Effect of Nitrogen on Precipitate Characteristics and Pitting Resistance of Martensitic Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
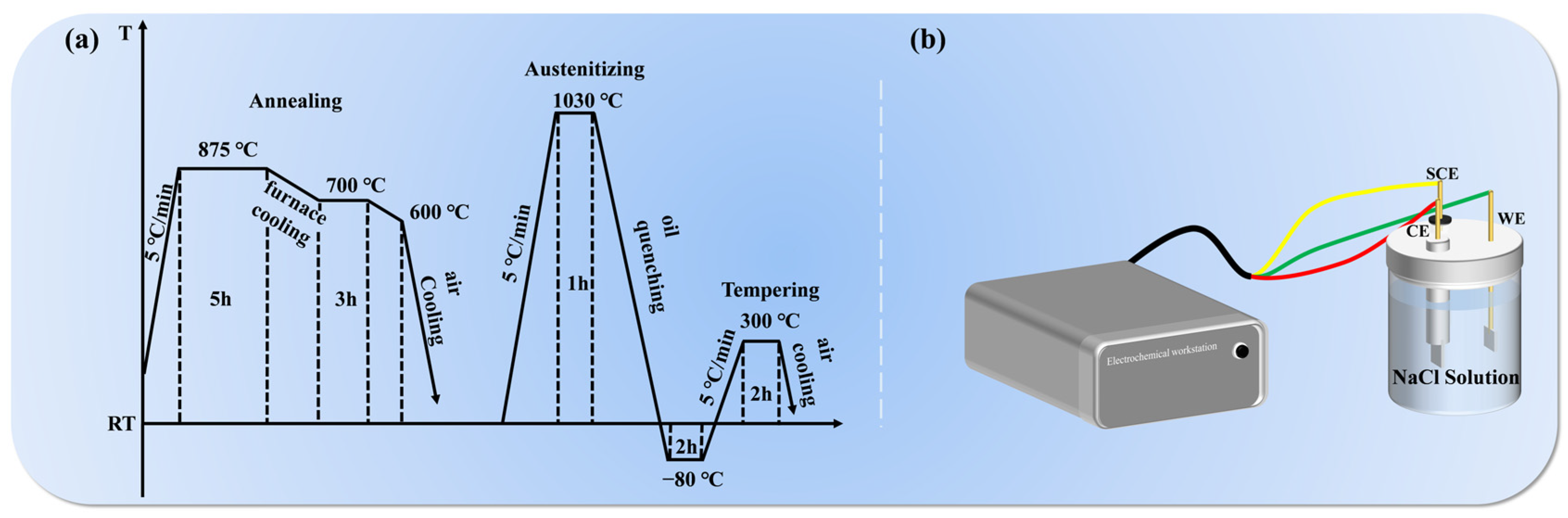
2.1. Materials Preparation
2.2. Microstructure Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
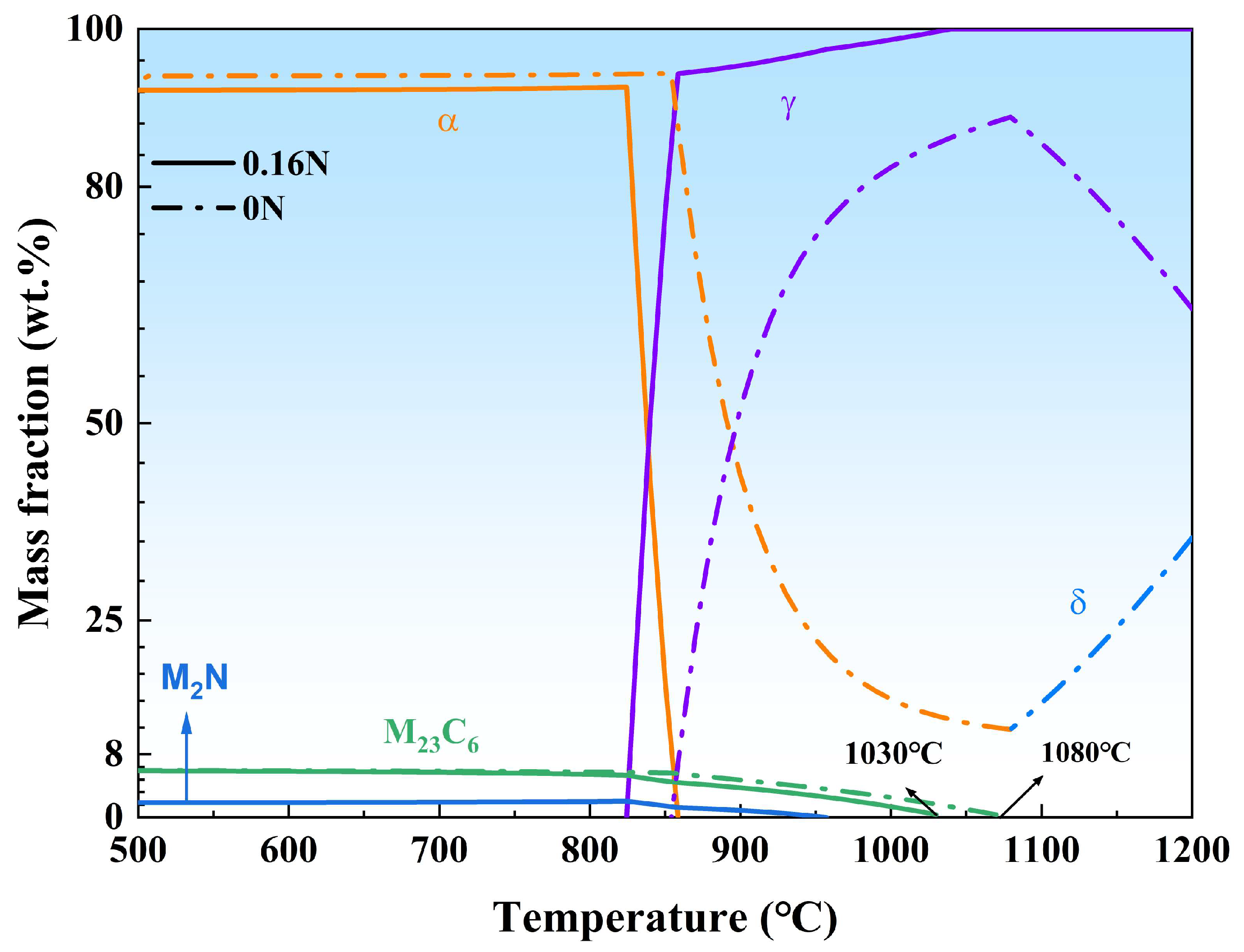
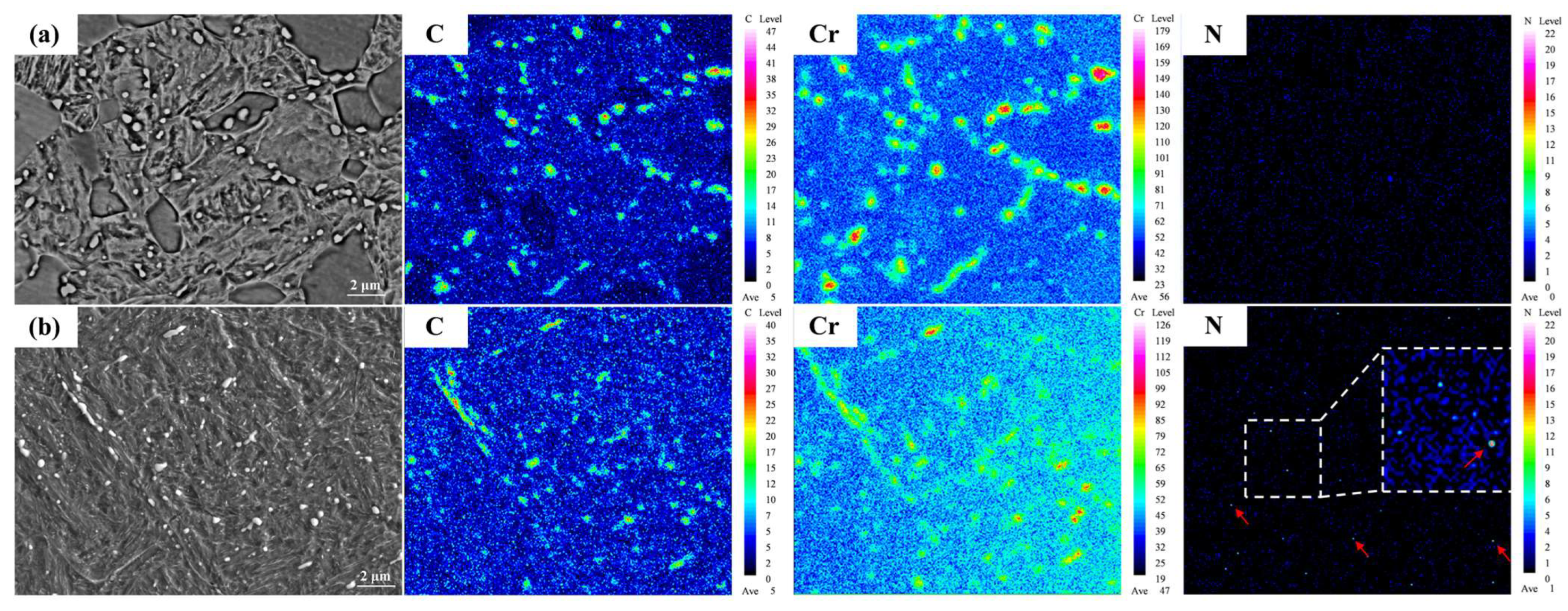
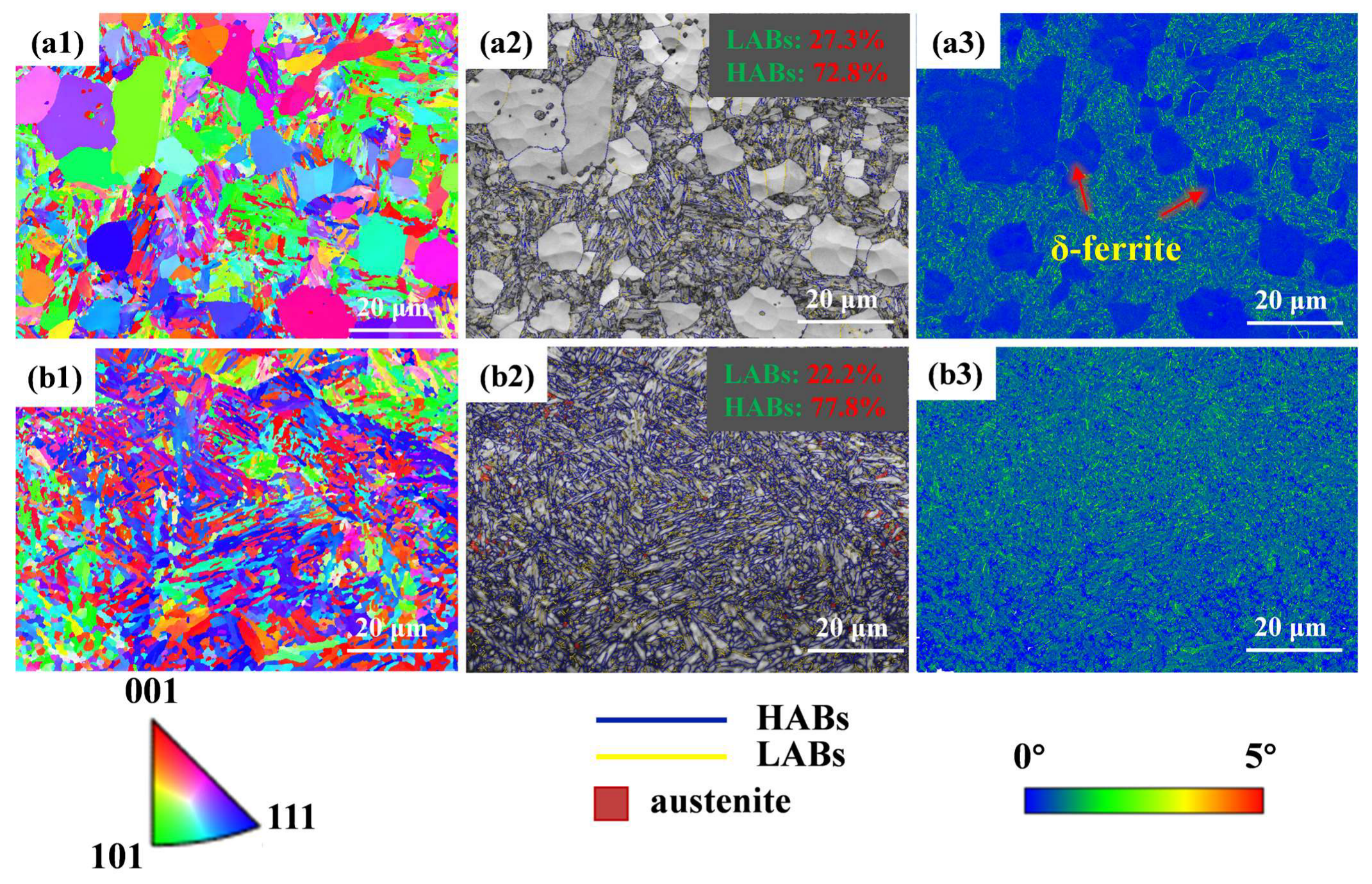
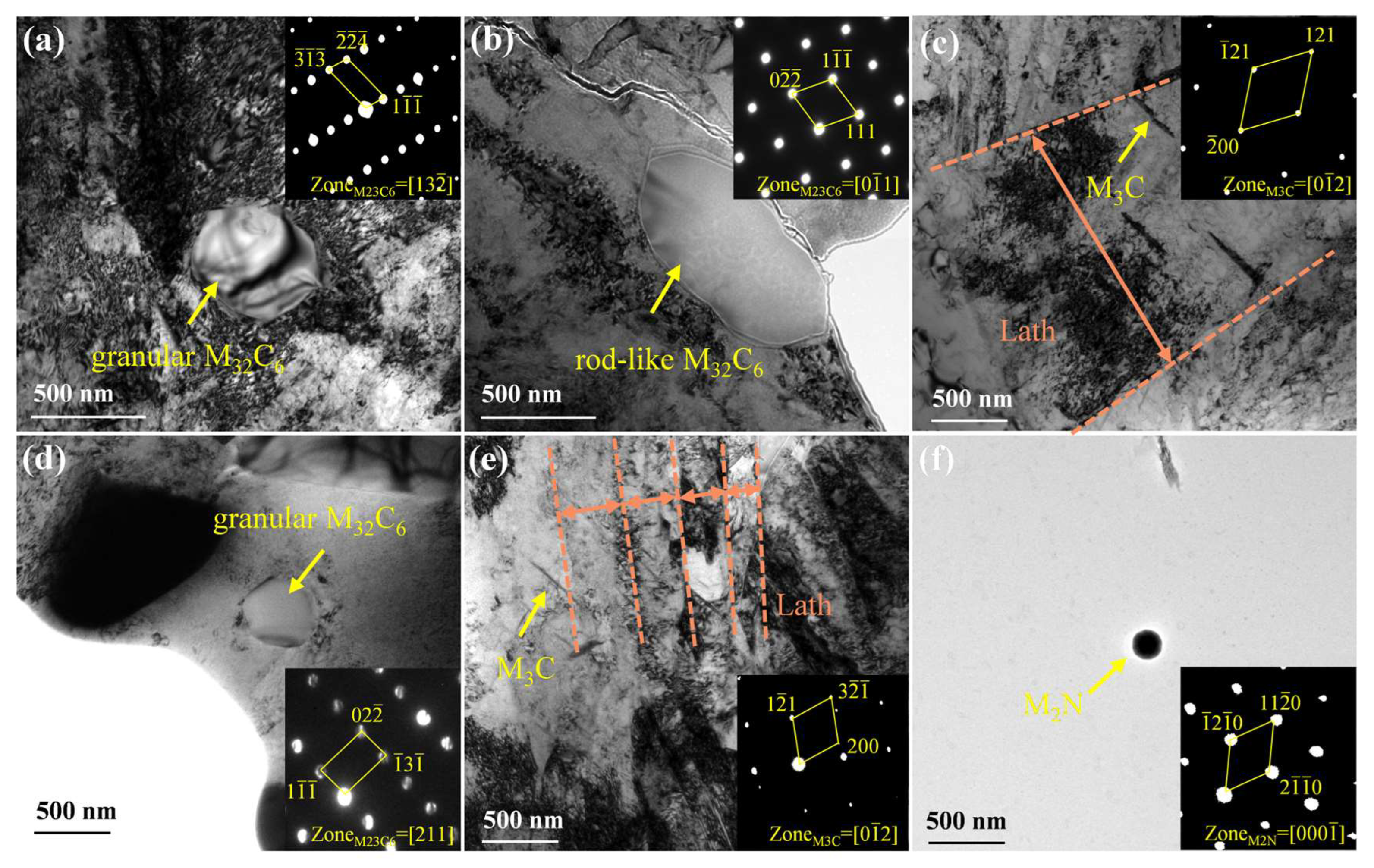
3.1. Microstructural Characteristics
3.2. Effect of Nitrogen on Electrochemical Behavior
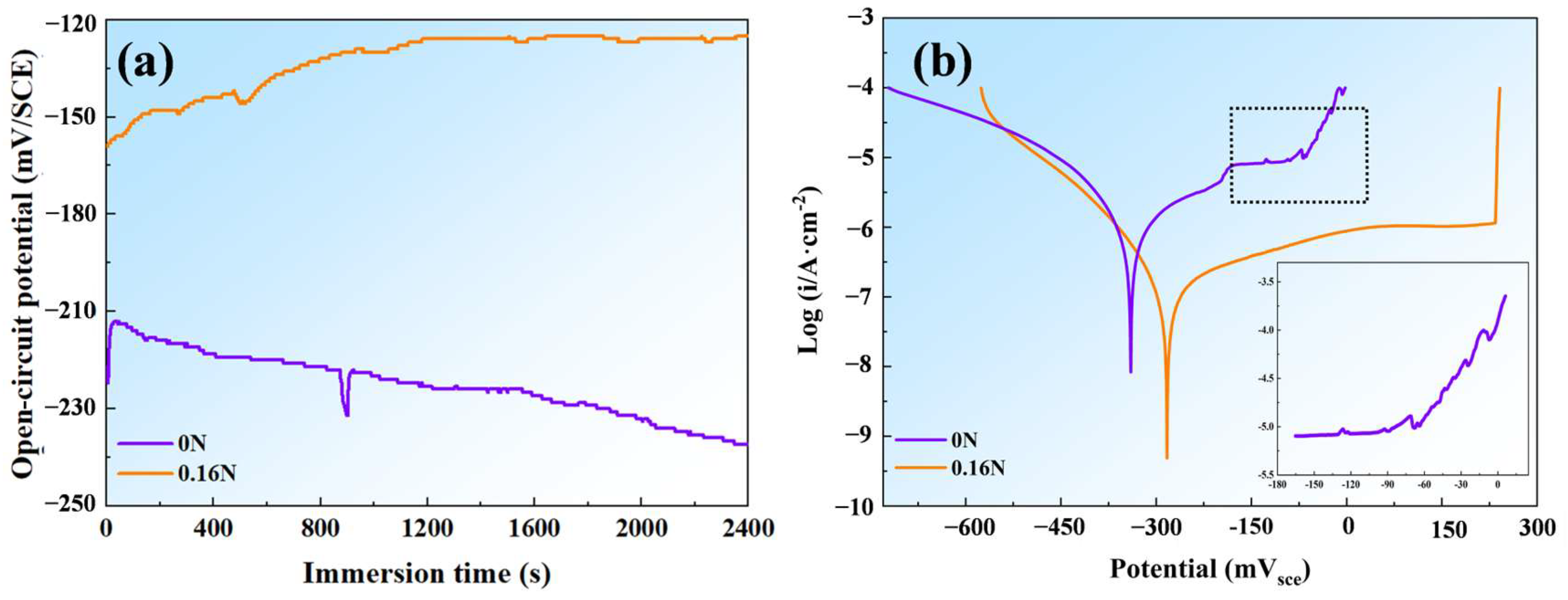
3.2.1. OCP and Potentiodynamic Polarization Measurement
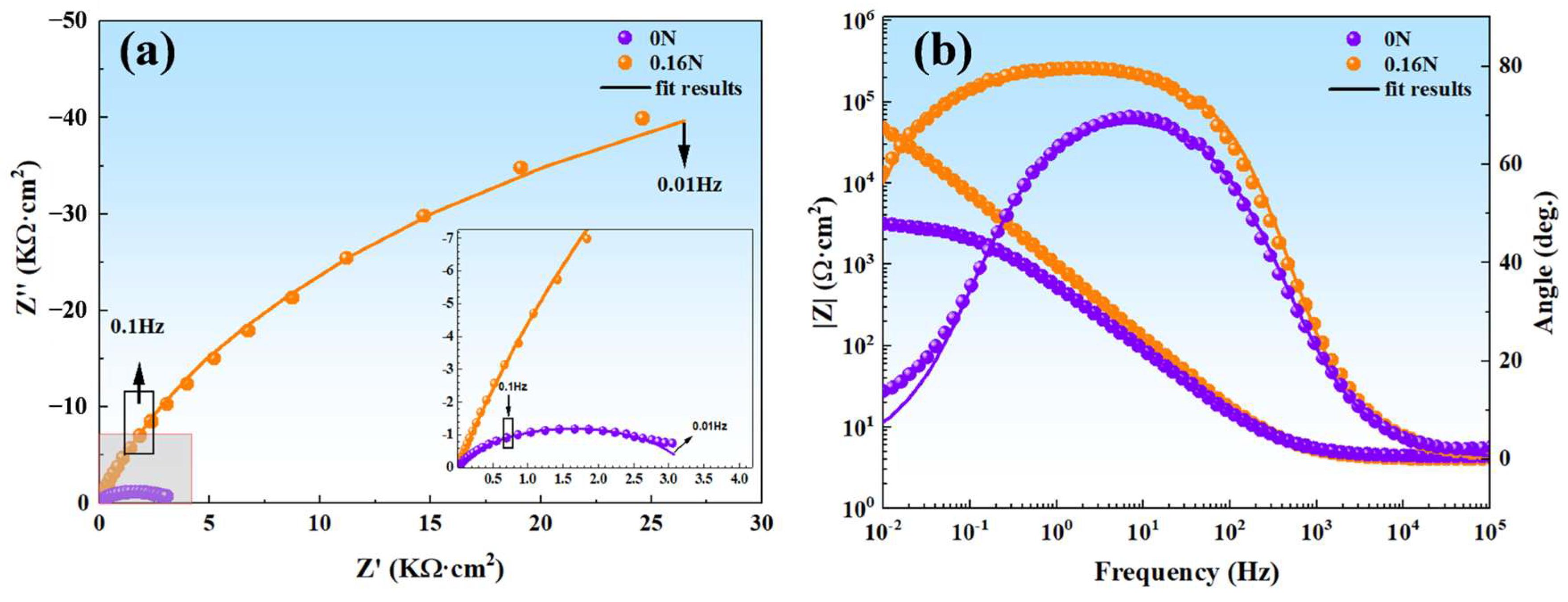
3.2.2. Electrochemical Impedance Spectroscopy Measurements
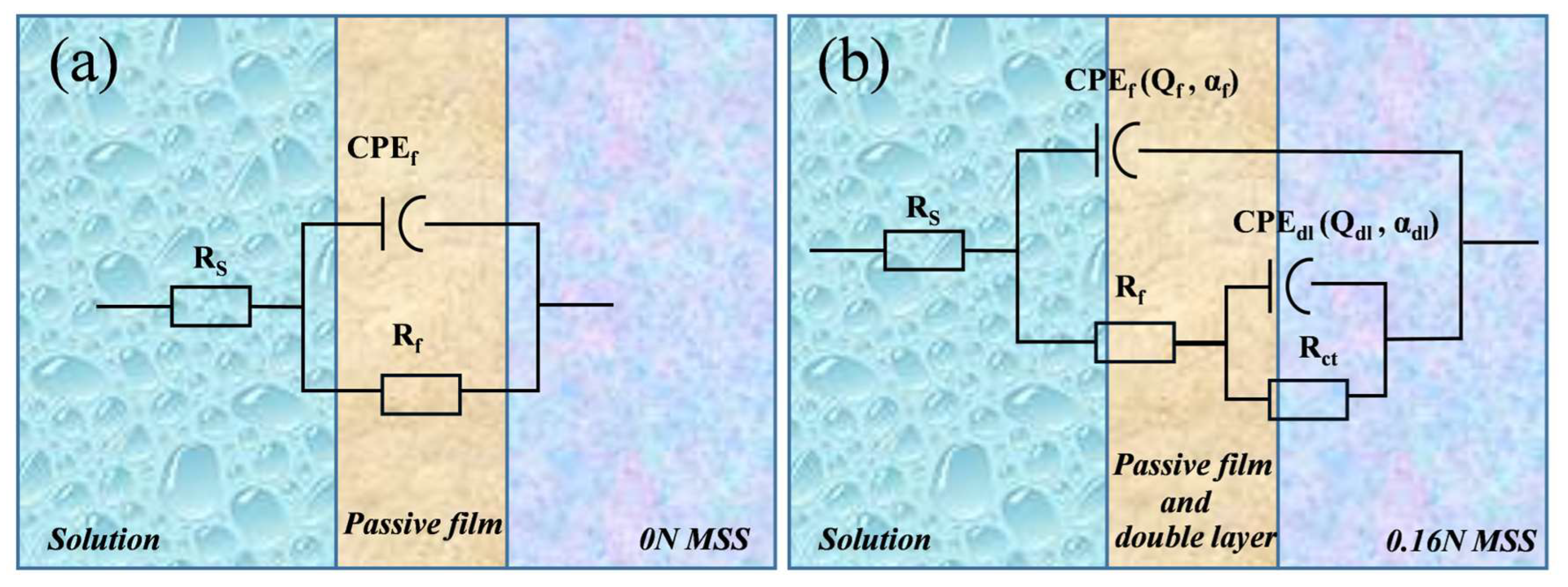
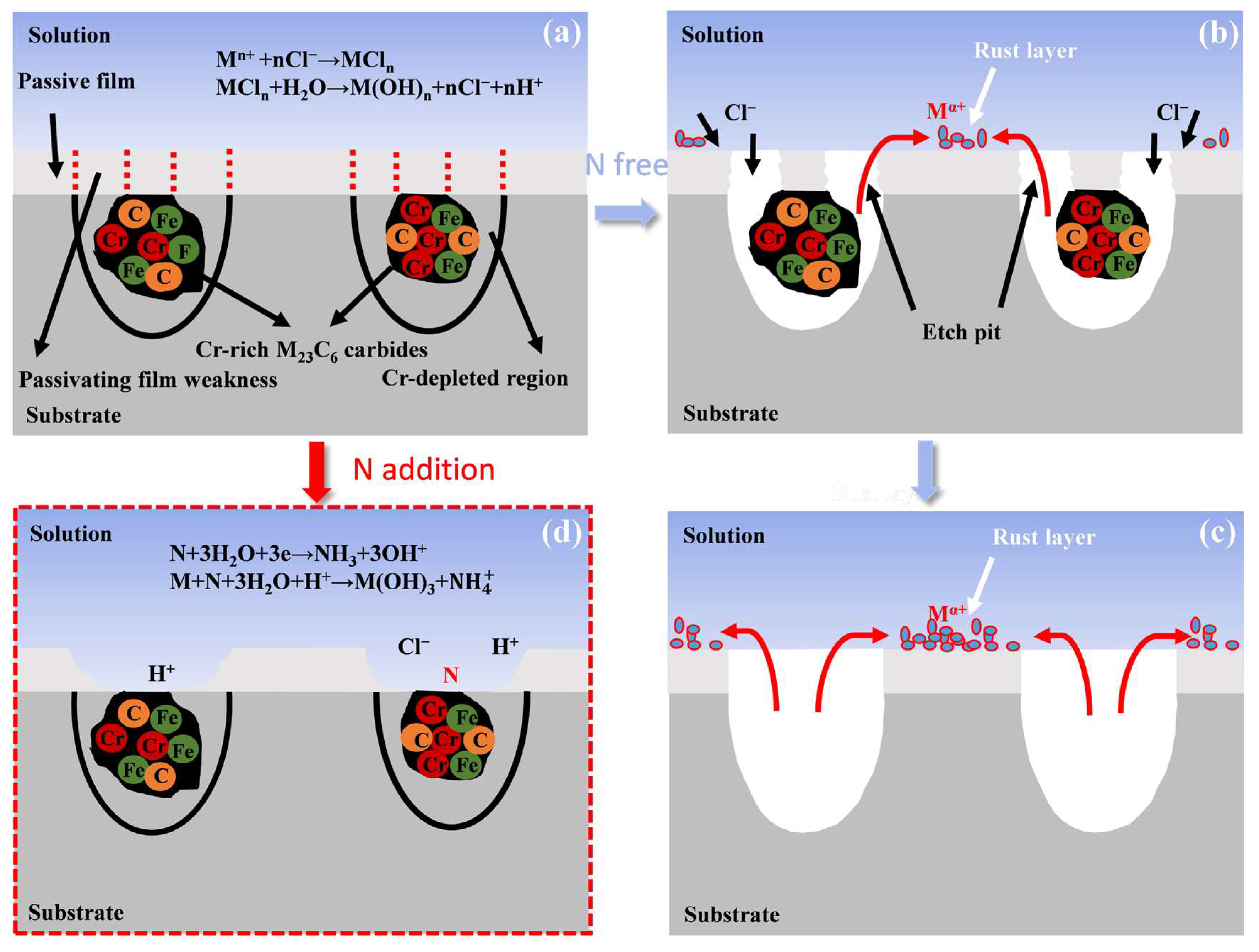
3.2.3. Corrosion Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishna, S.C.; Karthick, N.K.; Jha, A.K.; Pant, B.; Venkitakrishnan, P.V. Microstructure and Properties of Nitrogen-Alloyed Martensitic Stainless Steel. Metall. Microstruct. Anal. 2017, 6, 425–432. [Google Scholar] [CrossRef]
- Ebert, F.-J. An Overview of Performance Characteristics, Experiences and Trends of Aerospace Engine Bearings Technologies. Chin. J. Aeronaut. 2007, 20, 378–384. [Google Scholar] [CrossRef]
- Xu, H.-F.; Wu, G.-L.; Wang, C.; Li, J.; Cao, W.-Q. Microstructure, hardness and contact fatigue properties of X30N high nitrogen stainless bearing steel. J. Iron Steel Res. Int. 2018, 25, 954–967. [Google Scholar] [CrossRef]
- Xu, H.-F.; Yu, F.; Wang, C.; Zhang, W.-L.; Li, J.; Cao, W.-Q. Comparison of microstructure and property of high chromium bearing steel with and without nitrogen addition. J. Iron Steel Res. Int. 2017, 24, 206–213. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R.; Chen, M.; Yang, K.; Sun, Z.; Zhang, X.; Tang, S.; Sun, G. Enhanced wear and corrosion resistance of the laser direct metal deposited AISI 316L stainless steel by in-situ interstitial N alloying. Opt. Laser Technol. 2024, 171, 110381. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Wang, W.; Ma, B. Effect of nitrogen content on mechanical properties of 316L (N) austenitic stainless steel. Mater. Sci. Eng. A 2023, 884, 145549. [Google Scholar] [CrossRef]
- Hao, X.; Sun, X.; Zhao, T.; Zhang, M.; Wang, Y.; Zhang, F.; Zhao, J.; Wang, T. Effect of N on microstructure evolution and strain hardening of high-Mn austenitic steel during tensile deformation. Mater. Sci. Eng. A 2024, 896, 146291. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.-B.; Jiang, Z.-H.; Zhang, T.; Dong, N.; Zhang, S.-C.; Han, P.-D.; Zhao, S.; Chen, Z.-G. Designing for high corrosion-resistant high nitrogen martensitic stainless steel based on DFT calculation and pressurized metallurgy method. Corros. Sci. 2019, 158, 108081. [Google Scholar] [CrossRef]
- Chang, R.; Li, J.; Gu, J. Effect of nitrogen on microstructure and corrosion resistance of Cr15 super martensitic stainless steel. Corros. Eng. Sci. Technol. 2019, 54, 225–232. [Google Scholar] [CrossRef]
- Jiao, W.-C.; Li, H.-B.; Dai, J.; Feng, H.; Jiang, Z.-H.; Zhang, T.; Xu, D.-K.; Zhu, H.-C.; Zhang, S.-C. Effect of partial replacement of carbon by nitrogen on intergranular corrosion behavior of high nitrogen martensitic stainless steels. J. Mater. Sci. Technol. 2019, 35, 2357–2364. [Google Scholar] [CrossRef]
- Wang, R.; Li, F.; Yu, Z.; Kang, Y.; Li, M.; Hu, Y.; An, H.; Fan, J.; Miao, F.; Zhao, Y.; et al. Influences of partial substitution of C by N on the microstructure and mechanical properties of 9Cr18Mo martensitic stainless steel. Mater. Des. 2023, 236, 112497. [Google Scholar] [CrossRef]
- Li, S.-h.; Li, J.; Zhang, J.; Shi, C. Effect of nitrogen on microstructure and microsegregation of martensitic stainless steel 4Cr13 produced by electroslag remelting. J. Iron Steel Res. Int. 2022, 30, 1854–1861. [Google Scholar] [CrossRef]
- Bénéteau, A.; Weisbecker, P.; Geandier, G.; Aeby-Gautier, E.; Appolaire, B. Austenitization and precipitate dissolution in high nitrogen steels: An in situ high temperature X-ray synchrotron diffraction analysis using the Rietveld method. Mater. Sci. Eng. A 2005, 393, 63–70. [Google Scholar] [CrossRef]
- Lee, J.-B.; Yoon, S.-I. Effect of nitrogen alloying on the semiconducting properties of passive films and metastable pitting susceptibility of 316L and 316LN stainless steels. Mater. Chem. Phys. 2010, 122, 194–199. [Google Scholar] [CrossRef]
- Ha, H.; Jang, H.; Kwon, H.; Kim, S. Effects of nitrogen on the passivity of Fe–20Cr alloy. Corros. Sci. 2009, 51, 48–53. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Aspects of the effects of electrolyte composition on the occurrence of metastable pitting on stainless steel. Corros. Sci. 1994, 36, 525–538. [Google Scholar] [CrossRef]
- Olefjord, I.; Wegrelius, L. The influence of nitrogen on the passivation of stainless steels. Corros. Sci. 1996, 38, 1203–1220. [Google Scholar] [CrossRef]
- Saadi, S.A.; Yi, Y.; Cho, P.; Jang, C.; Beeley, P. Passivity breakdown of 316L stainless steel during potentiodynamic polarization in NaCl solution. Corros. Sci. 2016, 111, 720–727. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Zhang, H.X.; Li, H.; Shen, H.P. Effect of tempering temperature on the microstructure and mechanical properties in mooring chain steel. Mater. Sci. Eng. A 2015, 636, 164–171. [Google Scholar] [CrossRef]
- Leda, H. Nitrogen in martensitic stainless steels. J. Mater. Process. Technol. 1995, 53, 263–272. [Google Scholar] [CrossRef]
- Shimada, T.; Yamamoto, A.; Abe, S. The effect of nitrogen content on material properties of high carbon stainless steel SUS440A. Tetsu Hagane 1996, 82, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Mao, H.; Yang, Y. Corrosion behavior of nitrogen alloyed martensitic stainless steel in chloride containing solutions. Corros. Sci. 2017, 120, 90–98. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Feng, H.; Li, H.B.; Zhu, H.C.; Zhang, S.C.; Zhang, B.B.; Han, Y.; Zhang, T.; Xu, D.K. Relationship between Microstructure and Corrosion Behavior of Martensitic High Nitrogen Stainless Steel 30Cr15Mo1N at Different Austenitizing Temperatures. Materials 2017, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.P.; Wang, L.J.; Qin, B.; Liu, C.M.; Subramanian, S.V. Effect of N on microstructure and mechanical properties of 16Cr5Ni1Mo martensitic stainless steel. Mater. Des. 2012, 34, 74–81. [Google Scholar] [CrossRef]
- Gavriljuk VG, B.H. Precipitates in Tempered Stainless Martensitic Steels Alloyed with Nitrogen, Carbon or Both. Mater. Sci. Forum 1999, 318–320, 71–80. [Google Scholar] [CrossRef]
- Danielsen, H.K.; Villa, M.; Gutiérrez Guzmán, F.; Fæster, S.; Dahl, K.V.; Vegter, R.H.; Jensen, O.L.; Hummelshøj, T.S.; Lehmann, B.; Jacobs, G.; et al. New White Etch Cracking resistant martensitic stainless steel for bearing applications by high temperature solution nitriding. Wear 2023, 534–535, 205134. [Google Scholar] [CrossRef]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.-Y. Ni/Ni3Al interface: A density functional theory study. Appl. Surf. Sci. 2009, 255, 3669–3675. [Google Scholar] [CrossRef]
- Feng, H.; Jiang, Z.; Li, H.; Lu, P.; Zhang, S.; Zhu, H.; Zhang, B.; Zhang, T.; Xu, D.; Chen, Z. Influence of nitrogen on corrosion behaviour of high nitrogen martensitic stainless steels manufactured by pressurized metallurgy. Corros. Sci. 2018, 144, 288–300. [Google Scholar] [CrossRef]
- Reed, R.P. Nitrogen in austenitic stainless steels. JOM 1989, 41, 16–21. [Google Scholar] [CrossRef]
- Lewis, M.H.; Hattersley, B. Precipitation of M23C6 in austenitic steels. Acta Metall. 1965, 13, 1159–1168. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhou, Y.F.; Xing, X.L.; Liu, S.; Zhao, C.C.; Yang, Y.L.; Yang, Q.X. The effect of nitrogen alloying to the microstructure and mechanical properties of martensitic stainless steel hardfacing. Surf. Coat. Technol. 2016, 294, 115–121. [Google Scholar] [CrossRef]
- Li, K.; Zhan, J.; Yang, T.; To, A.C.; Tan, S.; Tang, Q.; Cao, H.; Murr, L.E. Homogenization timing effect on microstructure and precipitation strengthening of 17–4PH stainless steel fabricated by laser powder bed fusion. Addit. Manuf. 2022, 52, 102672. [Google Scholar] [CrossRef]
- Caballero, A.; Ding, J.; Ganguly, S.; Williams, S. Wire+ Arc Additive Manufacture of 17-4 PH stainless steel: Effect of different processing conditions on microstructure, hardness, and tensile strength. J. Mater. Process. Technol. 2019, 268, 54–62. [Google Scholar] [CrossRef]
- Kuzmina, M.; Ponge, D.; Raabe, D. Grain boundary segregation engineering and austenite reversion turn embrittlement into toughness: Example of a 9wt.% medium Mn steel. Acta Mater. 2015, 86, 182–192. [Google Scholar] [CrossRef]
- Raabe, D.; Sandlöbes, S.; Millán, J.; Ponge, D.; Assadi, H.; Herbig, M.; Choi, P.P. Segregation engineering enables nanoscale martensite to austenite phase transformation at grain boundaries: A pathway to ductile martensite. Acta Mater. 2013, 61, 6132–6152. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Jiang, L.; Yuan, C.; Zhang, J.; Yan, Q. Advancement of strength and toughness in ultra-low carbon martensitic stainless steel by reversed austenite. Nucl. Mater. Energy 2024, 38, 101601. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, W.; Dai, X.; Zhang, P.; Wang, Y. Prominent role of reversed austenite on corrosion property of super 13Cr martensitic stainless steel. J. Mater. Res. Technol. 2023, 22, 1753–1767. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Rong, L.; Li, Y. The influence of tempering temperature on the reversed austenite formation and tensile properties in Fe–13%Cr–4%Ni–Mo low carbon martensite stainless steels. Mater. Sci. Eng. A 2011, 528, 4075–4079. [Google Scholar] [CrossRef]
- Morito, S.; Tanaka, H.; Konishi, R.; Furuhara, T.; Maki, T. The morphology and crystallography of lath martensite in Fe-C alloys. Acta Mater. 2003, 51, 1789–1799. [Google Scholar] [CrossRef]
- Morito, S.; Huang, X.; Furuhara, T.; Maki, T.; Hansen, N. The morphology and crystallography of lath martensite in alloy steels. Acta Mater. 2006, 54, 5323–5331. [Google Scholar] [CrossRef]
- Fan, R.; Gao, M.; Ma, Y.; Zha, X.; Hao, X.; Liu, K. Effects of Heat Treatment and Nitrogen on Microstructure and Mechanical Properties of 1Cr12NiMo Martensitic Stainless Steel. J. Mater. Sci. Technol. 2012, 28, 1059–1066. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Yao, K.-F.; Chen, Y.-B.; Wang, M.-H.; Ge, X.-Y. Influence of Heat Treatment on the Microstructure and Corrosion Resistance of 13 Wt Pct Cr-Type Martensitic Stainless Steel. Metall. Mater. Trans. A 2015, 46, 6090–6102. [Google Scholar] [CrossRef]
- Ilevbare, G.O.; Burstein, G.T. The role of alloyed molybdenum in the inhibition of pitting corrosion in stainless steels. Corros. Sci. 2001, 43, 485–513. [Google Scholar] [CrossRef]
- Yuan, F.; Wei, G.; Gao, S.; Lu, S.; Liu, H.; Li, S.; Fang, X.; Chen, Y. Tuning the pitting performance of a Cr-13 type martensitic stainless steel by tempering time. Corros. Sci. 2022, 203, 110346. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Ni, H.; Hao, W.; Man, C.; Chen, S.; Wang, X.; Liu, Z.; Li, X. Influence of temperature on the electrochemical and passivation behavior of 2507 super duplex stainless steel in simulated desulfurized flue gas condensates. Corros. Sci. 2017, 118, 31–48. [Google Scholar] [CrossRef]
- Gao, B.; Xu, T.; Wang, L.; Liu, Y.; Liu, J.; Zhang, Y.; Sui, Y.; Sun, W.; Chen, X.; Li, X.; et al. Achieving a superior combination of tensile properties and corrosion resistance in AISI420 martensitic stainless steel by low-temperature tempering. Corros. Sci. 2023, 225, 111551. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, W.; Li, S.; Chowwanonthapunya, T.; Wongpat, B.; Zhao, Y.; Dong, B.; Zhang, T.; Li, X. Evolution of rust layers on carbon steel and weathering steel in high humidity and heat marine atmospheric corrosion. J. Mater. Sci. Technol. 2020, 39, 190–199. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Du, C.; Wang, H.; Li, C.; Li, X. Effect of cathodic potentials on the SCC behavior of E690 steel in simulated seawater. Mater. Sci. Eng. A 2015, 642, 22–31. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Z.; Du, C.; Sun, M.; Li, X. Effects of cathodic polarization on corrosion fatigue life of E690 steel in simulated seawater. Int. J. Fatigue 2018, 110, 105–114. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Shao, Y.; Meng, G.; Wang, F. Effect of hydrostatic pressure on the corrosion behaviour of Ni–Cr–Mo–V high strength steel. Corros. Sci. 2010, 52, 2697–2706. [Google Scholar] [CrossRef]
- Duan, Z.; Man, C.; Dong, C.; Cui, Z.; Kong, D.; Wang, L.; Wang, X. Pitting behavior of SLM 316L stainless steel exposed to chloride environments with different aggressiveness: Pitting mechanism induced by gas pores. Corros. Sci. 2020, 167, 108520. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, L.; Zhong, M.; Cheng, L.; Cui, Z. Understanding the effect of fluoride on corrosion behavior of pure titanium in different acids. Corros. Sci. 2021, 192, 109812. [Google Scholar] [CrossRef]
- Pan, L.; Kwok, C.T.; Niu, B.; Huang, X.; Cao, Y.; Zou, X.; Yi, J. Enhancement in hardness and corrosion resistance of directed energy deposited 17-4 PH martensitic stainless steel via heat treatment. J. Mater. Res. Technol. 2023, 23, 1296–1311. [Google Scholar] [CrossRef]
- Shahriari, A.; Khaksar, L.; Nasiri, A.; Hadadzadeh, A.; Amirkhiz, B.S.; Mohammadi, M. Microstructure and corrosion behavior of a novel additively manufactured maraging stainless steel. Electrochim. Acta 2020, 339, 135925. [Google Scholar] [CrossRef]
- Blanc, C.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V.; Wu, S. The origin of the complex character of the Ohmic impedance. Electrochim. Acta 2010, 55, 6313–6321. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Wu, M.; Huang, R. Study of depassivation of carbon steel in simulated concrete pore solution using different equivalent circuits. Constr. Build. Mater. 2017, 157, 357–362. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wen, T.-C.; Teng, H. Polyaniline-deposited porous carbon electrode for supercapacitor. Electrochim. Acta 2003, 48, 641–649. [Google Scholar] [CrossRef]
- Lu, G.; Liu, Y.; Beiersmann, C.; Feng, Y.; Cao, J.; Müller, O. Challenges in and lessons learned during the implementation of the 1-3-7 malaria surveillance and response strategy in China: A qualitative study. Infect. Dis. Poverty 2016, 5, 94. [Google Scholar] [CrossRef]
- Lei, X.; Feng, Y.; Zhang, J.; Fu, A.; Yin, C.; Macdonald, D.D. Impact of Reversed Austenite on the Pitting Corrosion Behavior of Super 13Cr Martensitic Stainless Steel. Electrochim. Acta 2016, 191, 640–650. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Yao, K.-F.; Chen, Y.-B.; Wang, M.-H.; Liu, X.; Ge, X. The effect of tempering temperature on the microstructure and electrochemical properties of a 13wt.% Cr-type martensitic stainless steel. Electrochim. Acta 2015, 165, 45–55. [Google Scholar] [CrossRef]
- Burstein, G.T.; Liu, C.; Souto, R.M.; Vines, S.P. Origins of pitting corrosion. Corros. Eng. Sci. Technol. 2004, 39, 25–30. [Google Scholar] [CrossRef]
- Gong, K.; Wu, M.; Xie, F.; Liu, G.; Sun, D. Effect of Cl− and rust layer on stress corrosion cracking behavior of X100 steel base metal and heat-affected zone in marine alternating wet/dry environment. Mater. Chem. Phys. 2021, 270, 124826. [Google Scholar] [CrossRef]
- Lothongkum, G.; Wongpanya, P.; Morito, S.; Furuhara, T.; Maki, T. Effect of nitrogen on corrosion behavior of 28Cr–7Ni duplex and microduplex stainless steels in air-saturated 3.5 wt% NaCl solution. Corros. Sci. 2006, 48, 137–153. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Shao, Y.; Zhang, T.; Wang, Y.; Wang, F. Effect of Cl− on the Properties of the Passive Films Formed on 316L Stainless Steel in Acidic Solution. J. Mater. Sci. Technol. 2014, 30, 253–258. [Google Scholar] [CrossRef]
- Grabke, H.J. The Role of Nitrogen in the Corrosion of Iron and Steels. ISIJ Int. 1996, 36, 777–786. [Google Scholar] [CrossRef]


| Steels | C | Cr | Mo | N | Mn | Si | Fe |
|---|---|---|---|---|---|---|---|
| 0N | 0.30 | 15.17 | 1.02 | / | 0.45 | 0.55 | Bal. |
| 0.16N | 0.31 | 15.16 | 0.97 | 0.16 | 0.42 | 0.51 | Bal. |
| Samples | OCP/mV | ipass/(μA/cm2) | Epit/mV | icorr/(μA/cm2) | Ecorr/mV |
|---|---|---|---|---|---|
| 0 N | −250.2 | 8.3 | −6.8 | 0.9 | −339.5 |
| 0.16 N | −124.7 | 1.2 | 241.6 | 0.1 | −281.0 |
| Samples | Rs (Ω·cm2) | CPEf | Rf (kΩ·cm2) | CPEdl | Rct (kΩ·cm2) | Χ2 | ||
|---|---|---|---|---|---|---|---|---|
| Qf (MΩ−1/cm−2sα) | αf | Qdl (MΩ−1/cm−2sα) | αdl | |||||
| 0 N | 4.86 | 4.77 × 10−4 | 0.80 | 4.05 | / | / | / | 9.31 × 10−4 |
| 0.16 N | 4.05 | 8.39 × 10−5 | 0.95 | 7.27 × 10−3 | 1.19 × 10−4 | 0.82 | 1.24 × 102 | 1.53 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Wang, J.; Li, Y.; Wang, B.; Li, H.; Liu, G. Effect of Nitrogen on Precipitate Characteristics and Pitting Resistance of Martensitic Stainless Steel. Materials 2024, 17, 3817. https://doi.org/10.3390/ma17153817
Xu H, Wang J, Li Y, Wang B, Li H, Liu G. Effect of Nitrogen on Precipitate Characteristics and Pitting Resistance of Martensitic Stainless Steel. Materials. 2024; 17(15):3817. https://doi.org/10.3390/ma17153817
Chicago/Turabian StyleXu, Hui, Jinbin Wang, Yugui Li, Bin Wang, Huaying Li, and Guangming Liu. 2024. "Effect of Nitrogen on Precipitate Characteristics and Pitting Resistance of Martensitic Stainless Steel" Materials 17, no. 15: 3817. https://doi.org/10.3390/ma17153817






