CO2 Adsorption by CMK-3 at Low Temperatures and High Pressure to Reduce the Greenhouse Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. CMK-3
2.2. Material Characterisation
2.3. Adsorption Isotherms
3. Results and Discussion
3.1. Characterisation
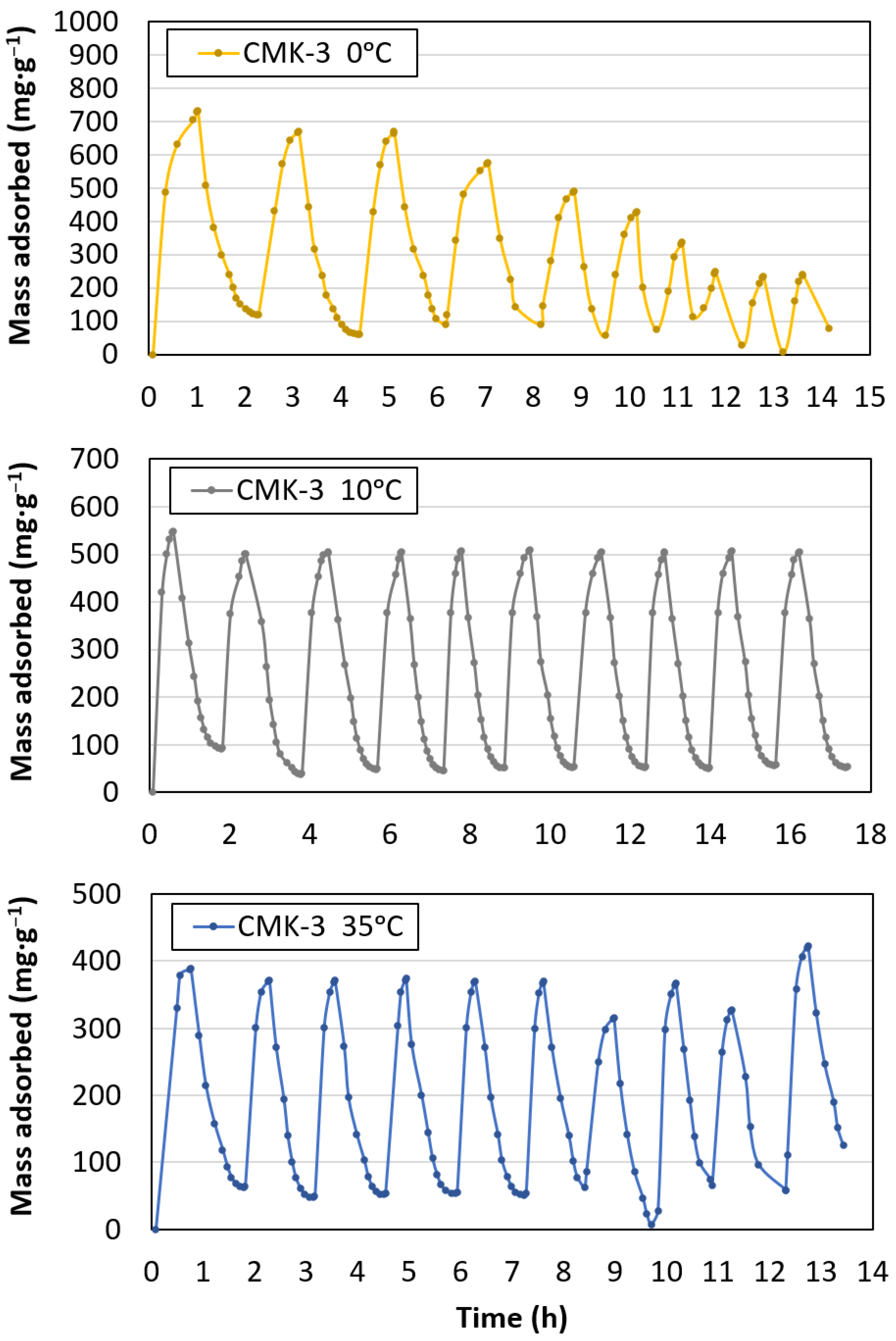
3.2. CO2 Adsorption
| Adsorbent | Tª Isotherm (°C) | Pressure (atm) | Capacity Adsorption (mg·g−1) | Ref. |
|---|---|---|---|---|
| Layered double hydroxide (LDH) | ||||
| Hydrotalcite MgAl-CO3 1 | 0 | ≈35 | 142.02 | [8] |
| Organohydrotalcite TDD 1 | 0 | 35 | 176.66 | [9] |
| Periodic mesoporous organosilica (PMO) | ||||
| PMO–ethane Np 2 iPrbipy 3 | 0 and 25 | 1 | 99.44 and 45.7 | [46] |
| PMO–ethane nanocubes | 0 | 1 | 62.48 | [10] |
| PMO-UDF 4 | 0 | ≈1 | 52.8 | [47] |
| PMO–benzene | 25 | 1 | 22 | [48] |
| PMO–benzene-modified 5 | 25 | 1 | 133.32 | |
| NH2–phenylene–PMO | 25 | ≈10 | 114.4 | [11] |
| PMO–ethane | 0 and 35 | ≈34 | 827.8 and 273.9 | [12] |
| Mesoporous metal–organic framework (MOF) | ||||
| MIL-53 (Al) | 31 | 29 | 462 | [49] |
| MIL-47 (V) | 31 | 20 | 506 | |
| MIL-101 | 25 | 30 | 1007.6 | [50] |
| MIL-101(Cr,Mg) | 25 | 1 | 145.2 | [51] |
| MOF-2 | 25 | 42 | 143 | [52] |
| MOF-74 | 25 | 42 | 457 | |
| MOF-177 | 25 | 42 | 1493 | |
| MOF-200 and MOF-210 | 25 | 50 | 2400 | [53] |
| Mesoporous silica (MS) | ||||
| SBA-15 | 45 | 1 | 21.2 | [5] |
| PEI-SBA-15 | 25 | 1 | 81.4 | [48] |
| PEI50-MS nanotube | 85 | 0.6 | 121 | [58] |
| PEI50–nano silica | 75 | 1 | 138.16 | [59] |
| Activated carbon (AC) | ||||
| Commercial carbon black | 28 | 1 | 13.2 | [17] |
| PEI-AC | 28 | 1 | 112.2 | |
| Modified AC (VR5-4:1) | 25 | 45 | 1388 | [19] |
| Modified AC (G-3.6-2) | 25 | 50 | 2160 | [21] |
| AC–hydrotalcite | 200 | 3 | 72 | [60] |
| AC-MIL-101(Cr) MOF | 27 | 9 | 1080 | [54] |
| Ordered mesoporous carbon | ||||
| CMK-3 | 0 | ≈34 | 682 | [29] |
| CMK-3-MDEA | 0 | ≈34 | 550 | |
| CMK-3 | 0 | ≈34 | 727 | This work |
| 10 | ≈34 | 571 | ||
| 20 | ≈34 | 447 | ||
| 35 | ≈34 | 404 | ||
4. Conclusions
- The laser particle size test showed a unimodal-type distribution curve located between 0.8 μm and 14 μm and a peak centred at 6.3 μm.
- The pore size range was 1.7 nm or less (micropores) and 6.7 nm in diameter (small mesopores). The pore volume was 0.77 cm3·g−1. The SBET was 990 m2·g−1, of which 198 m2·g−1 corresponded to micropores (20%).
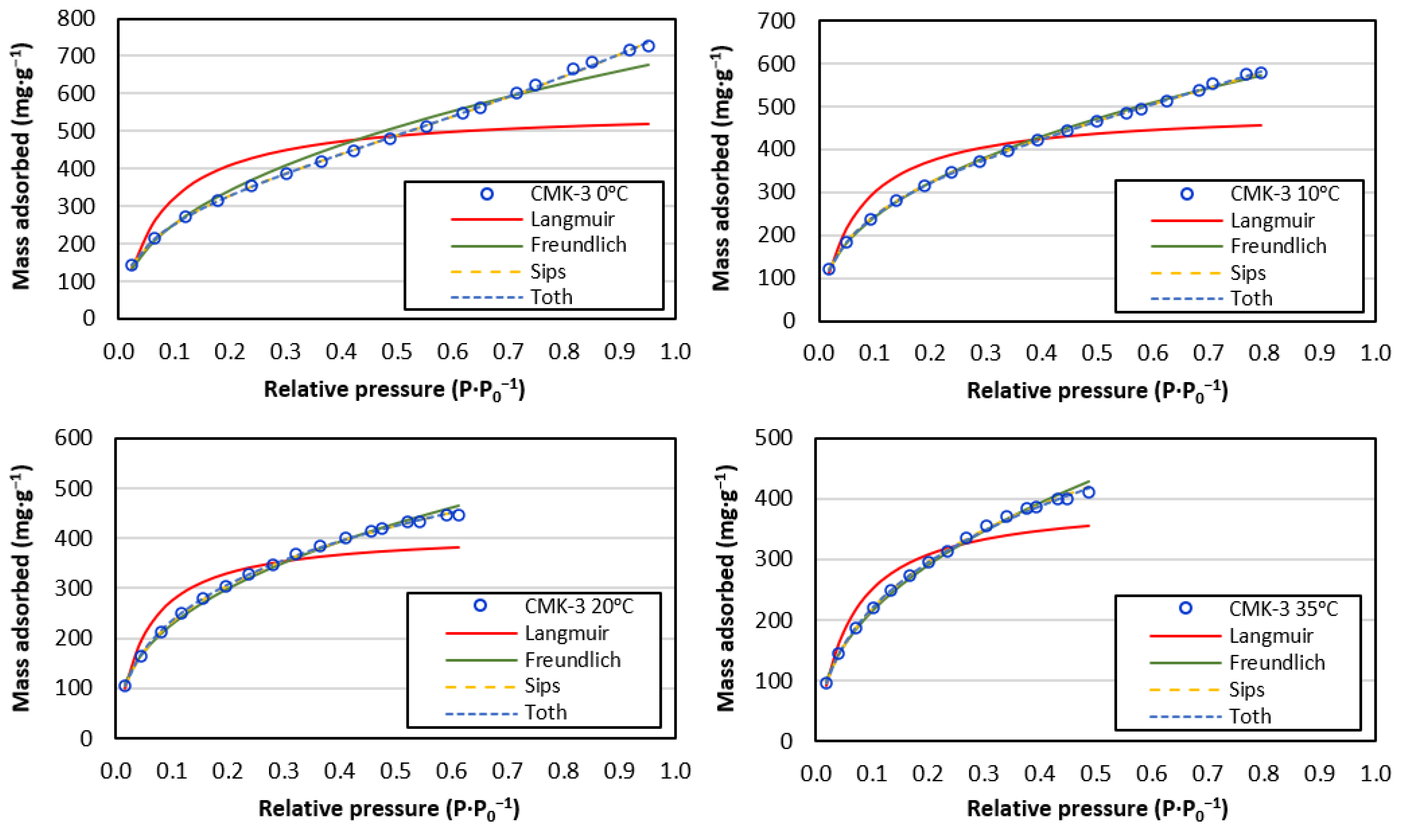
- All isotherms showed a good fit to the Freundlich model, and the Langmuir model obtained a worse fit. The obtained Freundlich values indicated a somewhat heterogeneous surface and a weak but favourable adsorbate–adsorbate interaction.
- The Sips and Toth models improved the Langmuir and Freundlich fit, especially for low temperatures (0 °C and 10 °C) where multilayers were formed.
- The free energy (E < 8 kJ·mol−1) obtained from the Dubinin–Radushkevich model was in agreement with the physical nature of adsorption. Temkin’s constant indicated a weak intermolecular adsorbent–adsorbate interaction.
- The highest capture capacity (726.7 mg·g−1) was obtained for working conditions of the minimum temperature studied (0 °C) and maximum pressure (34 atm).
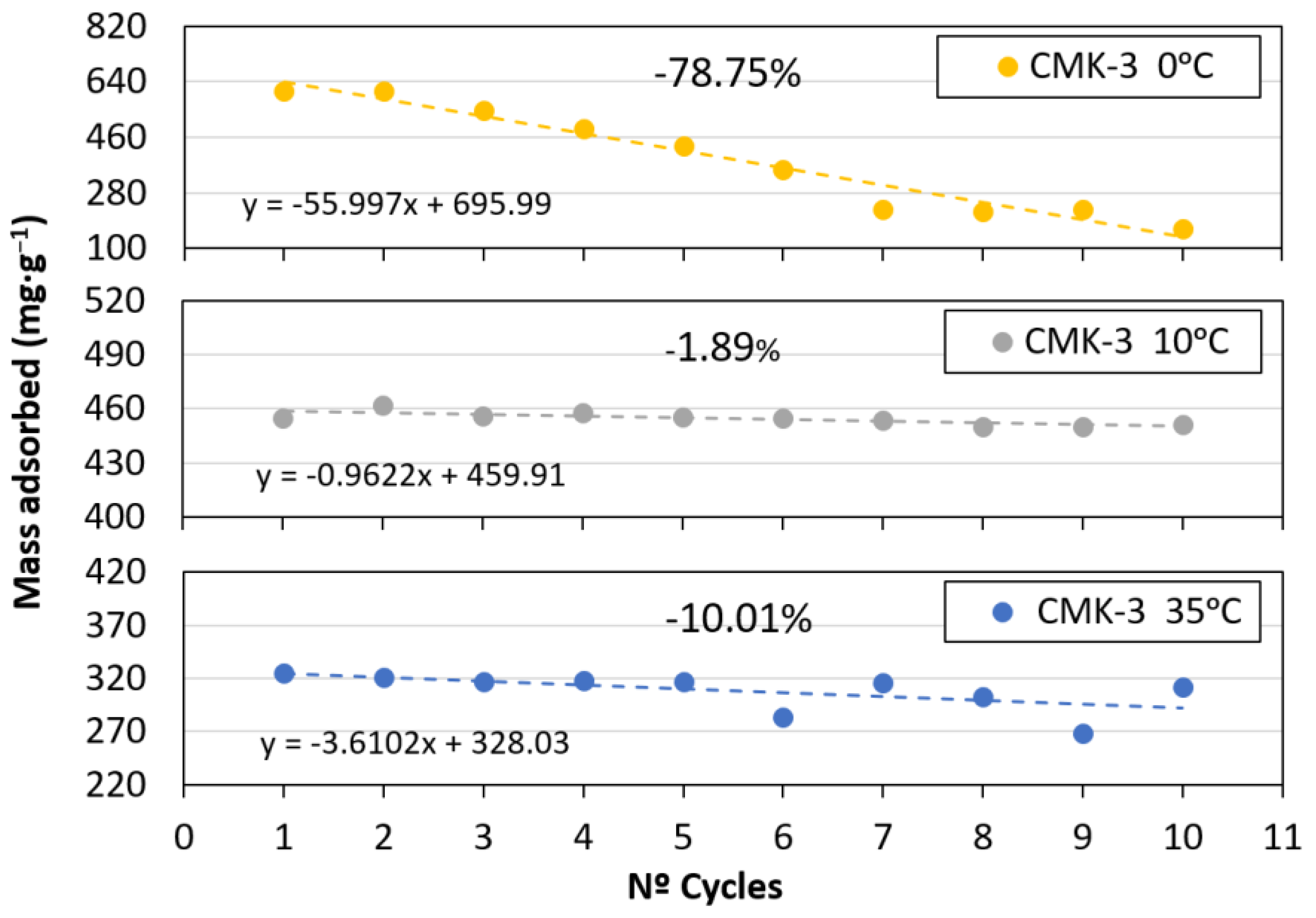
- The best performance, in terms of capture capacity loss after 10 adsorption–desorption cycles, was achieved at 10 °C and 34 atm, with only a 2% loss.
- The results showed that 0.478 g of CMK-3 would be enough to reduce the CO2 concentration in 1 m3 of air to pre-industrial levels. Consequently, 1243.2 kg of CMK-3 would be required to reduce the CO2 concentration within the Great Pyramid of Keops at Giza (Egypt) to pre-industrial levels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Department of Commerce, National Oceanic and Atmospheric Administration. Available online: https://www.noaa.gov/news-release/carbon-dioxide-now-more-than-50-higher-than-pre-industrial-levels#:~:text=Prior%20to%20the%20Industrial%20Revolution,atmosphere%20for%20thousands%20of%20years (accessed on 2 August 2024).
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- World Resources Institute. Climate Watch. Available online: https://www.climatewatchdata.org/ (accessed on 4 July 2023).
- United Nations. Paris Agreement. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 12 June 2023).
- Sanz-Pérez, E.S.; Arencibia, A.; Sanz, R.; Calleja, G. New Developments on Carbon Dioxide Capture Using Amine-Impregnated Silicas. Adsorption 2016, 22, 609–619. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. APTES Grafted Ordered Mesoporous Silica KIT-6 for CO2 Adsorption. Chem. Eng. J. 2015, 262, 882–890. [Google Scholar] [CrossRef]
- Licciulli, A.; Notaro, M.; De Santis, S.; Terreni, C.; Kunjalukkal Padmanabhan, S. CO2 Capture on Amine Impregnated Mesoporous Alumina-Silica Mixed Oxide Spheres. Fuel Process. Technol. 2017, 166, 202–208. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.R.; Fernández, J.M. Mitigation of CO2 Emissions by Hydrotalcites of Mg3Al-CO3 at 0 °C and High Pressure. Appl. Clay Sci. 2021, 202, 105950. [Google Scholar] [CrossRef]
- Cantador Fernandez, D.; Suescum Morales, D.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 Adsorption by Organohydrotalcites at Low Temperatures and High Pressure. Chem. Eng. J. 2022, 431, 134324. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Zhang, R.; Liu, Y.; Wang, W.; Ling, Y.; El-Toni, A.M.; Zhao, D. Periodic Mesoporous Organosilica Nanocubes with Ultrahigh Surface Areas for Efficient CO2 Adsorption. Sci. Rep. 2016, 6, 20769. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.A.O.; Siquet, C.; Sardo, M.; Mafra, L.; Pires, J.; Jorge, M.; Pinto, M.L.; Ferreira, P.; Gomes, J.R.B. Interaction of CO2 and CH4 with Functionalized Periodic Mesoporous Phenylene-Silica: Periodic DFT Calculations and Gas Adsorption Measurements. J. Phys. Chem. C 2016, 120, 3863–3875. [Google Scholar] [CrossRef]
- Cantador-Fernandez, D.; Suescum-Morales, D.; Esquivel, D.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 Adsorption by Ethane Periodic Mesoporous Organosilica at Low Temperatures and High Pressure. J. Environ. Chem. Eng. 2023, 11, 110582. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Hou, X.J. Amine-Functionalized Metal Organic Framework as a Highly Selective Adsorbent for CO2 over CO. J. Phys. Chem. C 2012, 116, 19814–19821. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon Capture and Conversion Using Metal–Organic Frameworks and MOF-Based Materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; De Luna, P.; Daff, T.D.; Rother, J.; Liu, M.; Buchanan, W.; Hawari, A.I.; Woo, T.K.; Vaidhyanathan, R. A Single-Ligand Ultra-Microporous MOF for Precombustion CO2 Capture and Hydrogen Purification. Sci. Adv. 2015, 1, e1500421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Huang, J.; Lu, J.; Zhao, D.; Lu, L.; Jin, S.; Zhou, Q. Worm-like Hierarchical Porous Carbon Derived from Bio-Renewable Lignin with High CO2 Capture Capacity. Int. J. Electrochem. Sci. 2017, 12, 11102–11107. [Google Scholar] [CrossRef]
- Pino, L.; Italiano, C.; Vita, A.; Fabiano, C.; Recupero, V. Sorbents with High Efficiency for CO2 Capture Based on Amines-Supported Carbon for Biogas Upgrading. J. Environ. Sci. 2016, 48, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Zhou, H.; Liu, X.; Qiao, W.; Long, D.; Ling, L. Carbon Dioxide Capture Using Polyethylenimine-Loaded Mesoporous Carbons. J. Environ. Sci. 2013, 25, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Casco, M.E.; Martínez-Escandell, M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Effect of the Porous Structure in Carbon Materials for CO2 Capture at Atmospheric and High-Pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef]
- Libbrecht, W.; Vandaele, K.; De Buysser, K.; Verberckmoes, A.; Thybaut, J.W.; Poelman, H.; De Clercq, J.; Van Der Voort, P. Tuning the Pore Geometry of Ordered Mesoporous Carbons for Enhanced Adsorption of Bisphenol-A. Materials 2015, 8, 1652–1665. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Al-Jumialy, A.S.M.; Fuertes, A.B.; Mokaya, R. Optimization of the Pore Structure of Biomass-Based Carbons in Relation to Their Use for CO2 Capture under Low- and High-Pressure Regimes. ACS Appl. Mater. Interfaces 2018, 10, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of Highly Ordered Carbon Molecular Sieves via Template-Mediated Structural Transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Ryoo, R.; Joo, S.H. Characterization of Ordered Mesoporous Carbons Synthesized Using MCM-48 Silicas as Templates. J. Phys. Chem. B 2000, 104, 7960–7968. [Google Scholar] [CrossRef]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of New, Nanoporous Carbon with Hexagonally Ordered Mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Martín-Martínez, J.M. Adsorción Física de Gases y Vapores Por Carbones; Universidad de Alicante, Secretariado de Publicaciones: Alicante, Spain, 1990; ISBN 84-86809-33-9. [Google Scholar]
- Vorokhta, M.; Morávková, J.; Dopita, M.; Zhigunov, A.; Šlouf, M.; Pilař, R.; Sazama, P. Effect of Micropores on CO2 Capture in Ordered Mesoporous CMK-3 Carbon at Atmospheric Pressure. Adsorption 2021, 27, 1221–1236. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.-W.; Su, W.; Zhou, Y.; Zhou, L. Studies on Ordered Mesoporous Materials for Potential Environmental and Clean Energy Applications. Appl. Surf. Sci. 2007, 253, 5650–5655. [Google Scholar] [CrossRef]
- Zhou, J.; Su, W.; Sun, Y.; Deng, S.; Wang, X. Enhanced CO2 Sorption on Ordered Mesoporous Carbon CMK-3 in the Presence of Water. J. Chem. Eng. Data 2016, 61, 1348–1352. [Google Scholar] [CrossRef]
- Su, W.; Lu, X.; Sheng, F.; Sun, Y.; Liu, C.; He, G.; Liu, J.; Wang, X. CO2 Sorption Properties over Ordered Mesoporous Carbon CMK-3 in the Presence of MDEA Solution. J. Chem. Eng. Data 2018, 63, 4779–4785. [Google Scholar] [CrossRef]
- Niebrzydowska, P.; Janus, R.; Kuśtrowski, P.; Jarczewski, S.; Wach, A.; Silvestre-Albero, A.M.; Rodríguez-Reinoso, F. A Simplified Route to the Synthesis of CMK-3 Replica Based on Precipitation Polycondensation of Furfuryl Alcohol in SBA-15 Pore System. Carbon 2013, 64, 252–261. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD). The Powder Diffraction; ICDD: Newtown Square, PA, USA, 2003. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Tóth, J. Uniform Interpretation of Gas/Solid Adsorption. Adv. Colloid Interface Sci. 1995, 55, 1–239. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Zaverina, E.D. Sorbtsiya I Struktura Aktivnykh Uglei. 6. Strukturnye Tipy Aktivnykh Uglei. J. Phys. Chem 1949, 23, 1129. [Google Scholar]
- Dubinin, M.M. Generalization of the Theory of Volume Filling of Micropores to Nonhomogeneous Microporous Structures. Carbon 1985, 23, 373–380. [Google Scholar] [CrossRef]
- Temkin, M.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Temkin, M. Adsorption Equilibrium on Heterogeneous Surfaces. J. Phys. Chem. 1946, 20, 1441. [Google Scholar]
- Serafin, J.; Dziejarski, B. Application of Isotherms Models and Error Functions in Activated Carbon CO2 Sorption Processes. Microporous Mesoporous Mater. 2023, 354, 112513. [Google Scholar] [CrossRef]
- Adsorción En Sólidos Mesoporosos. Available online: http://linux0.unsl.edu.ar/~rlopez/cap3new.pdf (accessed on 2 August 2024).
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. 786. Studies in Adsorption. Part XI. A System of Classification of Solution Adsorption Isotherms, and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. J. Chem. Soc. 1960, 14, 3973–3993. [Google Scholar] [CrossRef]
- Esfandiari, B.; Monajjemi, M. Physical Adsorption between Mono and Diatomic Gases inside of Carbon Nanotube with Respect to Potential Energy. J. Phys. Theor. Chem. 2013, 10, 31–42. [Google Scholar]
- Abd, A.A.; Othman, M.R.; Kim, J. A Review on Application of Activated Carbons for Carbon Dioxide Capture: Present Performance, Preparation, and Surface Modification for Further Improvement. Environ. Sci. Pollut. Res. Int. 2021, 28, 43329–43364. [Google Scholar] [CrossRef]
- Kirren, P.; Barka, L.; Rahmani, S.; Bondon, N.; Donzel, N.; Trens, P.; Bessière, A.; Raehm, L.; Charnay, C.; Durand, J.O. Periodic Mesoporous Organosilica Nanoparticles for CO2 Adsorption at Standard Temperature and Pressure. Molecules 2022, 27, 4245. [Google Scholar] [CrossRef]
- Liu, M.; Lu, X.; Shi, L.; Wang, F.; Sun, J. Periodic Mesoporous Organosilica with a Basic Urea-Derived Framework for Enhanced Carbon Dioxide Capture and Conversion Under Mild Conditions. ChemSusChem 2017, 10, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Lee, N.; Kim, J.; Cho, E.B.; Gunathilake, C.; Jaroniec, M. CO2 Adsorption on Amine-Functionalized Periodic Mesoporous Benzenesilicas. ACS Appl. Mater. Interfaces 2015, 7, 6792–6802. [Google Scholar] [CrossRef] [PubMed]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Férey, G. Different Adsorption Behaviors of Methane and Carbon Dioxide in the Isotypic Nanoporous Metal Terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, S.; Xian, S.; Xi, H.; Li, Z. Adsorption Equilibrium and Kinetics of CO2 on Chromium Terephthalate MIL-101. Energy Fuels 2011, 25, 835–842. [Google Scholar] [CrossRef]
- Zhou, Z.; Mei, L.; Ma, C.; Xu, F.; Xiao, J.; Xia, Q.; Li, Z. A Novel Bimetallic MIL-101(Cr, Mg) with High CO2 Adsorption Capacity and CO2/N2 Selectivity. Chem. Eng. Sci. 2016, 147, 109–117. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Kayal, S.; Chakraborty, A. Activated Carbon (Type Maxsorb-III) and MIL-101(Cr) Metal Organic Framework Based Composite Adsorbent for Higher CH4 Storage and CO2 Capture. Chem. Eng. J. 2018, 334, 780–788. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. Calcium-Looping for Thermochemical Energy Storage in Concentrating Solar Power Applications: Evaluation of the Effect of Acoustic Perturbation on the Fluidized Bed Carbonation. Chem. Eng. J. 2020, 392, 123658. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P. Sound-Assisted Fluidization for Temperature Swing Adsorption and Calcium Looping: A Review. Materials 2021, 14, 672. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-Combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-Impregnated Mesoporous Silica Nanotube as an Emerging Nanocomposite for CO2 Capture. ACS Appl. Mater. Interfaces 2016, 8, 17312–17320. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiang, J.; Tian, S.; Yan, F.; Chen, X. Polyethyleneimine–Nano Silica Composites: A Low-Cost and Promising Adsorbent for CO2 Capture. J. Mater. Chem. A 2015, 3, 2166–2175. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Q.; Shi, Y.; Cai, N. Layered Double Oxide/Activated Carbon-Based Composite Adsorbent for Elevated Temperature H2/CO2 Separation. Int. J. Hydrogen Energy 2015, 40, 9244–9253. [Google Scholar] [CrossRef]
- Scripps Institution of Oceanography, UC San Diego. The Keeling Curve. Available online: https://keelingcurve.ucsd.edu (accessed on 24 October 2022).
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon Capture and Storage (CCS): The Way Forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Lehner, M.; Hawass, Z. Giza and the Pyramids: The Definitive History; University of Chicago Press: Chicago, IL, USA, 2017; ISBN 9780226425696. [Google Scholar]

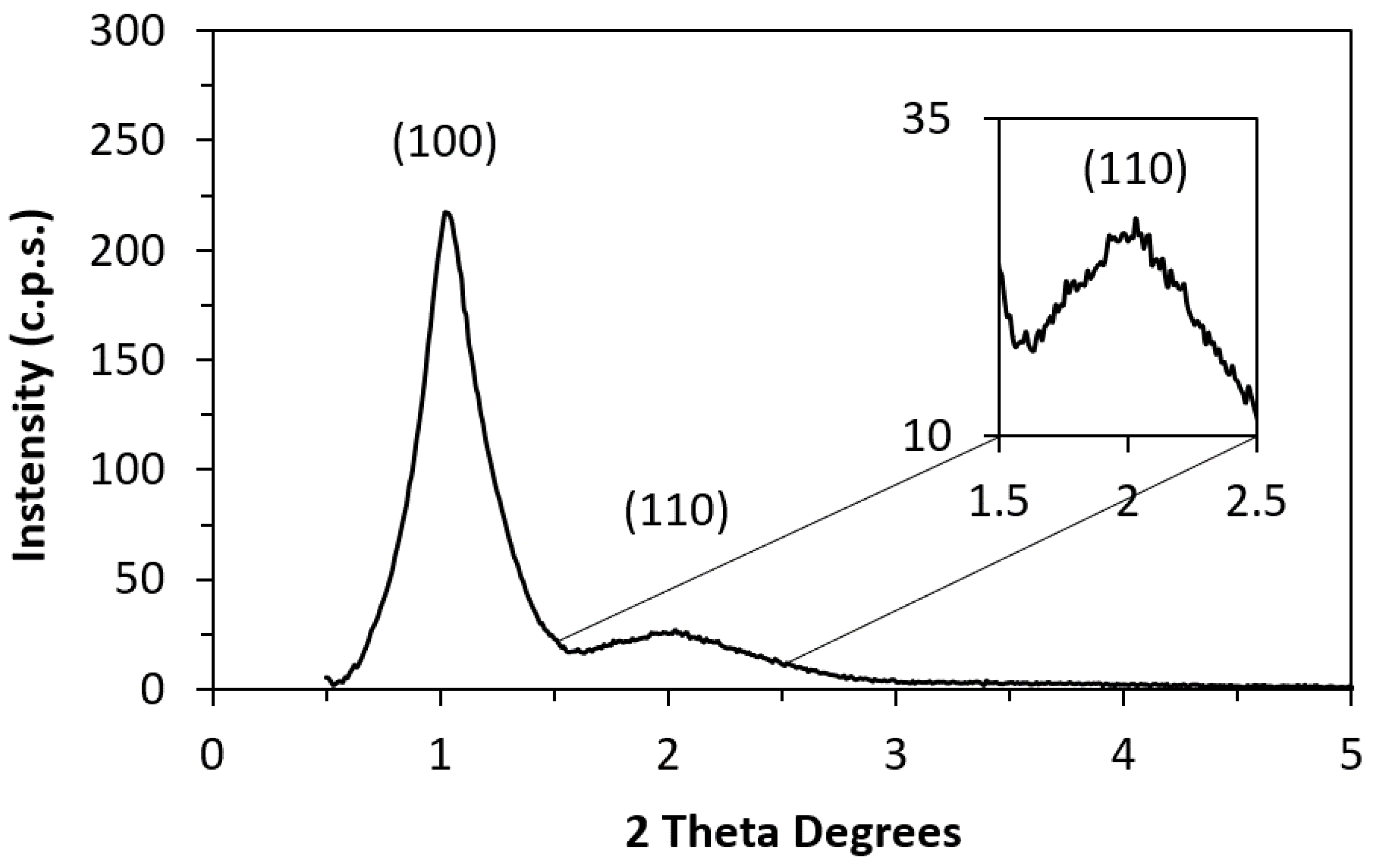

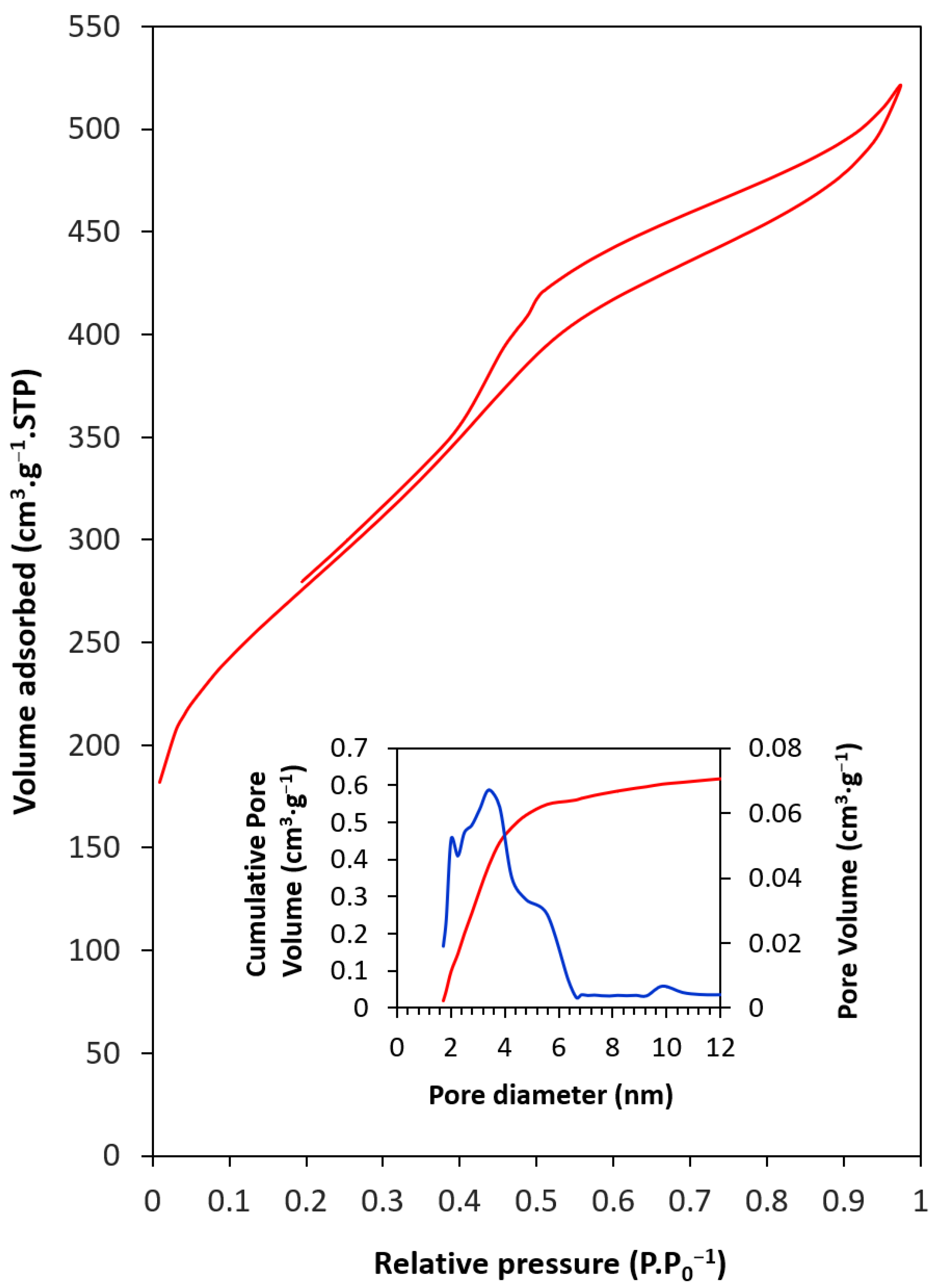
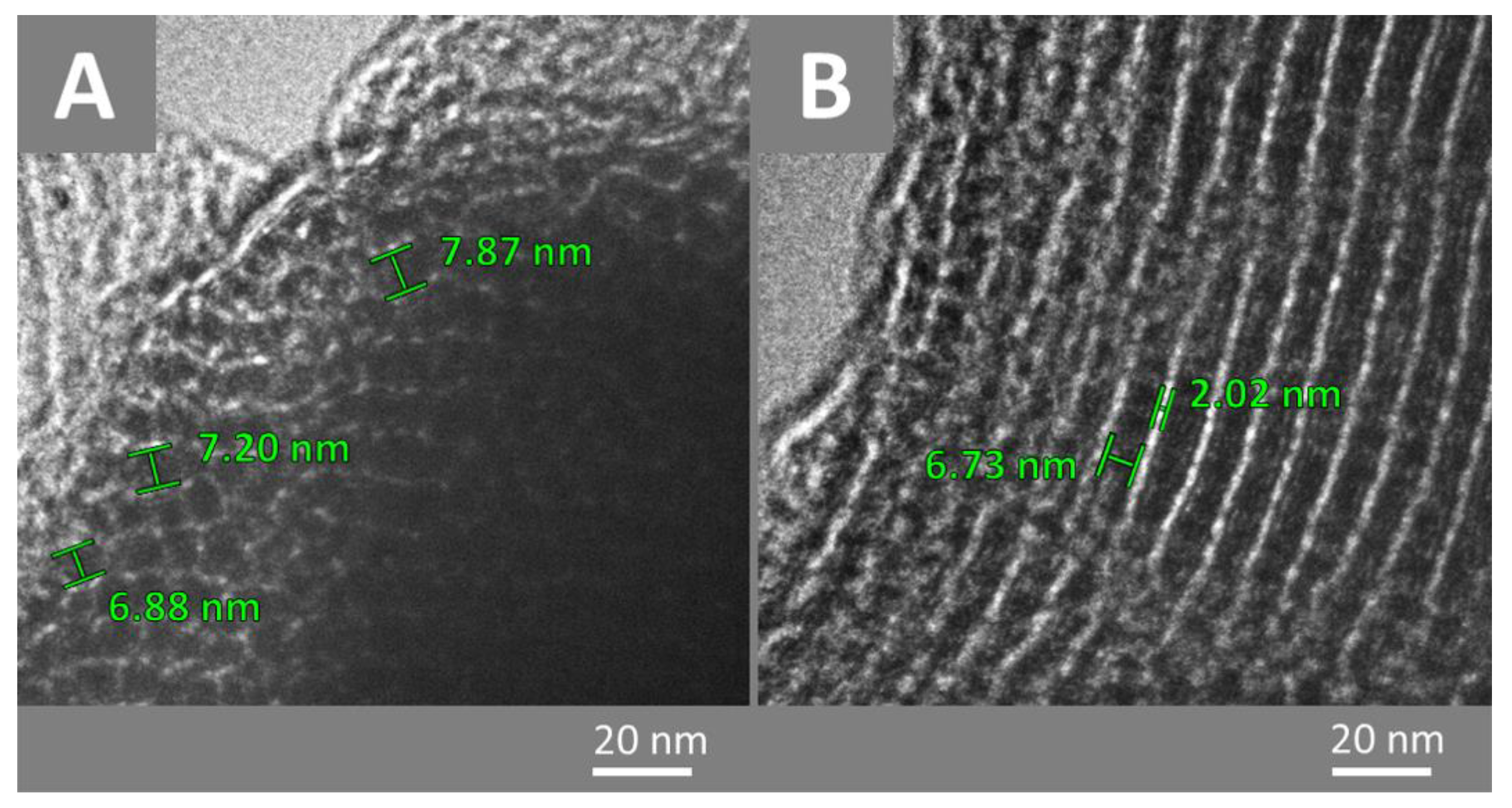

| SBET (m2·g−1) | Smc a (m2·g−1) | Vp b (cm3·g−1) | Dp c (nm) | |
|---|---|---|---|---|
| CMK-3 | 990 | 198 | 0.77 | 3.41 |
| Langmuir | Freundlich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm | XL | qmc | KL | SL | R2 | Kf | n | nf | R2 | |
| (mg·g−1) | (mg·g−1) | (atm−1) | (m2·g−1) | (mg·g−1·atm(−1/n)) | (1/n) | |||||
| CMK-3 0 °C | 560.87 | 1.073 | 522.61 | 13.66 | 1215.7 | 0.888 | 690.93 | 0.437 | 2.290 | 0.988 |
| CMK-3 10 °C | 492.67 | 1.065 | 462.61 | 15.39 | 1076.1 | 0.933 | 628.83 | 0.416 | 2.402 | 0.999 |
| CMK-3 20 °C | 413.29 | 1.050 | 393.61 | 20.00 | 915.6 | 0.947 | 564.14 | 0.392 | 2.549 | 0.997 |
| CMK-3 35 °C | 396.80 | 1.058 | 374.89 | 17.11 | 872.1 | 0.960 | 585.32 | 0.435 | 2.300 | 0.997 |
| Sips | Toth | |||||||
|---|---|---|---|---|---|---|---|---|
| qS | KS | nS | R2 | qT | KT | nT | R2 | |
| (mg·g−1) | (atm−1) | (mg·g−1) | (atm−1) | |||||
| CMK-3 0 °C | 600.25 | 1.073 | 0.460 | 1.000 | 609.05 | 2.955 | 0.170 | 1.000 |
| CMK-3 10 °C | 612.04 | 1.356 | 0.530 | 1.000 | 616.65 | 3.080 | 0.260 | 1.000 |
| CMK-3 20 °C | 530.06 | 0.647 | 0.490 | 1.000 | 529.44 | 2.267 | 0.180 | 1.000 |
| CMK-3 35 °C | 535.32 | 0.661 | 0.530 | 1.000 | 539.43 | 1.940 | 0.180 | 1.000 |
| Dubinin–Radushkevich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|
| qD | β | E | R2 | KTk | B | bTk | R2 | |
| (mg·g−1) | (mol2·kJ−2) | (kJ·mol−1) | (atm−1) | (kJ·mol−1) | ||||
| CMK-3 0 °C | 601.72 | 0.023 | 4.702 | 0.848 | 53.52 | 162.29 | 0.014 | 0.890 |
| CMK-3 10 °C | 525.74 | 0.019 | 5.109 | 0.922 | 79.30 | 129.04 | 0.018 | 0.949 |
| CMK-3 20 °C | 447.22 | 0.016 | 5.679 | 0.958 | 122.52 | 101.04 | 0.024 | 0.978 |
| CMK-3 35 °C | 428.65 | 0.015 | 5.774 | 0.968 | 104.71 | 101.52 | 0.025 | 0.976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantador-Fernandez, D.; Otero-Izquierdo, R.; Van Der Voort, P.; Jiménez, J.R.; Fernández-Rodríguez, J.M. CO2 Adsorption by CMK-3 at Low Temperatures and High Pressure to Reduce the Greenhouse Effect. Materials 2024, 17, 3845. https://doi.org/10.3390/ma17153845
Cantador-Fernandez D, Otero-Izquierdo R, Van Der Voort P, Jiménez JR, Fernández-Rodríguez JM. CO2 Adsorption by CMK-3 at Low Temperatures and High Pressure to Reduce the Greenhouse Effect. Materials. 2024; 17(15):3845. https://doi.org/10.3390/ma17153845
Chicago/Turabian StyleCantador-Fernandez, David, Rocio Otero-Izquierdo, Pascal Van Der Voort, José Ramón Jiménez, and José María Fernández-Rodríguez. 2024. "CO2 Adsorption by CMK-3 at Low Temperatures and High Pressure to Reduce the Greenhouse Effect" Materials 17, no. 15: 3845. https://doi.org/10.3390/ma17153845
APA StyleCantador-Fernandez, D., Otero-Izquierdo, R., Van Der Voort, P., Jiménez, J. R., & Fernández-Rodríguez, J. M. (2024). CO2 Adsorption by CMK-3 at Low Temperatures and High Pressure to Reduce the Greenhouse Effect. Materials, 17(15), 3845. https://doi.org/10.3390/ma17153845









