Progress in Additive Manufacturing of Magnesium Alloys: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Bed Fusion (PBF) of Magnesium
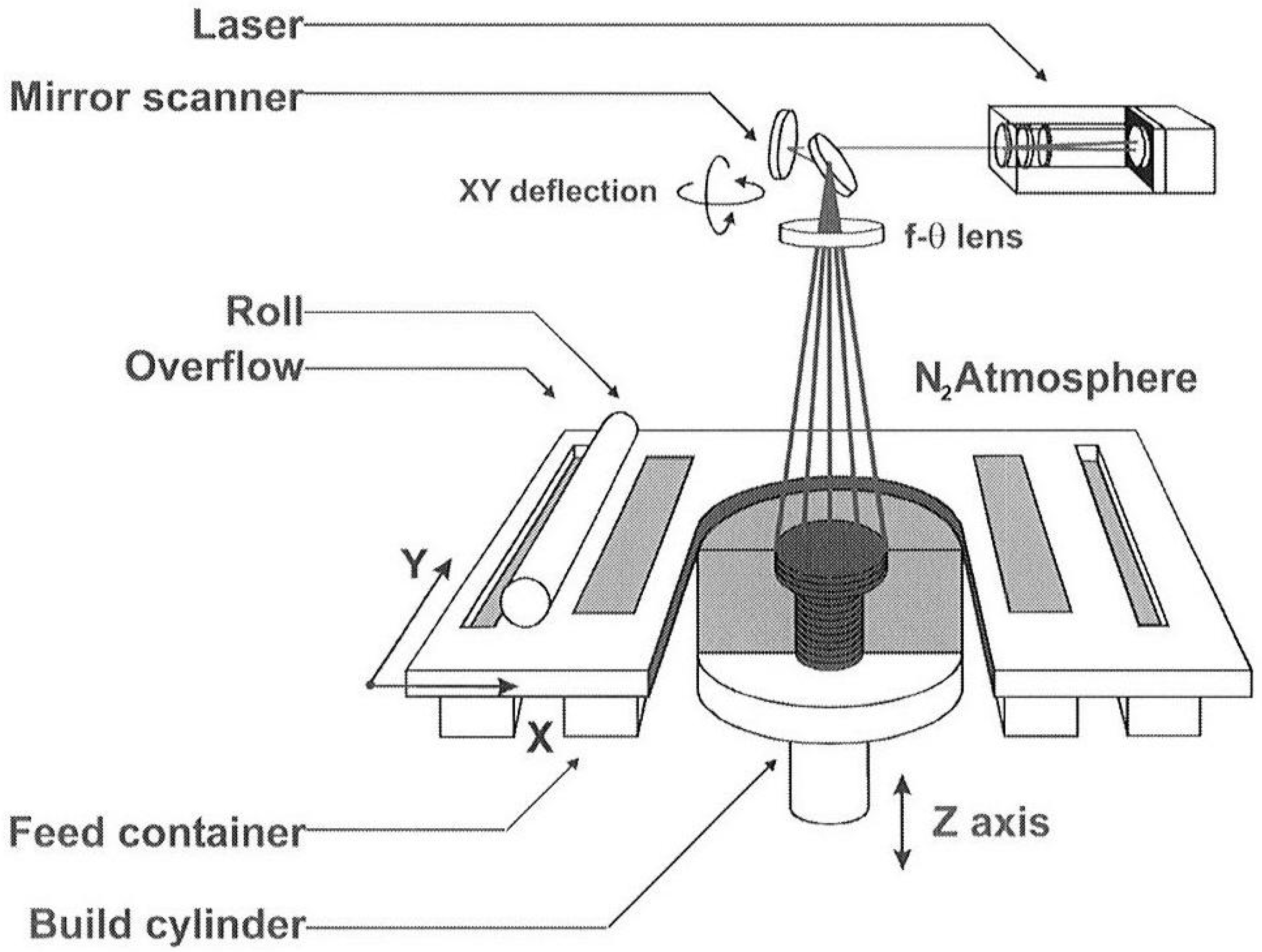
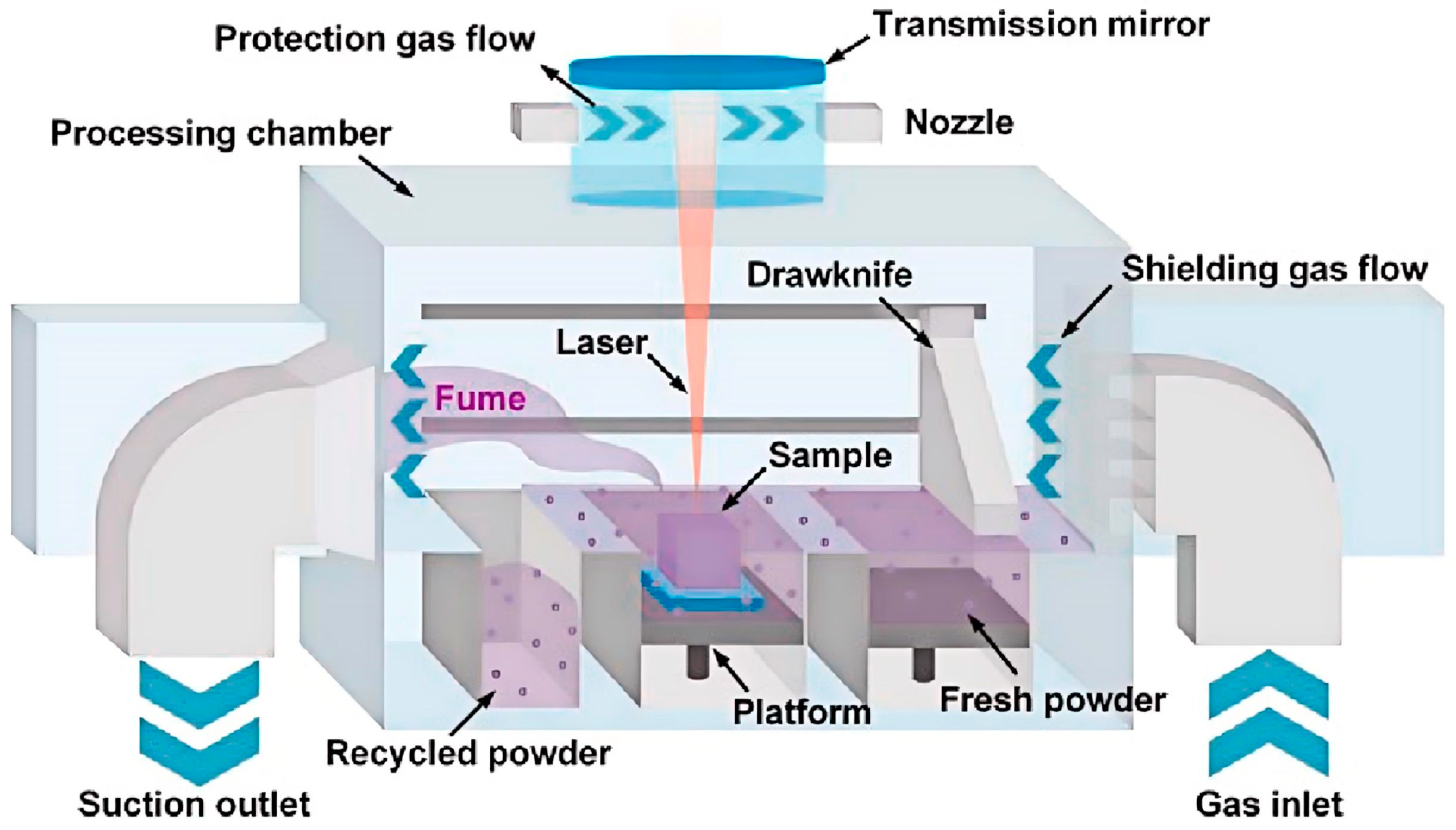
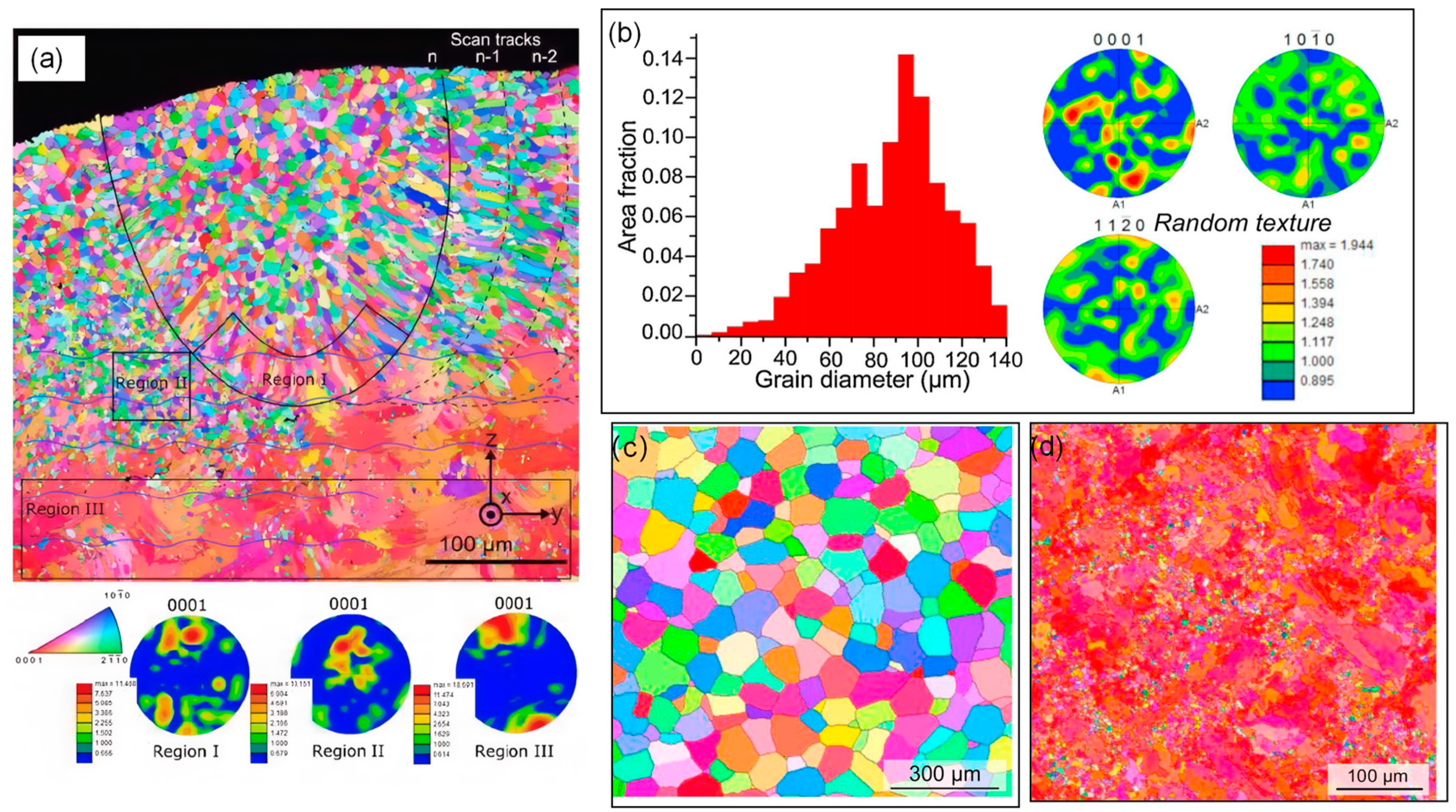
2.1.1. Selective Laser Melting
Magnesium Powder
Layer Thickness
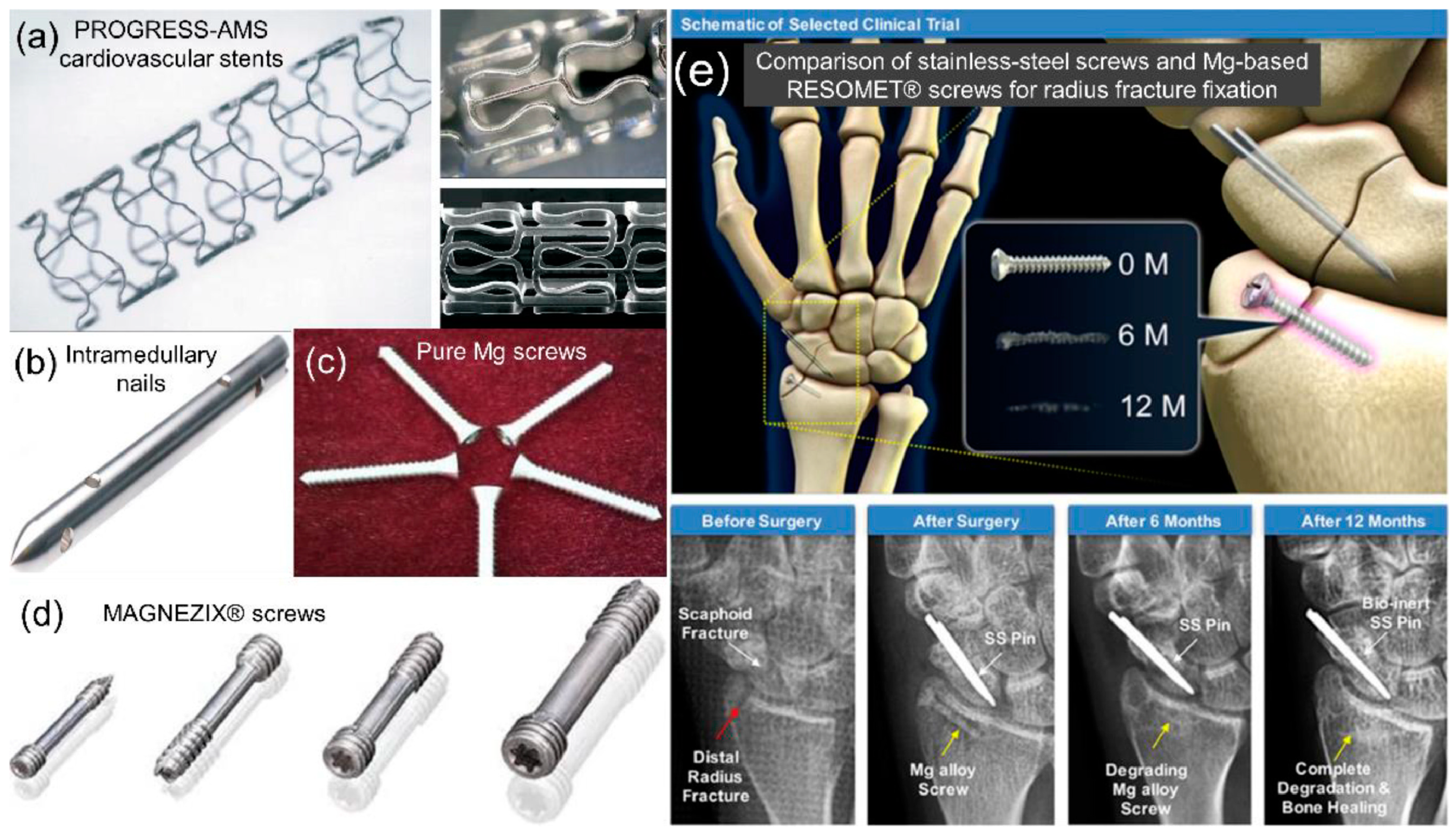
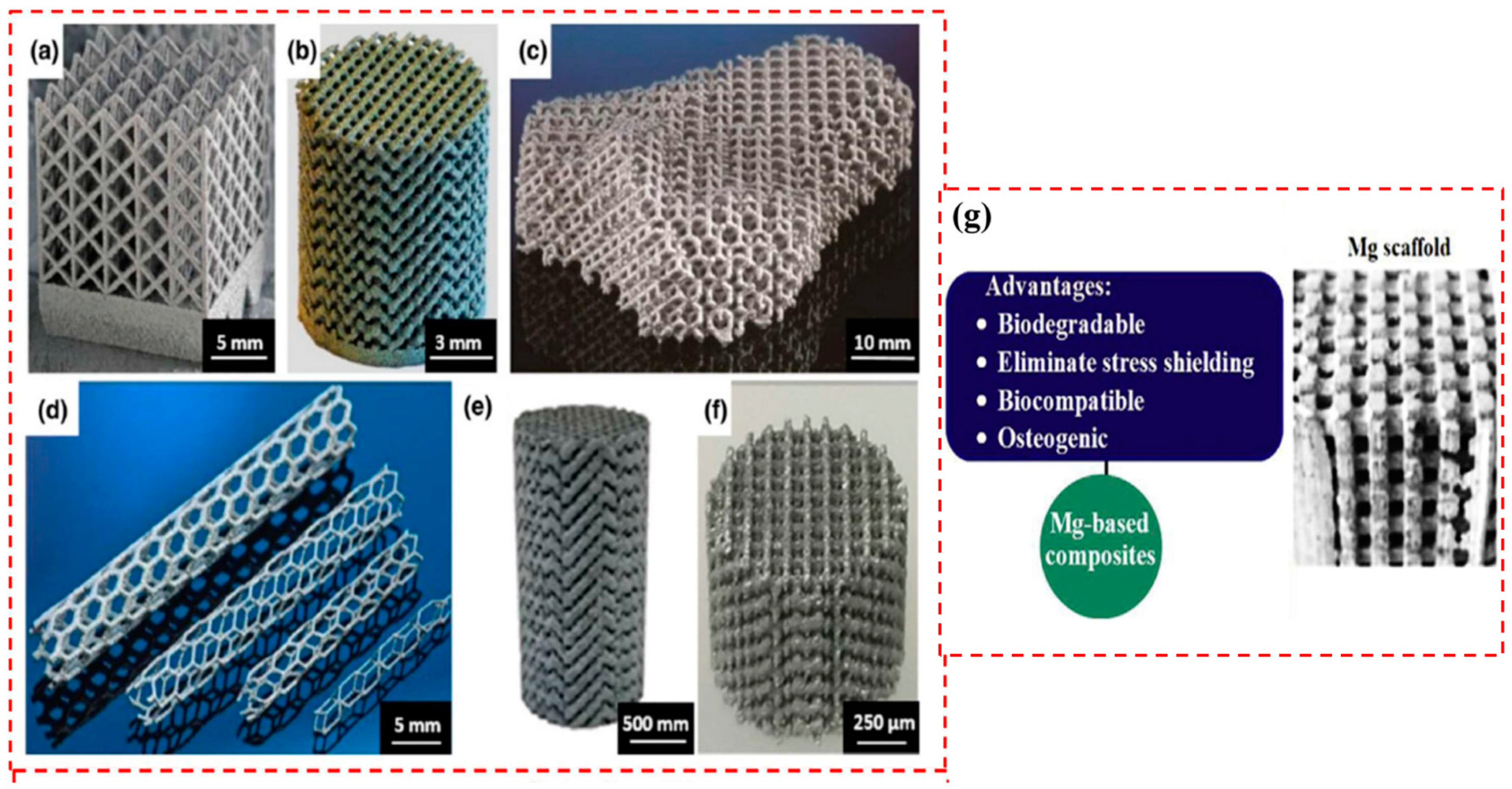
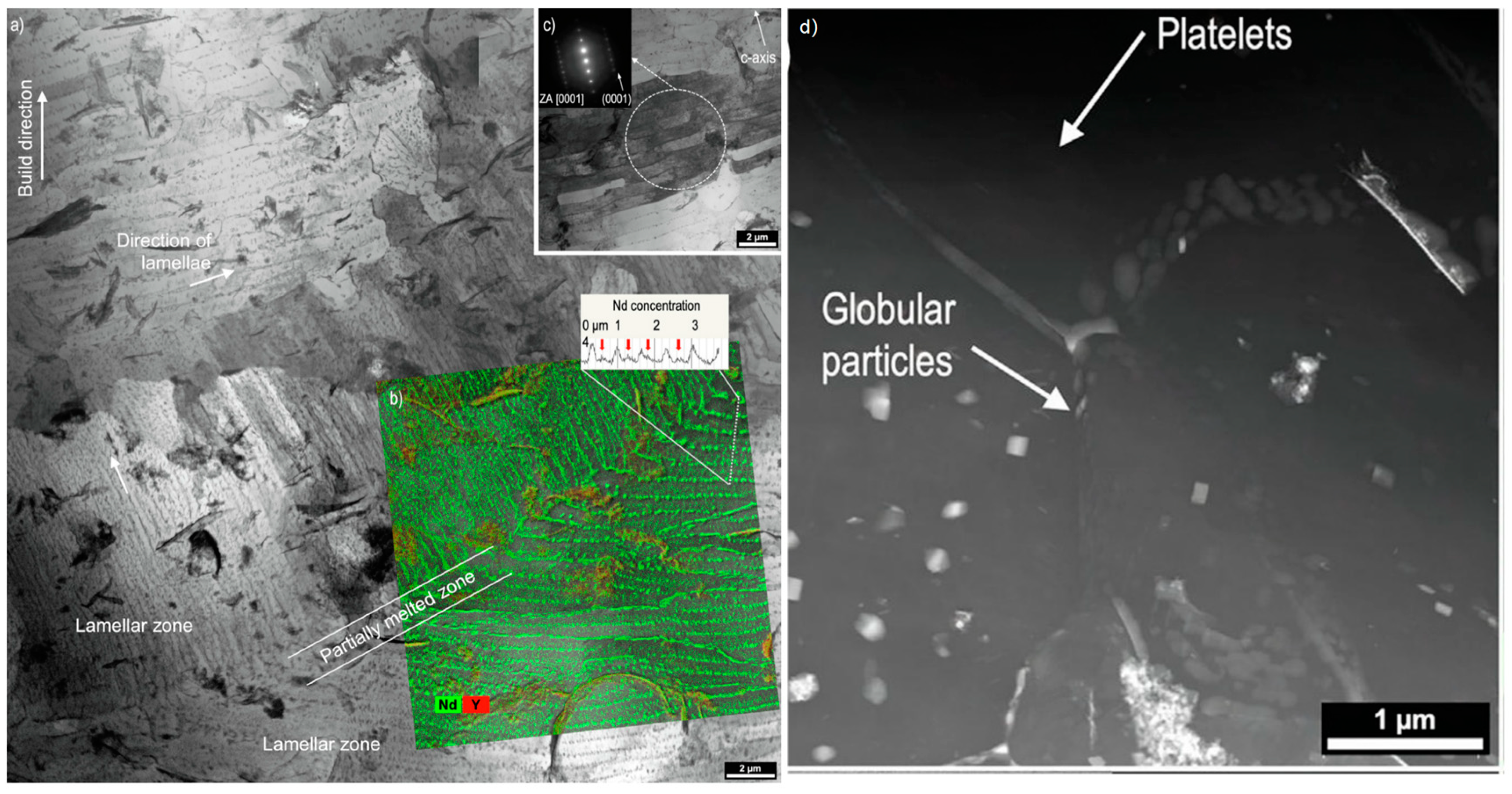
Degradation Behavior of LPBF Mg Alloys
Energy Density and Vaporization

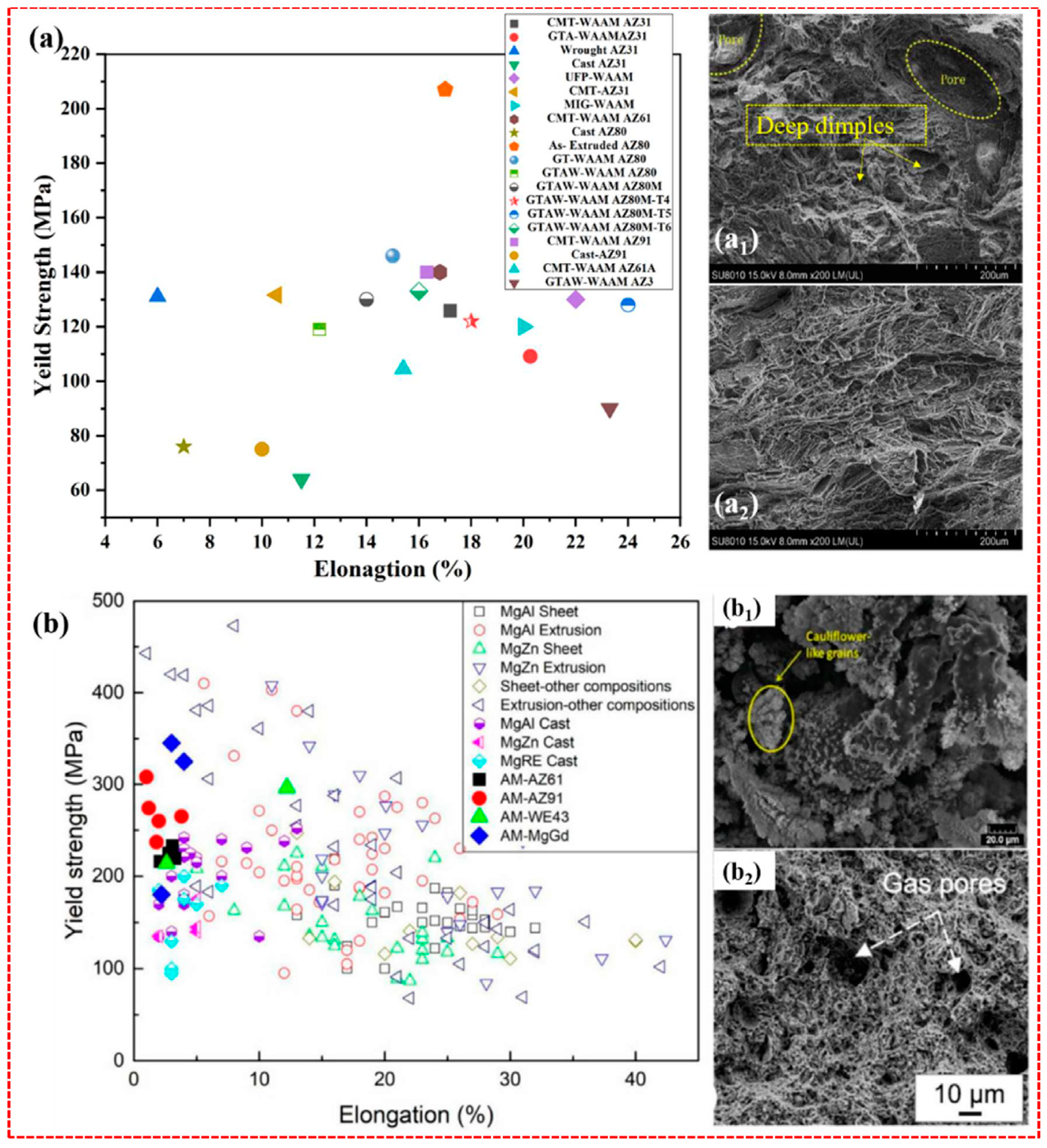
2.2. Wire Arc Additive Manufacturing
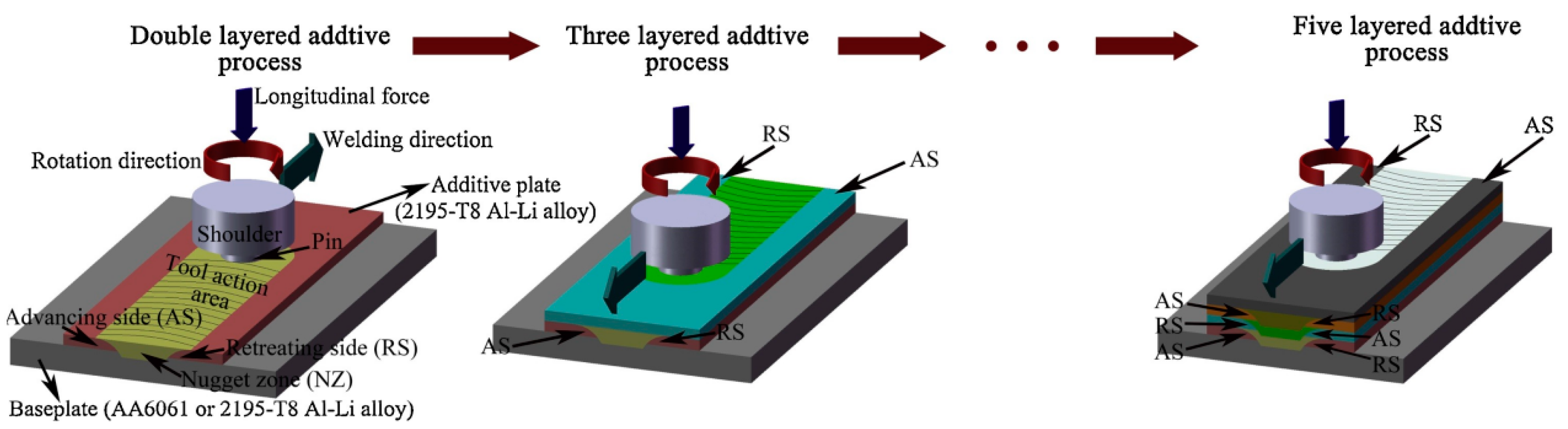
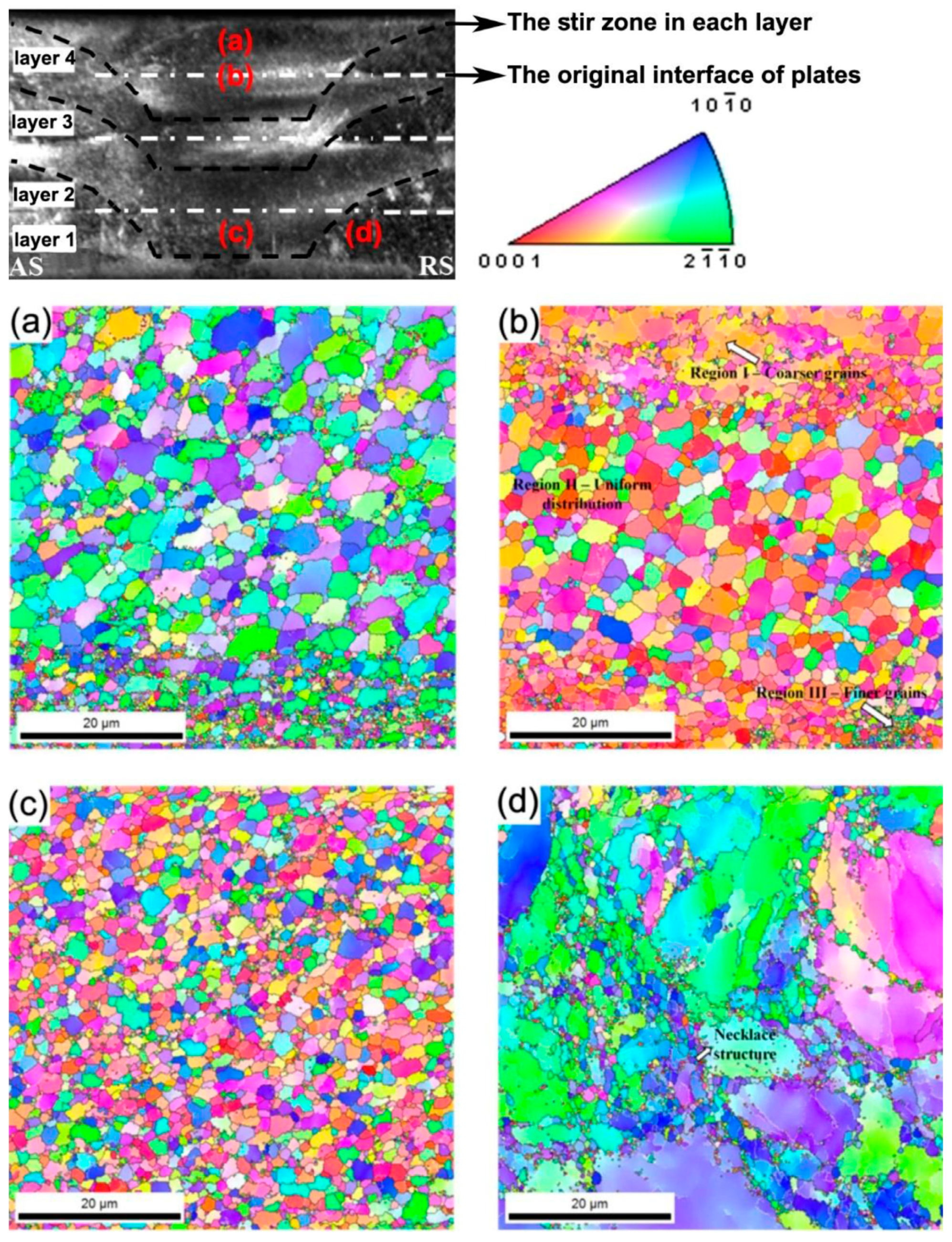
2.3. Friction Stir Additive Manufacturing
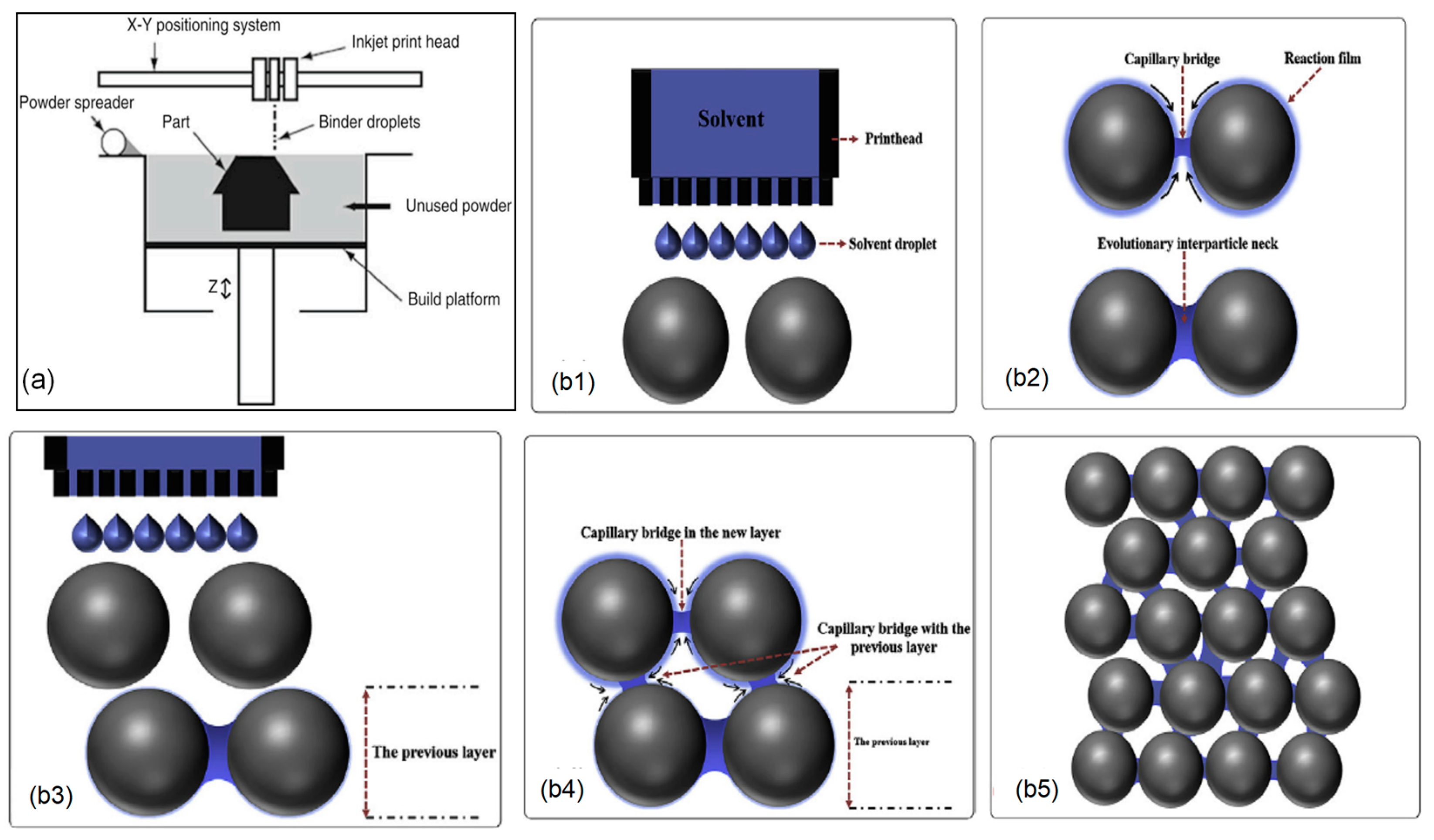
2.4. Binder Jetting
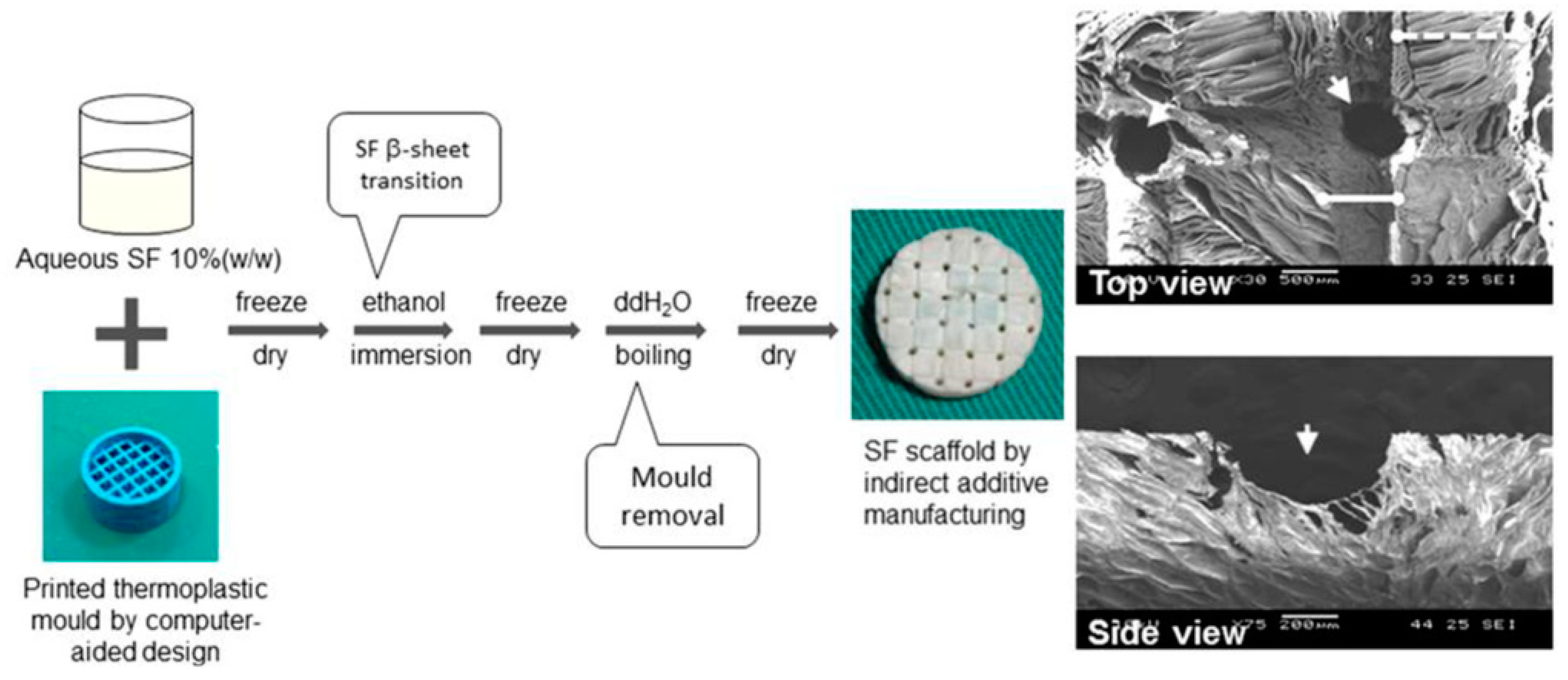
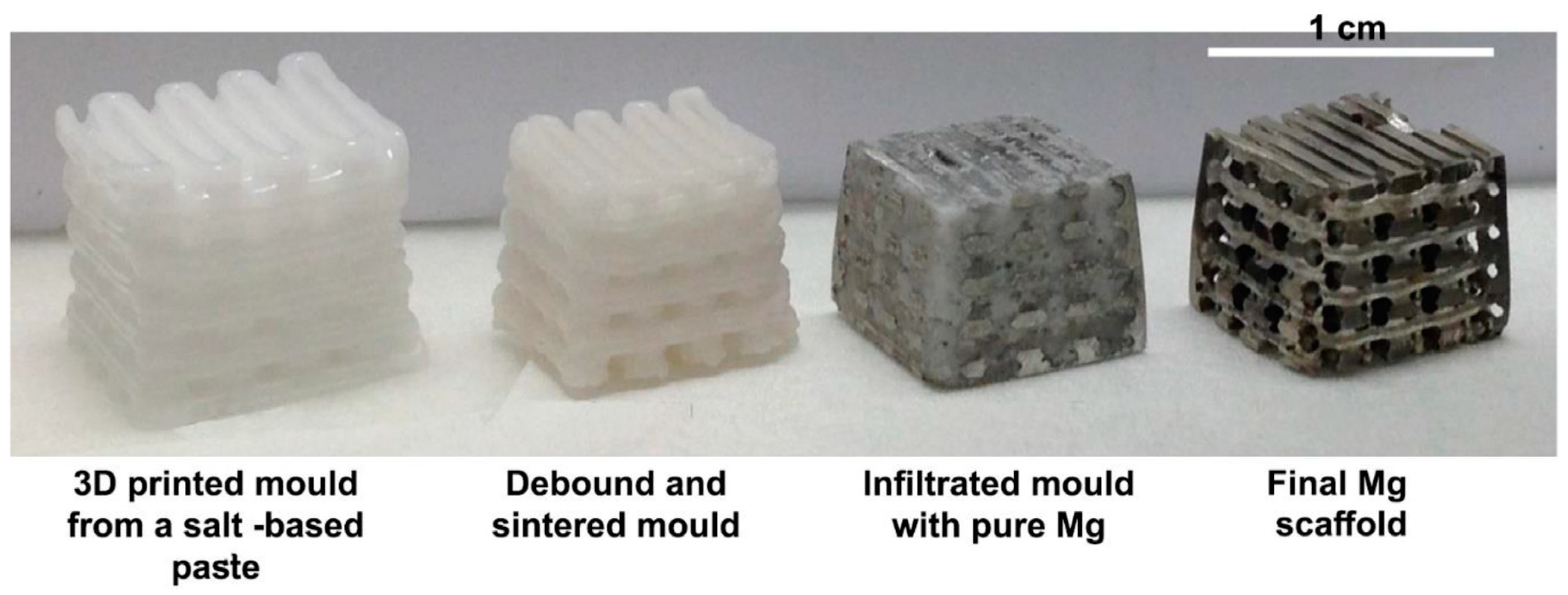
2.5. Indirect Additive Manufacturing
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| Mg AM | magnesium alloy additive manufacturing |
| SLM/LPBF | selective laser melting/laser powder bed fusion |
| WAAM | wire arc additive manufacturing |
| BJ | jetting technologies |
| FSAM | friction stir additive manufacturing |
| I-AM | indirect additive manufacturing |
| DOE | design of experiments |
| AZ31 | Mg-Al-Zn alloy |
| Ev | energy density |
| DLD | direct laser deposition |
| RD | Relative density |
| MBG | Mesoporous Bioactive Glass |
| ZK60 | Mg-Zn-Zr alloy |
| WE43 | Mg-Al-Zn-RE alloy |
| AZ61 | Mg-Al-Zn alloy |
| AZ91 | Mg-Al-Zn alloy |
| AZ91D | Mg-Al-Zn alloy |
| ZK30 | Mg-Zn-Zr alloy |
| DED | direct energy deposition |
| TIG | tungsten inert gas |
| PAW | plasma arc welding |
| MIG | metal inert gas |
| CMT(GMAW) | cold metal transfer(gas shielded metal arc welding) |
| GTAW | gas tungsten arc welding |
| AZ-series Mg-alloys | Mg-Al-Zn alloy |
| GW63K | Mg-Gd-Y alloy |
| TMAZ | Thermo-Mechanically Affected Zone |
| CIJ | both continuous inkjet |
| DOD | drop-on-demand |
| CAD | computer-aided design |
| EBM | electron beam melting |
References
- Ding, W.J.; Fu, P.H.; Peng, L.M.; Jiang, H.Y.; Wang, Y.X.; Wu, G.H.; Dong, J.; Guo, X.W. Advanced magnesium alloys and their applications in aerospace. Spacecr. Environ. Eng. 2011, 28, 103–109. [Google Scholar]
- Kulekci, M.K. Magnesium and its alloys applications in automotive industry. Int. J. Adv. Manuf. Technol. 2008, 39, 851–865. [Google Scholar] [CrossRef]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Asp. Med. 2003, 24, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pesode, P.; Barve, S. Magnesium Alloy for Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2022; pp. 133–158. [Google Scholar]
- Polmear, I.; StJohn, D.; Nie, J.F.; Qian, M. Light Alloys: Metallurgy of the Light Metals; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F.J. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of magnesium and its alloys: A review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Bettles, C.; Barnett, M. Advances in Wrought Magnesium Alloys: Fundamentals of Processing, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Moosbrugger, C. Engineering Properties of Magnesium Alloys; ASM International: Almere, The Netherlands, 2017. [Google Scholar]
- Smith, W.F. Structure and Properties of Engineering Alloys; McGraw-Hill: New York, NY, USA, 1993. [Google Scholar]
- Chen, F.; Cai, X.; Dong, B.; Lin, S. Effect of process modes on microstructure and mechanical properties of CMT wire arc additive manufactured WE43 magnesium alloy. J. Mater. Res. Technol. 2023, 27, 2089–2101. [Google Scholar] [CrossRef]
- Peng, B.; Xu, H.; Song, F.; Wen, P.; Tian, Y.; Zheng, Y. Additive manufacturing of porous magnesium alloys for biodegradable orthopedic implants: Process, design, and modification. J. Mater. Sci. Technol. 2024, 182, 79–110. [Google Scholar] [CrossRef]
- ASTM52900-15; Additive Manufacturing—General Principles—Terminology. ASTM International: West Conshohocken, PA, USA, 2015.
- Gehrmann, R.; Frommert, M.M.; Gottstein, G. Texture effects on plastic deformation of magnesium. Mater. Sci. Eng. A 2005, 395, 338–349. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Z.; Zeng, X. Influence of element vaporization on formability, composition, microstructure, and mechanical performance of the selective laser melted Mg–Zn–Zr components. Mater. Lett. 2015, 156, 187–190. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J. The role of twinning and untwinning in yielding behavior in hot-extruded Mg–Al–Zn alloy. Acta Mater. 2007, 55, 897–905. [Google Scholar] [CrossRef]
- Yan, Q.; Song, B.; Shi, Y. Comparative study of performance comparison of AlSi10Mg alloy prepared by selective laser melting and casting. J. Mater. Sci. Technol. 2020, 41, 199–208. [Google Scholar] [CrossRef]
- Zhu, H.; Windle, A. Effects of cell irregularity on the high strain compression of open-cell foams. Acta Mater. 2002, 50, 1041–1052. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Staiger, M.P.; Dias, G.J.; Woodfield, T.B.F. A novel manufacturing route for fabrication of topologically-ordered porous magnesium scaffolds. Adv. Eng. Mater. 2011, 13, 872–881. [Google Scholar] [CrossRef]
- Dobkowska, A.; Żrodowski, Ł.; Chlewicka, M.; Koralnik, M.; Adamczyk-Cieślak, B.; Ciftci, J.; Morończyk, B.; Kruszewski, M.; Jaroszewicz, J.; Kuc, D.; et al. A comparison of the microstructure-dependent corrosion of dual-structured Mg-Li alloys fabricated by powder consolidation methods: Laser powder bed fusion vs pulse plasma sintering. J. Magnes. Alloys 2022, 10, 3553–3564. [Google Scholar] [CrossRef]
- Han, S.; Zielewski, M.; Holguin, D.M.; Parra, M.M.; Kim, N. Optimization of AZ91D process and corrosion resistance using wire arc additive manufacturing. Appl. Sci. 2018, 8, 1306. [Google Scholar] [CrossRef]
- Zeng, Z.; Salehi, M.; Kopp, A.; Xu, S.; Esmaily, M.; Birbilis, N. Recent progress and perspectives in additive manufacturing of magnesium alloys. J. Magnes. Alloys 2022, 10, 1511–1541. [Google Scholar] [CrossRef]
- Manjhi, S.K.; Sekar, P.; Bontha, S.; Balan, A. Additive manufacturing of magnesium alloys: Characterization and Post-processing. Int. J. Light. Mater. Manuf. 2024, 7, 184–213. [Google Scholar] [CrossRef]
- Gneiger, S.; Österreicher, J.A.; Arnoldt, A.R.; Birgmann, A.; Fehlbier, M. Development of a high strength magnesium alloy for wire arc additive manufacturing. Metals 2020, 10, 778. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Additive manufacturing of metallic biomaterials: Sustainability aspect, opportunity, and challenges. J. Ind. Prod. Eng. 2023, 40, 464–505. [Google Scholar] [CrossRef]
- Takagi, H.; Sasahara, H.; Abe, T.; Sannomiya, H.; Nishiyama, S.; Ohta, S.; Nakamura, K. Material-property evaluation of magnesium alloys fabricated using wire-and-arc-based additive manufacturing. Addit. Manuf. 2018, 24, 498–507. [Google Scholar] [CrossRef]
- Hermawan, H.; Dubé, D.; Mantovani, D. Degradable metallic biomaterials: Design and development of Fe–Mn alloys for stents. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, R.; Ortgies, S.; Tamayol, A.; Bobaru, F.; Sealy, M.P. Additive manufacturing of magnesium alloys. Bioact. Mater. 2020, 5, 44–54. [Google Scholar] [CrossRef]
- Gangireddy, S.; Gwalani, B.; Liu, K.; Faierson, E.J.; Mishra, R.S. Microstructure and mechanical behavior of an additive manufactured (AM) WE43-Mg alloy. Addit. Manuf. 2019, 26, 53–64. [Google Scholar] [CrossRef]
- Kruth, J.-P.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Proaño, B.; Miyahara, H.; Matsumoto, T.; Hamada, S.; Sakai, H.; Ogawa, K.; Suyalatu; Noguchi, H. Weakest region analysis of non-combustible Mg products fabricated by selective laser melting. Theor. Appl. Fract. Mech. 2019, 103, 102291. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Min, S.; Yin, B.; Peng, B.; Yu, Z.; Wang, C.; Ma, X.; Wen, P.; Tian, Y.; et al. Biodegradable magnesium alloy WE43 porous scaffolds fabricated by laser powder bed fusion for orthopedic applications: Process optimization, in vitro and in vivo investigation. Bioact. Mater. 2022, 16, 301–319. [Google Scholar] [CrossRef]
- Wei, K.; Zeng, X.; Wang, Z.; Deng, J.; Liu, M.; Huang, G.; Yuan, X. Selective laser melting of Mg-Zn binary alloys: Effects of Zn content on densification behavior, microstructure, and mechanical property. Mater. Sci. Eng. A 2019, 756, 226–236. [Google Scholar] [CrossRef]
- Park, J.; Wyman, L. Phase Relationships in Magnesium Alloys. Period Covered: December 1954 to August 1957; National Bureau of Standards: Washington, DC, USA, 1957. [Google Scholar]
- Li, K.; Ji, C.; Bai, S.; Jiang, B.; Pan, F. Selective laser melting of magnesium alloys: Necessity, formability, performance, optimization and applications. J. Mater. Sci. Technol. 2023, 154, 65–93. [Google Scholar] [CrossRef]
- Alloy, N.B.; Tian, Z.; Zhang, C.; Wang, D.; Liu, W.; Fang, X. A Review on Laser Powder Bed Fusion of Inconel 625. Appl. Sci. 2020, 10, 81. [Google Scholar]
- Hong, C.; Gu, D.; Dai, D.; Alkhayat, M.; Urban, W.; Yuan, P.; Cao, S.; Gasser, A.; Weisheit, A.; Kelbassa, I.; et al. Laser additive manufacturing of ultrafine TiC particle reinforced Inconel 625 based composite parts: Tailored microstructures and enhanced performance. Mater. Sci. Eng. A 2015, 635, 118–128. [Google Scholar] [CrossRef]
- Pawlak, A.; Rosienkiewicz, M.; Chlebus, E. Design of experiments approach in AZ31 powder selective laser melting process optimization. Arch. Civ. Mech. Eng. 2017, 17, 9–18. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser rapid solidification improves corrosion behavior of Mg-Zn-Zr alloy. J. Alloys Compd. 2017, 691, 961–969. [Google Scholar] [CrossRef]
- Jiao, L.; Chua, Z.Y.; Moon, S.K.; Song, J.; Bi, G.; Zheng, H. Femtosecond laser produced hydrophobic hierarchical structures on additive manufacturing parts. Nanomaterials 2018, 8, 601. [Google Scholar] [CrossRef] [PubMed]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive manufactured WE43 magnesium: A comparative study of the microstructure and mechanical properties with those of powder extruded and as-cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, H.; Xi, X. Research status and prospects of selective laser melting forming of aluminum alloy and composites. Powder Metall. Ind. 2022, 32, 1–12. [Google Scholar]
- Bär, F.; Berger, L.; Jauer, L.; Kurtuldu, G.; Schäublin, R.; Schleifenbaum, J.H.; Löffler, J.F. Laser additive manufacturing of biodegradable magnesium alloy WE43: A detailed microstructure analysis. Acta Biomater. 2019, 98, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; De Cicco, M.P.; Li, X. Using diluted master nanocomposites to achieve grain refinement and mechanical property enhancement in as-cast Al–9Mg. Mater. Sci. Eng. A 2012, 532, 396–400. [Google Scholar] [CrossRef]
- Fu, P.H.; Wang, N.Q.; Liao, H.G.; Xu, W.Y.; Peng, L.M.; Juan, C.H.; Hu, G.Q.; Ding, W.J. Microstructure and mechanical properties of high strength Mg− 15Gd− 1Zn− 0.4 Zr alloy additive-manufactured by selective laser melting process. Trans. Nonferrous Met. Soc. China 2021, 31, 1969–1978. [Google Scholar] [CrossRef]
- Esmaily, M.; Zeng, Z.; Mortazavi, A.; Gullino, A.; Choudhary, S.; Derra, T.; Benn, F.; D'Elia, F.; Müther, M.; Thomas, S.; et al. A detailed microstructural and corrosion analysis of magnesium alloy WE43 manufactured by selective laser melting. Addit. Manuf. 2020, 35, 101321. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Chen, C. Effect of laser processing parameters on porosity, microstructure and mechanical properties of porous Mg-Ca alloys produced by laser additive manufacturing. Mater. Sci. Eng. A 2017, 703, 359–371. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Zhang, D.; Hao, L.; Jiang, J.; Li, Z.; Chen, Y. Experimental investigation on selective laser melting of bulk net-shape pure magnesium. Mater. Manuf. Process. 2015, 30, 1298–1304. [Google Scholar] [CrossRef]
- Savalani, M.M.; Pizarro, J.M. Effect of preheat and layer thickness on selective laser melting (SLM) of magnesium. Rapid Prototyp. J. 2016, 22, 115–122. [Google Scholar] [CrossRef]
- Niu, X.; Shen, H.; Fu, J.; Yan, J.; Wang, Y. Corrosion behaviour of laser powder bed fused bulk pure magnesium in hank’s solution. Corros. Sci. 2019, 157, 284–294. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Liu, B.; Zeng, R.-C.; Chen, X.-B.; Li, S.-Q.; Wang, Z.-L.; Han, E.-H. Corrosion resistance of glucose-induced hydrothermal calcium phosphate coating on pure magnesium. Appl. Surf. Sci. 2019, 465, 1066–1077. [Google Scholar] [CrossRef]
- Yin, B.; Liu, J.; Peng, B.; Zhou, M.; Liu, B.; Ma, X.; Wang, C.; Wen, P.; Tian, Y.; Zheng, Y. Influence of layer thickness on formation quality, microstructure, mechanical properties, and corrosion resistance of WE43 magnesium alloy fabricated by laser powder bed fusion. J. Magnes. Alloys 2024, 12, 1367–1385. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Additive manufacturing of magnesium alloys and its biocompatibility. Bioprinting 2023, 36, e00318. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.J. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf. -Green Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Z.; Zeng, X. Element loss of AZ91D magnesium alloy during selective laser melting process. Acta Met. Sin 2016, 52, 184–190. [Google Scholar]
- Liu, J.; Yin, B.; Sun, Z.; Wen, P.; Zheng, Y.; Tian, Y. Hot cracking in ZK60 magnesium alloy produced by laser powder bed fusion process. Mater. Lett. 2021, 301, 130283. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, Q.; Liang, L.; Hu, X.; Liu, T.; Weng, Y.; Long, T.; Liu, Y.; Li, Q.; Zhou, S.; et al. In vitro degradation behavior and cytocompatibility of ZK30/bioactive glass composites fabricated by selective laser melting for biomedical applications. J. Alloys Compd. 2019, 785, 38–45. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Gieseke, M.; Noelke, C.; Kaierle, S.; Maier, H.J.; Haferkamp, H. Selective laser melting of magnesium alloys for manufacturing individual implants. In Proceedings of the Fraunhofer Direct Digital Manufacturing Conference, Berlin, Germany, 12–13 March 2014. [Google Scholar]
- Committee F42 on Additive Manufacturing Technologies; ASTM International: West Conshohocken, PA, USA, 2012.
- Chen, C.-H.; Liu, J.M.-J.; Chua, C.-K.; Chou, S.-M.; Shyu, V.B.-H.; Chen, J.-P. Cartilage tissue engineering with silk fibroin scaffolds fabricated by indirect additive manufacturing technology. Materials 2014, 7, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Derekar, K.S. A review of wire arc additive manufacturing and advances in wire arc additive manufacturing of aluminium. Mater. Sci. Technol. 2018, 34, 895–916. [Google Scholar] [CrossRef]
- Jianwei, L.I.; Youmin, Q.I.; Junjie, Y.A.; Sheng, Y.; Yanliang, Y.I.; Xun, Z.E.; Lianxi, C.H.; Fengliang, Y.I.; Jiangzhou, S.U.; Zhang, T.; et al. Effect of grain refinement induced by wire and arc additive manufacture (WAAM) on the corrosion behaviors of AZ31 magnesium alloy in NaCl solution. J. Magnes. Alloys 2023, 11, 217–229. [Google Scholar]
- Hauser, T.; Reisch, R.T.; Breese, P.P.; Nalam, Y.; Joshi, K.S.; Bela, K.; Kamps, T.; Volpp, J.; Kaplan, A.F. Oxidation in wire arc additive manufacturing of aluminium alloys. Addit. Manuf. 2021, 41, 101958. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Wang, Z.; Lin, X.; Liu, F.; Huang, W.; Liang, E. Microstructure and mechanical properties of wire and arc additive manufactured AZ31 magnesium alloy using cold metal transfer process. Mater. Sci. Eng. A 2020, 774, 138942. [Google Scholar] [CrossRef]
- Dong, B.; Cai, X.; Lin, S.; Li, X.; Fan, C.; Yang, C.; Sun, H. Wire arc additive manufacturing of Al-Zn-Mg-Cu alloy: Microstructures and mechanical properties. Addit. Manuf. 2020, 36, 101447. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, Y.; Zhou, C.; Li, X.; Chen, Y. Microstructure and mechanical properties of Mg–Li alloys fabricated by wire arc additive manufacturing. J. Mater. Res. Technol. 2024, 29, 3487–3493. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, M.; Zhang, T.; Xie, J.; Wei, K.; Wang, S.; Yin, L.; He, P. Grain refinement and mechanical properties improvement of Inconel 625 alloy fabricated by ultrasonic-assisted wire and arc additive manufacturing. J. Alloys Compd. 2022, 910, 164957. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Farias, F.W.C.; Zhang, K.; Shamsolhodaei, A.; Shen, J.; Zhou, N.; Schell, N.; Capek, J.; Polatidis, E.; Santos, T.G.; et al. Wire and arc additive manufacturing of 316L stainless steel/Inconel 625 functionally graded material: Development and characterization. J. Mater. Res. Technol. 2022, 21, 237–251. [Google Scholar] [CrossRef]
- Srivastava, M.; Rathee, S.; Tiwari, A.; Dongre, M. Wire arc additive manufacturing of metals: A review on processes, materials and their behaviour. Mater. Chem. Phys. 2023, 294, 126988. [Google Scholar] [CrossRef]
- Song, J.; Chen, Y.; Hao, X.; Wang, M.; Ma, Y.; Xie, J. Microstructure and mechanical properties of novel Ni–Cr–Co-based superalloy GTAW joints. J. Mater. Res. Technol. 2024, 29, 2758–2767. [Google Scholar] [CrossRef]
- Kim, J.D.; Jun, H.U.; Cheon, J.; Kim, J.H.; Ji, C.; Kim, Y.D. Effect of additional side shielding on the wire arc additive manufacturing of AZ31 magnesium alloy. J. Mater. Res. Technol. 2023, 25, 6567–6577. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Bai, P.; Zhang, R.; Zhang, Z.; Wang, L.; Du, W.; Wang, F.; Huang, Z. Microstructure and mechanical properties of AZ31 magnesium alloy prepared using wire arc additive manufacturing. J. Alloys Compd. 2023, 939, 168665. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Liu, C.; Wu, Q.; Chen, X.; Lu, J. Wire arc additive manufacturing of AZ31 magnesium alloy: Grain refinement by adjusting pulse frequency. Materials 2016, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Arnoldt, A.; Schnall, M.; Gneiger, S. Microstructure formation and mechanical properties of a wire-arc additive manufactured magnesium alloy. JOM 2021, 73, 1126–1134. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, G.; Jiang, Y.; Ren, L.; Fan, L.; Pan, H. Formability, microstructure evolution and mechanical properties of wire arc additively manufactured AZ80M magnesium alloy using gas tungsten arc welding. J. Magnes. Alloys 2021, 9, 192–201. [Google Scholar] [CrossRef]
- Razavi, M.; Huang, Y. Assessment of magnesium-based biomaterials: From bench to clinic. Biomater. Sci. 2019, 7, 2241–2263. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Fang, X.; Li, Z.; Jiao, G.; Huang, K. Improved strength-ductility synergy of directed energy deposited AZ31 magnesium alloy with cryogenic cooling mode. Virtual Phys. Prototyp. 2023, 18, e2170252. [Google Scholar] [CrossRef]
- Li, X.; Fang, X.; Zhang, M.; Zhang, H.; Duan, Y.; Huang, K. Gradient microstructure and prominent performance of wire-arc directed energy deposited magnesium alloy via laser shock peening. Int. J. Mach. Tools Manuf. 2023, 188, 104029. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, G.; Celikin, M.; Ren, L.; Zhan, Y.; Fan, L.; Pan, H. Effect of heat treatment on the microstructure and mechanical properties of AZ80M magnesium alloy fabricated by wire arc additive manufacturing. J. Magnes. Alloys 2022, 10, 1930–1940. [Google Scholar] [CrossRef]
- Fang, X.; Yang, J.; Wang, S.; Wang, C.; Huang, K.; Li, H.; Lu, B. Additive manufacturing of high performance AZ31 magnesium alloy with full equiaxed grains: Microstructure, mechanical property, and electromechanical corrosion performance. J. Mater. Process. Technol. 2022, 300, 117430. [Google Scholar] [CrossRef]
- Cao, Q.; Qi, B.; Zeng, C.; Zhang, R.; He, B.; Qi, Z.; Wang, F.; Wang, H.; Cong, B. Achieving equiaxed microstructure and isotropic mechanical properties of additively manufactured AZ31 magnesium alloy via ultrasonic frequency pulsed arc. J. Alloys Compd. 2022, 909, 164742. [Google Scholar] [CrossRef]
- Yi, H.; Wang, Q.; Cao, H. Wire-arc directed energy deposition of magnesium alloys: Microstructure, properties and quality optimization strategies. J. Mater. Res. Technol. 2022, 20, 627–649. [Google Scholar] [CrossRef]
- Wan, Y.; Zeng, Y.; Dou, Y.; Hu, D.; Qian, X.; Zeng, Q.; Sun, K.; Quan, G. Improved mechanical properties and strengthening mechanism with the altered precipitate orientation in magnesium alloys. J. Magnes. Alloys 2022, 10, 1256–1267. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Xiao, Z.; Liu, Y.; Huang, Y. Comparative study on dislocation slip activity of the solid-solution and as-aged Mg-9.8Gd-3.5Y-2.0Zn-0.4Zr (wt%) alloys during ambient temperature quasi-in-situ compression. Mater. Charact. 2022, 194, 112398. [Google Scholar] [CrossRef]
- Bi, J.; Shen, J.; Hu, S.; Zhen, Y.; Yin, F.; Bu, X. Microstructure and mechanical properties of AZ91 Mg alloy fabricated by cold metal transfer additive manufacturing. Mater. Lett. 2020, 276, 128185. [Google Scholar] [CrossRef]
- Tong, X.; Wu, G.; Easton, M.A.; Sun, M.; Wang, Q.; Zhang, L. Microstructural evolution and strengthening mechanism of Mg-Y-RE-Zr alloy fabricated by quasi-directed energy deposition. Addit. Manuf. 2023, 67, 103487. [Google Scholar] [CrossRef]
- Wei, J.; He, C.; Qie, M.; Li, Y.; Tian, N.; Qin, G.; Zuo, L. Achieving high performance of wire arc additive manufactured Mg–Y–Nd alloy assisted by interlayer friction stir processing. J. Mater. Process. Technol. 2023, 311, 117809. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Lin, X.; Feng, L.; Ouyang, L.; Zhang, T.; Dai, C.; Yang, W.; Huang, W.; Pan, F. Microstructure and mechanical properties of a dilute Mg-Gd-Y-Zr alloy fabricated using wire-arc directed energy deposition additive manufacturing. J. Mater. Res. Technol. 2024, 29, 4881–4894. [Google Scholar] [CrossRef]
- Zheng, D.; Li, Z.; Jiang, Y.; Li, R.; Wu, Y.; Tu, Y.; Cheng, X.; Fu, P.; Peng, L.; Tang, H. Effect of multiple thermal cycles on the microstructure evolution of GA151K alloy fabricated by laser-directed energy deposition. Addit. Manuf. 2022, 57, 102957. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Li, Q.; Ayas, C. A computationally efficient thermo-mechanical model for wire arc additive manufacturing. Addit. Manuf. 2021, 46, 102090. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, X.; Zhang, Y.; Ren, S.; Yin, C.; Cao, Y.; Zhang, D. Modeling of whole-phase heat transport in laser-based directed energy deposition with multichannel coaxial powder feeding. Addit. Manuf. 2022, 59, 103161. [Google Scholar] [CrossRef]
- Hooper, P.A. Melt pool temperature and cooling rates in laser powder bed fusion. Addit. Manuf. 2018, 22, 548–559. [Google Scholar] [CrossRef]
- Nopová, K.; Jaroš, J.; Červinek, O.; Pantělejev, L.; Gneiger, S.; Senck, S.; Koutný, D. Processing of AZ91D Magnesium Alloy by Laser Powder Bed Fusion. Appl. Sci. 2023, 13, 1377. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Zhu, H.; Li, Q.; Feng, M. Wire-arc additive manufacturing of AZ31 magnesium alloy fabricated by cold metal transfer heat source: Processing, microstructure, and mechanical behavior. J. Mater. Process. Technol. 2021, 288, 116895. [Google Scholar] [CrossRef]
- Wu, B.; Pan, Z.; Ding, D.; Cuiuri, D.; Li, H.; Xu, J.; Norrish, J. A review of the wire arc additive manufacturing of metals: Properties, defects and quality improvement. J. Manuf. Process. 2018, 35, 127–139. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Lin, X.; Kang, N.; Zhang, T.; Wang, Y.; Wang, L.; Dang, C.; Huang, W. Solidification microstructure evolution and its correlations with mechanical properties and damping capacities of Mg-Al-based alloy fabricated using wire and arc additive manufacturing. J. Mater. Sci. Technol. 2023, 144, 28–44. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, B.; Zhang, Y.; Wang, H.; Liu, Y.; Zhang, X.; Chen, C. Microstructural evolution and strengthening mechanisms of CMT directed energy deposition-arc with interlayer ultrasonic impact treatment manufactured AZ31 magnesium alloy. Mater. Sci. Eng. A 2023, 879, 145267. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, F.; Xu, Y.; Li, C.; Liu, F.; Huang, C.; Lin, X.; Zheng, H. Effects of arc oscillation on microstructure and mechanical properties of AZ31 magnesium alloy prepared by CMT wire-arc directed energy deposition. Mater. Sci. Eng. A 2023, 864, 144539. [Google Scholar] [CrossRef]
- Yi, H.; Wang, Q.; Zhang, W.; Cao, H. Wire-arc directed energy deposited Mg-Al alloy assisted by ultrasonic vibration: Improving properties via controlling grain structures. J. Mater. Process. Technol. 2023, 321, 118134. [Google Scholar] [CrossRef]
- Palanivel, S.; Nelaturu, P.; Glass, B.; Mishra, R. Friction stir additive manufacturing for high structural performance through microstructural control in an Mg based WE43 alloy. Mater. Des. 2015, 65, 934–952. [Google Scholar] [CrossRef]
- McClelland, Z.; Avery, D.Z.; Williams, M.B.; Mason, C.J.; Rivera, O.G.; Leah, C.; Allison, P.G.; Jordon, J.B.; Martens, R.L.; Hardwick, N. Microstructure and mechanical properties of high shear material deposition of rare earth magnesium alloys WE43. In Magnesium Technology 2019; Springer: Berlin/Heidelberg, Germany, 2019; pp. 277–282. [Google Scholar]
- Robinson, T.; Williams, M.; Rao, H.; Kinser, R.P.; Allison, P.G.; Jordon, J.B. Microstructural and mechanical properties of a solid-state additive manufactured magnesium alloy. J. Manuf. Sci. Eng. 2022, 144, 061013. [Google Scholar] [CrossRef]
- Williams, M.B.; Robinson, T.W.; Williamson, C.J.; Kinser, R.P.; Ashmore, N.A.; Allison, P.G.; Jordon, J.B. Elucidating the effect of additive friction stir deposition on the resulting microstructure and mechanical properties of magnesium alloy we43. Metals 2021, 11, 1739. [Google Scholar] [CrossRef]
- Wlodarski, S.; Avery, D.Z.; White, B.C.; Mason, C.J.T.; Cleek, C.; Williams, M.B.; Allison, P.G.; Jordon, J.B. Evaluation of grain refinement and mechanical properties of additive friction stir layer welding of AZ31 magnesium alloy. J. Mater. Eng. Perform. 2021, 30, 964–972. [Google Scholar] [CrossRef]
- Mishra, R.S.; Ma, Z.Y. Friction stir welding and processing. Mater. Sci. Eng. R Rep. 2005, 50, 1–78. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, X.; Li, S.; Li, D. Interfacial bonding features of friction stir additive manufactured build for 2195-T8 aluminum-lithium alloy. J. Manuf. Process. 2019, 38, 396–410. [Google Scholar] [CrossRef]
- Ren, X.; Jiang, X.; Yuan, T.; Zhao, X.; Chen, S. Microstructure and properties research of Al-Zn-Mg-Cu alloy with high strength and high elongation fabricated by wire arc additive manufacturing. J. Mater. Process. Technol. 2022, 307, 117665. [Google Scholar] [CrossRef]
- Heidarzadeh, A.; Mironov, S.; Kaibyshev, R.; Çam, G.; Simar, A.; Gerlich, A.; Khodabakhshi, F.; Mostafaei, A.; Field, D.; Robson, J.; et al. Friction stir welding/processing of metals and alloys: A comprehensive review on microstructural evolution. Prog. Mater. Sci. 2021, 117, 100752. [Google Scholar] [CrossRef]
- Singh, K.; Singh, G.; Singh, H. Review on friction stir welding of magnesium alloys. J. Magnes. Alloys 2018, 6, 399–416. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Huang, Y.; Han, C.; Chen, J.; Xiao, Y.; Li, Y.; Wang, D. Study on powder particle behavior in powder spreading with discrete element method and its critical implications for binder jetting additive manufacturing processes. Virtual Phys. Prototyp. 2023, 18, e2158877. [Google Scholar] [CrossRef]
- Mei, J.; Lovell, M.R.; Mickle, M.H. Formulation and processing of novel conductive solution inks in continuous inkjet printing of 3-D electric circuits. IEEE Trans. Electron. Packag. Manuf. 2005, 28, 265–273. [Google Scholar]
- Vorndran, E.; Moseke, C.; Gbureck, U. 3D printing of ceramic implants. Mrs Bull. 2015, 40, 127–136. [Google Scholar] [CrossRef]
- Meininger, S.; Moseke, C.; Spatz, K.; März, E.; Blum, C.; Ewald, A.; Vorndran, E. Effect of strontium substitution on the material properties and osteogenic potential of 3D powder printed magnesium phosphate scaffolds. Mater. Sci. Eng. C 2019, 98, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Meininger, S.; Mandal, S.; Kumar, A.; Groll, J.; Basu, B.; Gbureck, U. Strength reliability and in vitro degradation of three-dimensional powder printed strontium-substituted magnesium phosphate scaffolds. Acta Biomater. 2016, 31, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, J.; Li, H.; You, Z.; Li, J. Binder-jetting additive manufacturing of Mg alloy densified by two-step sintering process. J. Manuf. Process. 2021, 72, 71–79. [Google Scholar] [CrossRef]
- Stevens, E.; Schloder, S.; Bono, E.; Schmidt, D.; Chmielus, M. Density variation in binder jetting 3D-printed and sintered Ti-6Al-4V. Addit. Manuf. 2018, 22, 746–752. [Google Scholar] [CrossRef]
- Rishmawi, I.; Salarian, M.; Vlasea, M. Tailoring green and sintered density of pure iron parts using binder jetting additive manufacturing. Addit. Manuf. 2018, 24, 508–520. [Google Scholar] [CrossRef]
- Kumar, A.Y.; Bai, Y.; Eklund, A.; Williams, C.B. The effects of Hot Isostatic Pressing on parts fabricated by binder jetting additive manufacturing. Addit. Manuf. 2018, 24, 115–124. [Google Scholar] [CrossRef]
- Biegler, M.; Marko, A.; Graf, B.; Rethmeier, M. Finite element analysis of in-situ distortion and bulging for an arbitrarily curved additive manufacturing directed energy deposition geometry. Addit. Manuf. 2018, 24, 264–272. [Google Scholar] [CrossRef]
- Kuah, K.X.; Blackwood, D.J.; Ong, W.K.; Salehi, M.; Seet, H.L.; Nai, M.L.S.; Wijesinghe, S. Analysis of the corrosion performance of binder jet additive manufactured magnesium alloys for biomedical applications. J. Magnes. Alloys 2022, 10, 1296–1310. [Google Scholar] [CrossRef]
- Mirzababaei, S.; Pasebani, S. A review on binder jet additive manufacturing of 316L stainless steel. J. Manuf. Mater. Process. 2019, 3, 82. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Bin Sapari, M.A.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. Additive manufacturing of magnesium–zinc–zirconium (ZK) alloys via capillary-mediated binderless three-dimensional printing. Mater. Des. 2019, 169, 107683. [Google Scholar] [CrossRef]
- Minárik, P.; Zemková, M.; Veselý, J.; Lukáč, F.; Bohlen, J.; Knapek, M.; Král, R. Synergic effect of high temperature and high pressure on consolidation of Mg-4Y-3Nd powder by spark plasma sintering. Mater. Lett. 2021, 292, 129647. [Google Scholar] [CrossRef]
- Munir, Z.A. Analytical treatment of the role of surface oxide layers in the sintering of metals. J. Mater. Sci. 1979, 14, 2733–2740. [Google Scholar] [CrossRef]
- Munir, Z.A. Surface oxides and sintering of metals. Powder Met. 1981, 24, 177–180. [Google Scholar] [CrossRef]
- Li, M.; Yang, Q.; Zhao, Z.; Zhang, J.; Li, J. Full liquid phase sintering of binder jetting printed magnesium alloy. J. Manuf. Process. 2023, 108, 194–203. [Google Scholar] [CrossRef]
- Konda Gokuldoss, P.; Kolla, S.; Eckert, J. Additive manufacturing processes: Selective laser melting, electron beam melting and binder jetting—Selection guidelines. Materials 2017, 10, 672. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Raj, R.; Rixecker, G.; Valentinotti, M. A phenomenological model (and experiments) for liquid phase sintering. Metall. Mater. Trans. A 2007, 38, 628–637. [Google Scholar] [CrossRef]
- Podor, R.; Clavier, N.; Ravaux, J.; Claparede, L.; Dacheux, N.; Bernache-Assollant, D. Dynamic aspects of cerium dioxide sintering: HT-ESEM study of grain growth and pore elimination. J. Eur. Ceram. Soc. 2012, 32, 353–362. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, Y.; Zhang, J.; Conejo, A.N.; Liu, Z. The grain growth and grain boundary migrations during solid-phase sintering of Fe2O3: Experiments and simulations. Chem. Eng. Sci. 2022, 262, 118038. [Google Scholar] [CrossRef]
- Yin, H.-M.; Xu, W.-J.; Zhou, H.-W.; Zhao, X.-Y.; Huang, Y.-N. Effects of phase composition and grain size on the piezoelectric properties of HfO2-doped barium titanate ceramics. J. Mater. Sci. 2019, 54, 12392–12400. [Google Scholar] [CrossRef]
- Puchmark, C.; Rujijanagul, G. Relationships between Processing parameters and properties of PZT ceramics. Adv. Mater. Res. 2008, 55, 57–60. [Google Scholar] [CrossRef]
- Xu, L.Q.; Huang, C.Z.; Wang, S.L.; Liu, Z.W. Effect of sintering temperature on the mechanical properties of rare earth oxides toughened cermet tool materials. Key Eng. Mater. 2006, 315, 706–709. [Google Scholar] [CrossRef]
- Lin, S.J.; Feng, D.P.; Shi, Q.N. Microstructure and mechanical properties of vacuum sintered austenitic stainless steel parts. Adv. Mater. Res. 2011, 160, 915–920. [Google Scholar] [CrossRef]
- Belnou, F.; Bernard, J.; Houivet, D.; Haussonne, J.-M. Low temperature sintering of MgTiO3 with bismuth oxide based additions. J. Eur. Ceram. Soc. 2005, 25, 2785–2789. [Google Scholar] [CrossRef]
- Wheat, E.; Vlasea, M.; Hinebaugh, J.; Metcalfe, C. Sinter structure analysis of titanium structures fabricated via binder jetting additive manufacturing. Mater. Des. 2018, 156, 167–183. [Google Scholar] [CrossRef]
- Robertson, I.M.; Schaffer, G.B. Swelling during sintering of titanium alloys based on titanium hydride powder. Powder Metall. 2010, 53, 27–33. [Google Scholar] [CrossRef]
- Makovetskii, A.A. Discrete model for the swelling of a sealed capillary in a high-temperature furnace with a nonuniform axial temperature profile. J. Appl. Mech. Tech. Phys. 2011, 52, 965–974. [Google Scholar] [CrossRef]
- Wolff, M.; Schaper, J.G.; Dahms, M.; Ebel, T.; Kainer, K.U.; Klassen, T. Magnesium powder injection moulding for biomedical application. Powder Met. 2014, 57, 331–340. [Google Scholar] [CrossRef]
- Wolff, M.; Schaper, J.G.; Suckert, M.R.; Dahms, M.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. Magnesium powder injection molding (MIM) of orthopedic implants for biomedical applications. JOM 2016, 68, 1191–1197. [Google Scholar] [CrossRef]
- Salehi, M.; Kuah, K.X.; Huang, Z.; Blackwood, D.J.; Zhang, S.X.; Seet, H.L.; Nai, M.L.S. Enhancing densification in binder jet additive manufacturing of magnesium via nanoparticles as sintering aids. J. Manuf. Process. 2023, 99, 705–717. [Google Scholar] [CrossRef]
- Su, C.; Wang, J.; Li, H.; You, Z.; Li, J. High strength and rapid solution Mg alloy by adding Fe element fabricated by binder jetting additive manufacturing. J. Manuf. Process. 2022, 84, 652–659. [Google Scholar] [CrossRef]
- Salehi, M.; Seet, H.L.; Gupta, M.; Farnoush, H.; Maleksaeedi, S.; Nai, M.L.S. Rapid densification of additive manufactured magnesium alloys via microwave sintering. Addit. Manuf. 2021, 37, 101655. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.; Lin, P.; Leeflang, M.; van Asperen, S.; Yu, K.; Tümer, N.; Norder, B.; Zadpoor, A.; Zhou, J. Solvent-cast 3D printing of magnesium scaffolds. Acta Biomater. 2020, 114, 497–514. [Google Scholar] [CrossRef] [PubMed]
- German, R. Sintering: From Empirical Observations to Scientific Principles; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Salehi, M.; Maleksaeedi, S.; Nai, M.L.S.; Gupta, M. Towards additive manufacturing of magnesium alloys through integration of binderless 3D printing and rapid microwave sintering. Addit. Manuf. 2019, 29, 100790. [Google Scholar] [CrossRef]
- Staiger, M.P.; Kolbeinsson, I.; Kirkland, N.T.; Nguyen, T.; Dias, G.; Woodfield, T.B. Synthesis of topologically-ordered open-cell porous magnesium. Mater. Lett. 2010, 64, 2572–2574. [Google Scholar] [CrossRef]
- Yusop, A.H.; Bakir, A.A.; Shaharom, N.A.; Abdul Kadir, M.R.; Hermawan, H. Porous biodegradable metals for hard tissue scaffolds: A review. Int. J. Biomater. 2012, 2012, 641430. [Google Scholar] [CrossRef]
- Liu, M.; Chou, S.; Chua, C.; Tay, B.; Ng, B. The development of silk fibroin scaffolds using an indirect rapid prototyping approach: Morphological analysis and cell growth monitoring by spectral-domain optical coherence tomography. Med. Eng. Phys. 2013, 35, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.E.; van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W.J.A.; de Bruijn, J.D. Scaffolds with a standardized macro-architecture fabricated from several calcium phosphate ceramics using an indirect rapid prototyping technique. J. Mater. Sci. Mater. Med. 2011, 22, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Deisinger, U.; Detsch, R.; Ziegler, G. Indirect rapid prototyping of biphasic calcium phosphate scaffolds as bone substitutes: Influence of phase composition, macroporosity and pore geometry on mechanical properties. J. Mater. Sci. Mater. Med. 2010, 21, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.; Puppi, D.; Dinucci, D.; Errico, C.; Bártolo, P.; Chiellini, F. Dual-scale polymeric constructs as scaffolds for tissue engineering. Materials 2011, 4, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Sezer, N.; Evis, Z.; Koc, M. Additive manufacturing of biodegradable magnesium implants and scaffolds: Review of the recent advances and research trends. J. Magnes. Alloys 2021, 9, 392–415. [Google Scholar] [CrossRef]
- Kleger, N.; Cihova, M.; Masania, K.; Studart, A.R.; Löffler, J.F. 3D printing of salt as a template for magnesium with structured porosity. Adv. Mater. 2019, 31, 1903783. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Chen, B. Progress in Additive Manufacturing of Magnesium Alloys: A Review. Materials 2024, 17, 3851. https://doi.org/10.3390/ma17153851
Chen J, Chen B. Progress in Additive Manufacturing of Magnesium Alloys: A Review. Materials. 2024; 17(15):3851. https://doi.org/10.3390/ma17153851
Chicago/Turabian StyleChen, Jiayu, and Bin Chen. 2024. "Progress in Additive Manufacturing of Magnesium Alloys: A Review" Materials 17, no. 15: 3851. https://doi.org/10.3390/ma17153851




