Zirconia and Crofer Joint Made by Reactive Air Brazing Using the Silver Base Paste and Cu-Ti Coating Layer
Abstract
1. Introduction
2. Materials and Experimental Procedures
2.1. Materials Preparation
2.2. RAB Process
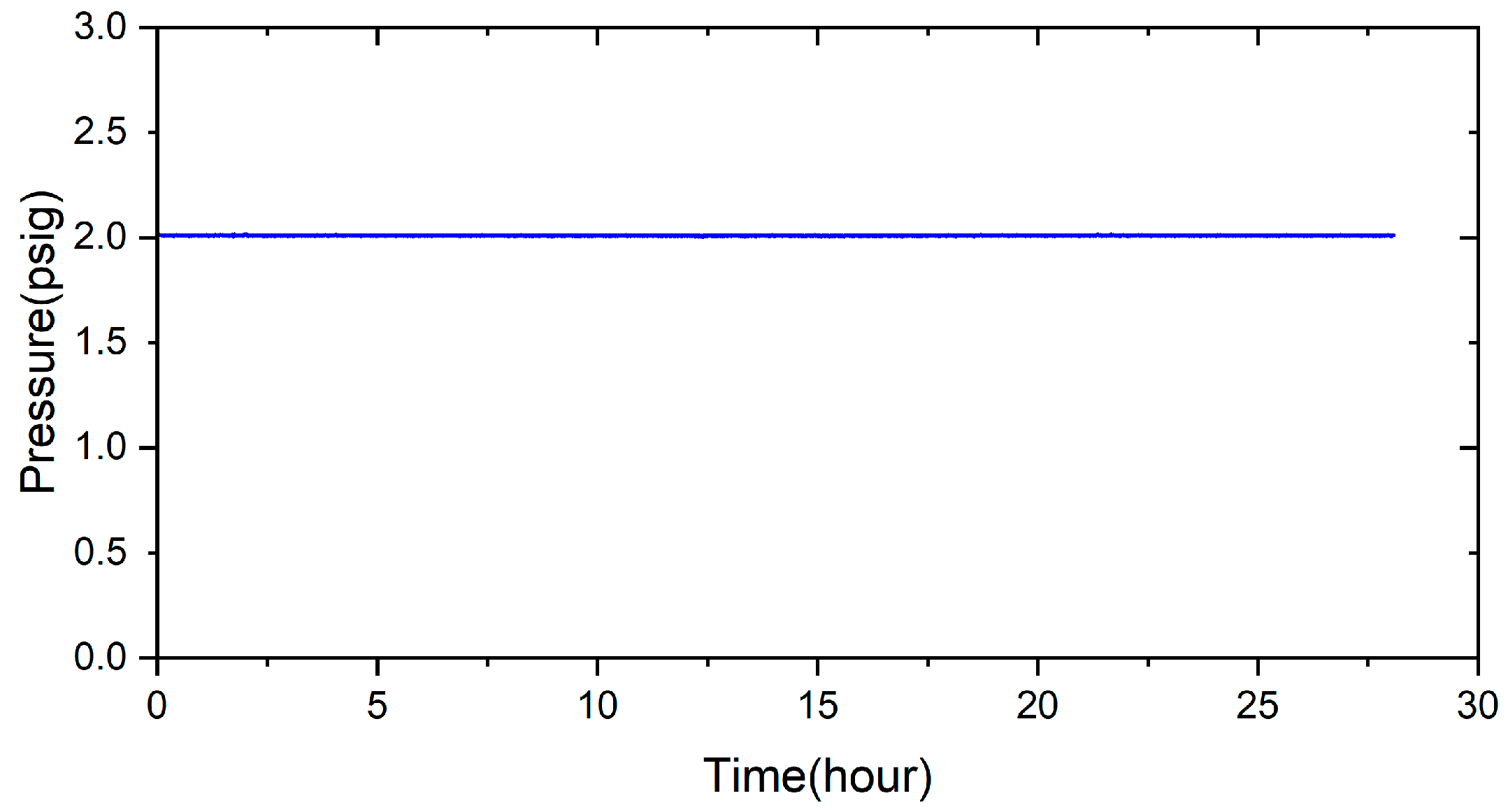
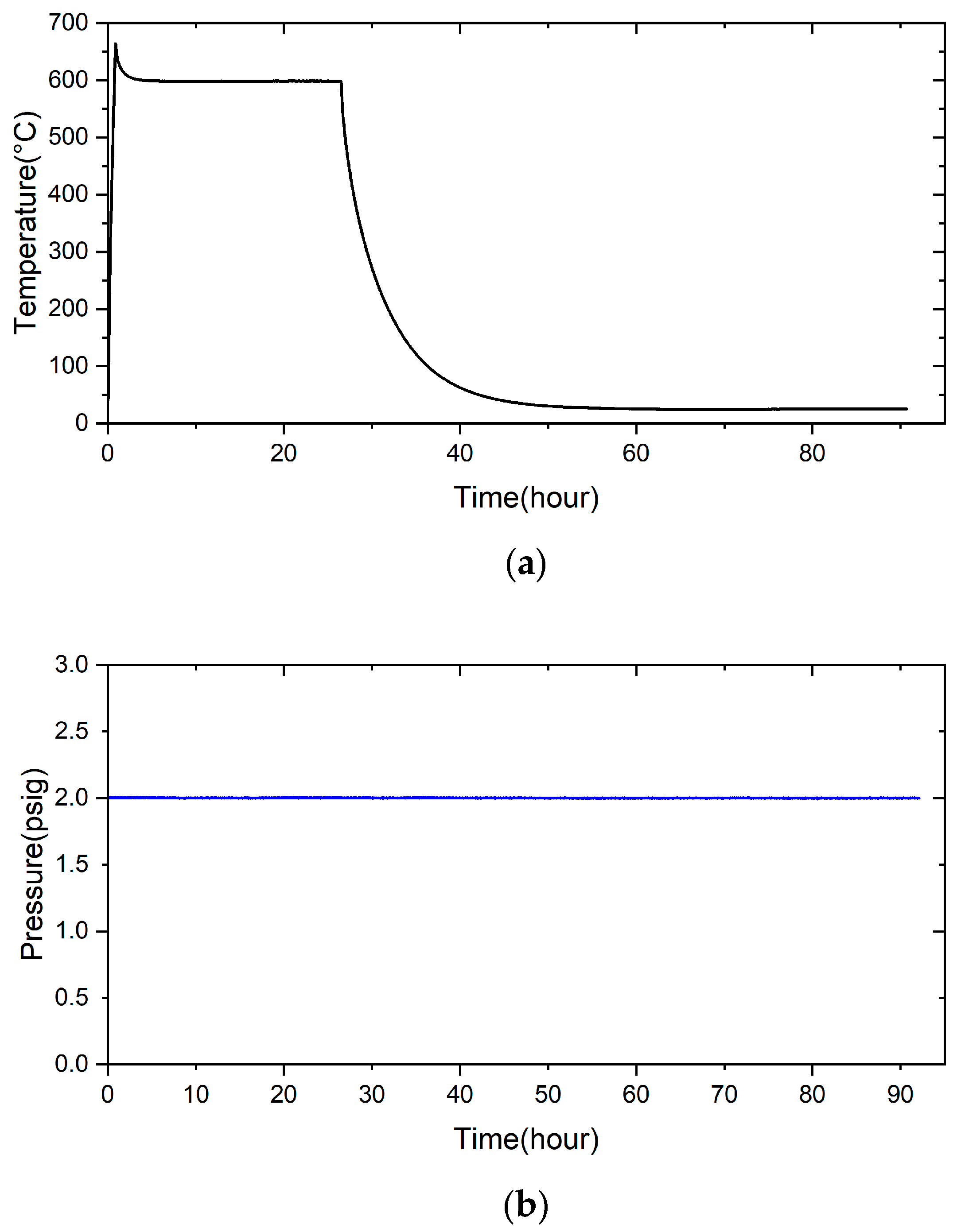
2.3. Pressure Drop Test
3. Results and Discussion
4. Conclusions
- Applying 70Cu-30Ti (at%) active coating layer on both substrates can effectively improve the bonding between pure silver and ZrO2 and pure silver and Crofer alloy.
- The interfacial reaction of the ZrO2 side is very different from that of the Crofer side. There are two immiscible liquids, Ag-rich and Cu/Ti-rich liquids, at 960 °C. The activity of the Ag-rich liquid is much less than that of the Cu/Ti-rich liquid. The Ag-rich liquid acts as a barrier against the oxidation of the braze alloy during the RAB. The ZrO2 is readily wetted by alloying the Cu/Ti liquid. For the Crofer side, the Cu/Ti-rich liquid reacted with Fe and Cr in the Crofer substrate and oxidized during the RAB.
- The oxidation of the interface for the Crofer side was more vigorous than that of the ZrO2 side. The thickness of the interfacial reaction layer on the Crofer side was approximately 10 and 20 μm. In contrast, the interfacial thickness of the ZrO2 side was approximately 5 μm.
- The coating thickness of the 70Cu-30Ti active layer is optimized into 3 μm in the experiment. ZrO2/Ag and Ag/Crofer interfaces are free of pores and cracks.
- There is no pressure drop under 2 psig at room temperature for 28 h. The pressure condition can still be maintained even under the high-temperature condition of 600 °C for 24 h. It can potentially be used in solid oxide fuel cells in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Li, Y. Micro gas turbine: Developments, applications, and critical technologies on components. Propuls. Power Res. 2023, 12, 1–43. [Google Scholar] [CrossRef]
- Moskal, G.; Niemiec, D.; Chmiela, B.; Kałamarz, P.; Durejko, T.; Ziętala, M.; Czujko, T. Microstructural characterization of laser cladding NiCrAlY coatings on Inconel 625 Ni-based superalloy and 316L stainless steel. Surf. Coat. Technol. 2020, 387, 125317. [Google Scholar] [CrossRef]
- Murayama, N.; Hirao, K.; Sando, M.; Tsuchiya, T.; Yamaguchi, H. High-temperature electro-ceramics and their application to SiC power modules. Ceram. Int. 2018, 44, 3523–3530. [Google Scholar] [CrossRef]
- Hirao, K.; Zhou, Y.; Fukushima, M.; Wakasugi, N.; Suganuma, K. Evaluation of in-plane effective thermal conductivity for metalized ceramic substrates using a micro heater chip. Ceram. Int. 2023, 49, 28593–28606. [Google Scholar] [CrossRef]
- Ntasi, A.; Jabbari, Y.S.A.; Mueller, W.D.; Eliades, T.; Zinelis, S. Electrochemical characterization of novel Ag-based brazing alloys for dental applications. Dent. Mater. 2019, 35, 163–174. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, L.; Yang, W.; Ji, H.; Li, M.; Li, Y. Microstructure evolution and bonding mechanism of ZrO2 ceramic and Ti-6Al-4V alloy joints brazed by Bi2O3-B2O3-ZnO glass paste. J. Eur. Ceram. Soc. 2022, 42, 5953–5963. [Google Scholar] [CrossRef]
- Hirata, Y.; Daio, S.; Kai, A.; Shimonosono, T.; Yano, R.; Sameshima, S.; Yamaji, K. Performance of yttria-stabilized zirconia fuel cell using H2-CO2 gas system and CO-O2 gas system. Ceram. Int. 2016, 42, 18373–18379. [Google Scholar] [CrossRef]
- Mir, F.A.; Khan, N.Z.; Parvez, S. Recent advances and development in joining ceramics to metals. Mater. Today Proc. 2021, 46, 6570–6575. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.K.; Yu, D.S.; Sun, D.Q.; Li, H.M. A review paper on the effect of the welding process of ceramics and metals. J. Mater. Res. Technol. 2020, 9, 16214–16236. [Google Scholar] [CrossRef]
- Caron, N.; Bianchi, L.; Méthout, S. Development of a functional sealing layer for SOFC applications. J. Therm. Spray Technol. 2008, 17, 598–602. [Google Scholar] [CrossRef]
- Darsell, J.T.; Weil, K.S. High-temperature strength of YSZ joints brazed with palladium silver copper oxide filler metals. Int. J. Hydrogen Energy. 2011, 36, 4519–4524. [Google Scholar] [CrossRef]
- Hardy, J.S.; Kim, J.Y.; Thomsen, E.C.; Weil, K.S. Improved wetting of mixed ionic/electronic conductors used in electrochemical devices with ternary air braze filler metals. J. Electrochem. Soc. 2007, 154, 32. [Google Scholar] [CrossRef]
- Kiebach, R.; Engelbrecht, K.; Grahl-Madsen, L.; Sieborg, B.; Chen, M.; Hjelm, J.; Norrman, K.; Chatzichristodoulou, C.; Hendriksen, P. An Ag-based brazing system with a tunable thermal expansion for the use as sealant for solid oxide cells. J. Power Sources 2016, 315, 339–350. [Google Scholar] [CrossRef]
- Ayoub, G.; Veljovic, D.; Zebic, M.L.; Miletic, V.; Palcevskis, E.; Petrovic, R.; Janackovic, D. Composite nanostructured hydroxyapatite/yttrium stabilized zirconia dental inserts—The processing and application as dentin substitutes. Ceram. Int. 2018, 44, 18200–18208. [Google Scholar] [CrossRef]
- Tsipas, S.A.; Golosnoy, I.O. Effect of substrate temperature on the microstructure and properties of thick plasma-sprayed YSZ TBCs. J. Eur. Ceram. 2011, 31, 2923–2929. [Google Scholar] [CrossRef]
- Tucker, M.C.; Jacobson, C.P.; De Jonghe, L.; Visco, S. A braze system for sealing metal-supported solid oxide fuel cells. J. Power Sources 2006, 160, 1049–1057. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, K.; Yan, Y.; Le, S.; Zhang, N.; Sun, W.; Wang, P. Investigation on silver electric adhesive doped with Al2O3 ceramic particles for sealing planar solid oxide fuel cell. J. Power Sources 2009, 192, 408–413. [Google Scholar] [CrossRef]
- Si, X.; Cao, J.; Talic, B.; Ritucci, I.; Li, C.; Qi, J.; Feng, J.; Kiebach, R. A novel Ag-based sealant for solid oxide cells with a fully tunable thermal expansion. J. Alloys Compd. 2020, 831, 154608. [Google Scholar] [CrossRef]
- Huang, L.W.; Wu, Y.Y.; Shiue, R.K. The effect of oxygen pressure in active brazing 8YSZ and Crofer 22H alloy. J. Mater. Res. Technol. 2021, 10, 1382–1388. [Google Scholar] [CrossRef]
- Huang, L.W.; Shiue, R.K.; Liu, C.K.; Cheng, Y.N.; Lee, R.Y.; Tsay, L.W. Vacuum Brazing of Metallized YSZ and Crofer Alloy Using 72Ag-28Cu Filler Foil. Materials 2022, 15, 939. [Google Scholar] [CrossRef]
- Xin, C.; Liu, W.; Li, N.; Yan, J.; Shi, S. Metallization of Al2O3 ceramic by magnetron sputtering Ti/Mo bilayer thin films for robust brazing to Kovar alloy. Ceram. Int. 2016, 42, 9599–9604. [Google Scholar] [CrossRef]
- Song, X.G.; Niu, C.N.; Hu, S.P.; Liu, D.; Cao, J.; Feng, J.C. Contact reactive brazing of Al7075 alloy using Cu layer deposited by magnetron sputtering. J. Mater. Process. Technol. 2018, 252, 469–476. [Google Scholar] [CrossRef]
- Singh, M.; Shpargel, T.P.; Asthana, R. Brazing of yttria-stabilized zirconia (YSZ) to stainless steel using Cu, Ag, and Ti-based brazes. J. Mater. Sci. 2008, 43, 23–32. [Google Scholar] [CrossRef]
- Yang, Z.W.; Xiong, Z.; Wang, J.L.; Wang, Y.; Wang, D.P. Microstructural evolution and high-temperature oxidation resistance of YSZ/Crofer 22H brazed joints using Ag-based filler for solid-oxide fuel cell applications. Mater. Charact. 2023, 200, 112888. [Google Scholar] [CrossRef]
- Lin, P.; Lin, T.; He, P.; Wang, M.; Yang, J. Microstructure evolution and mechanical properties of a vacuum-brazed Al2O3/Ti joint with Mo-coating on Al2O3 and Ti surfaces. Ceram. Int. 2019, 45, 11195–11203. [Google Scholar] [CrossRef]
- Laik, A.; Mishra, P.; Bhanumurthy, K.; Kale, G.B.; Kashyap, B.P. Microstructural evolution during reactive brazing of alumina to Inconel 600 using Ag-based alloy. Acta Mater. 2013, 61, 126–138. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, Z.W.; Wang, Y.; Wang, D.P.; Li, H.J. Microstructural stability and mechanical properties of Al2O3/Kovar 4 J34 joint vacuum brazed using Ag-5Cu-1Al-1.25Ti (wt%) filler metal. J. Manuf. Process. 2021, 72, 553–564. [Google Scholar] [CrossRef]
- Massalski, T.B. Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Materials Park, OH, USA, 1992. [Google Scholar]
- Humpston, G.; Jacobson, D.M. Principles of Soldering and Brazing; ASM International: Materials Park, OH, USA, 1993. [Google Scholar]
- Shiue, R.K.; Wu, S.K.O.J.; Wang, J.Y. Microstructural evolution at the bonding interface during the early-stage infrared active brazing of alumina. Metall. Mater. Trans. 2000, 31, 2527–2536. [Google Scholar] [CrossRef]
- Villars, P.; Prince, A.; Okamoto, H. Handbook of Ternary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1995. [Google Scholar]


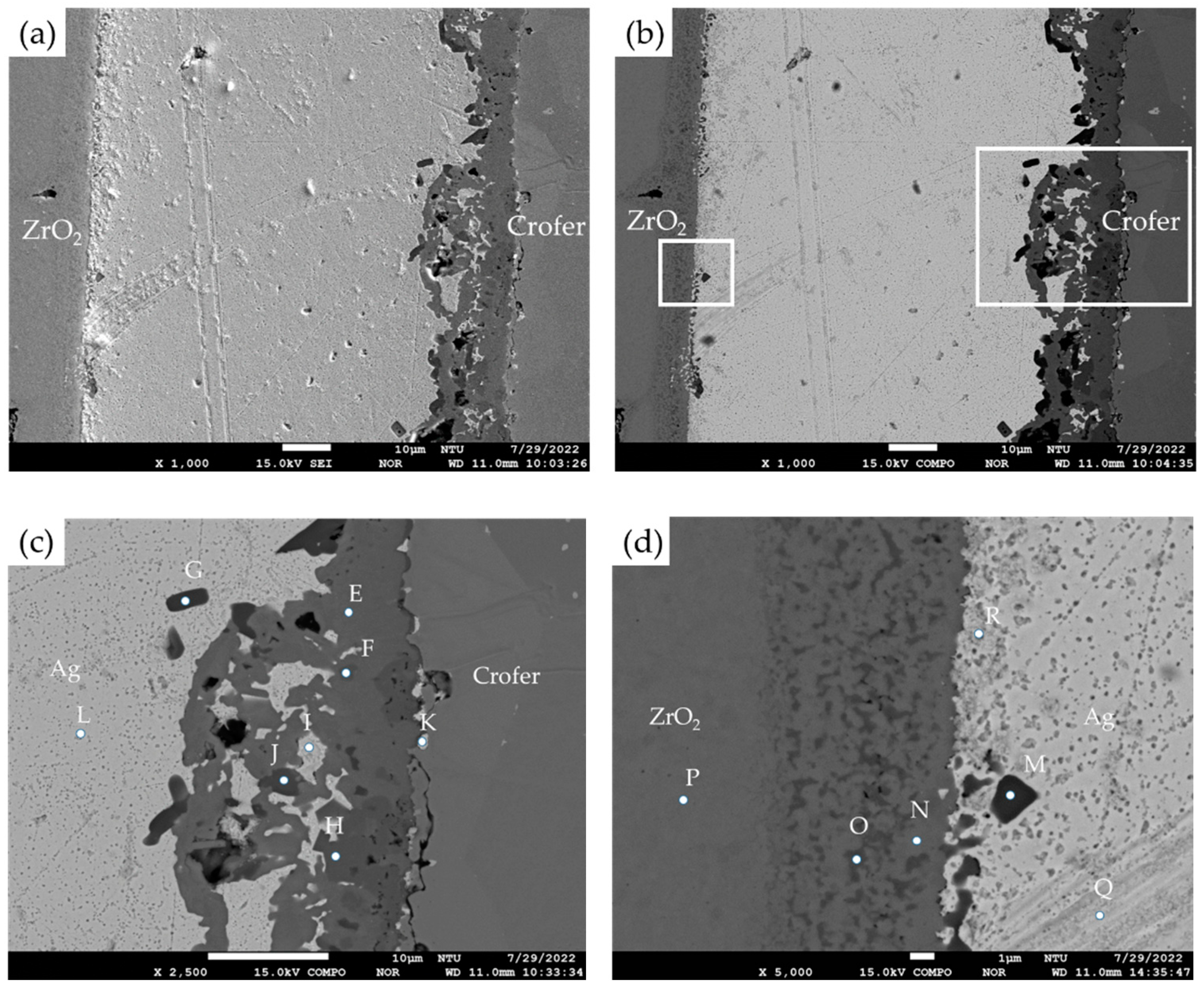
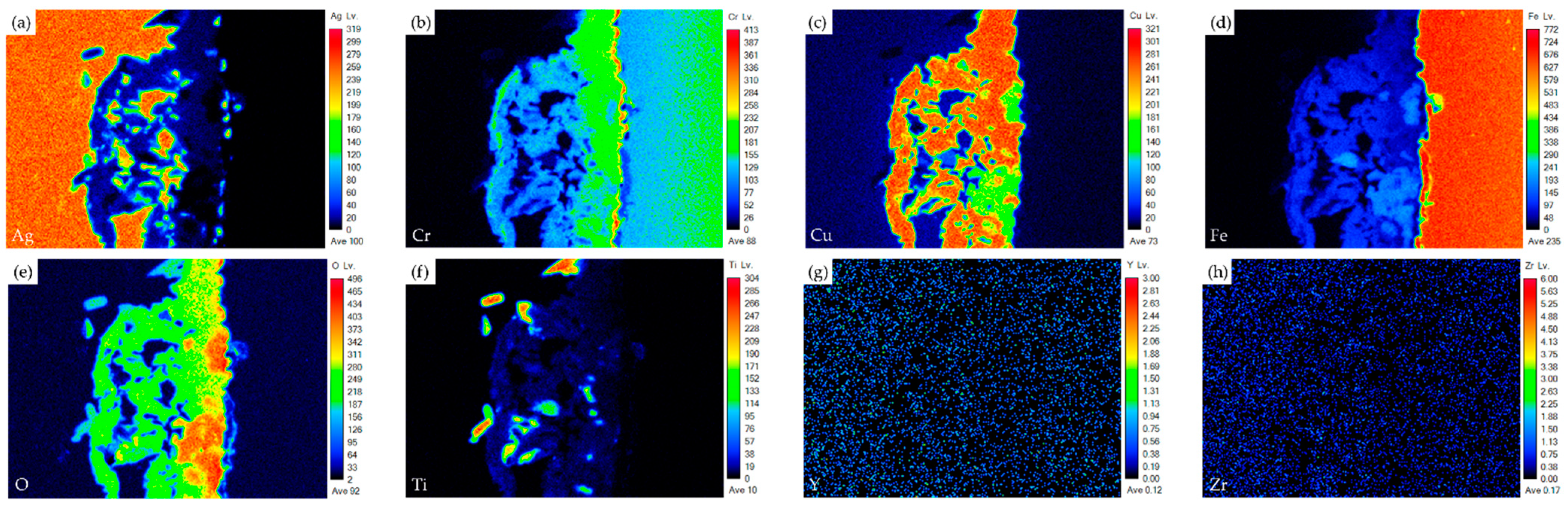
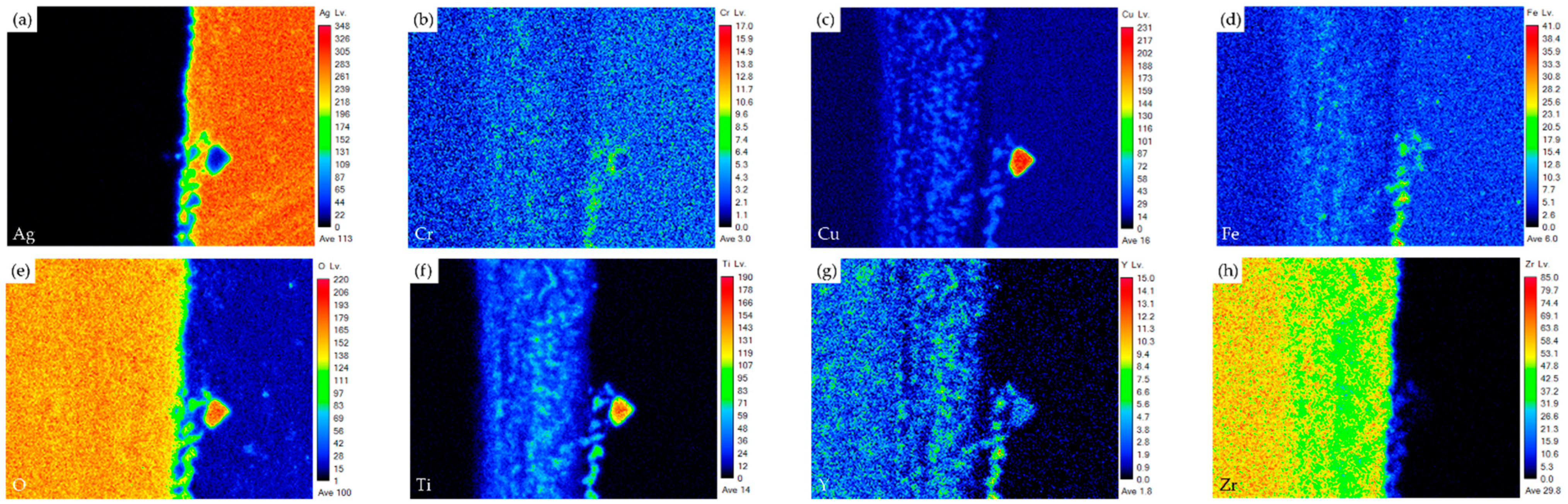

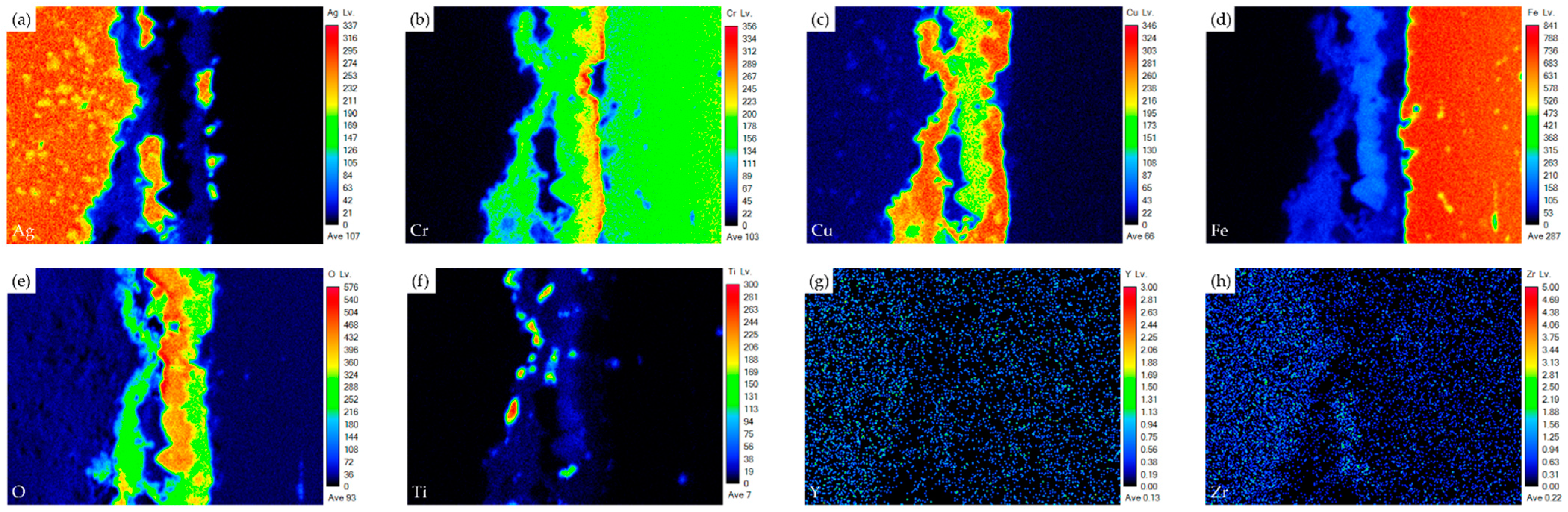
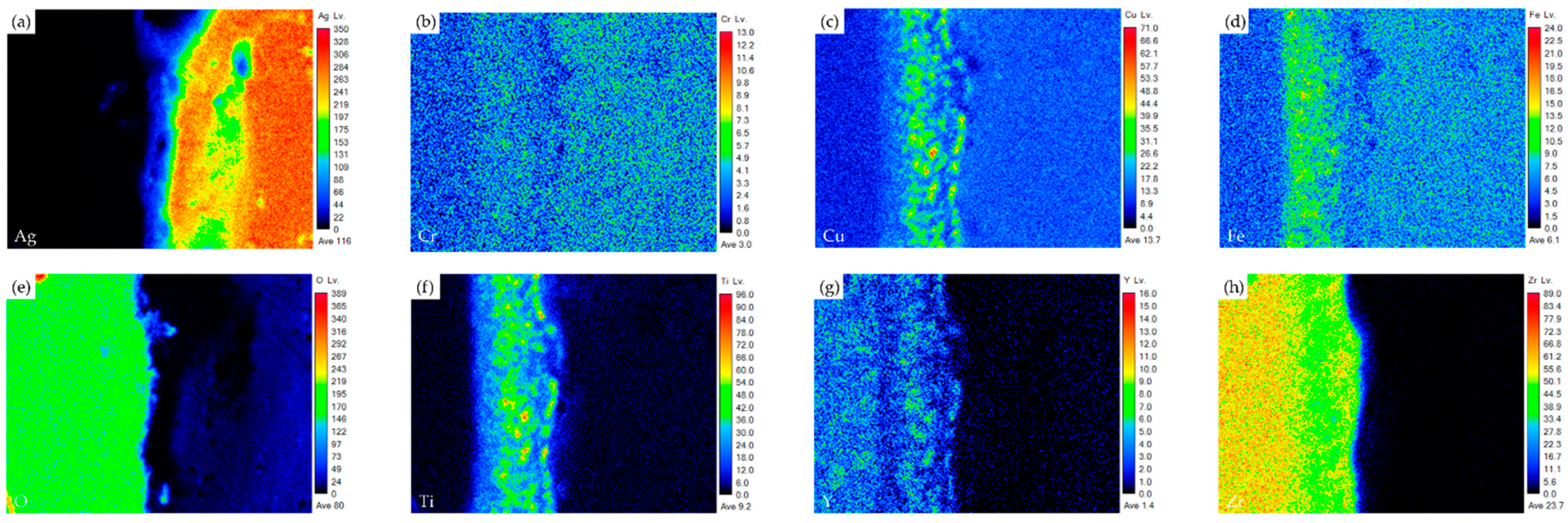
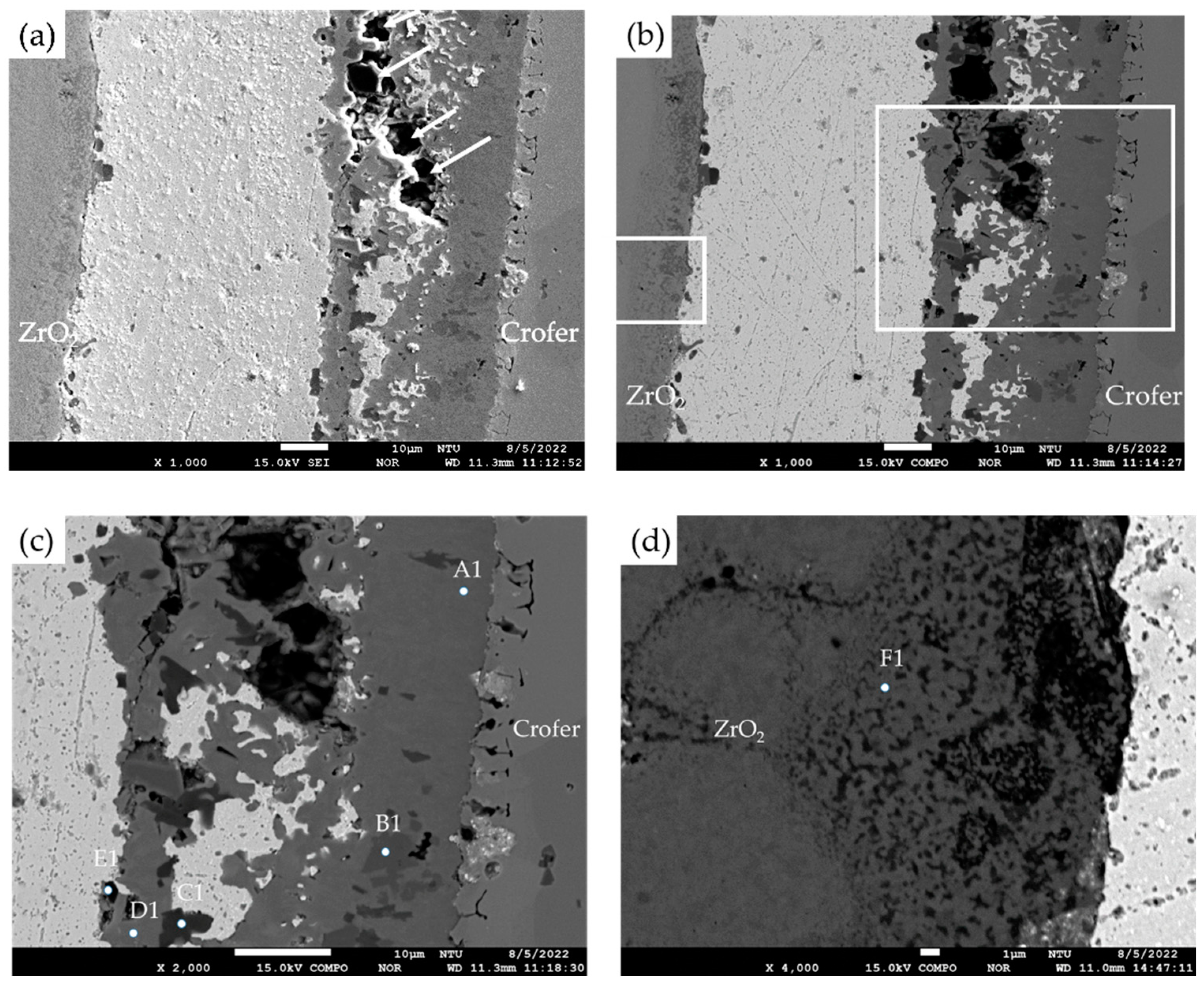

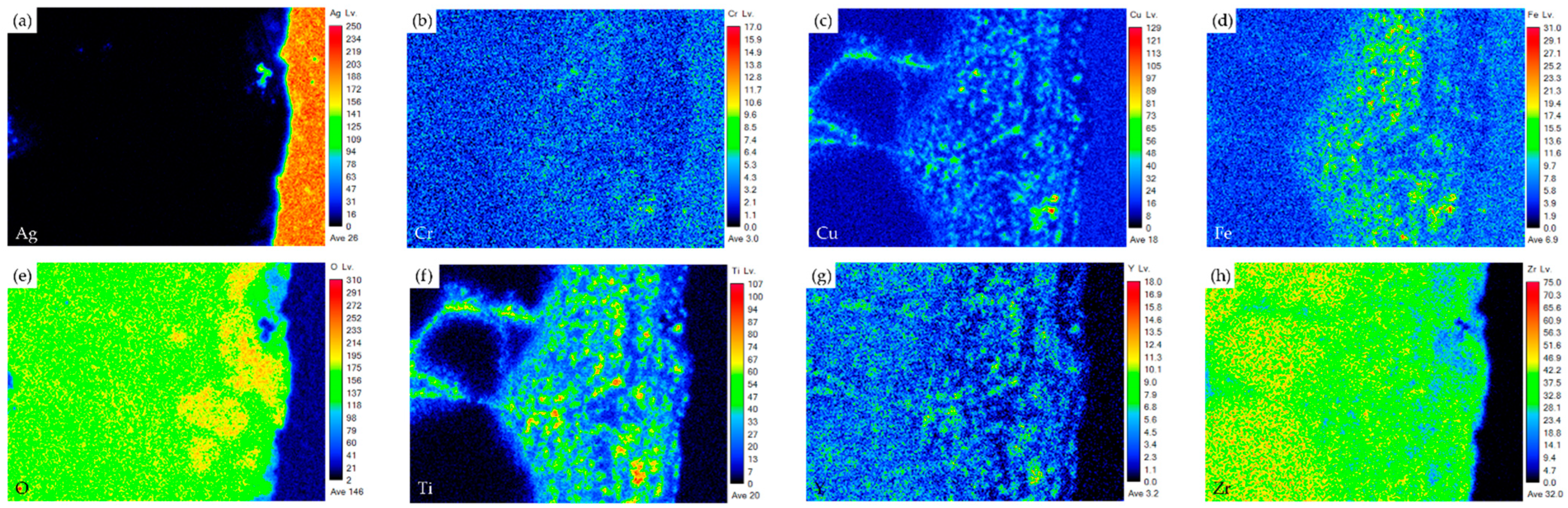
| Element/at% | Ag | Cu | Cr | Fe | O | Ti | Y | Zr | Alloy/Phase |
|---|---|---|---|---|---|---|---|---|---|
| A | 0.0 | 0.0 | 23.9 | 73.8 | 0.00 | 0.0 | 0.0 | 0.0 | Crofer (substrate) |
| B | 1.2 | 0.0 | 35.0 | 2.1 | 59.2 | 0.0 | 0.0 | 0.0 | Cr2O3 |
| C | 97.6 | 0.0 | 0.5 | 1.0 | 0.5 | 0.0 | 0.0 | 0.0 | Ag-rich |
| D | 0.0 | 0.0 | 0.0 | 0.0 | 63.3 | 0.0 | 2.0 | 34.6 | ZrO2 (substrate) |
| Element/at% | Ag | Cu | Cr | Fe | O | Ti | Y | Zr | Alloy/Phase |
|---|---|---|---|---|---|---|---|---|---|
| Crofer | 0.0 | 0.1 | 16.8 | 81.2 | 0.0 | 0.0 | 0.0 | 0.0 | Crofer substrate |
| E | 1.7 | 23.5 | 19.6 | 4.2 | 49.7 | 0.8 | 0.0 | 0.0 | Cr/Cu-rich oxide |
| F | 0.4 | 14.3 | 13.9 | 11.1 | 57.0 | 1.4 | 0.0 | 0.0 | Cr/Cu/Fe-rich oxide |
| G | 2.2 | 0.0 | 0.6 | 0.5 | 64.5 | 31.3 | 0.0 | 0.0 | TiO2 |
| H | 0.2 | 13.3 | 12.7 | 13.3 | 57.8 | 1.5 | 0.0 | 0.0 | Cr/Cu/Fe-rich oxide |
| I | 94.5 | 2.2 | 0.8 | 0.9 | 1.2 | 0.1 | 0.0 | 0.0 | Ag-rich |
| J | 0.5 | 4.5 | 1.7 | 16.7 | 57.6 | 18.5 | 0.0 | 0.0 | Fe/Ti-rich Oxide |
| K | 81.2 | 2.7 | 4.6 | 8.4 | 2.2 | 0.1 | 0.0 | 0.0 | Ag-rich |
| L | 99.6 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | Ag-rich |
| M | 3.4 | 14.6 | 0.2 | 0.0 | 58.4 | 20.3 | 2.5 | 0.4 | Ti-rich oxide |
| N | 0.1 | 0.3 | 0.0 | 0.0 | 63.8 | 3.1 | 0.6 | 32.1 | ZrO2 alloyed with Ti |
| O | 0.1 | 2.7 | 0.2 | 0.0 | 62.5 | 8.5 | 3.5 | 21.8 | ZrO2 alloyed with Ti/Cu |
| P | 0.0 | 0.0 | 0.0 | 0.0 | 64.3 | 0.1 | 1.9 | 33.7 | ZrO2 substrate |
| Q | 99.6 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | Ag-rich |
| R | 93.3 | 0.2 | 0.0 | 0.1 | 1.1 | 0.2 | 0.0 | 4.5 | Ag-rich |
| Element/at% | Ag | Cu | Cr | Fe | O | Ti | Y | Zr | Phase/Alloy |
|---|---|---|---|---|---|---|---|---|---|
| S | 6.4 | 37.9 | 28.7 | 5.2 | 20.5 | 0.8 | 0.0 | 0.0 | Cr/Cu-rich Oxide |
| T | 0.3 | 25.4 | 22.1 | 19.7 | 27.7 | 2.4 | 0.0 | 0.0 | Cr/Cu/Fe-rich Oxide |
| U | 8.9 | 37.4 | 19.9 | 10.3 | 20.9 | 1.6 | 0.0 | 0.0 | Cr/Cu/Fe-rich Oxide |
| V | 0.5 | 25.8 | 21.4 | 19.1 | 27.2 | 2.9 | 0.0 | 0.0 | Cr/Cu/Fe-rich Oxide |
| W | 0.1 | 3.6 | 0.0 | 0.7 | 61.6 | 10.6 | 4.8 | 17.6 | ZrO2 alloyed with Ti/Cu |
| X | 0.1 | 0.6 | 0.0 | 0.1 | 64.9 | 3.9 | 1.3 | 28.7 | ZrO2 alloyed with Ti |
| Y | 0.1 | 0.4 | 0.0 | 0.3 | 64.8 | 2.9 | 0.9 | 30.5 | ZrO2 alloyed with Ti |
| Z | 0.0 | 0.0 | 0.0 | 0.0 | 62.4 | 0.0 | 3.2 | 34.3 | ZrO2 (substrate) |
| Element/at% | Ag | Cu | Cr | Fe | O | Ti | Y | Zr | Alloy/Phase |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 1.9 | 24.2 | 19.3 | 5.9 | 47.3 | 0.9 | 0.0 | 0.0 | Cr/Cu-rich Oxide |
| B1 | 0.1 | 15.3 | 10.4 | 15.7 | 55.5 | 1.9 | 0.0 | 0.0 | Cr/Cu/Fe-rich Oxide |
| C1 | 0.7 | 0.3 | 0.8 | 1.4 | 62.4 | 32.2 | 0.0 | 0.0 | TiO2 |
| D1 | 4.6 | 22.8 | 8.3 | 10.3 | 50.5 | 3.2 | 0.0 | 0.0 | Cr/Cu/Fe-rich Oxide |
| E1 | 1.2 | 0.2 | 0.6 | 1.1 | 62.8 | 32.4 | 0.0 | 0.0 | TiO2 |
| F1 | 0.0 | 0.4 | 0.0 | 0.0 | 64.9 | 3.2 | 1.0 | 29.9 | ZrO2 alloyed with Ti |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-W.; Shiue, R.-K.; Huang, L.-W. Zirconia and Crofer Joint Made by Reactive Air Brazing Using the Silver Base Paste and Cu-Ti Coating Layer. Materials 2024, 17, 3822. https://doi.org/10.3390/ma17153822
Chang S-W, Shiue R-K, Huang L-W. Zirconia and Crofer Joint Made by Reactive Air Brazing Using the Silver Base Paste and Cu-Ti Coating Layer. Materials. 2024; 17(15):3822. https://doi.org/10.3390/ma17153822
Chicago/Turabian StyleChang, Shu-Wei, Ren-Kae Shiue, and Liang-Wei Huang. 2024. "Zirconia and Crofer Joint Made by Reactive Air Brazing Using the Silver Base Paste and Cu-Ti Coating Layer" Materials 17, no. 15: 3822. https://doi.org/10.3390/ma17153822
APA StyleChang, S.-W., Shiue, R.-K., & Huang, L.-W. (2024). Zirconia and Crofer Joint Made by Reactive Air Brazing Using the Silver Base Paste and Cu-Ti Coating Layer. Materials, 17(15), 3822. https://doi.org/10.3390/ma17153822







