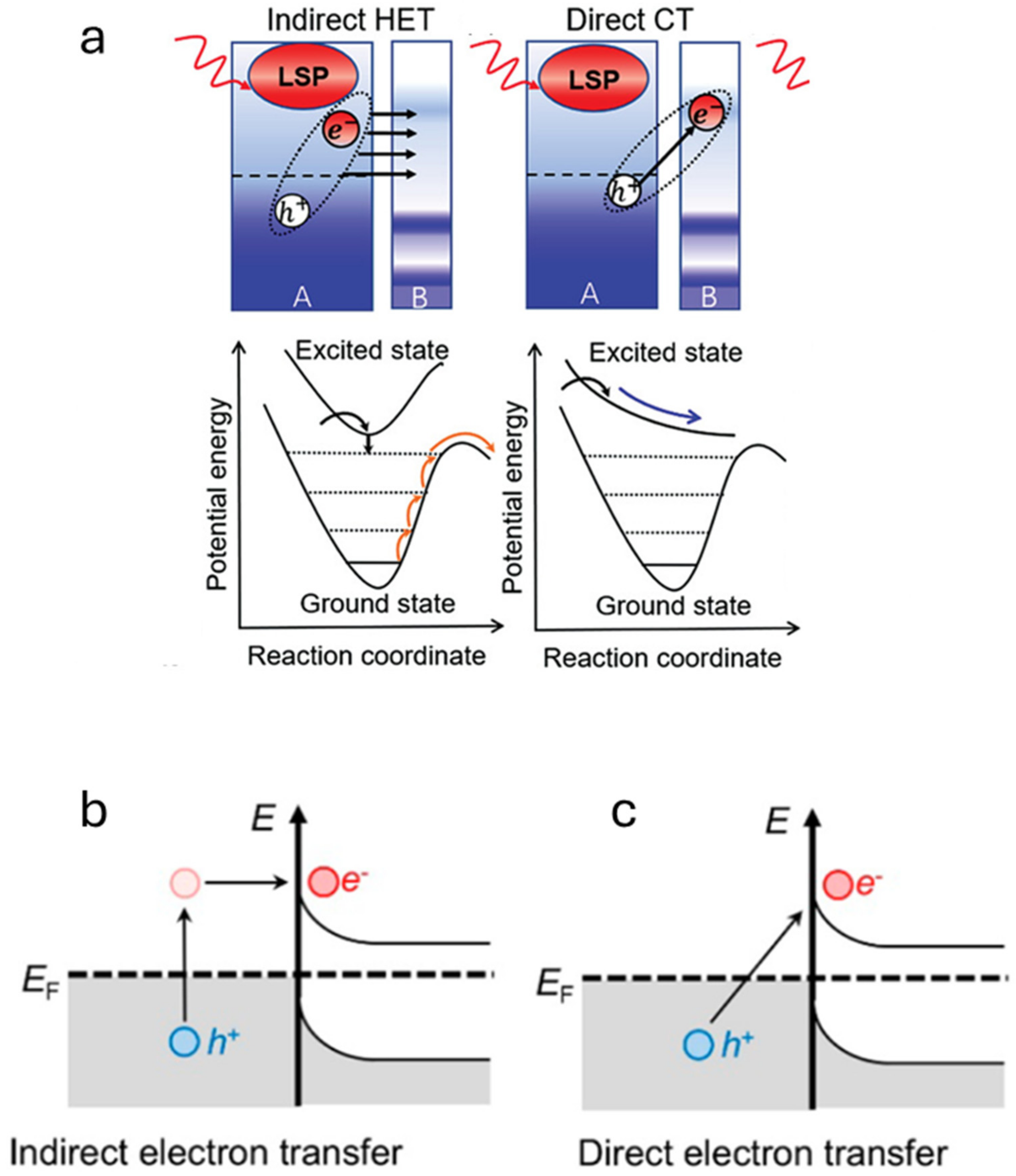
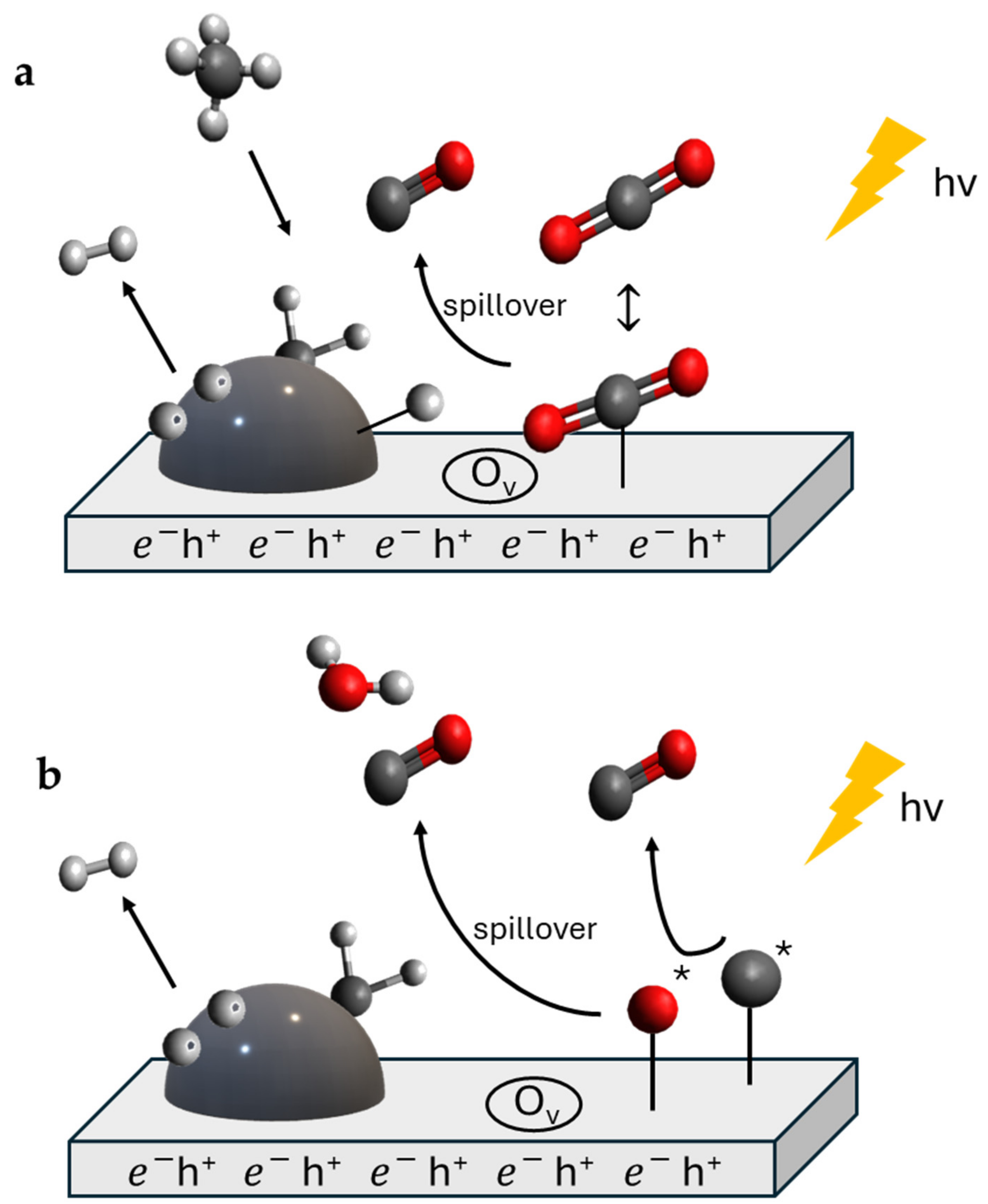
5. Photo-Thermal Dry Reforming of Methane
- -
- high visible light absorption;
- -
- good match between the band structure of the catalyst and the redox potentials of the reaction;
- -
- good thermocatalytic activity.
6. Catalysts for PTC-DRM Reaction
6.1. PGM-Free Catalysts
6.2. PGM-Based Catalysts for Photo-Thermal DRM
- -
- the dispersion of Ni decreases;
- -
- sintering becomes more significant, and this leads, inevitably, to a decrease in the BET surface area.
| Name Sample | Temperature °C | Light Intensity W/cm2 | BET Surface Area m2/g | Pore Volume cm3/g | Average Pore Diameter nm | Catalytic Performances | References |
|---|---|---|---|---|---|---|---|
| P25 | 500 | 4.6 | 63.0 | 0.12 | 7.9 | N.A. | [105] |
| Pt/P25 | 500 | 4.6 | 62.0 | 0.14 | 9.1 | r (H2) = 65 mmol·g−1·h−1 r (CO) = 162 mmol·g−1·h−1 H2/CO = 0.40 | [105] |
| Au/P25 | 500 | 4.6 | 60.0 | 0.14 | 9.3 | N.A. | [105] |
| Pt-Au/P25 | 500 | 4.6 | 55.0 | 0.12 | 8.7 | r (H2) = 86 mmol·g−1·h−1 r (CO) = 203 mmol·g−1·h−1 H2/CO = 0.42 | [105] |
| mes-TiO2 | 500 | 3.8 | 91.8 | 0.25 | 10.7 | N.A. | [104] |
| P25 | 500 | 3.8 | 62.0 | 0.12 | 7.5 | N.A. | [104] |
| Pt/P25 | 500 | 3.8 | 54.3 | 0.11 | 8.4 | r (H2) = 40 mmol·g−1·h−1 r (CO) = 106 mmol·g−1·h−1 H2/CO = 0.38 | [104] |
| Ni/mes-TiO2 | 500 | 3.8 | 85.1 | 0.24 | 11.1 | r (H2) = 31 mmol·g−1·h−1 r (CO) = 81 mmol·g−1·h−1 H2/CO = 0.38 | [104] |
| Pt/mes-TiO2 | 500 | 3.8 | 77.5 | 0.22 | 11.4 | r (H2) = 211 mmol·g−1·h−1 r (CO) = 309 mmol·g−1·h−1 H2/CO = 0.68 | [104] |
| 0.3 Ni | 650 | 0 | 580.7 | 0.39 | 2.2 | CH4 = 16% CO2 = 25% H2/CO = 0.69 | [64] |
| 0.2 PdNi | 650 | 0 | 523.6 | 0.38 | 2.4 | CH4 = 23% CO2 = 31% H2/CO = 0.83 | [64] |
| 0.3 PdNi | 650 | 0 | 514.7 | 0.35 | 2.2 | CH4 = 36% CO2 = 52% H2/CO = 0.81 | [64] |
| 0.4 PdNi | 650 | 0 | 506.6 | 0.35 | 2.1 | CH4 = 30% CO2 = 42% H2/CO = 0.81 | [64] |
| Ni/CaO-Al2O3 | 700 | 0 | 76.1 | 0.21 | 11.1 | CH4 conv. = 54% CO2 conv. = 59% | [81] |
| CeO2-Ni/CaO-Al2O3 | 700 | 0 | 90.7 | 0.29 | 13.2 | CH4 conv. = 82% CO2 conv. = 84% | [81] |
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiraiwa, M. Facing Global Climate and Environmental Change. ACS Environ. Au 2023, 3, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Yoro, K.O.; Daramola, M.O. CO2 Emission Sources, Greenhouse Gases, and the Global Warming Effect. In Advances in Carbon Capture: Methods, Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–28. ISBN 9780128196571. [Google Scholar]
- Global Climate Change Impacts in the United States: Highlights—Citations, Rights, Re-Use—UNT Digital Library. Available online: https://digital.library.unt.edu/ark:/67531/metadc11959/citation/#responsibilities-of-use (accessed on 4 June 2024).
- McCulloch, M.T.; Winter, A.; Sherman, C.E.; Trotter, J.A. 300 Years of Sclerosponge Thermometry Shows Global Warming Has Exceeded 1.5 °C. Nat. Clim. Change 2024, 14, 171–177. [Google Scholar] [CrossRef]
- Pearce, J.M.; Parncutt, R. Quantifying Global Greenhouse Gas Emissions in Human Deaths to Guide Energy Policy. Energies 2023, 16, 6074. [Google Scholar] [CrossRef]
- Painter, D.S. Burning Up: A Global History of Fossil Fuel Consumption. J. Interdiscip. Hist. 2019, 50, 442–443. [Google Scholar] [CrossRef]
- Hulme, M. 1.5 °C and climate research after the Paris Agreement. Nature Clim Change 2016, 6, 222–224. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Chatterjee, S.; Huang, K.W. The Insignificant Role of Dry Reforming of Methane in CO2 emission Relief. ACS Energy Lett. 2020, 5, 2881–2885. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Hu, Y.H. Thermo-Photo Coupled Catalytic CO2 Reforming of Methane: A Review. Chem. Eng. J. 2022, 428, 131222. [Google Scholar] [CrossRef]
- Ji, G.; Wu, S.; Song, X.; Meng, L.; Jia, Y.; Tian, J. Recent Progress in Photo-Thermal Synergistic Catalysis for Methane Dry Reforming. Int. J. Hydrogen Energy 2024, 57, 696–708. [Google Scholar] [CrossRef]
- Lei, Y.; Ye, J.; García-Antón, J.; Liu, H. Recent Advances in the Built-in Electric-Field-Assisted Photocatalytic Dry Reforming of Methane. Chin. J. Catal. 2023, 53, 72–101. [Google Scholar] [CrossRef]
- Kotkowski, T.; Cherbański, R.; Stankiewicz, A.I. Electrifying the Dry Reforming of Methane. Shall We Target the Chemistry or the Heat Supply? Chem. Eng. Process. 2024, 202, 109875. [Google Scholar] [CrossRef]
- Cho, Y.; Shoji, S.; Yamaguchi, A.; Hoshina, T.; Fujita, T.; Abe, H.; Miyauchi, M. Visible-Light-Driven Dry Reforming of Methane Using a Semiconductor-Supported Catalyst. Chem. Commun. 2020, 56, 4611–4614. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, K. Tuning combined steam and dry reforming of methane for “metgas” production: A thermodynamic approach and state-of-the-art catalysts. J. Energy 2020, 48, 54–91. [Google Scholar] [CrossRef]
- He, Z.; Huang, M.; Lin, T.; Zhong, L. Recent Advances in Dry Reforming of Methane via Photothermocatalysis. Acta Phys. Chim. Sin. 2023, 39, 2212060. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Wang, L.; Zhang, J. Recent Advances in Photo-Enhanced Dry Reforming of Methane: A Review. J. Photochem. Photobiol. C 2022, 51, 100468. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Luyben, W.L. Design and Control of the Dry Methane Reforming Process. Ind. Eng. Chem. Res. 2014, 53, 14423–14439. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon Dioxide Reforming of Methane to Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A Review of Dry (CO2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Standard Thermodynamic Quantities for Chemical Substances at 25 °C. In CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2004.
- Kulandaivalu, T.; Mohamed, A.R.; Ali, K.A.; Mohammadi, M. Photocatalytic Carbon Dioxide Reforming of Methane as an Alternative Approach for Solar Fuel Production—A Review. Renew. Sustain. Energy Rev. 2020, 134, 110363. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. Sci. Eng. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent Progress in Ceria-Based Catalysts for the Dry Reforming of Methane: A Review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, K.; Jiang, Y.; Li, M.; Tan, X.; Yang, Y.; Zhao, X.; Wang, L.; Wang, Y.; Wang, X.; et al. Photoinducing Different Mechanisms on a Co-Ni Bimetallic Alloy in Catalytic Dry Reforming of Methane. ACS Catal. 2023, 13, 10855–10865. [Google Scholar] [CrossRef]
- Liu, X.; Shi, H.; Meng, X.; Sun, C.; Zhang, K.; Gao, L.; Ma, Y.; Mu, Z.; Ling, Y.; Cheng, B.; et al. Solar-Enhanced CO2 Conversion with CH4 over Synergetic NiCo Alloy Catalysts with Light-to-Fuel Efficiency of 33.8%. Sol. RRL 2021, 5, 2100185. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, Z.Y.; Zheng, H.Y.; Xu, K.D.; Hu, Z.; Lei, Y. Enhanced Photothermal Catalytic Performance of Dry Reforming of Methane over Ni/Mesoporous TiO2 Composite Catalyst. Chem. Eng. J. 2022, 429, 132507. [Google Scholar] [CrossRef]
- Fang, S.; Hu, Y.H. Thermo-Photo Catalysis: A Whole Greater than the Sum of Its Parts. Chem. Soc. Rev. 2022, 51, 3609–3647. [Google Scholar] [CrossRef] [PubMed]
- De Caprariis, B.; De Filippis, P.; Palma, V.; Petrullo, A.; Ricca, A.; Ruocco, C.; Scarsella, M. Rh, Ru and Pt Ternary Perovskites Type Oxides BaZr(1-x)MexO3 for Methane Dry Reforming. Appl. Catal. A Gen. 2016, 517, 47–55. [Google Scholar] [CrossRef]
- Rego de Vasconcelos, B.; Pham Minh, D.; Martins, E.; Germeau, A.; Sharrock, P.; Nzihou, A. Highly-Efficient Hydroxyapatite-Supported Nickel Catalysts for Dry Reforming of Methane. Int. J. Hydrogen Energy 2020, 45, 18502–18518. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Bao, W.; Yan, W.; Zhang, J.; Huang, Y.; Li, H.; Wang, Z.; Liu, M.; Yu, F. Enhanced Dry Reforming of CO2 and CH4 on Photothermal Catalyst Ru/SrTiO3. Appl. Catal. B 2023, 338, 123054. [Google Scholar] [CrossRef]
- Sokefun, Y.O.; Trottier, J.; Yung, M.M.; Joseph, B.; Kuhn, J.N. Low Temperature Dry Reforming of Methane Using Ru-Ni-Mg/Ceria-Zirconia Catalysts: Effect of Ru Loading and Reduction Temperature. Appl. Catal. A Gen. 2022, 645, 118842. [Google Scholar] [CrossRef]
- Fresno, F.; Iglesias-Juez, A.; Coronado, J.M. Photothermal Catalytic CO2 Conversion: Beyond Catalysis and Photocatalysis. Top. Curr. Chem. 2023, 381, 21. [Google Scholar] [CrossRef] [PubMed]
- Ghoussoub, M.; Xia, M.; Duchesne, N.P.; Segal, D.; Ozin, G. Principles of photothermal gas-phase heterogenous CO2 catalysis. Energy Environ. Sci. 2019, 12, 1122–1142. [Google Scholar] [CrossRef]
- Entesari, N.; Goeppert, A.; Prakash, G.K.S. Renewable Methanol Synthesis through Single Step Bi-Reforming of Biogas. Ind. Eng. Chem. Res. 2020, 59, 10542–10551. [Google Scholar] [CrossRef]
- Farooqi, A.S.; Yusuf, M.; Mohd Zabidi, N.A.; Saidur, R.; Sanaullah, K.; Farooqi, A.S.; Khan, A.; Abdullah, B. A Comprehensive Review on Improving the Production of Rich-Hydrogen via Combined Steam and CO2 Reforming of Methane over Ni-Based Catalysts. Int. J. Hydrogen Energy 2021, 46, 31024–31040. [Google Scholar] [CrossRef]
- Tahir, M.; Ali Khan, A.; Bafaqeer, A.; Kumar, N.; Siraj, M.; Fatehmulla, A. Highly Stable Photocatalytic Dry and Bi-Reforming of Methane with the Role of a Hole Scavenger for Syngas Production over a Defective Co-Doped g-C3N4 Nanotexture. Catalysts 2023, 13, 1140. [Google Scholar] [CrossRef]
- Lino, A.V.P.; Colmenares Calderon, Y.N.; Mastelaro, V.R.; Assaf, E.M.; Assaf, J.M. Syngas for Fischer-Tropsch Syn-thesis by Methane Tri-Reforming Using Nickel Supported on MgAl2O4 Promoted with Zr, Ce and Ce-Zr. Appl. Surf. Sci. 2019, 481, 747–760. [Google Scholar] [CrossRef]
- Minh, D.P.; Siang, T.J.; Vo, D.V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Hydrogen Production from Biogas Reforming: An Overview of Steam Reforming, Dry Reforming, Dual Reforming, and Tri-Reforming of Methane. Hydrog. Supply Chain Des. Deploy. Oper. 2018, 111–166. [Google Scholar] [CrossRef]
- Alhassan, M.; Jalil, A.A.; Nabgan, W.; Hamid, M.Y.S.; Bahari, M.B.; Ikram, M. Bibliometric Studies and Impediments to Valorization of Dry Reforming of Methane for Hydrogen Production. Fuel 2022, 328, 125240. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Z.; Kadirova, Z.C.; Guo, M.; Fang, R.; He, J.; Yan, Y.; Ran, J. Photothermal Functional Material and Structure for Photothermal Catalytic CO2 Reduction: Recent Advance, Application and Prospect. Coord. Chem. Rev. 2022, 473, 214794. [Google Scholar] [CrossRef]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical Transformations on Plasmonic Metal Nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Duan, X.; Sun, H.; Wang, S. Photothermal Catalysis: From Fundamentals to Practical Applications. Mater. Today 2023, 68, 234–253. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and Applications of Photo-Thermal Catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Z.; Yin, Z.; Xiao, D.; Ma, D. Principles and Applications of Photothermal Catalysis. Chem. Catal. 2022, 2, 52–83. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Fresno, F.; Coronado, J.M.; Highfield, J.; Ruppert, A.M.; Keller, N. Emerging High-Prospect Applications in Photothermal Catalysis. Curr. Opin. Green. Sustain. Chem. 2022, 37, 100652. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, L.; Guan, M.; Chen, D.; Xu, Z.; Guo, H.; Hu, S.; Zhang, S.; Liu, X.; Guo, Z.; et al. Indirect to Direct Charge Transfer Transition in Plasmon-Enabled CO2 Photoreduction. Adv. Sci. 2022, 9, 102978. [Google Scholar] [CrossRef]
- Zhu, Z.; Guo, W.; Zhang, Y.; Pan, C.; Xu, J.; Zhu, Y.; Lou, Y. Research Progress on Methane Conversion Coupling Photocatalysis and Thermocatalysis. Carbon. Energy 2021, 3, 519–540. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Sun, J.; Chen, H.; Zhu, Y.; Zhao, X.; Zhang, L.C.; Wang, S.; Zhang, H.; Duan, X.; et al. Regulation of Energetic Hot Carriers on Pt/TiO2 with Thermal Energy for Photothermal Catalysis. Appl. Catal. B 2022, 309, 121263. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning Selectivity of CO2 Hydrogenation Reactions at the Metal/Oxide Interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef]
- Wang, N.; Qian, W.; Chu, W.; Wei, F. Crystal-Plane Effect of Nanoscale CeO2 on the Catalytic Performance of Ni/CeO2 Catalysts for Methane Dry Reforming. Catal. Sci. Technol. 2016, 6, 3594–3605. [Google Scholar] [CrossRef]
- Tavasoli, A.V.; Preston, M.; Ozin, G. Photocatalytic Dry Reforming: What Is It Good For? Energy Environ. Sci. 2021, 14, 3098–3109. [Google Scholar] [CrossRef]
- Pan, F.; Xiang, X.; Du, Z.; Sarnello, E.; Li, T.; Li, Y. Integrating Photocatalysis and Thermocatalysis to Enable Efficient CO2 Reforming of Methane on Pt Supported CeO2 with Zn Doping and Atomic Layer Deposited MgO Overcoating. Appl. Catal. B 2020, 260, 118189. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Dry Reforming of Methane for Syngas Production: A Review and Assessment of Catalyst Development and Efficacy. J. Indian Chem. Soc. 2021, 98, 100002. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, L. Role and Mechanism of Calcium-Based Catalysts for Methane Dry Reforming: A Review. Fuel 2024, 355, 129329. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, T.; Yao, J.L.; Xie, T.; Xiao, Q. Mechanism and Kinetic Characteristics of Photo-Thermal Dry Reforming of Methane on Pt/Mesoporous-TiO2 Catalyst. Mol. Catal. 2023, 535, 112828. [Google Scholar] [CrossRef]
- Xu, Z.; Park, E.D. Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2024, 14, 176. [Google Scholar] [CrossRef]
- Grams, J.; Ruppert, A.M. Catalyst Stability—Bottleneck of Efficient Catalytic Pyrolysis. Catalysts 2021, 11, 265. [Google Scholar] [CrossRef]
- Study on the Critical Raw Materials for the EU 2023—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/57318397-fdd4-11ed-a05c-01aa75ed71a1/language-en (accessed on 3 June 2024).
- Anderson, C.G. The Production of Critical Materials as By Products. Asp. Min. Miner. Sci. 2018, 2, 532. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Improved Pt-Ni Nanocatalysts for Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 191–199. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G.; Sener, C.; Dogu, T. MCM-41 Supported PdNi Catalysts for Dry Reforming of Methane. Appl. Catal. B 2009, 92, 250–261. [Google Scholar] [CrossRef]
- Pinheiro, A.N.; Valentini, A.; Sasaki, J.M.; Oliveira, A.C. Highly Stable Dealuminated Zeolite Support for the Production of Hydrogen by Dry Reforming of Methane. Appl. Catal. A Gen. 2009, 355, 156–168. [Google Scholar] [CrossRef]
- Alabi, W.O. CO2 Reforming of CH4 on Ni-Al-Ox Catalyst Using Pure and Coal Gas Feeds: Synergetic Effect of CoO and MgO in Mitigating Carbon Deposition. Environ. Pollut. 2018, 242, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto-Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13, 550. [Google Scholar] [CrossRef]
- Daily Metal Price: Cobalt Price (USD/Pound) Chart for the Last Year. Available online: https://www.dailymetalprice.com/metalpricecharts.php?c=co&u=lb&d=240 (accessed on 7 January 2024).
- Hu, Q.; Li, Y.; Wu, J.; Hu, Y.; Cao, H.; Yang, Y. Extraordinary Catalytic Performance of Nickel Half-Metal Clusters for Light-Driven Dry Reforming of Methane. Adv. Energy Mater. 2023, 13, 2300071. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of Nano CaO–Based Catalysts in Biodiesel Synthesis. Renew. Sustain. Energy Rev. 2017, 72, 746–760. [Google Scholar] [CrossRef]
- Wang, L.; Pu, Z.; Shi, Y.; Wu, M.; Shi, W.; Wang, F. Photothermal Methane Dry Reforming Catalyzed by Multifunctional (Ni-Cu/CeO2)@SiO2 Catalyst. ACS Sustain. Chem. Eng. 2023, 11, 17384–17399. [Google Scholar] [CrossRef]
- Wang, E.; Zhu, Z.; Li, R.; Wu, J.; Ma, K.; Zhang, J. Ni/CaO-Based Dual-Functional Materials for Calcium-Looping CO2 Capture and Dry Reforming of Methane: Progress and Challenges. Chem. Eng. J. 2024, 482, 148476. [Google Scholar] [CrossRef]
- Han, K.; Wang, Y.; Wang, S.; Liu, Q.; Deng, Z.; Wang, F. Narrowing Band Gap Energy of CeO2 in (Ni/CeO2)@SiO2 Catalyst for Photothermal Methane Dry Reforming. Chem. Eng. J. 2021, 421, 129989. [Google Scholar] [CrossRef]
- Yao, Y.; Li, B.; Gao, X.; Yang, Y.; Yu, J.; Lei, J.; Li, Q.; Meng, X.; Chen, L.; Xu, D. Highly Efficient Solar-Driven Dry Reforming of Methane on a Rh/LaNiO3 Catalyst through a Light-Induced Metal-To-Metal Charge Transfer Process. Adv. Mater. 2023, 35, 2303654. [Google Scholar] [CrossRef] [PubMed]
- Lorber, K.; Djinović, P. Accelerating Photo-Thermal CO2 Reduction to CO, CH4 or Methanol over Metal/Oxide Semiconductor Catalysts. iScience 2022, 25, 104107. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Dao, T.D.; Liu, Y.; Zhou, W.; Liu, L.; Meng, X.; Nagao, T.; Ye, J. Design of PdAu Alloy Plasmonic Nanoparticles for Improved Catalytic Performance in CO2 Reduction with Visible Light Irradiation. Nano Energy 2016, 26, 398–404. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A Review on Dry Reforming of Methane over Perovskite Derived Catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Moogi, S.; Hyun Ko, C.; Hoon Rhee, G.; Jeon, B.H.; Ali Khan, M.; Park, Y.K. Influence of Catalyst Synthesis Methods on Anti-Coking Strength of Perovskites Derived Catalysts in Biogas Dry Reforming for Syngas Production. Chem. Eng. J. 2022, 437, 135348. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, Y.; Li, C.; Ren, K.; Zhu, Y.; Dou, W. High-Performance Photothermal Catalytic CO2 Reduction to CH4 and CO by ABO3 (A = La, Ce; B = Ni, Co, Fe) Perovskite Nanomaterials. Ceram. Int. 2023, 49, 20907–20919. [Google Scholar] [CrossRef]
- Costilla, I.O.; Sánchez, M.D.; Gigola, C.E. Palladium Nanoparticle’s Surface Structure and Morphology Effect on the Catalytic Activity for Dry Reforming of Methane. Appl. Catal. A Gen. 2014, 478, 38–44. [Google Scholar] [CrossRef]
- Taherian, Z.; Shahed Gharahshiran, V.; Khataee, A.; Meshkani, F.; Orooji, Y. Comparative Study of Modified Ni Catalysts over Mesoporous CaO-Al2O3 Support for CO2/Methane Reforming. Catal. Commun. 2020, 145, 106100. [Google Scholar] [CrossRef]
- Takami, D.; Ito, Y.; Kawaharasaki, S.; Yamamoto, A.; Yoshida, H. Low Temperature Dry Reforming of Methane over Plasmonic Ni Photocatalysts under Visible Light Irradiation. Sustain. Energy Fuels 2019, 3, 2968–2971. [Google Scholar] [CrossRef]
- Fertout, R.I.; Ghelamallah, M.; Helamallah, M.; Kacimi, S.; López, P.N.; Corberán, V.C. Nickel Supported on Alkaline Earth Metal–Doped γ-Al2O3–La2O3 as Catalysts for Dry Reforming of Methane. Russ. J. Appl. Chem. 2020, 93, 289–298. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Well-Designed 2D/2D Ti3C2TA/R MXene Coupled g-C3N4 Heterojunction with in-Situ Growth of Anatase/Rutile TiO2 Nucleates to Boost Photocatalytic Dry-Reforming of Methane (DRM) for Syngas Production under Visible Light. Appl. Catal. B 2021, 285, 119777. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Photo-Induced CO2 Reduction by CH4/H2O to Fuels over Cu-Modified g-C3N4 Nanorods under Simulated Solar Energy. Appl. Surf. Sci. 2017, 419, 875–885. [Google Scholar] [CrossRef]
- Taherian, Z.; Gharahshiran, V.S.; Fazlikhani, F.; Yousefpour, M. Catalytic Performance of Samarium-Modified Ni Catalysts over Al2O3–CaO Support for Dry Reforming of Methane. Int. J. Hydrogen Energy 2021, 46, 7254–7262. [Google Scholar] [CrossRef]
- Ranjbar, A.; Rezaei, M. Preparation of Nickel Catalysts Supported on CaO.2Al2O3 for Methane Reforming with Carbon Dioxide. Int. J. Hydrogen Energy 2012, 37, 6356–6362. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Zhao, N.; Wei, W.; Sun, Y.; Sun, C.; Liu, H.; Snape, C.E. Coking and Deactivation of a Mesoporous Ni-CaO-ZrO2 Catalyst in Dry Reforming of Methane: A Study under Different Feeding Compositions. Fuel 2015, 143, 527–535. [Google Scholar] [CrossRef]
- Dama, S.; Ghodke, S.R.; Bobade, R.; Gurav, H.R.; Chilukuri, S. Active and Durable Alkaline Earth Metal Substituted Perovskite Catalysts for Dry Reforming of Methane. Appl. Catal. B 2018, 224, 146–158. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, X.; Shi, R.; Waterhouse, G.I.N.; Zhang, X.; Dai, Q.; Zhang, T. NiFe Nanoalloys Derived from Layered Double Hydroxides for Photothermal Synergistic Reforming of CH4 with CO2. Adv. Funct. Mater. 2022, 32, 2204056. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Tailoring Performance of La-Modified TiO2 Nanocatalyst for Continuous Photocatalytic CO2 Reforming of CH4 to Fuels in the Presence of H2O. Energy Convers. Manag. 2018, 159, 284–298. [Google Scholar] [CrossRef]
- Rao, Z.; Cao, Y.; Huang, Z.; Yin, Z.; Wan, W.; Ma, M.; Wu, Y.; Wang, J.; Yang, G.; Cui, Y.; et al. Insights into the Nonthermal Effects of Light in Dry Reforming of Methane to Enhance the H2/CO Ratio near Unity over Ni/Ga2O3. ACS Catal. 2021, 11, 4730–4738. [Google Scholar] [CrossRef]
- Boudart, M. Turnover Rates in Heterogeneous Catalysis. Chem. Rev. 1995, 95, 661–666. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. “Turning over” Definitions in Catalytic Cycles. ACS Catal. 2012, 2, 2787–2794. [Google Scholar] [CrossRef]
- Crampton, A.S.; Rötzer, M.D.; Ridge, C.J.; Yoon, B.; Schweinberger, F.F.; Landman, U.; Heiz, U. Assessing the Concept of Structure Sensitivity or Insensitivity for Sub-Nanometer Catalyst Materials. Surf. Sci. 2016, 652, 7–19. [Google Scholar] [CrossRef]
- Vogt, C.; Kranenborg, J.; Monai, M.; Weckhuysen, B.M. Structure Sensitivity in Steam and Dry Methane Reforming over Nickel: Activity and Carbon Formation. ACS Catal. 2020, 10, 1428–1438. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the Size and Support Effects of Ni Catalysts for Dry Reforming of Methane. Appl. Catal. B 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.; Zhao, Q.; Mao, D.; Hu, C. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Si/ZrO2 Catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry Reforming of Methane: Influence of Process Parameters—A Review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Z.; Qin, X.; Zhang, Z.; Muhetaer, A.; Wang, C.; Huang, H.; Yang, C.; Ma, D.; Li, Q.; et al. Light-Induced Redox Looping of a Rhodium/Cex WO3 Photocatalyst for Highly Active and Robust Dry Reforming of Methane. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200567. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Shen, T.; Li, J.; Ning, C.; Xue, Y.; Chen, G.; Xu, M.; Wang, F.; Song, Y.F.; Zhao, Y.; et al. Solar-Driven Dry Reforming of Methane Using RuNi Single-Atom Alloy Catalyst Coupled with Thermal Decomposition of Carbonates. Chem. Eng. J. 2023, 470, 144416. [Google Scholar] [CrossRef]
- Song, H.; Meng, X.; Dao, T.D.; Zhou, W.; Liu, H.; Shi, L.; Zhang, H.; Nagao, T.; Kako, T.; Ye, J. Light-Enhanced Carbon Dioxide Activation and Conversion by Effective Plasmonic Coupling Effect of Pt and Au Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, Y.; Hu, Q.; Wu, J.; Zhang, Q. Photothermocatalytic Dry Reforming of Methane for Efficient CO2 Reduction and Solar Energy Storage. ACS Sustain. Chem. Eng. 2021, 9, 11635–11651. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, T.; Liang, W.P.; Bai, P.W.; Zheng, H.Y.; Lei, Y.; Hu, Z.; Xie, T. Promoted Solar-Driven Dry Reforming of Methane with Pt/Mesoporous-TiO2 Photo-Thermal Synergistic Catalyst: Performance and Mechanism Study. Energy Convers. Manag. 2022, 258, 115496. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Zhang, T.; Wang, R.-K.; Yu, B.; Tang, Z.-Y.; Zheng, H.-Y.; He, D.; Xie, T.; Hu, Z. Photo-Enhanced Dry Reforming of Methane over Pt-Au/P25 Composite Catalyst by Coupling Plasmonic Effect. J. Catal. 2022, 413, 829–842. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhao, X.; Shi, L.; Chen, H.; Zhang, S.; Zhang, P.; Wang, S.; Zhang, L.; Wang, Y.; et al. The Nature of Active Sites for Plasmon-Mediated Photothermal Catalysis and Heat-Coupled Photocatalysis in Dry Reforming of Methane. Energy Environ. Mater. 2023, 6, e12416. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Yang, B.; Li, H.F.; Zhang, Y.; Yang, Y.; Liu, Q.Y.; Xu, H.G.; Zheng, W.J.; He, S.G. Photoassisted Selective Steam and Dry Reforming of Methane to Syngas Catalyzed by Rhodium–Vanadium Bimetallic Oxide Cluster Anions at Room Temperature. Angew. Chem. Int. Ed. 2020, 59, 21216–21223. [Google Scholar] [CrossRef] [PubMed]


| Catalysts | GHSV h−1 | τ s | Temperature °C | Catalytic Performances | References |
|---|---|---|---|---|---|
| CeO2-Ni/CaO-Al2O3 | 9000 | 0.4 | 700 | CH4 conv. = 81% CO2 conv. = 84% | [81] |
| Sm/Ni/Al2O3-CaO | 12,000 | 0.3 | 700 | CH4 conv. = 68% CO2 conv. = 43% H2/CO = 0.64 | [86] |
| 7%Ni/CaO-Al2O3 | 1800 | 0.5 | 700 | CH4 conv. = 66% CO2 conv. =62% H2/CO = 0.85 | [87] |
| Ni-CaO-ZrO2 | 48,000 | 0.075 | 750 | CH4 conv. = 82% H2/CO = 0.94 | [88] |
| CaZr0.8Ni0.2O3-δ | 28,800 | 0.125 | 800 | CH4 conv. = 95% CO2 conv. = 96% H2/CO = 0.98 | [89] |
| Catalyst | Surface Temperature °C | Light Intensity mW·cm−2 | Catalytic Performances | Reference |
|---|---|---|---|---|
| CN/TNT | 25 | 20 | r (H2) = 49 μmol·g−1·h−1 r (CO) = 75 μmol·g−1·h−1 H2/CO = 0.64 | [84] |
| (Ni/CeO2)-SiO2 | 750 | - | CH4 conv. = 66% CO2 conv. = 80% H2/CO = 0.90 | [73] |
| Ni3Fe1 nanoalloy | 350 | 3.62 | r (H2) = 326 × 103 μmol·g−1·h−1 r (CO) = 632 × 103 μmol·g−1·h−1 H2/CO = 0.52 | [90] |
| La/TiO2 | 100 | 150 | r(H2) = 74 μmol·h−1·gcat−1 r (CO) = 183 μmol·h−1·gcat−1 H2/CO = 0.40 | [91] |
| Cu-g-C3N4 | 100 | 100 | r(H2) = 76 μmol·h−1·gcat−1 r(CO) = 142 μmol·h−1·gcat−1 H2/CO = 0.54 | [85] |
| Ni/Ga2O3 | 391 | 1.9–3 | r (H2) = 194 μmol·g−1·h−1 r (CO) = 206 μmol·g−1·h−1 H2/CO = 0.94 | [92] |
| Samples | Temperature °C | T.O.F. (CH4) s−1 | T.O.F. (CO2) s−1 | CH4conv % | CO2conv % | References |
|---|---|---|---|---|---|---|
| Ni/SiO2@Al2O3 | 800 | 135.2 | 177.2 | 62.8 | 82.3 | [97] |
| Ni/SiO2@MgO | 800 | 120.0 | 171.1 | 50.0 | 71.3 | [97] |
| Ni/SiO2@ZrO2 | 800 | 29.5 | 37.8 | 29.1 | 37.3 | [97] |
| Ni/SiO2@TiO2 | 800 | 12.9 | 18.1 | 17.0 | 23.8 | [97] |
| Ni-Zr/SiO2 | 400 | 0.32 | 0.32 | 2 | 2 | [98] |
| Ni-Zr/SiO2 | 450 | 1.06 | 1.48 | 0.8 | 1.2 | [98] |
| Ni-Si/ZrO2 | 400 | 0.50 | 0.44 | 4.3 | 3.8 | [98] |
| Ni-Si/ZrO2 | 450 | 1.38 | 1.30 | 1.6 | 2.4 | [98] |
| Catalyst | Surface Temperature °C | Light Intensity W·cm−2 | Catalytic Performances | Reference |
|---|---|---|---|---|
| Rh/La2O3 | 340 | 1.5 | r (H2) = 452 mmol·g−1·h−1 r (CO) = 527 mmol·g−1·h−1 H2/CO = 0.86 | [74] |
| Pt/TiO2 | 500 | 4.67 | r (H2) = 134 mmol·g−1·h−1 r (CO) = 221 mmol·g−1·h−1 H2/CO = 0.61 | [58] |
| Pt/TiO2 | 350 | 4.67 | r (H2) = 7 mmol·g−1·h−1 r (CO) = 22 mmol·g−1·h−1 H2/CO = 0.32 | [58] |
| Pt/TiO2 | 500 | 3.72 | r (H2) = 121 mmol·g−1·h−1 r (CO) = 200 mmol·g−1·h−1 H2/CO = 0.61 | [58] |
| MgO/Pt/Zn-CeO2 | 600 | 3 | r (H2) = 356 mmol·g−1·h−1 r (CO) = 516 mmol·g−1·h−1 H2/CO = 0.69 | [55] |
| Ru-Al/LDH | 350 | 13.5 | r (H2) = 9 mmol·g−1·h−1 r (CO) = 11 mmol·g−1·h−1 H2/CO = 0.76 | [101] |
| Pt/TiO2 | 500 | 0.4 | r (H2) = 598 mmol·g−1·h−1 r (CO) = 902 mmol·g−1·h−1 H2/CO = 0.66 | [51] |
| Pt/TiO2 | 700 | 0.4 | r (H2) = 1103 mmol·g−1·h−1 r (CO) = 1495 mmol·g−1·h−1 H2/CO = 0.74 | [51] |
| Pt/TiO2 | 500 | 3.76 | r(H2) = 211 mmol·g−1·h−1 r(CO) = 309 mmol·g−1·h−1 H2/CO = 0.68 | [104] |
| Pt/TiO2 | 500 | 2.2 | r (H2) = 103 mmol·g−1·h−1 r (CO) = 179 mmol·g−1·h−1 H2/CO = 0.58 | [104] |
| Catalyst | Temperature °C | Light Intensity mW·cm−2 | GHSV h−1 | Catalytic Performances | References |
|---|---|---|---|---|---|
| Ru/SrTiO3 | 600 | – | 4592 | r(H2) = 320 mmol·g−1·h−1 r(CO) = 387 mmol·g−1·h−1 H2/CO = 0.83 | [32] |
| Pt/TiO2 | 500 | 3.22 | 40,000 | r(H2) = 211 mmol·g−1·h−1 r(CO) = 309 mmol·g−1·h−1 H2/CO = 0.68 | [104] |
| Pt/P25 | 500 | 3.22 | 40,000 | r(H2) = 40 mmol·g−1·h−1 r(CO) = 106 mmol·g−1·h−1 H2/CO = 0.38 | [104] |
| Pt/TiO2 | 500 | 4.67 | 320,000 | r (H2) = 55 mmol·g−1·h−1 r (CO) = 73 mmol·g−1·h−1 H2/CO = 0.75 | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varotto, A.; Pasqual Laverdura, U.; Feroci, M.; Grilli, M.L. Photo-Thermal Dry Reforming of Methane with PGM-Free and PGM-Based Catalysts: A Review. Materials 2024, 17, 3809. https://doi.org/10.3390/ma17153809
Varotto A, Pasqual Laverdura U, Feroci M, Grilli ML. Photo-Thermal Dry Reforming of Methane with PGM-Free and PGM-Based Catalysts: A Review. Materials. 2024; 17(15):3809. https://doi.org/10.3390/ma17153809
Chicago/Turabian StyleVarotto, Alessio, Umberto Pasqual Laverdura, Marta Feroci, and Maria Luisa Grilli. 2024. "Photo-Thermal Dry Reforming of Methane with PGM-Free and PGM-Based Catalysts: A Review" Materials 17, no. 15: 3809. https://doi.org/10.3390/ma17153809
APA StyleVarotto, A., Pasqual Laverdura, U., Feroci, M., & Grilli, M. L. (2024). Photo-Thermal Dry Reforming of Methane with PGM-Free and PGM-Based Catalysts: A Review. Materials, 17(15), 3809. https://doi.org/10.3390/ma17153809








