Using Zeolite Materials to Remove Pharmaceuticals from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Adsorption and Desorption Experiments
2.3. Adsorption and Desorption Experiments
3. Results and Discussion
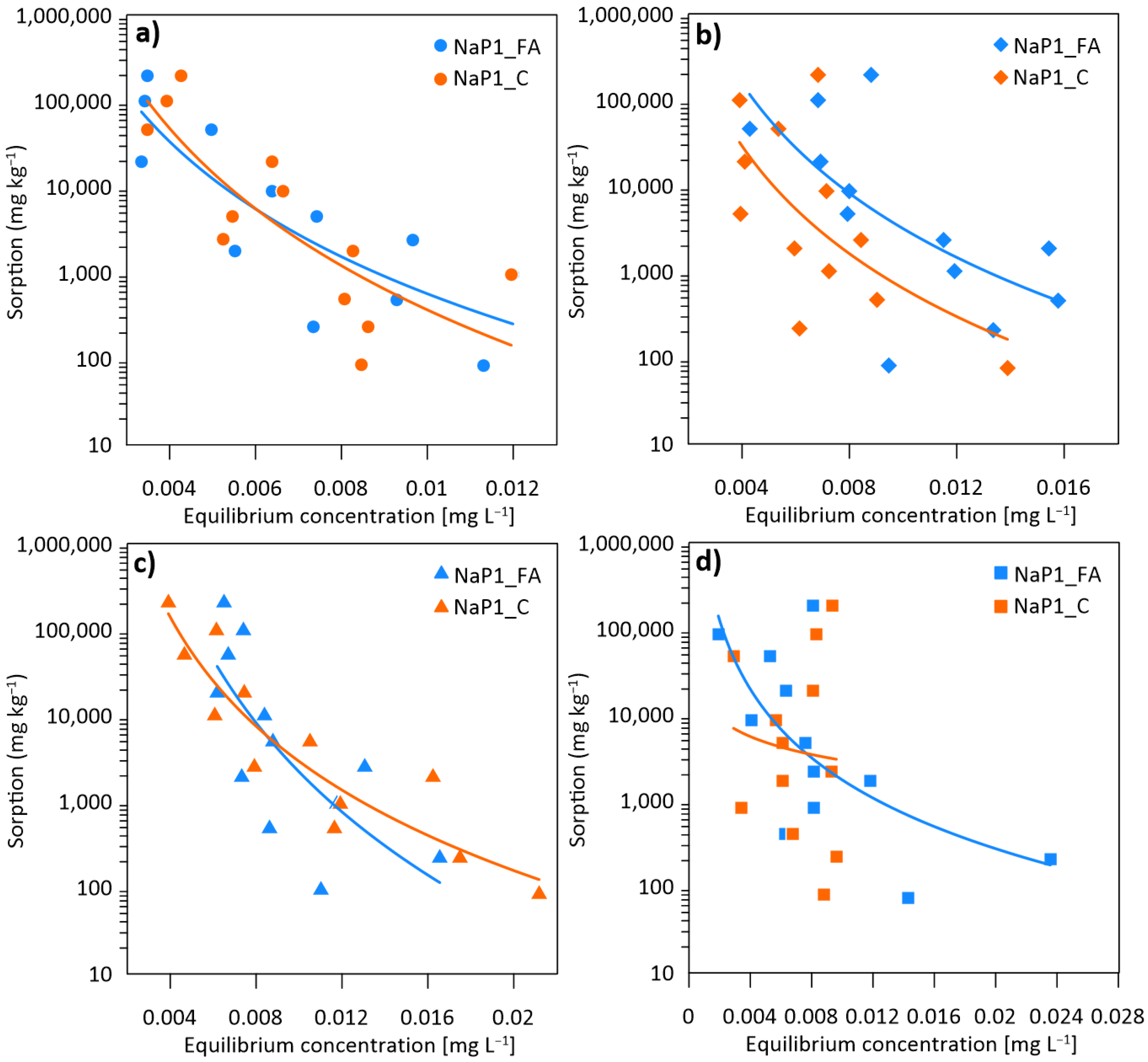
3.1. The Effect of the Initial Concentrations
3.2. The Effect of Dosage
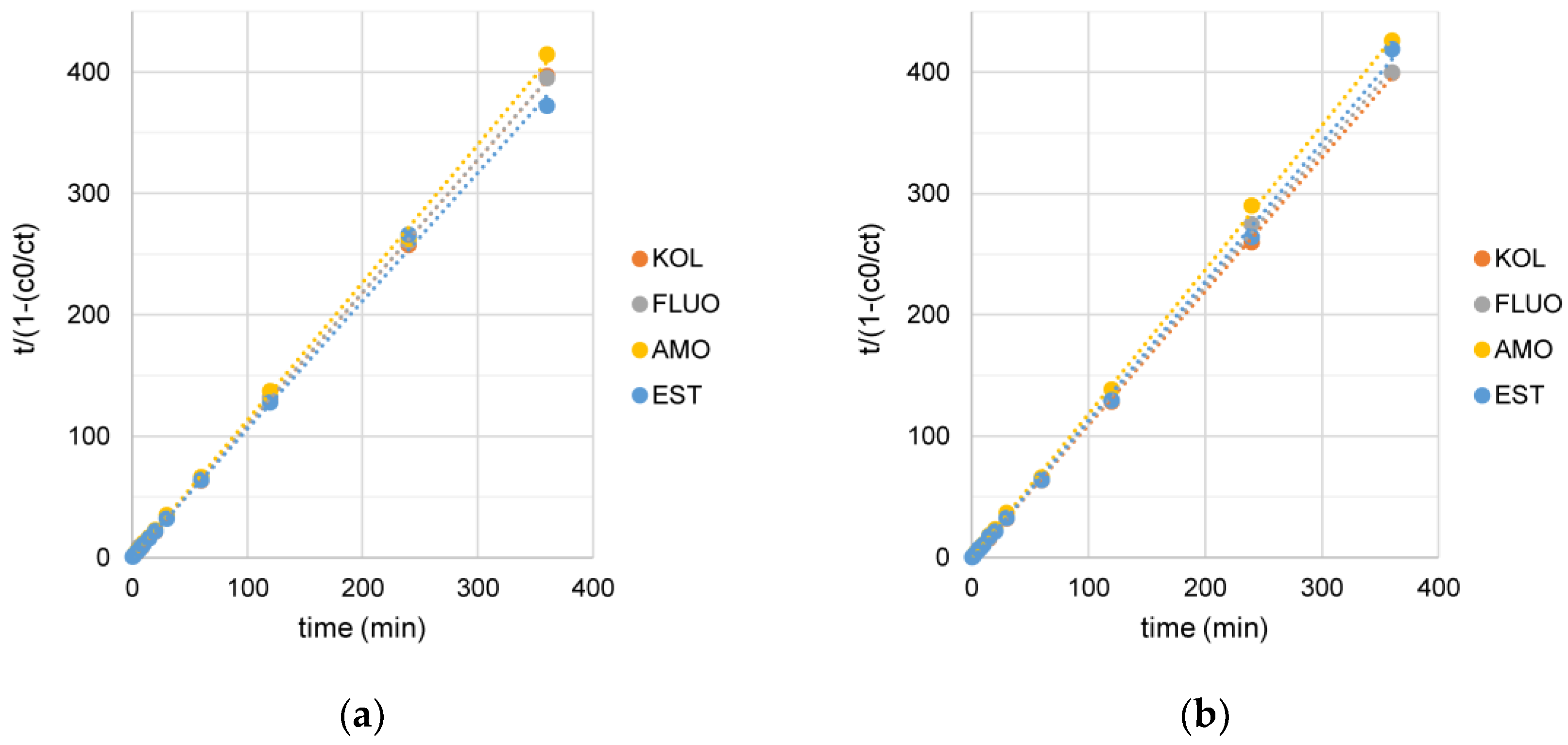
3.3. The Effect of Time Reaction
3.4. The Effect of pH
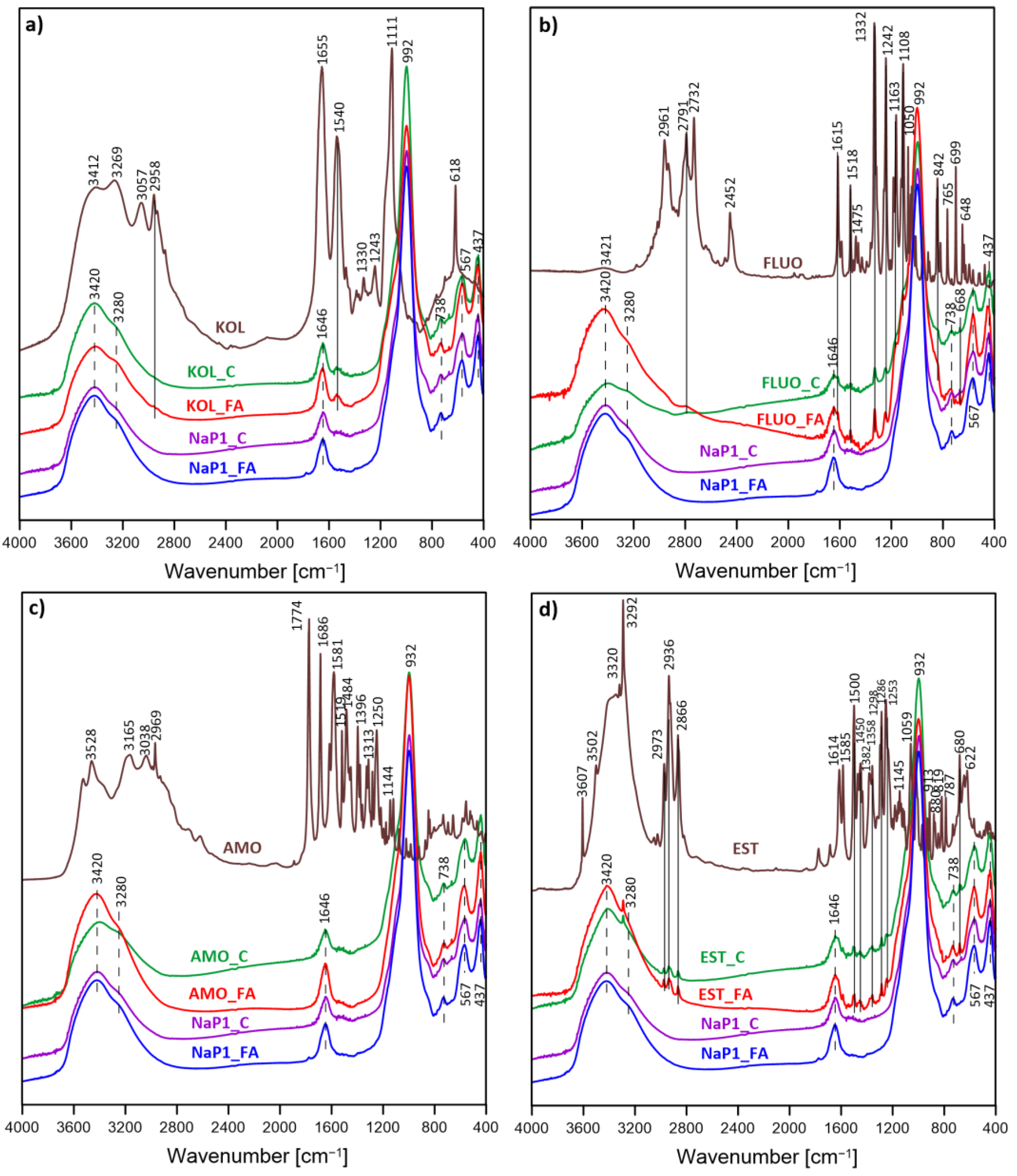
3.5. FTIR Results
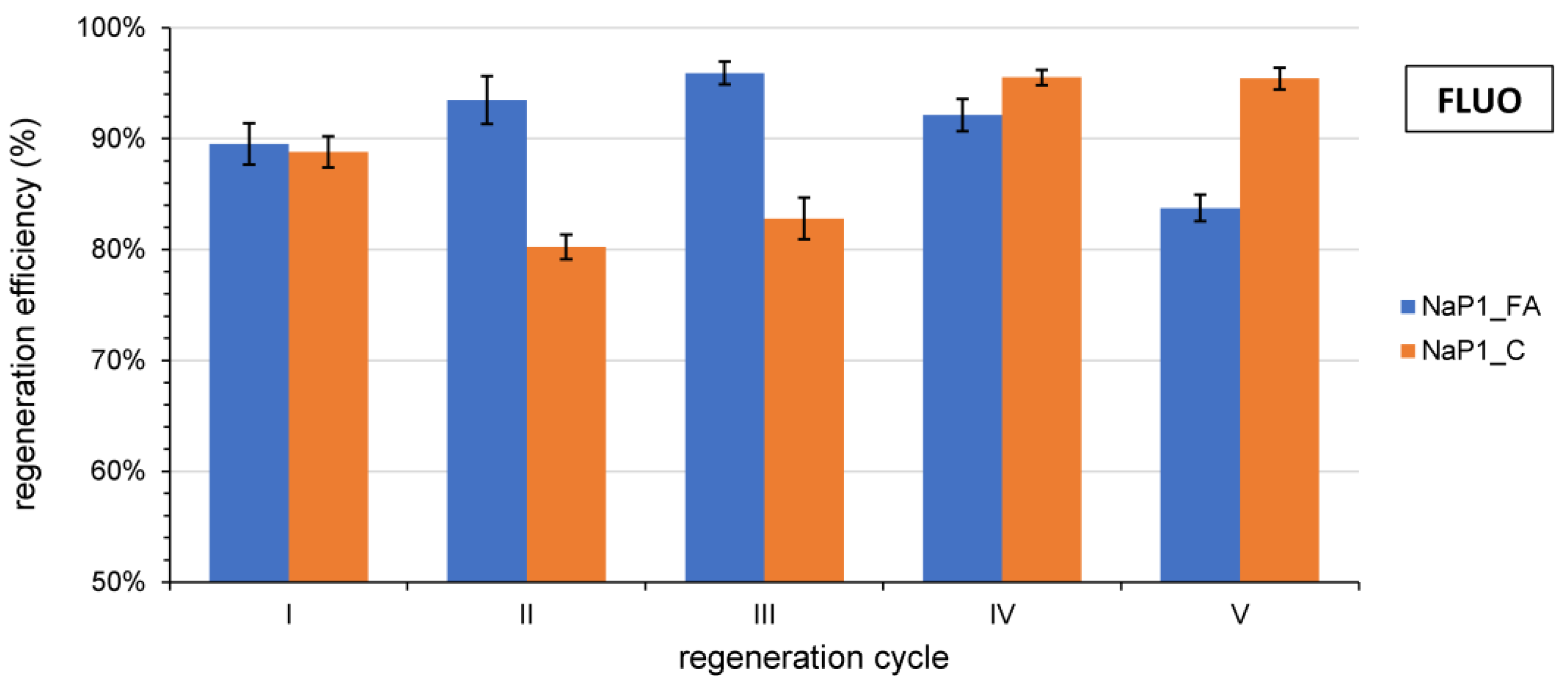
3.6. Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arlos, M.J.; Schürz, F.; Fu, Q.; Lauper, B.B.; Stamm, C.; Hollender, J. Coupling River Concentration Simulations with a Toxicokinetic Model Effectively Predicts the Internal Concentrations of Wastewater-Derived Micropollutants in Field Gammarids. Environ. Sci. Technol. 2020, 54, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, C.; Hong, Y.; Lee, W.; Chung, H.; Jeong, D.H.; Kim, H. Distribution and Removal of Pharmaceuticals in Liquid and Solid Phases in the Unit Processes of Sewage Treatment Plants. Int. J. Environ. Res. Public Health 2020, 17, 687. [Google Scholar] [CrossRef]
- Müller, M.E.; Werneburg, M.; Glaser, C.; Schwientek, M.; Zarfl, C.; Escher, B.I.; Zwiener, C. Influence of Emission Sources and Tributaries on the Spatial and Temporal Patterns of Micropollutant Mixtures and Associated Effects in a Small River. Environ. Toxicol. Chem. 2020, 39, 1382–1391. [Google Scholar] [CrossRef]
- Tosun, J.; Schaub, S.; Fleig, A. What Determines Regulatory Preferences? Insights from Micropollutants in Surface Waters. Environ. Sci. Policy 2020, 106, 136–144. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Azapagic, A. Life Cycle Environmental Impacts of Advanced Wastewater Treatment Techniques for Removal of Pharmaceuticals and Personal Care Products (PPCPs). J. Environ. Manag. 2018, 215, 258–272. [Google Scholar] [CrossRef]
- Zahn, D.; Neuwald, I.J.; Knepper, T.P. Analysis of Mobile Chemicals in the Aquatic Environment—Current Capabilities, Limitations and Future Perspectives. Anal. Bioanal. Chem. 2020, 412, 4763–4784. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Development of a Multi-Residue Analytical Methodology Based on Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) for Screening and Trace Level Determination of Pharmaceuticals in Surface and Wastewaters. Talanta 2006, 70, 678–690. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of Human Pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for the Release of Antibiotics in the Environment: A Review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- de Andrade Neto, J.B.; Josino, M.A.A.; da Silva, C.R.; de Sousa Campos, R.; do Nascimento, F.B.S.A.; Sampaio, L.S.; do Amaral Valente Sá, L.G.; de Sá Carneiro, I.; Barroso, F.D.D.; da Silva, L.J.; et al. A Mechanistic Approach to the In-Vitro Resistance Modulating Effects of Fluoxetine against Meticillin Resistant Staphylococcus Aureus Strains. Microb. Pathog. 2019, 127, 335–340. [Google Scholar] [CrossRef]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Removal of Emerging Pharmaceutical Contaminants by Adsorption in a Fixed-Bed Column: A Review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Novembre, D.; Gimeno, D.; Del Vecchio, A. Synthesis and Characterization of Na-P1 (GIS) Zeolite Using a Kaolinitic Rock. Sci. Rep. 2021, 11, 4872. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.F.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; St. Michael, F.; Cox, A.D.; et al. Colistin Resistance in Acinetobacter Baumannii Is Mediated by Complete Loss of Lipopolysaccharide Production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef]
- Sjölund, M.; Backhans, A.; Greko, C.; Emanuelson, U.; Lindberg, A. Antimicrobial Usage in 60 Swedish Farrow-to-Finish Pig Herds. Prev. Vet. Med. 2015, 121, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Vounba, P.; Rhouma, M.; Arsenault, J.; Bada Alambédji, R.; Fravalo, P.; Fairbrother, J.M. Prevalence of Colistin Resistance and Mcr-1/Mcr-2 Genes in Extended-Spectrum β-Lactamase/AmpC-Producing Escherichia Coli Isolated from Chickens in Canada, Senegal and Vietnam. J. Glob. Antimicrob. Resist. 2019, 19, 222–227. [Google Scholar] [CrossRef]
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin Update on Its Mechanism of Action and Resistance, Present and Future Challenges. Microorganisms 2020, 8, 1716. [Google Scholar] [CrossRef] [PubMed]
- Ngogang, M.P.; Ernest, T.; Kariuki, J.; Mouiche, M.M.M.; Ngogang, J.; Wade, A.; van der Sande, M.A.B. Microbial Contamination of Chicken Litter Manure and Antimicrobial Resistance Threat in an Urban Area Setting in Cameroon. Antibiotics 2021, 10, 20. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Murphy, M.; Mendola, N.; Wong, V.; Carlson, D.; Waring, L. Characterization of Pharmaceuticals and Personal Care Products in Hospital Effluent and Waste Water Influent/Effluent by Direct-Injection LC-MS-MS. Sci. Total Environ. 2015, 518–519, 459–478. [Google Scholar] [CrossRef]
- Oakes, K.D.; Coors, A.; Escher, B.I.; Fenner, K.; Garric, J.; Gust, M.; Knacker, T.; Küster, A.; Kussatz, C.; Metcalfe, C.D.; et al. Environmental Risk Assessment for the Serotonin Re Uptake Inhibitor Fluoxetine: Case Study Using the European Risk Assessment Framework. Integr. Environ. Assess. Manag. 2010, 6, 524–539. [Google Scholar] [CrossRef]
- Van Nuijs, A.L.N.; Tarcomnicu, I.; Simons, W.; Bervoets, L.; Blust, R.; Jorens, P.G.; Neels, H.; Covaci, A. Optimization and Validation of a Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry Method for the Determination of 13 Top-Prescribed Pharmaceuticals in Influent Wastewater. Anal. Bioanal. Chem. 2010, 398, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of Basic Antidepressants and Their N-Desmethyl Metabolites in Raw Sewage and Wastewater Using Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef]
- Vasskog, T.; Anderssen, T.; Pedersen-Bjergaard, S.; Kallenborn, R.; Jensen, E. Occurrence of Selective Serotonin Reuptake Inhibitors in Sewage and Receiving Waters at Spitsbergen and in Norway. J. Chromatogr. A 2008, 1185, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999-2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Tracing Pharmaceutical Residues of Different Therapeutic Classes in Environmental Waters by Using Liquid Chromatography/Quadrupole-Linear Ion Trap Mass Spectrometry and Automated Library Searching. Anal. Chem. 2009, 81, 898–912. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and Endocrine Disrupting Compounds in U.S. Drinking Water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef]
- Aksu, Z.; Tunç, Ö. Application of Biosorption for Penicillin G Removal: Comparison with Activated Carbon. Process Biochem. 2005, 40, 831–847. [Google Scholar] [CrossRef]
- Adriano, W.S.; Veredas, V.; Santana, C.C.; Gonçalves, L.R.B. Adsorption of Amoxicillin on Chitosan Beads: Kinetics, Equilibrium and Validation of Finite Bath Models. Biochem. Eng. J. 2005, 27, 132–137. [Google Scholar] [CrossRef]
- Órfão, J.J.M.; Silva, A.I.M.; Pereira, J.C.V.; Barata, S.A.; Fonseca, I.M.; Faria, P.C.C.; Pereira, M.F.R. Adsorption of a Reactive Dye on Chemically Modified Activated Carbons—Influence of PH. J. Colloid. Interface Sci. 2006, 296, 480–489. [Google Scholar] [CrossRef]
- Ali, I.; ALOthman, Z.A.; Mbianda, X.Y.; Arsh Basheer, A. Preparation and Characterization of Nanoporous Carbon for Removal of Amoxicillin Antibiotic from Water: Modelling, Kinetics and Thermodynamic Studies. Inorg. Chem. Commun. 2023, 155, 111006. [Google Scholar] [CrossRef]
- Terra, A. Robles Use of Oral Contraceptives. In Decision Making in Medicine, an Algorithmic Approach, 3rd ed.; Mushlin, S.B., Greene, H.L., Eds.; Elsevier: Philadelphia PA, USA, 2010; pp. 616–619. [Google Scholar]
- Lorphensri, O.; Intravijit, J.; Sabatini, D.A.; Kibbey, T.C.G.; Osathaphan, K.; Saiwan, C. Sorption of Acetaminophen, 17α-Ethynyl Estradiol, Nalidixic Acid, and Norfloxacin to Silica, Alumina, and a Hydrophobic Medium. Water Res. 2006, 40, 1481–1491. [Google Scholar] [CrossRef]
- The European Commission. Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/495. Implementing Decision (EU) 2018/840. 5 June 2018. Available online: https://eur-lex.europa.eu/eli/dec_impl/2018/840/oj (accessed on 31 July 2024).
- Li, J.; Jiang, L.; Liu, X.; Lv, J. Adsorption and Aerobic Biodegradation of Four Selected Endocrine Disrupting Chemicals in Soil-Water System. Int. Biodeterior. Biodegrad. 2013, 76, 3–7. [Google Scholar] [CrossRef]
- Holbrook, R.D.; Love, N.G.; Novak, J.T. Sorption of 17β-Estradiol and 17α-Ethinylestradiol by Colloidal Organic Carbon Derived from Biological Wastewater Treatment Systems. Environ. Sci. Technol. 2004, 38, 3322–3329. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Cicek, N. Removal Mechanisms of 17β-Estradiol and 17α-Ethinylestradiol in Membrane Bioreactors. Water Sci. Technol. 2012, 66, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef]
- Sangion, A.; Gramatica, P. PBT Assessment and Prioritization of Contaminants of Emerging Concern: Pharmaceuticals. Environ. Res. 2016, 147, 297–306. [Google Scholar] [CrossRef]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Franus, W. The Conversion Technology of Fly Ash into Zeolites. Clean. Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef]
- Grela, A.; Kuc, J.; Klimek, A.; Matusik, J.; Pamuła, J.; Franus, W.; Urbański, K.; Bajda, T. Erythromycin Scavenging from Aqueous Solutions by Zeolitic Materials Derived from Fly Ash. Molecules 2023, 28, 798. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.; Gonçalves, J.; Conceição, C.; Câmara, H.M.T.; Câmara, J.S. An Improved Analytical Strategy Combining Microextraction Bypacked Sorbent Combined with Ultra High Pressure Liquidchromatography for the Determination of Fluoxetine, Clomipramineand Their Active Metabolites in Human Urine. J. Chromatogr. A 2015, 1408, 30–40. [Google Scholar] [CrossRef]
- Orfanidis, A.; Gika, H.; Theodoridis, G.; Mastrogianni, O.; Raikos, N. Development of a UHPLC-MS/MS Method for the Determination of 84 Pharmaceuticals and Drugs of Abuse in Human Liver. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1151, 122192. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek, R.; Koteja, A.; Franus, W. Influence of the Reaction Time on the Crystal Structure of Na-P1 Zeolite Obtained from Coal Fly Ash Microspheres. Microporous Mesoporous Mater. 2018, 266, 102–108. [Google Scholar] [CrossRef]
- Bandura, L.; Białoszewska, M.; Malinowski, S.; Franus, W. Adsorptive Performance of Fly Ash-Derived Zeolite Modified by β-Cyclodextrin for Ibuprofen, Bisphenol A and Caffeine Removal from Aqueous Solutions—Equilibrium and Kinetic Study. Appl. Surf. Sci. 2021, 562, 150160. [Google Scholar] [CrossRef]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An Update on the Antibiotic of the 21st Century. Expert. Rev. Anti Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Hurst, M.; Lamb, H.M. Fluoxetine A Review of Its Use in Anxiety Disorders and Mixed Anxiety and Depression. CNS Drugs 2000, 14, 51–80. [Google Scholar] [CrossRef]
- Bajda, T.; Grela, A.; Kuc, J.; Klimek, A.; Matusik, J.; Pamuła, J.; Franus, W. Zeolite Materials for the Removal of Pharmaceuticals from Aqueous Medium. In Frontiers in Membrane Technology; Mannina, G., Ng, H.Y., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 42–47. [Google Scholar]
- Mariem, S.B.; Mabrouk, S.B. Moisture Sorption Isotherms and Isosteric Heats of Sorption of Tomato Slices. Am. J. Renew. Sustain. Energy 2015, 1, 140–155. [Google Scholar]
- Sevim, F.; Lacin, O.; Ediz, E.F.; Demir, F. Adsorption Capacity, Isotherm, Kinetic, and Thermodynamic Studies on Adsorption Behavior of Malachite Green onto Natural Red Clay. Environ. Prog. Sustain. Energy 2021, 40, e13471. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Ghanbary, F. A Kinetic Model for the Decolorization of C.I. Acid Yellow 23 by Fenton Process. J. Hazard. Mater. 2007, 148, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Bandura, L.; Białoszewska, M.; Leiviskä, T.; Franus, M. The Role of Zeolite Structure in Its β-Cyclodextrin Modification and Tetracycline Adsorption from Aqueous Solution: Characteristics and Sorption Mechanism. Materials 2022, 15, 6317. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Heo, J.; Yoon, Y. Oxidative Degradation of Bisphenol A and 17α-Ethinyl Estradiol by Fenton-like Activity of Silver Nanoparticles in Aqueous Solution. Chemosphere 2017, 168, 617–622. [Google Scholar] [CrossRef]
- Doǧan, M.; Özdemir, Y.; Alkan, M. Adsorption Kinetics and Mechanism of Cationic Methyl Violet and Methylene Blue Dyes onto Sepiolite. Dye. Pigment. 2007, 75, 701–713. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of Methylene Blue by a High-Efficiency Adsorbent (Polydopamine Microspheres): Kinetics, Isotherm, Thermodynamics and Mechanism Analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Chen, L.; Bai, B. Equilibrium, Kinetic, Thermodynamic, and in Situ Regeneration Studies about Methylene Blue Adsorption by the Raspberry-like TiO2@yeast Microspheres. Ind. Eng. Chem. Res. 2013, 52, 15568–15577. [Google Scholar] [CrossRef]
- Mishra, P.; Gong, Z.; Kelly, B.C. Assessing PH-Dependent Toxicity of Fluoxetine in Embryonic Zebrafish Using Mass Spectrometry-Based Metabolomics. Sci. Total Environ. 2019, 650, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, S.; Wang, X.; Liu, B.; Wang, Z. Ferrate (VI) Oxidation Is an Effective and Safe Way to Degrade Residual Colistin—A Last Resort Antibiotic—In Wastewater. Front. Vet. Sci. 2021, 8, 773089. [Google Scholar] [CrossRef]
- Sharma, E.; Kelso, C.; Zhang, S.; Guo, Y.; Sivakumar, M.; Jiang, G. Stability of Colistin and Carbapenems in Water and Wastewater. ACS ES T Water 2023, 3, 3496–3504. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Z.; Gao, S.; Luo, Z.; An, W.; Li, P.; Ping, S.; Ren, Y. Evaluating the Stability of Prescription Drugs in Municipal Wastewater and Sewers Based on Wastewater-Based Epidemiology. Sci. Total Environ. 2021, 754, 142414. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Peng, C.; Fu, S.; Qiu, Y. Adsorption-Desorption and Degradation of Colistin in Soils under Aerobic Conditions. Ecotoxicol. Environ. Saf. 2022, 243, 113989. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lago, A.; Neves, I.C.; Tavares, T. Waste-Based Biosorbents as Cost-Effective Alternatives to Commercial Adsorbents for the Retention of Fluoxetine from Water. Sep. Purif. Technol. 2020, 235, 116139. [Google Scholar] [CrossRef]
- Rad, L.R.; Anbia, M.; Vatanpour, V. Adsorption and Photocatalytic Degradation of Fluoxetine Using TiO2-Supported-Clinoptilolite, NaX and MIL-101 (Fe) Metal Organic Framework. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2154–2171. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Ledesma, B.; Laginhas, C.; Titirici, M.M. Surface Interactions during the Removal of Emerging Contaminants by Hydrochar-Based Adsorbents. Molecules 2020, 25, 2264. [Google Scholar] [CrossRef]
- Escudero-Curiel, S.; Acevedo-García, V.; Sanromán, M.Á.; Pazos, M. Eco-Approach for Pharmaceutical Removal: Thermochemical Waste Valorisation, Biochar Adsorption and Electro-Assisted Regeneration. Electrochim. Acta 2021, 389, 138694. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Moreira, M.M.; Paíga, P.; Dias, D.; Bernardo, M.; Carvalho, M.; Lapa, N.; Fonseca, I.; Morais, S.; Figueiredo, S.; et al. Evaluation of the Adsorption Potential of Biochars Prepared from Forest and Agri-Food Wastes for the Removal of Fluoxetine. Bioresour. Technol. 2019, 292, 121973. [Google Scholar] [CrossRef] [PubMed]
- Barrera, D.; Villarroel-Rocha, J.; Tara, J.C.; Basaldella, E.I.; Sapag, K. Synthesis and Textural Characterization of a Templated Nanoporous Carbon from MCM-22 Zeolite and Its Use as Adsorbent of Amoxicillin and Ethinylestradiol. Adsorption 2014, 20, 967–976. [Google Scholar] [CrossRef]
- Bilgin, N.; Bulut, E.; Sabah, E. Mechanistic Insight into Amoxicillin Removal by Natural Sepiolite. Int. J. Environ. Sci. Technol. 2023, 20, 8897–8912. [Google Scholar] [CrossRef]
- Rodrigues, D.L.C.; Machado, F.M.; Osório, A.G.; de Azevedo, C.F.; Lima, E.C.; da Silva, R.S.; Lima, D.R.; Gonçalves, F.M. Adsorption of Amoxicillin onto High Surface Area–Activated Carbons Based on Olive Biomass: Kinetic and Equilibrium Studies. Environ. Sci. Pollut. Res. 2020, 27, 41394–41404. [Google Scholar] [CrossRef] [PubMed]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Activated Carbon (as a Waste Plant Sources)–Clay Micro/Nanocomposite as Effective Adsorbent: Process Optimization for Ultrasound-Assisted Adsorption Removal of Amoxicillin Drug. Plant Arch. 2019, 19, 915–919. [Google Scholar]
- Homem, V.; Alves, A.; Santos, L. Amoxicillin Removal from Aqueous Matrices by Sorption with Almond Shell Ashes1. Int. J. Environ. Anal. Chem. 2010, 90, 1063–1084. [Google Scholar] [CrossRef]
- Bonenfant, D.; Mimeault, M.; Niquette, P.; Hausler, R. Adsorption Study of a Commonly Used Antidepressant Drug, Fluoxetine Hydrochloride, onto a Crosslinked β-Cyclodextrin-Carboxymethylcellulose Polymer. Water Sci. Technol. 2012, 66, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Jaria, G.; Calisto, V.; Gil, M.V.; Otero, M.; Esteves, V.I. Removal of Fluoxetine from Water by Adsorbent Materials Produced from Paper Mill Sludge. J. Colloid. Interface Sci. 2015, 448, 32–40. [Google Scholar] [CrossRef]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of Activated Carbon and Bentonite for Adsorption of Amoxicillin from Wastewater: Mechanisms, Isotherms and Kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar] [CrossRef]
- Dávila-Estrada, M.; Ramírez-García, J.J.; Díaz-Nava, M.C.; Solache-Ríos, M. Sorption of 17α-Ethinylestradiol by Surfactant-Modified Zeolite-Rich Tuff from Aqueous Solutions. Water Air Soil. Pollut. 2016, 227, 157. [Google Scholar] [CrossRef]
- de Almeida, A.D.S.V.; Mastelaro, V.R.; da Silva, M.G.C.; Prediger, P.; Vieira, M.G.A. Adsorption of 17α-Ethinylestradiol onto a Novel Nanocomposite Based on Graphene Oxide, Magnetic Chitosan and Organoclay (GO/MCS/OC): Kinetics, Equilibrium, Thermodynamics and Selectivity Studies. J. Water Process Eng. 2022, 47, 102729. [Google Scholar] [CrossRef]
- Joseph, L.; Zaib, Q.; Khan, I.A.; Berge, N.D.; Park, Y.G.; Saleh, N.B.; Yoon, Y. Removal of Bisphenol A and 17α-Ethinyl Estradiol from Landfill Leachate Using Single-Walled Carbon Nanotubes. Water Res. 2011, 45, 4056–4068. [Google Scholar] [CrossRef] [PubMed]
- Gallouze, H.; Akretche, D.E.; Daniel, C.; Coelhoso, I.; Crespo, J.G. Removal of Synthetic Estrogen from Water by Adsorption on Modified Bentonites. Environ. Eng. Sci. 2021, 38, 4–14. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhang, Z.; Wang, H.; Zhou, S.; Zhou, W. A Comparative Study on Fly Ash, Geopolymer and Faujasite Block for Pb Removal from Aqueous Solution. Fuel 2016, 185, 181–189. [Google Scholar] [CrossRef]
- Wang, M.; Xie, R.; Chen, Y.; Pu, X.; Jiang, W.; Yao, L. A Novel Mesoporous Zeolite-Activated Carbon Composite as an Effective Adsorbent for Removal of Ammonia-Nitrogen and Methylene Blue from Aqueous Solution. Bioresour. Technol. 2018, 268, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Provis, J.L. Quantitative Study of the Reactivity of Fly Ash in Geopolymerization by Ftir. J. Sustain. Cem. Based Mater. 2012, 1, 154–166. [Google Scholar] [CrossRef]
- Huo, Z.; Xu, X.; Lü, Z.; Song, J.; He, M.; Li, Z.; Wang, Q.; Yan, L. Synthesis of Zeolite NaP with Controllable Morphologies. Microporous Mesoporous Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Freudenthal, O.; Quilès, F.; Francius, G.; Wojszko, K.; Gorczyca, M.; Korchowiec, B.; Rogalska, E. Nanoscale Investigation of the Interaction of Colistin with Model Phospholipid Membranes by Langmuir Technique, and Combined Infrared and Force Spectroscopies. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2592–2602. [Google Scholar] [CrossRef]
- Mangal, S.; Xu, R.; Park, H.; Zemlyanov, D.; Shetty, N.; Lin, Y.W.; Morton, D.; Chan, H.K.; Li, J.; Zhou, Q.T. Understanding the Impacts of Surface Compositions on the In-Vitro Dissolution and Aerosolization of Co-Spray-Dried Composite Powder Formulations for Inhalation. Pharm. Res. 2019, 36, 6. [Google Scholar] [CrossRef]
- Mahgoub, S.M.; Essam, D.; Eldin, Z.E.; Moaty, S.A.A.; Shehata, M.R.; Farghali, A.; Abdalla, S.E.B.; Othman, S.I.; Allam, A.A.; El-Ela, F.I.A.; et al. Carbon Supported Ternary Layered Double Hydroxide Nanocomposite for Fluoxetine Removal and Subsequent Utilization of Spent Adsorbent as Antidepressant. Sci. Rep. 2024, 14, 3990. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.D.M.; Fernandes, D.F.; Figueiredo, S.A.; Freitas, O.M.; Delerue-Matos, C. Fluoxetine and Nutrients Removal from Aqueous Solutions by Phycoremediation. Int. J. Environ. Res. Public Health 2022, 19, 6081. [Google Scholar] [CrossRef] [PubMed]
- Oraby, M.; Ahmed, A.S.; Abdel-Lateef, M.A.; Mostafa, M.A.H.; Hassan, A.I. Employ FTIR Spectroscopic Method for Determination of Certain Multiple Sclerosis Medications in Plasma and Pharmaceutical Formulations. Microchem. J. 2021, 167, 106329. [Google Scholar] [CrossRef]
- San Román, M.S.; Holgado, M.J.; Salinas, B.; Rives, V. Characterisation of Diclofenac, Ketoprofen or Chloramphenicol Succinate Encapsulated in Layered Double Hydroxides with the Hydrotalcite-Type Structure. Appl. Clay Sci. 2012, 55, 158–163. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Ashok, A.; Sengupta, I.; Pal, S.K.; Chakraborty, S. Synthesis of Cu-Al LDH Nanofluid and Its Application in Spray Cooling Heat Transfer of a Hot Steel Plate. Powder Technol. 2018, 335, 285–300. [Google Scholar] [CrossRef]
- Nabipour, H.; Hosaini Sadr, M.; Thomas, N. Synthesis, Characterisation and Sustained Release Properties of Layered Zinc Hydroxide Intercalated with Amoxicillin Trihydrate. J. Exp. Nanosci. 2015, 10, 1269–1284. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Zhang, G.; Li, Z.; Yang, P.; Jing, X.; Zhang, M.; Liu, T.; Jiang, Z. Synthesis, Sustained Release Properties of Magnetically Functionalized Organic-Inorganic Materials: Amoxicillin Anions Intercalated Magnetic Layered Double Hydroxides via Calcined Precursors at Room Temperature. Solid. State Sci. 2009, 11, 1597–1601. [Google Scholar] [CrossRef]
- Moreira, C.G.; Santos, H.G.; Bila, D.M.; da Fonseca, F.V. Assessment of Fouling Mechanisms on Reverse Osmosis (RO) Membrane during Permeation of 17α-Ethinylestradiol (EE2) Solutions. Environ. Technol. 2022, 43, 3084–3096. [Google Scholar] [CrossRef]
- Simu, S.; Ledeţi, A.; Moacă, E.A.; Păcurariu, C.; Dehelean, C.; Navolan, D.; Ledeţi, I. Thermal Degradation Process of Ethinylestradiol—Kinetic Study. Processes 2022, 10, 1518. [Google Scholar] [CrossRef]
- Minaeva, V.A.; Minaev, B.F.; Hovorun, D.M. Vibrational Spectra of the Steroid Hormones, Estradiol and Estriol, Calculated by Density Functional Theory. The Role of Low-Frequency Vibrations. Ukr. Biokhim. Zh 2008, 80, 82–95. [Google Scholar]
- Osmari, T.A.; Gallon, R.; Schwaab, M.; Barbosa-Coutinho, E.; Severo, J.B.; Pinto, J.C. Statistical Analysis of Linear and Non-Linear Regression for the Estimation of Adsorption Isotherm Parameters. Adsorp. Sci. Technol. 2013, 31, 433–458. [Google Scholar] [CrossRef]
- Ho, Y.S. Isotherms for the Sorption of Lead onto Peat: Comparison of Linear and Non-Linear Methods. Polish J. Environ. Studies 2006, 15, 81–86. [Google Scholar]
- Mudhoo, A.; Pittman, C.U., Jr. The Dubinin-Radushkevich models: Dissecting the ps/p to cs/ce replacement in solid-aqueous interfacial adsorption and tracking the validity of E = 8 kJ mol–1 for assigning sorption type. Design 2023, 198, 370–402. [Google Scholar] [CrossRef]
- Tran, H.N. Applying Linear Forms of Pseudo-Second-Order Kinetic Model for Feasibly Identifying Errors in the Initial Periods of Time-Dependent Adsorption Datasets. Water 2023, 15, 1231. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Lian, Q.; Yao, L.; Ahmad, Z.U.; Gang, D.D.; Konggidinata, M.I.; Gallo, A.A.; Zappi, M.E. Enhanced Pb(II) adsorption onto functionalized ordered mesoporous carbon (OMC) from aqueous solutions: The important role of surface property and adsorption mechanism. Environ. Sci. Pollut. Res. 2020, 27, 23616–23630. [Google Scholar] [CrossRef]



| Pharmaceutical/CAS Number a | Molecular Formula | Weight/Exact Mass g mol−1 | Solubility in Water b mg L−1 | Log Kow c | pKa d |
|---|---|---|---|---|---|
| KOL/1264-72-8 | C52H98N16O13 | 1155.455/ 1154.74992724 | 283 | −2.4 | 10.23 |
| FLUO/54910-89-3 | C17H18F3NO | 309.33/ 309.134049 | 1.7 | 4.05 | 9.8 |
| AMO/26787-78-0 | C16H19N3O5S | 365.404/ 365.10454189 | 958 | 0.87 | 7.22 |
| EST/57-63-6 | C20H24O2 | 296.4034/ 296.177630004 | 6.77 | 3.67 | −1.7 |
| Model | Parameters | NaP1_C KOL | NaP1_C FLUO | NaP1_C AMO | NaP1_C EST | NaP1_FA KOL | NaP1_FA FLUO | NaP1_FA AMO | NaP1_FA EST |
|---|---|---|---|---|---|---|---|---|---|
| PFO | qe (µg g−1) exp. | 85.99 | 82.71 | 56.91 | 79.48 | 86.76 | 82.26 | 54.16 | 56.31 |
| qe (µg g−1) cal. | 3.42 | 7.21 | 1.98 | 1.56 | 3.77 | 3.09 | 3.61 | 2.44 | |
| k1 (min−1) | 0.0034 | 0.0077 | 0.0019 | −0.0014 | −0.0008 | 0.0032 | 0.0092 | 0.0028 | |
| R2 | 0.1534 | 0.3059 | 0.0278 | 0.0075 | 0.0044 | 0.0624 | 0.7318 | 0.0787 | |
| PSO | qe (µg g−1) cal. | 84.75 | 82.64 | 56.50 | 80.65 | 84.75 | 81.30 | 53.76 | 53.19 |
| k2 (g (µg min) | −0.0422 | 0.0542 | −0.4476 | −0.0177 | −0.0232 | −0.0344 | −0.0532 | −0.0113 | |
| R2 | 0.9998 | 0.9999 | 0.9993 | 0.9987 | 0.9998 | 0.9999 | 0.9997 | 0.998 | |
| BMG | 1/m | 1.9869 | 2.2952 | −22.7273 | 1.3526 | 2.2396 | −24.0964 | −2.3776 | −0.7151 |
| 1/b | 0.9466 | 0.8759 | 0.8811 | 0.9479 | 0.9358 | 0.8748 | 0.8411 | 0.8735 | |
| R2 | 0.9999 | 0.9999 | 0.9993 | 0.9987 | 0.9999 | 0.9999 | 0.9997 | 0.9989 | |
| WID | ki 1 (µg (g min1/2) | −0.7650 | 8.6716 | 0.2383 | 0.1919 | 23.1320 | 10.764 | 0.136 | −0.0877 |
| ki 2 (µg (g min1/2) | −0.0314 | −0.0475 | - | - | −0.0294 | −0.0918 | - | - | |
| c1 (µg g−1) | 77.21 | 51.80 | 53.41 | 76.95 | 53.63 | 57.26 | 52.78 | 56.56 | |
| c2 (µg g−1) | 86.04 | 83.12 | - | - | 85.85 | 83.34 | - | - | |
| R12 | 0.7632 | 0.5634 | 0.3271 | 0.1812 | 0.9810 | 0.8614 | 0.0539 | 0.0224 | |
| R22 | 0.0215 | 0.0814 | - | - | 0.0122 | 0.2590 | - | - |
| Pharmaceutical | Adsorbent Type | Adsorption Capacity [mg g−1] | References |
|---|---|---|---|
| KOL | Sandy loam | 4.467 | [60] |
| Sand | 4.467 | ||
| Loam | 4.600 | ||
| NaP1_FA | 199.3 | This work | |
| NaP1_C | 199.3 | ||
| FLUO | Synthetic zeolites (Zaolite 13x) | 32.11 | [61] |
| Synthetic zeolites (Zeolite 4A) | 21.8 | ||
| Granular activated carbon (GAC) | 233.5 | ||
| Spent coffee grounds | 14.31 | ||
| Pine bark | 6.53 | ||
| Cork waste | 4.74 | ||
| NaX/TiO2 | 87.50 | [62] | |
| Hydrochar, activated carbons | 44.1 | [63] | |
| Rice bran biochar | 67.58 | [64] | |
| Eucalyptus biochar | 6.41 | [65] | |
| NaP1_FA | 198.3 | This work | |
| NaP1_C | 198.2 | ||
| AMO | Nanoporous carbon from MCM-22 zeolite | 116.1 | [66] |
| Natural beige sepiolite | 100 | [67] | |
| Activated carbon from olive biomass prepared by muffle furnace (ACF) | 237.02 | [68] | |
| Activated carbon from olive biomass prepared by microwave-induced (ACMW) | 166.96 | ||
| Clay decorated carbon nanocomposite | 68.3 | [69] | |
| Almond shell ashes | 2.5 | [70] | |
| Chitosan beads | 8.71 | [28] | |
| Crosslinked β-cyclodextrin-carboxymethylcellulose polymer | 5.076 | [71] | |
| Paper mill sludge-based activated carbon with KOH | 191.6 | [72] | |
| Bentonite | 53.9315 | [73] | |
| NaP1_FA | 206.4 | This work | |
| NaP1_C | 206.4 | ||
| EST | Nanoporous carbon from MCM-22 zeolite | 116.1 | [66] |
| Surface-modified zeolitic tuff with hexadecyltrimethylammonium 25 mM (HDTMA) | 0.7073 | [74] | |
| Nanocomposite of graphene oxide, magnetic chitosan, and organophilic clay (GO/mCS/OC) | 50.5 | [75] | |
| Single-walled carbon nanotubes (SWCNTs) | 120 | [76] | |
| Na-bent, untreated bentonite | 4.07 | [77] | |
| Modification bentonite, NaCl treatment | 4.48 | ||
| Fe-Na-bent, Na-bent modified with FeCl2∙4H2O | 5.83 | ||
| NaP1_FA | 188.0 | This work | |
| NaP1_C | 188.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajda, T.; Grela, A.; Pamuła, J.; Kuc, J.; Klimek, A.; Matusik, J.; Franus, W.; Alagarsamy, S.K.K.; Danek, T.; Gara, P. Using Zeolite Materials to Remove Pharmaceuticals from Water. Materials 2024, 17, 3848. https://doi.org/10.3390/ma17153848
Bajda T, Grela A, Pamuła J, Kuc J, Klimek A, Matusik J, Franus W, Alagarsamy SKK, Danek T, Gara P. Using Zeolite Materials to Remove Pharmaceuticals from Water. Materials. 2024; 17(15):3848. https://doi.org/10.3390/ma17153848
Chicago/Turabian StyleBajda, Tomasz, Agnieszka Grela, Justyna Pamuła, Joanna Kuc, Agnieszka Klimek, Jakub Matusik, Wojciech Franus, Santhana Krishna Kumar Alagarsamy, Tomasz Danek, and Paweł Gara. 2024. "Using Zeolite Materials to Remove Pharmaceuticals from Water" Materials 17, no. 15: 3848. https://doi.org/10.3390/ma17153848
APA StyleBajda, T., Grela, A., Pamuła, J., Kuc, J., Klimek, A., Matusik, J., Franus, W., Alagarsamy, S. K. K., Danek, T., & Gara, P. (2024). Using Zeolite Materials to Remove Pharmaceuticals from Water. Materials, 17(15), 3848. https://doi.org/10.3390/ma17153848











