Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 2: Biochar Characterization and Application in the Remediation of Heavy Metal-Contaminated Soils
Abstract
:1. Introduction
2. Properties of MSS Biochar from Co-Pyrolysis
2.1. Physical Properties of Biochar from MSS Co-Pyrolysis
2.2. Chemical Properties of Biochar from MSS Co-Pyrolysis
2.2.1. Proximate Analysis
2.2.2. Ultimate Analysis
3. Effect of MSS Pyrolysis and Co-Pyrolysis on Micropollutants in Biochar
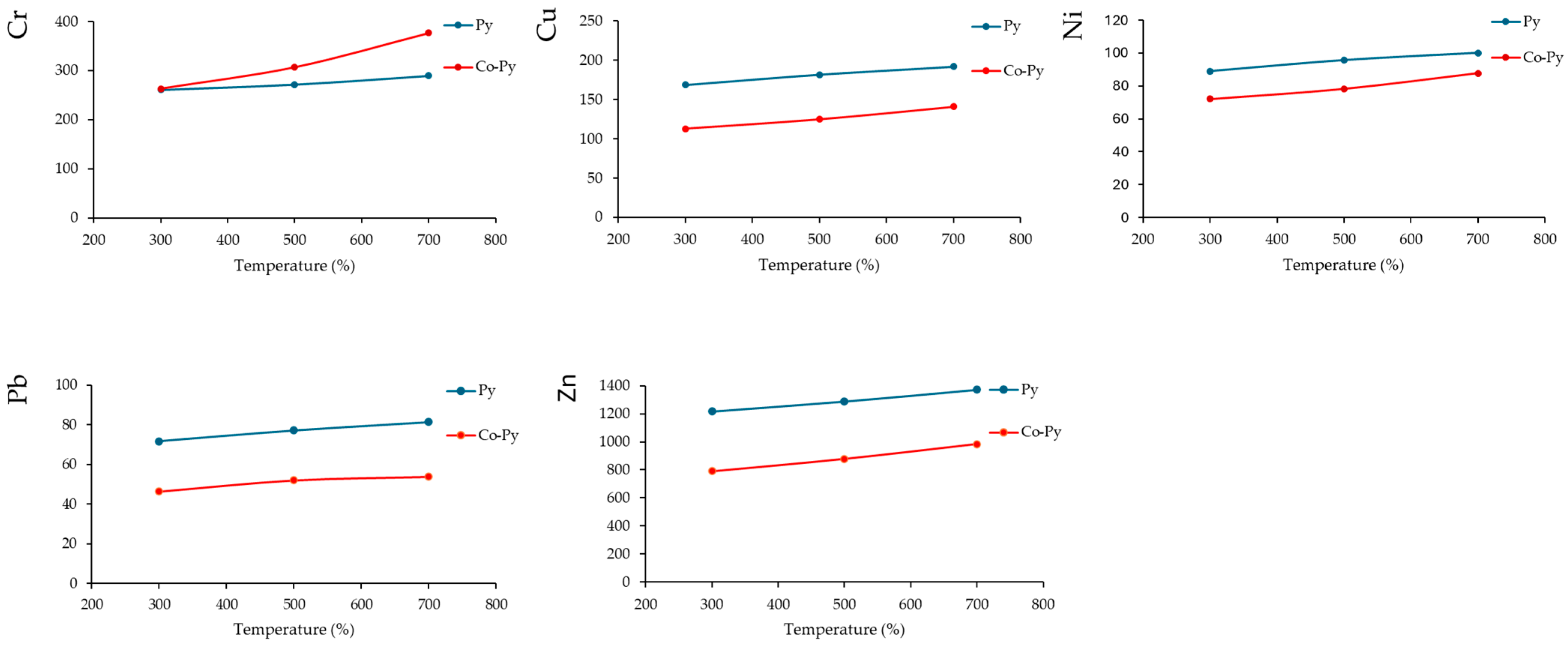
3.1. HM in MSS Biochar
3.2. PAHs in MSS Biochar
3.3. PCDD/Fs in MSS Biochar
3.4. PFASs (PFOS/PFOA) in MSS Biochar
3.5. Other Contaminants in MSS Biochar
4. Application of MSS Pyrolysis/Co-Pyrolysis Biochar in Remediation of HM-Contaminated Soils
4.1. Framework Conditions for the Remediation of HM-Contaminated Soils with MSS Pyrolysis/Co-Pyrolysis Biochar
4.1.1. Temperature
4.1.2. Biochar Rate
4.1.3. Experimental Duration
4.1.4. Soil Moisture
4.1.5. Soil pH
4.1.6. Cation Exchange Capacity (CEC) and Total Organic Carbon (TOC) of Soil
4.2. Effect of MSS Pyrolysis/Co-Pyrolysis Biochar Application on HM Immobilization in Soil
| Substrate Type/Temperature of Pyrolysis | Soil Type/Contamination Source/ Location | Crop Type | Type of HM | Experimental Conditions | Soil Indicators | Assessment of HM Immobilization | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Scale, Type of Experiment | Biochar Rate | Time of Experiment (Days) | Soil Moisture | |||||||
| Pyrolysis | ||||||||||
| MSS from mechanical-biological with/without tertiary treatment and with/without sludge stabilization/600–650 °C | Real contaminated soils/Zn-rich, smelter, As-rich, brownfield, garden/Czech Republic | Absent | As, Cd, Cu, Pb, Sb, Zn | Laboratory pots (150 mL soil and 4.5 mL MSS biochar) | 3% (v/v) | 30 | 70% of WHC | Analyses in pore water: pH, Eh, EC, anion, total carbon, DOC, major and trace elements | Exchangeable fraction for Zn, Pb, Cd, Cu with 0.11 M acetic acid, exchangeable fraction Sb and As with ammonium sulphate | [121] |
| Dewatered SS/500 °C | Real contaminated soil/reservoir area/China | Absent | Cr, Pb | Laboratory polymethyl methacrylate columns | nr | 35 | 25% or 35% | pH | HM fractionation with TSEP | [12] |
| Anaerobically digested, dewatered MSS/600 °C | Natural infertile oxisol soil (silty clay)/USA | Maize (Zea mays L., cv. Super Sweet #9) | As, Cd, Co, Cr, Ni, Pb, Se | Greenhouse bioassays (2.1 kg of soil) | 2.5% (w/w) | 35 | 50% (w/w) | Soil pH, TOC, TN, P, extractable basic cations (Ca, Mg), biomass yield | HM accumulation in above ground of maize biomass | [13] |
| MSS/550 °C | Chromosol/recycled organic agriculture site/Australia | Cherry tomato (Lycopersicon esculentum) | Ag, As, Ba, Be, Bo, Cd, Cr, Co, Cu, Ni, Pb, Sb, Sn, Se, Sr, Zn, | Glasshouse pots (6 kg soil) at 20–26 °C | 10 t/ha | 112 | nr | EC, pH, TN, extractable P, CEC, exchangeable cations, micronutrients | Accumulation of HMs in fruit by ICP according to the US EPA 6010 | [14] |
| MSS/400 °C | Real contaminated soil/mining site, battery manufacturing waste site, electroplating site, and industrial waste disposal site/China | Absent | As, Cd, Cr, Ni, Pb | Laboratory glass dishes, at different soil temperatures (4, 25, 45 °C) | 1–5% based on dry mass | 100 | 50 | pH | HM stability by acid batch extraction. HM fractionation with sequential extraction | [96] |
| MSS/300 °C, 400 °C, 500 °C | Real contaminated soil/mining site/Iran | Absent | Pb, Zn | Laboratory plastic containers (40 g of soil) | 3% (w/w) | 60 | 60% of WHC | pH, T | Estimation of bioavailable fraction with ammonium bicarbonate-diethylene triamine pentaacetic acid (AB-DTPA) method | [100] |
| MSS/500 °C | Haplic cambisol (sandy-loam)/mediterranean landscape/Spain | Absent | Cd, Cu, Ni, Pb, Zn | Laboratory glass vessels (100 g of soil) at 28 °C | 4% and 8% | 200 | 60% | TMC, soil perspiration (cumulative CO2 evolution) | Estimation of bioavailable fraction with diethylenetriaminepentaacetic acid-CaCl2-triethanolamine (DTPA) | [106] |
| MSS/400 °C | Spiked Alumi-Plinthic Acrisols/agricultural site/China | Absent | Cr, Cu, Mn, Zn | Laboratory pots (100 g of soil) | 5% (w/w) | 360 | 60% of WHC | pH | HM fractionation with BCR sequential extraction | [105] |
| MSS/500 °C | Real contaminated soil/mine site (acidic and basic)/Spain | Sarcocornia fruticosa (L.) | Cd, Pb, Zn | Laboratory PVC columns | 6% based on dried weight | 303 | nr | pH, Eh, T, EC, WSOC evolution | HM fractionation with BCR sequential extraction | [104] |
| MSS/650 °C | Real contaminated soil/composite soil/China | Maize (Zea mays L.) | Cr, Ni, Pb | Laboratory pots (1 kg of soil) | 2% (w/w) | 90 | 60–70% FC | nr | Modified sequential extraction procedure (8-steps). Acid digestion method used to measure HM in plants parts | [107] |
| MSS/300 °C, 500 °C | Real contaminated soil/paddy soil/rice field/China | Absent | Cd, Pb | Laboratory containers (600 g of soil) at 25 °C | 1%, 3%, 5% | 60 | 70% | nr | Estimation of bioavailable fraction with DTPA and HM fractionation with BCR sequential extraction | [99] |
| Co-pyrolysis | ||||||||||
| MSS + Rice straw/400 °C | Real contaminated soil/paddy soil (clay)/China | Absent | Cd | Greenhouse plastic pots (500 g of soil) at 25 °C | 3% (w/w) | 60 | 60% | pH, CEC, SOC, AP, chemical speciation of Cd | HM fractionation with TSEP, estimation of bioavailable fraction with 0.1 mol/L CaCl2 solution | [6] |
| MSS + Cotton stalks/650 °C | Real contaminated soil/sandy loam/farm near a Pb–Zn mining site/China | Ryegrass | Cu, Pb, Zn | Laboratory pots at 18–23 °C | 7.5 t/ha | 60 | 60% of WHC | pH, EC, CEC, AP, AK, AN, TOC | Estimation of fraction with DTPA and CaCl2 and mobility, HM fractionation with BCR sequential extraction | [108] |
4.2.1. MSS Biochar from Pyrolysis
4.2.2. MSS Biochar from Co-Pyrolysis
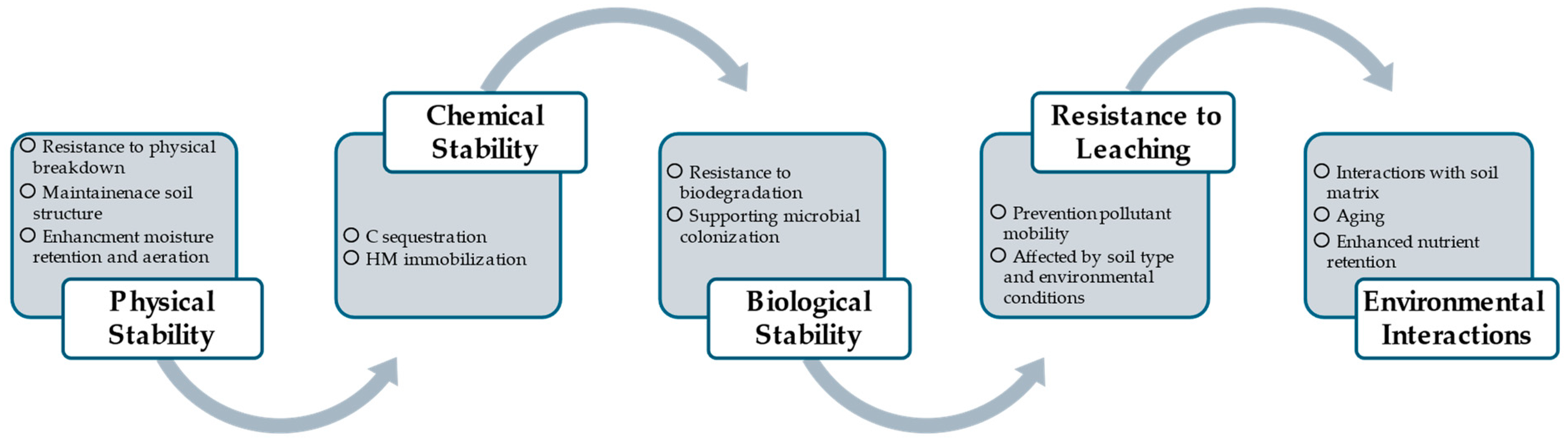
4.2.3. Long-Term Stability of MSS Biochar in Soil
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Yang, T.; Lai, F.; Wu, G. Co-Pyrolysis of Sewage Sludge and Sawdust/Rice Straw for the Production of Biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ruan, R.; Bilal, M.; Khan, N.A.; Awasthi, M.K.; Amer, M.A.; Leng, L.; Hamouda, M.A.; Vo, D.N.; Li, J. Co-Pyrolysis of Sewage Sludge and Biomass for Stabilizing Heavy Metals and Reducing Biochar Toxicity: A Review. Environ. Chem. Lett. 2023, 21, 1231–1250. [Google Scholar] [CrossRef]
- Yi, L.M.; Xin, Y.H.; Yuan, S.X. Sludge-Derived Biochar: A Review on the Influence of Synthesis Conditions on Environmental Risk Reduction and Removal Mechanism of Wastewater Pollutants. Arch. Environ. Prot. 2023, 49, 3–15. [Google Scholar]
- Li, B.; Ding, S.; Fan, H.; Ren, Y. Experimental Investigation into the Effect of Pyrolysis on Chemical Forms of Heavy Metals in Sewage Sludge Biochar (SSB), with Brief Ecological Risk Assessment. Materials 2021, 14, 447. [Google Scholar] [CrossRef]
- Kujawska, J. Content of Heavy Metals in Various Biochar and Assessment Environmental Risk. J. Ecol. Eng. 2023, 24, 287–295. [Google Scholar] [CrossRef]
- Sun, J.; Wang, P.; Guo, Y.; Hu, B.; Wang, X. Effect of Biochar Derived from Co-Pyrolysis of Sewage Sludge and Rice Straw on Cadmium Immobilization in Paddy Soil. Environ. Sci. Pollut. Res. 2023, 30, 74808–74819. [Google Scholar] [CrossRef]
- Biney, M.; Gusiatin, M.Z. Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 1: Evaluating Types of Co-Substrates and Co-Pyrolysis Conditions. Materials 2024, 17, 3603. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of Bamboo and Rice Straw Biochars on the Mobility and Redistribution of Heavy Metals (Cd, Cu, Pb and Zn) in Contaminated Soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, L.; Liu, K.; Wang, J.; Zhu, H.; Song, Q.; Shu, X. Co-Pyrolysis of Sewage Sludge and Cotton Stalks. Waste Manag. 2019, 89, 430–438. [Google Scholar] [CrossRef]
- Gbouri, I.; Yu, F.; Wang, X.; Wang, J.; Cui, X.; Hu, Y.; Yan, B.; Chen, G. Co-Pyrolysis of Sewage Sludge and Wetland Biomass Waste for Biochar Production: Behaviors of Phosphorus and Heavy Metals. Int. J. Environ. Res. Public Health 2022, 19, 2818. [Google Scholar] [CrossRef]
- Jin, J.; Wang, M.; Cao, Y.; Wu, S.; Liang, P.; Li, Y.; Zhang, J.; Zhang, J.; Wong, M.H.; Shan, S.; et al. Cumulative Effects of Bamboo Sawdust Addition on Pyrolysis of Sewage Sludge: Biochar Properties and Environmental Risk from Metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ni, Q.; Wu, Y.; Fu, C.; Ping, W.; Bai, H.; Li, M.; Huang, H.; Liu, H. Passivation and Remediation of Pb and Cr in Contaminated Soil by Sewage Sludge Biochar Tubule. Environ. Sci. Pollut. Res. 2021, 28, 49102–49111. [Google Scholar] [CrossRef] [PubMed]
- Deenik, J.; Cooney, M. The Potential Benefits and Limitations of Corn Cob and Sewage Sludge Biochars in an Infertile Oxisol. Sustainability 2016, 8, 131. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Yin Chan, K.; Nelson, P.F. Agronomic Properties of Wastewater Sludge Biochar and Bioavailability of Metals in Production of Cherry Tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Chagas, J.K.M.; de Figueiredo, C.C.; Paz-Ferreiro, J. Sewage Sludge Biochars Effects on Corn Response and Nutrition and on Soil Properties in a 5-Yr Field Experiment. Geoderma 2021, 401, 115323. [Google Scholar] [CrossRef]
- Yue, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. Efficiency of Sewage Sludge Biochar in Improving Urban Soil Properties and Promoting Grass Growth. Chemosphere 2017, 173, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wang, Y.; Sheng, Y.; Wu, C.; Lu, S. Ameliorating Soil Acidity and Physical Properties of Two Contrasting Texture Ultisols with Wastewater Sludge Biochar. Environ. Sci. Pollut. Res. 2018, 25, 25726–25733. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.; Guo, M. Efficacy of Sewage Sludge Derived Biochar on Enhancing Soil Health and Crop Productivity in Strongly Acidic Soil. Front. Soil Sci. 2023, 3, 1066547. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil Biochemical Activities and the Geometric Mean of Enzyme Activities after Application of Sewage Sludge and Sewage Sludge Biochar to Soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and Negative Carbon Mineralization Priming Effects among a Variety of Biochar-Amended Soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Ali, L.; Palamanit, A.; Techato, K.; Ullah, A.; Chowdhury, M.S.; Phoungthong, K. Characteristics of Biochars Derived from the Pyrolysis and Co-Pyrolysis of Rubberwood Sawdust and Sewage Sludge for Further Applications. Sustainability 2022, 14, 3829. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wang, M.; Naidu, R.; Liu, Y.; Man, Y.B.; Liang, X.; Wong, M.H.; Christie, P.; Zhang, Y.; et al. Co-Pyrolysis of Sewage Sludge and Rice Husk/ Bamboo Sawdust for Biochar with High Aromaticity and Low Metal Mobility. Environ. Res. 2020, 191, 110034. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.C.; Li, Z.; Chen, Z.; Wang, Y. Co-Pyrolysis of Sewage Sludge and Organic Fractions of Municipal Solid Waste: Synergistic Effects on Biochar Properties and the Environmental Risk of Heavy Metals. J. Hazard. Mater. 2021, 412, 125200. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Cui, M.-H.; Ren, Y.-G.; Guo, J.-C.; Zheng, Z.-Y.; Liu, H. Towards Understanding the Mechanism of Heavy Metals Immobilization in Biochar Derived from Co-Pyrolysis of Sawdust and Sewage Sludge. Bull. Environ. Contam. Toxicol. 2020, 104, 489–496. [Google Scholar] [CrossRef]
- Wang, S.; Mandfloen, P.; Jönsson, P.; Yang, W. Synergistic Effects in the Copyrolysis of Municipal Sewage Sludge Digestate and Salix: Reaction Mechanism, Product Characterization and Char Stability. Appl. Energy 2021, 289, 116687. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast Co-Pyrolysis of Sewage Sludge and Lignocellulosic Biomass in a Conical Spouted Bed Reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Walnut Shell for Ammonium and Phosphate Adsorption from Water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, X.; Zhu, H.; Xie, L.; Cheng, S.; Zhang, Y. Characteristics of Biochars Prepared by Co-Pyrolysis of Sewage Sludge and Cotton Stalk Intended for Use as Soil Amendments. Environ. Technol. 2020, 41, 1347–1357. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, S.; Wu, B.; Pi, M.; Xiong, Y.; Zhang, H. Co-Pyrolysis of Sewage Sludge and Rice Straw: Thermal Behavior and Char Characteristic Evaluations. Energy Fuels 2020, 34, 607–615. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Y.; Lan, G.; Xia, Y.; Yan, B.; Li, Y.; Zhang, Y.; Yu, Y.; Fu, C.; Xu, A.; et al. Effect of Co-Pyrolysis of Sewage Sludge with Different Plastics on the Nitrogen, Sulfur, and Chlorine Releasing Characteristics and the Heavy Metals Ecological Risk of Biochar. J. Environ. Chem. Eng. 2023, 11, 110406. [Google Scholar] [CrossRef]
- Ruiz-Gómez, N.; Quispe, V.; Ábrego, J.; Atienza-Martínez, M.; Murillo, M.B.; Gea, G. Co-Pyrolysis of Sewage Sludge and Manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef]
- Fachini, J.; de Figueiredo, C.C. Pyrolysis of Sewage Sludge: Physical, Chemical, Morphological and Mineralogical Transformations. Braz. Arch. Biol. Technol. 2022, 65, 22210592. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.-F.; Pan, X.-W.; Tan, J.-Y.; Yang, S.-S.; Wu, J.-T.; Chen, C.; Yuan, Y.; Ren, N.-Q. Sewage Sludge Derived Biochar for Environmental Improvement: Advances, Challenges, and Solutions. Water Res. X 2023, 18, 100167. [Google Scholar] [CrossRef]
- Yu, F.; Lv, H.; Fan, L.; Chen, L.; Hu, Y.; Wang, X.; Guo, Q.; Cui, X.; Zhou, N.; Jiao, L. Co-Pyrolysis of Sewage Sludge and Poplar Sawdust under Controlled Low-Oxygen Conditions: Biochar Properties and Heavy Metals Behavior. J. Anal. Appl. Pyrolysis 2023, 169, 105868. [Google Scholar] [CrossRef]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Tomozei, C.; Panainte-Lehadus, M.; Mosnegutu, E. Evaluation of the Use of Sewage Sludge Biochar as a Soil Amendment—A Review. Sustainability 2022, 14, 5309. [Google Scholar] [CrossRef]
- Benavente, V.; Pérez, C.; Jansson, S. Co-Hydrothermal Carbonization of Microalgae and Digested Sewage Sludge: Assessing the Impact of Mixing Ratios on the Composition of Primary and Secondary Char. Waste Manag. 2024, 174, 429–438. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P. The Conversion of Sewage Sludge into Biochar Reduces Polycyclic Aromatic Hydrocarbon Content and Ecotoxicity but Increases Trace Metal Content. Biomass Bioenergy 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Xie, L.; Zhu, H.; Shu, X. Influence of the Addition of Cotton Stalk during Co-Pyrolysis with Sewage Sludge on the Properties, Surface Characteristics, and Ecological Risks of Biochars. J. Therm. Sci. 2019, 28, 755–762. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Cui, M.-H.; Guo, J.-C.; Du, J.-J.; Zheng, Z.-Y.; Liu, H. Effects of Co-Pyrolysis of Rice Husk and Sewage Sludge on the Bioavailability and Environmental Risks of Pb and Cd. Environ. Technol. 2021, 42, 2304–2312. [Google Scholar] [CrossRef]
- Wang, X.; Wei-Chung Chang, V.; Li, Z.; Song, Y.; Li, C.; Wang, Y. Co-Pyrolysis of Sewage Sludge and Food Waste Digestate to Synergistically Improve Biochar Characteristics and Heavy Metals Immobilization. Waste Manag. 2022, 141, 231–239. [Google Scholar] [CrossRef]
- Bai, J.; Fu, X.; Lv, Q.; Chen, F.; Yang, Y.; Wang, J.; Gan, W.; Deng, F.; Zhu, C. Co-Pyrolysis of Sewage Sludge and Pinewood Sawdust: The Synergistic Effect and Bio-Oil Characteristics. Biomass Convers. Biorefin. 2023, 13, 9205–9212. [Google Scholar] [CrossRef]
- Kończak, M.; Oleszczuk, P. Co-Pyrolysis of Sewage Sludge and Biomass in Carbon Dioxide as a Carrier Gas Affects the Total and Leachable Metals in Biochars. J. Hazard. Mater. 2020, 400, 123144. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Pan, L.; Li, C.; You, F.; Wang, Y. Ciprofloxacin Adsorption by Biochar Derived from Co-Pyrolysis of Sewage Sludge and Bamboo Waste. Environ. Sci. Pollut. Res. 2020, 27, 22806–22817. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, Z.; Du, H.; Zheng, X.; Zhang, B.; Jin, B. Co-Pyrolysis of Sludge and Kaolin/Zeolite in a Rotary Kiln: Analysis of Stabilizing Heavy Metals. Front. Environ. Sci. Eng. 2022, 16, 85. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Herath, H.M.S.K. Polycyclic Aromatic Hydrocarbons (PAHs) in Biochar—Their Formation, Occurrence and Analysis: A Review. Org. Geochem. 2017, 114, 1–11. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Pyrolysis of Hydrocarbons. In Pyrolysis of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–161. [Google Scholar]
- Luo, F.; Song, J.; Xia, W.; Dong, M.; Chen, M.; Soudek, P. Characterization of Contaminants and Evaluation of the Suitability for Land Application of Maize and Sludge Biochars. Environ. Sci. Pollut. Res. 2014, 21, 8707–8717. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, L.; Myneni, S.C.B.; Deng, Y. Leaching of Polycyclic Aromatic Hydrocarbons (PAHs) from Sewage Sludge-Derived Biochar. Chem. Eng. J. 2019, 373, 840–845. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P. Effect of Pyrolysis Temperatures on Freely Dissolved Polycyclic Aromatic Hydrocarbon (PAH) Concentrations in Sewage Sludge-Derived Biochars. Chemosphere 2016, 153, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the Total and Bioavailable Polycyclic Aromatic Hydrocarbons and Dioxins in Biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef]
- Hilber, I.; Mayer, P.; Gouliarmou, V.; Hale, S.E.; Cornelissen, G.; Schmidt, H.P.; Bucheli, T.D. Bioavailability and Bioaccessibility of Polycyclic Aromatic Hydrocarbons from (Post-Pyrolytically Treated) Biochars. Chemosphere 2017, 174, 700–707. [Google Scholar] [CrossRef]
- Home—International Biochar Initiative. Available online: https://biochar-international.org/ (accessed on 6 May 2024).
- EBC and WBC Guidelines & Documents. Available online: https://www.european-biochar.org/en/ct/2-EBC-and-WBC-guidelines-documents (accessed on 24 January 2024).
- Fan, Z.; Zhou, X.; Peng, Z.; Wan, S.; Gao, Z.F.; Deng, S.; Tong, L.; Han, W.; Chen, X. Co-Pyrolysis Technology for Enhancing the Functionality of Sewage Sludge Biochar and Immobilizing Heavy Metals. Chemosphere 2023, 317, 137929. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, M.; Passaseo, D.; Bonaccorso, G.; Fichera, M.; Renai, L.; Venturini, L.; Colzi, I.; Fibbi, D.; Del Bubba, M. Biochar from Co-Pyrolysis of Biological Sludge and Sawdust in Comparison with the Conventional Filling Media of Vertical-Flow Constructed Wetlands for the Treatment of Domestic-Textile Wastewater. Water Sci. Technol. 2024, 89, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Kończak, M.; Pan, B.; Ok, Y.S.; Oleszczuk, P. Carbon Dioxide as a Carrier Gas and Mixed Feedstock Pyrolysis Decreased Toxicity of Sewage Sludge Biochar. Sci. Total Environ. 2020, 723, 137796. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Hilber, I.; Graham, M.C.; Masěk, O. Composition of PAHs in Biochar and Implications for Biochar Production. ACS Sustain. Chem. Eng. 2022, 10, 6755–6765. [Google Scholar] [CrossRef] [PubMed]
- Schlederer, F.; Martín-Hernández, E.; Vaneeckhaute, C. Micropollutants in Biochar Produced from Sewage Sludge: A Systematic Review on the Impact of Pyrolysis Operating Conditions. Waste Manag. 2024, 174, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Hilber, I.; Bastos, A.C.; Loureiro, S.; Soja, G.; Marsz, A.; Cornelissen, G.; Bucheli, T.D. The Different Faces of Biochar: Contamination Risk versus Remediation Tool. J. Environ. Eng. Landsc. Manag. 2017, 25, 86–104. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, B.; Wei, J.; Zhang, T.; Yu, Q.; Zhang, W. Effects of Chlorine on the Volatilization of Heavy Metals during the Co-Combustion of Sewage Sludge. Waste Manag. 2017, 62, 204–210. [Google Scholar] [CrossRef]
- Han, H.; Buss, W.; Zheng, Y.; Song, P.; Khalid Rafiq, M.; Liu, P.; Mašek, O.; Li, X. Contaminants in Biochar and Suggested Mitigation Measures—A Review. Chem. Eng. J. 2022, 429, 132287. [Google Scholar] [CrossRef]
- Lopes, E.J.; Okamura, L.A.; Yamamoto, C.I. Formation of Dioxins and Furans during Municipal Solid Waste Gasification. Braz. J. Chem. Eng. 2015, 32, 87–97. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Metcalf, J. The Formation of Polyaromatic Hydrocarbons and Dioxins during Pyrolysis: A Review of the Literature with Descriptions of Biomass Composition, Fast Pyrolysis Technologies and Thermochemical Reactions; Washington State University Extention: Pullman, WA, USA, 2008. [Google Scholar]
- Stanmore, B.R. The Formation of Dioxins in Combustion Systems. Combust. Flame 2004, 136, 398–427. [Google Scholar] [CrossRef]
- Hu, Z.B.; Saman Wijesekara, R.G.; Navarro, R.R.; Wu, D.Y.; Zhang, D.L.; Matsumura, M.; Kong, H.N. Removal of PCDD/Fs and PCBs from Sediment by Oxygen Free Pyrolysis. J. Environ. Sci. 2006, 18, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Wielgosiński, G. The Reduction of Dioxin Emissions from the Processes of Heat and Power Generation. J. Air Waste Manag. Assoc. 2011, 61, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Nganai, S.; Lomnicki, S. Surface Catalysed PCDD/F Formation from Precursors—High PCDF Yield Does Not Indicate de Novo Mechanism! Int. J. Environ. Pollut. 2017, 61, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Sobol, Ł.; Dyjakon, A.; Soukup, K. Dioxins and Furans in Biochars, Hydrochars and Torreficates Produced by Thermochemical Conversion of Biomass: A Review. Environ. Chem. Lett. 2023, 21, 2225–2249. [Google Scholar] [CrossRef]
- Sørmo, E.; Krahn, K.M.; Flatabø, G.Ø.; Hartnik, T.; Arp, H.P.H.; Cornelissen, G. Distribution of PAHs, PCBs, and PCDD/Fs in Products from Full-Scale Relevant Pyrolysis of Diverse Contaminated Organic Waste. J. Hazard. Mater. 2024, 461, 132546. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wen, J.; Jiang, X.; Dai, L.; Jin, Y.; Wang, F.; Chi, Y.; Yan, J. Distribution of PCDD/Fs over the Three Product Phases in Wet Sewage Sludge Pyrolysis. J. Anal. Appl. Pyrolysis 2018, 133, 169–175. [Google Scholar] [CrossRef]
- Schmidt, H.; Bucheli, T.; Kammann, C.; Glaser, B. European Biochar Certificate-Guidelines for a Sustainable Production of Biochar; European Biochar Certification: Arbaz, Switzerland, 2016. [Google Scholar]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Winchell, L.J.; Ross, J.J.; Brose, D.A.; Pluth, T.B.; Fonoll, X.; Norton, J.W.; Bell, K.Y. Pyrolysis and Gasification at Water Resource Recovery Facilities: Status of the Industry. Water Environ. Res. 2022, 94, 10701. [Google Scholar] [CrossRef]
- Kundu, S.; Patel, S.; Halder, P.; Patel, T.; Hedayati Marzbali, M.; Pramanik, B.K.; Paz-Ferreiro, J.; De Figueiredo, C.C.; Bergmann, D.; Surapaneni, A.; et al. Removal of PFASs from Biosolids Using a Semi-Pilot Scale Pyrolysis Reactor and the Application of Biosolids Derived Biochar for the Removal of PFASs from Contaminated Water. Environ. Sci. Water Res. Technol. 2021, 7, 638–649. [Google Scholar] [CrossRef]
- Kim, J.H.; Ok, Y.S.; Choi, G.H.; Park, B.J. Residual Perfluorochemicals in the Biochar from Sewage Sludge. Chemosphere 2015, 134, 435–437. [Google Scholar] [CrossRef]
- Gehling, W.; Dellinger, B. Environmentally Persistent Free Radicals and Their Lifetimes in PM 2.5. Environ. Sci. Technol. 2013, 47, 8172–8178. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gong, M.; Wang, J.; Bassi, A. Current Research Trends on Microplastic Pollution from Wastewater Systems: A Critical Review. Rev. Environ. Sci. Biotechnol. 2019, 18, 207–230. [Google Scholar] [CrossRef]
- Xu, X.; Hou, Q.; Xue, Y.; Jian, Y.; Wang, L.P. Pollution Characteristics and Fate of Microfibers in the Wastewater from Textile Dyeing Wastewater Treatment Plant. Water Sci. Technol. 2018, 78, 2046–2054. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Zhang, J.; Li, Q.; Wang, Y.; Wang, M.; Huang, X. Distribution and Characteristics of Microplastics in Urban Waters of Seven Cities in the Tuojiang River Basin, China. Environ. Res. 2020, 189, 109893. [Google Scholar] [CrossRef]
- Ni, B.J.; Zhu, Z.R.; Li, W.H.; Yan, X.; Wei, W.; Xu, Q.; Xia, Z.; Dai, X.; Sun, J. Microplastics Mitigation in Sewage Sludge through Pyrolysis: The Role of Pyrolysis Temperature. Environ. Sci. Technol. Lett. 2020, 7, 961–967. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.P.J.; Gascó, G. Biochar from Biosolids Pyrolysis: A Review. Int. J. Environ. Res. Public Health 2018, 15, 956. [Google Scholar] [CrossRef]
- Mujtaba Munir, M.A.; Yousaf, B.; Ali, M.U.; Dan, C.; Abbas, Q.; Arif, M.; Yang, X. In Situ Synthesis of Micro-Plastics Embedded Sewage-Sludge Co-Pyrolyzed Biochar: Implications for the Remediation of Cr and Pb Availability and Enzymatic Activities from the Contaminated Soil. J. Clean. Prod. 2021, 302, 127005. [Google Scholar] [CrossRef]
- Kończak, M.; Oleszczuk, P.; Różyło, K. Application of Different Carrying Gases and Ratio between Sewage Sludge and Willow for Engineered (Smart) Biochar Production. J. CO2 Util. 2019, 29, 20–28. [Google Scholar] [CrossRef]
- Moško, J.; Pohořelý, M.; Cajthaml, T.; Jeremiáš, M.; Robles-Aguilar, A.A.; Skoblia, S.; Beňo, Z.; Innemanová, P.; Linhartová, L.; Michalíková, K.; et al. Effect of Pyrolysis Temperature on Removal of Organic Pollutants Present in Anaerobically Stabilized Sewage Sludge. Chemosphere 2021, 265, 129082. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal—A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Zhang, W.; Tsang, D.C.W. Sludge-Derived Biochar and Its Application in Soil Fixation. In Biochar from Biomass and Waste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 239–253. [Google Scholar]
- Grutzmacher, P.; Puga, A.P.; Bibar, M.P.S.; Coscione, A.R.; Packer, A.P.; de Andrade, C.A. Carbon Stability and Mitigation of Fertilizer Induced N2O Emissions in Soil Amended with Biochar. Sci. Total Environ. 2018, 625, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, M.I.S.; de Souza, D.C.F.; de Almeida, A.Q.; Mackowiak, C.; da Silva Lima, I.; de Jesus Santos, J.C.; de Andrade, R.S. Nitrogen and Phosphorus Uptake Efficiency in Indian Mustard Cultivated during Three Growth Cycles in a Copper Contaminated Soil Treated with Biochar. Ciênc. Rural 2019, 49, 1. [Google Scholar]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Baran, A.; Bajda, T. Sewage Sludge Biochars Management—Ecotoxicity, Mobility of Heavy Metals, and Soil Microbial Biomass. Environ. Toxicol. Chem. 2018, 37, 1197–1207. [Google Scholar] [CrossRef]
- de Figueiredo, C.C.; Chagas, J.K.M.; da Silva, J.; Paz-Ferreiro, J. Short-Term Effects of a Sewage Sludge Biochar Amendment on Total and Available Heavy Metal Content of a Tropical Soil. Geoderma 2019, 344, 31–39. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The Dark Side of Black Gold: Ecotoxicological Aspects of Biochar and Biochar-Amended Soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and Limitations of Biochar Amendment in Agricultural Soils: A Review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Ong, W.S.; Lu, D.; Zhou, Y. A Potential Phosphorus Fertilizer to Alleviate the Coming “Phosphorus Crisis”-Biochar Derived from Enhanced Biological Phosphorus Removal Sludge. Sci. Total Environ. 2022, 838, 156559. [Google Scholar] [CrossRef] [PubMed]
- Penido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; do Rosário Guimarães, I.; Guilherme, L.R.G. Combining Biochar and Sewage Sludge for Immobilization of Heavy Metals in Mining Soils. Ecotoxicol. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef]
- Fang, S.; Tsang, D.C.W.; Zhou, F.; Zhang, W.; Qiu, R. Stabilization of Cationic and Anionic Metal Species in Contaminated Soils Using Sludge-Derived Biochar. Chemosphere 2016, 149, 263–271. [Google Scholar] [CrossRef]
- Hu, X.; Qu, C.; Han, Y.; Sun, P.; Cai, P.; Chen, W.; Huang, Q. Elevated Temperature Induces Contrasting Transformation of Exogenous Copper to Soil Solution and Solid Phases in an Arable Soil. Ecotoxicol. Environ. Saf. 2023, 255, 114744. [Google Scholar] [CrossRef]
- Hasanzad, S.; Pirkharrati, H.; Dovlati, B.; Farhadi, K. Investigation about Cd(II) Immobility in Contaminated Soils Using Phosphate Fertilizer, Heat, and Lime (CaCO3). J. Environ. Stud. 2014, 40, 321–330. [Google Scholar]
- Wang, H.; Zhou, L.; Dan, Y.; Wang, X.; Diao, Y.; Liu, F.; Sang, W. Impact of Pyrolysis Temperature and Application Amount of Sewage Sludge Biochar on the Speciation and Bioavailability of Cd and Pb in Paddy Soil. Water Air Soil Pollut. 2022, 233, 209. [Google Scholar] [CrossRef]
- Fathianpour, A.; Taheriyoun, M.; Soleimani, M. Lead and Zinc Stabilization of Soil Using Sewage Sludge Biochar: Optimization through Response Surface Methodology. CLEAN–Soil Air Water 2018, 46, 1700429. [Google Scholar] [CrossRef]
- Namdari, M.; Soleimani, M.; Mirghaffari, N.; Kharrazi, S.M. Effect of Biological Sewage Sludge and Its Derived Biochar on Accumulation of Potentially Toxic Elements by Corn (Zea mays L.). Sci. Rep. 2024, 14, 5985. [Google Scholar] [CrossRef]
- He, E.; Yang, Y.; Xu, Z.; Qiu, H.; Yang, F.; Peijnenburg, W.J.G.M.; Zhang, W.; Qiu, R.; Wang, S. Two Years of Aging Influences the Distribution and Lability of Metal(Loid)s in a Contaminated Soil Amended with Different Biochars. Sci. Total Environ. 2019, 673, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, M.; Zhou, F.; Huang, X.; Tsang, D.C.W.; Zhang, W. Effects of Atmospheric Ageing under Different Temperatures on Surface Properties of Sludge-Derived Biochar and Metal/Metalloid Stabilization. Chemosphere 2017, 184, 176–184. [Google Scholar] [CrossRef]
- Álvarez-Rogel, J.; Tercero Gómez, M.D.C.; Conesa, H.M.; Párraga-Aguado, I.; González-Alcaraz, M.N. Biochar from Sewage Sludge and Pruning Trees Reduced Porewater Cd, Pb and Zn Concentrations in Acidic, but Not Basic, Mine Soils under Hydric Conditions. J. Environ. Manag. 2018, 223, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, D.; Gao, F.; Li, M.; Luo, X. Effects of Biochar-Derived Sewage Sludge on Heavy Metal Adsorption and Immobilization in Soils. Int. J. Environ. Res. Public Health 2017, 14, 681. [Google Scholar] [CrossRef]
- Méndez, A.; Gómez, A.; Paz-Ferreiro, J.; Gascó, G. Effects of Sewage Sludge Biochar on Plant Metal Availability after Application to a Mediterranean Soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef]
- Rashid, M.S.; Liu, G.; Yousaf, B.; Hamid, Y.; Rehman, A.; Munir, M.A.M.; Arif, M.; Ahmed, R.; Song, Y. Assessing the Influence of Sewage Sludge and Derived-Biochar in Immobilization and Transformation of Heavy Metals in Polluted Soil: Impact on Intracellular Free Radical Formation in Maize. Environ. Pollut. 2022, 309, 119768. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, R.; Ji, S.; Xie, L.; Zhang, H. Effects of Biochar Derived from Sewage Sludge and Sewage Sludge/Cotton Stalks on the Immobilization and Phytoavailability of Pb, Cu, and Zn in Sandy Loam Soil. J. Hazard. Mater. 2021, 419, 126468. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Q.; Xu, Q.; Zhou, Z.; Shi, J. Wheat (Triticum aestivum L.) Grains Uptake of Lead (Pb), Transfer Factors and Prediction Models for Various Types of Soils from China. Ecotoxicol. Environ. Saf. 2020, 206, 111387. [Google Scholar] [CrossRef]
- Taraqqi-A-Kamal, A.; Atkinson, C.J.; Khan, A.; Zhang, K.; Sun, P.; Akther, S.; Zhang, Y. Biochar Remediation of Soil: Linking Biochar Production with Function in Heavy Metal Contaminated Soils. Plant Soil Environ. 2021, 67, 183–201. [Google Scholar] [CrossRef]
- Hong, Y.K.; Kim, J.W.; Lee, S.P.; Yang, J.E.; Kim, S.C. Effect of Combined Soil Amendment on Immobilization of Bioavailable As and Pb in Paddy Soil. Toxics 2022, 10, 90. [Google Scholar] [CrossRef]
- Zheng, X.J.; Chen, M.; Wang, J.F.; Liu, Y.; Liao, Y.Q.; Liu, Y.C. Assessment of Zeolite, Biochar, and Their Combination for Stabilization of Multimetal-Contaminated Soil. ACS Omega 2020, 5, 27374–27382. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Lahori, A.H.; Awasthi, M.K.; Wang, Z.; Guo, Z.; Wang, Q.; Feng, S.; Li, R.; et al. (Im)Mobilization of Soil Heavy Metals Using CaO, FA, Sulfur, and Na2S: A 1-Year Incubation Study. Int. J. Environ. Sci. Technol. 2018, 15, 607–620. [Google Scholar] [CrossRef]
- Liu, G. Effect of Citric Acid on Immobilization of Heavy Metals. IOP Conf. Ser. Earth Environ. Sci. 2019, 242, 052020. [Google Scholar] [CrossRef]
- Jiang, K.; Zhou, K. Chemical Immobilization of Lead, Cadmium, and Arsenic in a Smelter-Contaminated Soil Using 2,4,6-Trimercaptotriazine, Trisodium Salt, Nonahydrate and Ferric Sulfate. J. Soils Sediments 2018, 18, 1060–1065. [Google Scholar] [CrossRef]
- Liu, D.D.; Miao, D.R.; Liu, F. Evaluation of the As, Cu and Pb Immobilizing Efficiency by Tessier, TCLP and SBET Method. Adv. Mater. Res. 2014, 878, 520–531. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, W.Q.; Shen, L. Immobilization Efficiency Evaluation by Using Tessier, TCLP and SBET Method for As, Cu and Pb Contaminated Soils. Adv. Mater. Res. 2014, 909, 95–99. [Google Scholar] [CrossRef]
- Rizwan, M.S.; Imtiaz, M.; Chhajro, M.A.; Huang, G.; Fu, Q.; Zhu, J.; Aziz, O.; Hu, H. Influence of Pyrolytic and Non-Pyrolytic Rice and Castor Straws on the Immobilization of Pb and Cu in Contaminated Soil. Environ. Technol. 2016, 37, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Rizwan, M.S.; Salam, A.; Fu, Q.; Zhu, J.; Shaaban, M.; Hu, H. Cadmium Immobilization Potential of Rice Straw-Derived Biochar, Zeolite and Rock Phosphate: Extraction Techniques and Adsorption Mechanism. Bull. Environ. Contam. Toxicol. 2018, 100, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, S.; Ning, X.; Yang, M.; Liu, M.; Zang, F.; Nan, Z. A Promising Amendment for the Immobilization of Heavy Metal(Loid)s in Agricultural Soil, Northwest China. J. Soils Sediments 2021, 21, 2273–2286. [Google Scholar] [CrossRef]
- Mitzia, A.; Böserle Hudcová, B.; Vítková, M.; Kunteová, B.; Casadiego Hernandez, D.; Moško, J.; Pohořelý, M.; Grasserová, A.; Cajthaml, T.; Komárek, M. Pyrolysed Sewage Sludge for Metal(Loid) Removal and Immobilisation in Contrasting Soils: Exploring Variety of Risk Elements across Contamination Levels. Sci. Total Environ. 2024, 918, 170572. [Google Scholar] [CrossRef]
- Hernández-Arenas, R.; Beltrán-Sanahuja, A.; Navarro-Quirant, P.; Sanz-Lazaro, C. The Effect of Sewage Sludge Containing Microplastics on Growth and Fruit Development of Tomato Plants. Environ. Pollut. 2021, 268, 115779. [Google Scholar] [CrossRef]
- Hazrati, S.; Farahbakhsh, M.; Cerdà, A.; Heydarpoor, G. Functionalization of Ultrasound Enhanced Sewage Sludge-Derived Biochar: Physicochemical Improvement and Its Effects on Soil Enzyme Activities and Heavy Metals Availability. Chemosphere 2021, 269, 128767. [Google Scholar] [CrossRef]
- Moon, D.H.; Park, J.W.; Chang, Y.Y.; Ok, Y.S.; Lee, S.S.; Ahmad, M.; Koutsospyros, A.; Park, J.H.; Baek, K. Immobilization of Lead in Contaminated Firing Range Soil Using Biochar. Environ. Sci. Pollut. Res. 2013, 20, 8464–8471. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Rajapaksha, A.U.; Lim, J.E.; Kim, B.Y.; Ahn, J.H.; Lee, Y.H.; Al-Wabel, M.I.; Lee, S.E.; Lee, S.S. Lead and Copper Immobilization in a Shooting Range Soil Using Soybean Stover- and Pine Needle-Derived Biochars: Chemical, Microbial and Spectroscopic Assessments. J. Hazard. Mater. 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Wang, S.L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G.; et al. Enhanced Cu and Cd Sorption after Soil Aging of Woodchip-Derived Biochar: What Were the Driving Factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef]
- Sumaraj; Padhye, L.P. Influence of Surface Chemistry of Carbon Materials on Their Interactions with Inorganic Nitrogen Contaminants in Soil and Water. Chemosphere 2017, 184, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Lee, S.S.; Lee, S.E.; Al-Wabel, M.I.; Tsang, D.C.W.; Ok, Y.S. Biochar-Induced Changes in Soil Properties Affected Immobilization/Mobilization of Metals/Metalloids in Contaminated Soils. J. Soils Sediments 2017, 17, 717–730. [Google Scholar] [CrossRef]
- You, J.; Sun, L.; Liu, X.; Hu, X.; Xu, Q. Effects of Sewage Sludge Biochar on Soil Characteristics and Crop Yield in Loamy Sand Soil. Pol. J. Environ. Stud. 2019, 28, 2973–2980. [Google Scholar] [CrossRef]
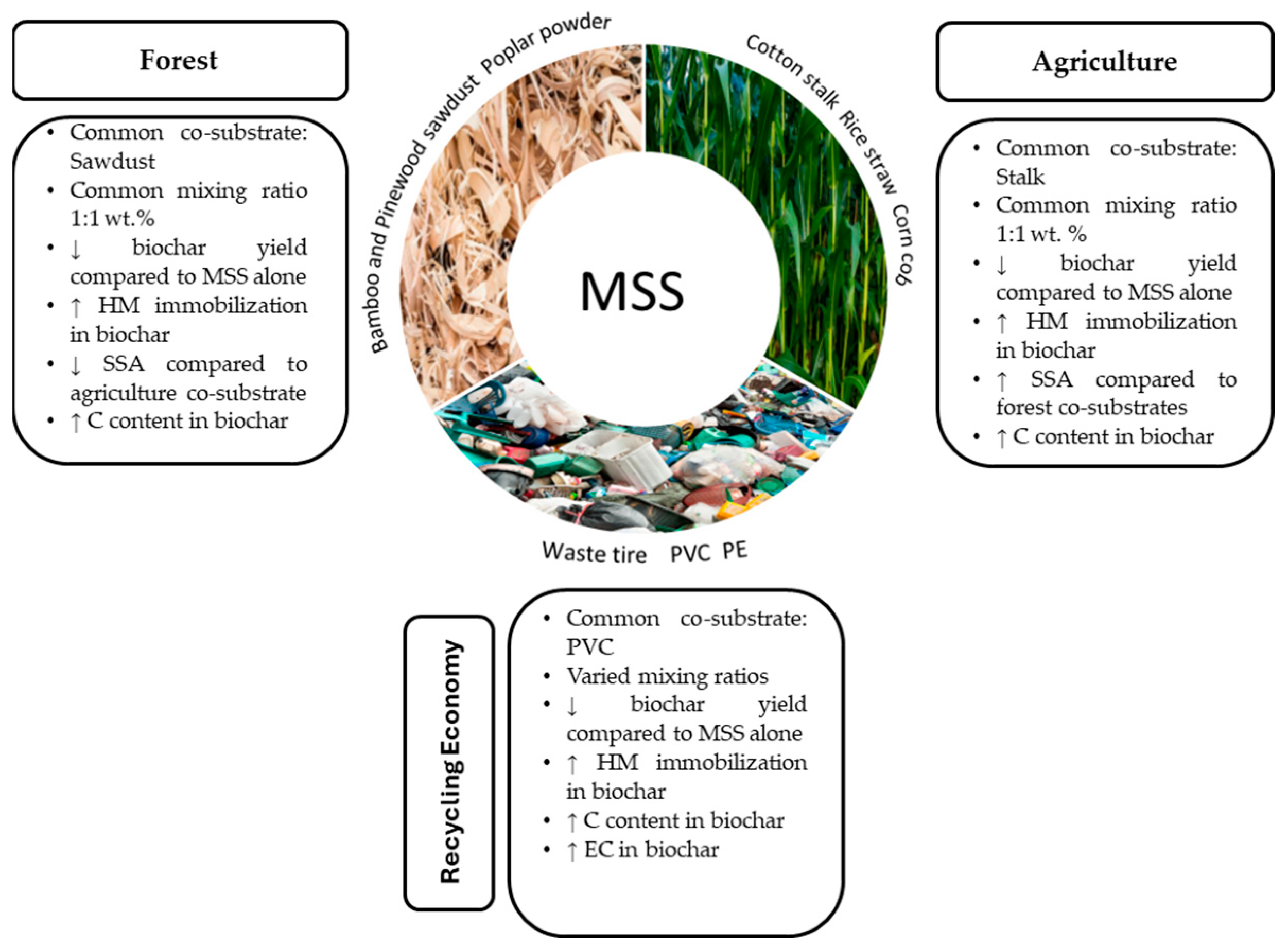


| Feedstock | Temp (°C) | Mixing Ratio | Physical Properties | Proximate Analysis | Ultimate Analysis | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSA (m2/g) | TPV (cm3/g) | APW (nm) | VM | A | FC | C | H | N | O | S | H/C Molar | O/C Molar | ||||||
| MSS + Bamboo Sawdust (BS) | ||||||||||||||||||
| MSS | 400 | 1:0 * | 5.5 | nr | nr | nr | 64.2 | nr | 21.9 | 1.9 | 3.1 | 72.3 | 0.9 | 1.0 | 2.5 | [11] | ||
| 500 | 1:0 * | 7.7 | nr | nr | nr | 69.8 | nr | 21.2 | 1.2 | 2.8 | 73.9 | 0.8 | 0.7 | 2.6 | ||||
| 600 | 1:0 * | 6.0 | nr | nr | nr | 74.0 | nr | 19.9 | 0.7 | 2,0 | 76.5 | 0.9 | 0.4 | 2.9 | ||||
| MSS + BS | 400 | 1:1 * | 2.8 | nr | nr | nr | 52.6 | nr | 31.2 | 1.8 | 2.1 | 64.3 | 0.5 | 0.7 | 1.5 | |||
| 500 | 1:1 * | 4.5 | nr | nr | nr | 58.3 | nr | 30.4 | 1.6 | 2.0 | 65.4 | 0.6 | 0.6 | 1.6 | ||||
| 600 | 1:1 * | 8.5 | nr | nr | nr | 61.9 | nr | 30.2 | 1.2 | 1.8 | 66.4 | 0.5 | 0.5 | 1.6 | ||||
| MSS | 1:0 * | 5.5 | nr | nr | nr | nr | nr | nr | 21.9 | 1.9 | 3.1 | 8.1 | 0.9 | 1.0 | 0.3 | [22] | ||
| 1:0 * | 7.1 | nr | nr | nr | nr | nr | nr | 32.4 | 0.6 | 1.7 | 4.2 | 1.1 | 0.2 | 0.1 | ||||
| MSS + BS | 400 | 1:1 ** | 2.8 | nr | nr | nr | nr | nr | 31.2 | 1.8 | 2.1 | 11.7 | 0.5 | 0.7 | 0.3 | |||
| 700 | 1:1 ** | 7.6 | nr | nr | nr | nr | nr | 32.4 | 0.9 | 1.4 | 0.8 | 0.6 | 0.3 | 0.02 | ||||
| MSS | 550 | 1:0 * | 31.3 | nr | nr | nr | 78.4 | nr | 14.4 | 0.9 | 2.0 | 2.4 | 2.0 | 0.7 | 0.1 | [23] | ||
| MSS + BS | 550 | 4:1 * | 20.4 | nr | nr | nr | 70.0 | nr | 21.1 | 0.9 | 1.9 | 4.3 | 1.9 | 0.5 | 0.2 | |||
| MSS + Sawdust from Furniture (SD) | ||||||||||||||||||
| MSS | 300 | 10:0 * | nr | nr | nr | nr | 60.7 | nr | 20.9 | 7.4 | 3.5 | 7.4 | nr | 4.3 | 0.3 | [24] | ||
| 400 | 10:0 * | nr | nr | nr | nr | 65.6 | nr | 17.7 | 6.1 | 2.5 | 8.2 | nr | 4.1 | 0.3 | ||||
| 500 | 10:0 * | nr | nr | nr | nr | 68.5 | nr | 17.0 | 6.0 | 2.0 | 6.5 | nr | 4.3 | 0.3 | ||||
| 600 | 10:0 * | nr | nr | nr | nr | 70.4 | nr | 16.3 | 6.0 | 1.7 | 5.6 | nr | 4.4 | 0.3 | ||||
| MSS + SD | 300 | 9:1 * | nr | nr | nr | nr | 55.2 | nr | 22.0 | 7.9 | 3.3 | 11.6 | nr | 4.3 | 0.4 | |||
| 300 | 7:3 * | nr | nr | nr | nr | 46.1 | nr | 24.3 | 6.5 | 3.9 | 19.2 | nr | 3.2 | 0.6 | ||||
| 300 | 5:5 * | nr | nr | nr | nr | 41.0 | nr | 26.7 | 8.01 | 3.0 | 21.4 | nr | 3.6 | 1.0 | ||||
| 400 | 9:1 * | nr | nr | nr | nr | 56.9 | nr | 23.8 | 7.0 | 2.5 | 9.8 | nr | 3.5 | 0.3 | ||||
| 400 | 7:3 * | nr | nr | nr | nr | 47.6 | nr | 34.8 | 4.3 | 2.6 | 10.7 | nr | 1.5 | 0.2 | ||||
| 400 | 5:5 * | nr | nr | nr | nr | 43.7 | nr | 43.4 | 7.6 | 2.2 | 3.1 | nr | 2.1 | 0.1 | ||||
| 500 | 9:1 * | nr | nr | nr | nr | 61.9 | nr | 23.9 | 4.9 | 2.3 | 7.0 | nr | 2.5 | 0.2 | ||||
| 500 | 7:3 * | nr | nr | nr | nr | 50.6 | nr | 40.6 | 3.7 | 2.4 | 3.7 | nr | 1.1 | 0.1 | ||||
| 500 | 5:5 * | nr | nr | nr | nr | 44.6 | nr | 50.8 | 3.9 | 2.1 | 1.4 | nr | 0.9 | 0.02 | ||||
| 600 | 9:1 * | nr | nr | nr | nr | 67.2 | nr | 21.1 | 3.8 | 1.9 | 6.2 | nr | 2.2 | 0.2 | ||||
| 600 | 7:3 * | nr | nr | nr | nr | 59.8 | nr | 31.7 | 2.7 | 1.7 | 6.0 | nr | 1.0 | 0.1 | ||||
| 600 | 5:5 * | nr | nr | nr | nr | 45.1 | nr | 42.6 | 3.0 | 1.7 | 0.4 | nr | 0.8 | 0.01 | ||||
| MSS + Wood Sawdust (WS) | ||||||||||||||||||
| MSS | 550 | 5:0 * | 31.3 | nr | nr | nr | 78.4 | nr | 14.4 | 0.9 | 2.0 | 2.4 | 2.0 | 0.7 | 0.1 | [23] | ||
| MSS + WS | 550 | 4:1 * | 14.7 | nr | nr | nr | 69.0 | nr | 21.3 | 0.9 | 1.9 | 5.1 | 1.9 | 0.5 | 0.2 | |||
| MSS + Salix (SX) | ||||||||||||||||||
| MSS | 500 | 100:0 nr | nr | nr | nr | 20.4 | 61.9 | 17.7 | 30.1 | 1.2 | 3.9 | 1.9 | 0.9 | 0.5 | 0.1 | [25] | ||
| MSS + SX | 500 | 75:25 nr | nr | nr | nr | 19.3 | 54.8 | 25.5 | 37.6 | 1.4 | 3.7 | 1.6 | 0.7 | 0.4 | 0.03 | |||
| 500 | 50:50 nr | nr | nr | nr | 19.2 | 41.5 | 39.3 | 46.4 | 1.6 | 3.2 | 6.7 | 0.5 | 0.4 | 0.1 | ||||
| 500 | 25:75 nr | nr | nr | nr | 18.1 | 30.1 | 51.8 | 60.3 | 2 | 2.3 | 5 | 0.3 | 0.4 | 0.1 | ||||
| MSS + Pinewood Sawdust (PS) | ||||||||||||||||||
| MSS | 500 | 100:0 *** | 42.9 | nr | nr | 19.0 | 69.3 | 11.7 | 18.5 | 1.0 | 2.7 | 7.4 | 1.2 | 0.6 | 0.3 | [26] | ||
| MSS + PS | 500 | 50:50 *** | 30.7 | nr | nr | 19.7 | 50.7 | 29.6 | 37.0 | 1.4 | 1.5 | 8.0 | 0.9 | 0.5 | 0.2 | |||
| MSS + Walnut Shell (WS) | ||||||||||||||||||
| MSS | 600 | 1:0 nr | 31.4 | nr | 18.1 | nr | nr | nr | 10.9 | 1.1 | 0.7 | 42.9 | 1.1 | 1.2 | 3.0 | [27] | ||
| MSS + WS | 600 | 3:1 nr | 47.1 | nr | 14.2 | nr | nr | nr | 21.0 | 1.2 | 0.9 | 38.2 | 0.9 | 0.7 | 1.4 | |||
| 600 | 1:1 nr | 75.5 | nr | 7.4 | nr | nr | nr | 32.9 | 1.2 | 1.0 | 32.1 | 0.6 | 0.4 | 0.7 | ||||
| 600 | 1:3 nr | 122.9 | nr | 4.0 | nr | nr | nr | 47.9 | 1.4 | 1.0 | 26.8 | 0.4 | 0.3 | 0.4 | ||||
| MSS + Cotton Stalk (CS) | ||||||||||||||||||
| MSS | 300 | 1:0 * | 15.6 | 0.1 | 16.0 | nr | nr | nr | 25.2 | 2.2 | 3.2 | nr | nr | 1.0 | nr | [28] | ||
| 400 | 1:0 * | 16.3 | 0.1 | 15.0 | nr | nr | nr | 24.3 | 1.7 | 3 | nr | nr | 0.8 | nr | ||||
| 500 | 1:0 * | 9.4 | 0.1 | 20.0 | nr | nr | nr | 20.2 | 1.1 | 2.4 | nr | nr | 0.6 | nr | ||||
| 600 | 1:0 * | 24.7 | 0.1 | 9.8 | nr | nr | nr | 22.6 | 0.4 | 1.3 | nr | nr | 0.2 | nr | ||||
| MSS + CS | 300 | 1:1 * | 14.4 | 0.1 | 13.8 | nr | nr | nr | 42.5 | 3.5 | 3.6 | nr | nr | 1.0 | nr | |||
| 400 | 1:1 * | 15.8 | 0.1 | 12.8 | nr | nr | nr | 35.2 | 2.2 | 3 | nr | nr | 0.7 | nr | ||||
| 500 | 1:1 * | 10.8 | 0.04 | 14.3 | nr | nr | nr | 33.6 | 1.8 | 2.3 | nr | nr | 0.7 | nr | ||||
| 600 | 1:1 * | 22.3 | 0.1 | 10.1 | nr | nr | nr | 32.2 | 1.7 | 2.1 | nr | nr | 0.6 | nr | ||||
| MSS + Rice Husk (RH) | ||||||||||||||||||
| MSS | 550 | 5:0 | 31.3 | nr | nr | nr | 78.4 | nr | 14.4 | 0.9 | 2.0 | 2.4 | 2.0 | 0.7 | 0.1 | [23] | ||
| MSS + RH | 550 | 4:1 * | 16.1 | nr | nr | nr | 70.7 | nr | 20.3 | 0.9 | 2.0 | 4.2 | 2.0 | 0.5 | 0.2 | |||
| MSS | 400 | 1:0 * | 5.5 | nr | nr | nr | nr | nr | 21.9 | 1.9 | 3.1 | 8.1 | 0.9 | 1.0 | 0.3 | [22] | ||
| 700 | 1:0 * | 7.1 | nr | nr | nr | nr | nr | 32.4 | 0.6 | 1.7 | 4.2 | 1.1 | 0.2 | 0.1 | ||||
| MSS + RH | 400 | 1:1 * | 4.4 | nr | nr | nr | nr | nr | 30.1 | 2.0 | 2.4 | 14.0 | 0.5 | 0.8 | 0.3 | |||
| 700 | 1:1 * | 10.7 | nr | nr | nr | nr | nr | 26.7 | 0.7 | 1.3 | 9.7 | 0.6 | 0.3 | 0.3 | ||||
| MSS + Rice Straw (RS) | ||||||||||||||||||
| MSS | 800 | 10:0 nr | 15.7 | 0.1 | 28.7 | 7.2 | 82.3 | 10.5 | 6.9 | 1.2 | 1.8 | 7.5 | 0.4 | 1.8 | 0.8 | [29] | ||
| MSS + RS | 800 | 7:3 nr | 27.7 | 0.1 | 13.0 | 8.1 | 72.3 | 19.6 | 17.8 | 1.4 | 1.9 | 6.4 | 0.3 | 0.7 | 0.3 | |||
| 800 | 5:5 nr | 58.5 | 0.1 | 5.2 | 8.8 | 64.8 | 26.5 | 25.6 | 1.5 | 1.7 | 6.3 | 0.2 | 0.5 | 0.2 | ||||
| 800 | 3:7 nr | 65.3 | 0.1 | 4.6 | 8.4 | 53.4 | 38.3 | 37.2 | 1.7 | 1.5 | 6.1 | 0.1 | 0.3 | 0.1 | ||||
| MSS + Plastics (PP, PVC, PA6) | ||||||||||||||||||
| MSS | 800 | 10:0 ** | nr | nr | nr | nr | nr | nr | 23.0 | 0.5 | 2.0 | 2.7 | 0.1 | 0.3 | 0.1 | [30] | ||
| MSS + PP | 800 | 8:2 ** | nr | nr | nr | nr | nr | nr | 23.3 | 0.5 | 2.8 | 0.6 | 0.3 | 0.2 | 0.02 | |||
| MSS + PVC | 800 | 8:2 ** | nr | nr | nr | nr | nr | nr | 31.4 | 0.5 | 2.6 | 1.7 | 0.1 | 0.2 | 0.04 | |||
| MSS + PA6 | 800 | 8:2 ** | nr | nr | nr | nr | nr | nr | 25.7 | 0.4 | 2.7 | 2.7 | 0.2 | 0.2 | 0.1 | |||
| MSS | 550 | 5:0 * | 31.3 | nr | nr | nr | 78.4 | nr | 14.4 | 0.9 | 2.0 | 2.4 | 2.0 | 0.7 | 0.1 | [23] | ||
| MSS + PVC | 550 | 4:1 * | 2.2 | nr | nr | nr | 70.1 | nr | 20.0 | 1.0 | 1.8 | 5.8 | 1.3 | 0.6 | 0.2 | |||
| MSS + Exhausted Tea (ET) or Kitchen Waste (KW) | ||||||||||||||||||
| MSS | 550 | 5:0 * | 31.3 | nr | nr | nr | 78.4 | nr | 14.4 | 0.9 | 2.0 | 2.4 | 2.0 | 0.7 | 0.1 | [23] | ||
| MSS + ET | 550 | 4:1 * | 22.2 | nr | nr | nr | 68.8 | nr | 19.7 | 0.9 | 2.3 | 6.3 | 2.1 | 0.5 | 0.2 | |||
| MSS + KW | 550 | 4:1 * | 12.1 | nr | nr | nr | 73.2 | nr | 19.2 | 0.9 | 2.6 | 2.0 | 2.1 | 0.6 | 0.1 | |||
| MSS + Digested Manure (DM) | ||||||||||||||||||
| MSS | 525 | 100:0 nr | nr | nr | nr | 20.2 | 74 | 5.7 | 20 | 0.9 | 2.5 | 75.3 | 1.3 | 0.6 | 2.8 | [31] | ||
| MSS + DM | 525 | 50:50 nr | nr | nr | nr | 19.5 | 62.2 | 18.2 | 30 | 1.2 | 2.4 | 65.5 | 0.9 | 0.5 | 1.6 | |||
| Categories of co-substrates: | ||||||||||||||||||
| Forest | Agriculture | Recycling economy | Food residues | Digestate | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biney, M.; Gusiatin, M.Z. Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 2: Biochar Characterization and Application in the Remediation of Heavy Metal-Contaminated Soils. Materials 2024, 17, 3850. https://doi.org/10.3390/ma17153850
Biney M, Gusiatin MZ. Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 2: Biochar Characterization and Application in the Remediation of Heavy Metal-Contaminated Soils. Materials. 2024; 17(15):3850. https://doi.org/10.3390/ma17153850
Chicago/Turabian StyleBiney, Michael, and Mariusz Z. Gusiatin. 2024. "Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 2: Biochar Characterization and Application in the Remediation of Heavy Metal-Contaminated Soils" Materials 17, no. 15: 3850. https://doi.org/10.3390/ma17153850







