Block Copoly (Ester-Carbonate) Electrolytes for LiFePO4|Li Batteries with Stable Cycling Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PCL-b-PPCP and PCL-b-PPC
2.3. Cathode Fabrication and Cell Assembly
2.4. Electrochemical Measurements
3. Results and Discussion
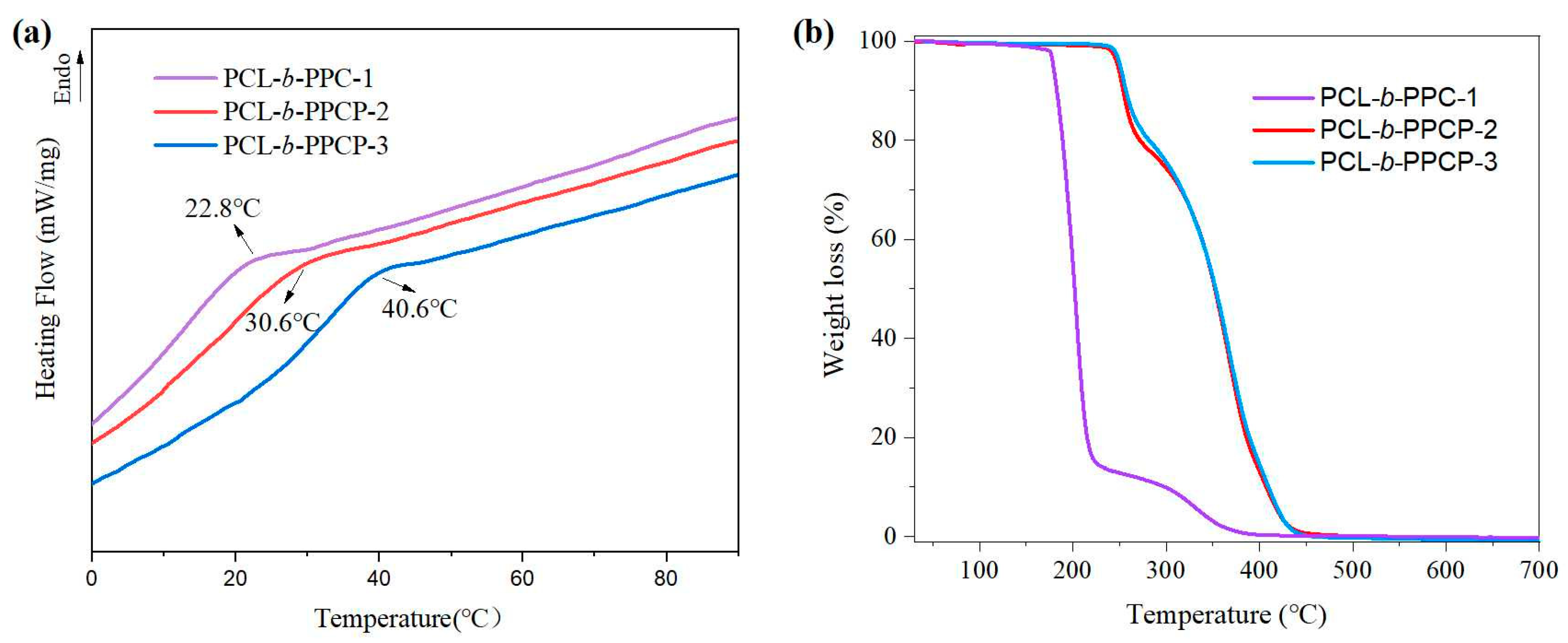
3.1. Analysis of the Structure and Thermal Properties of the Polymers
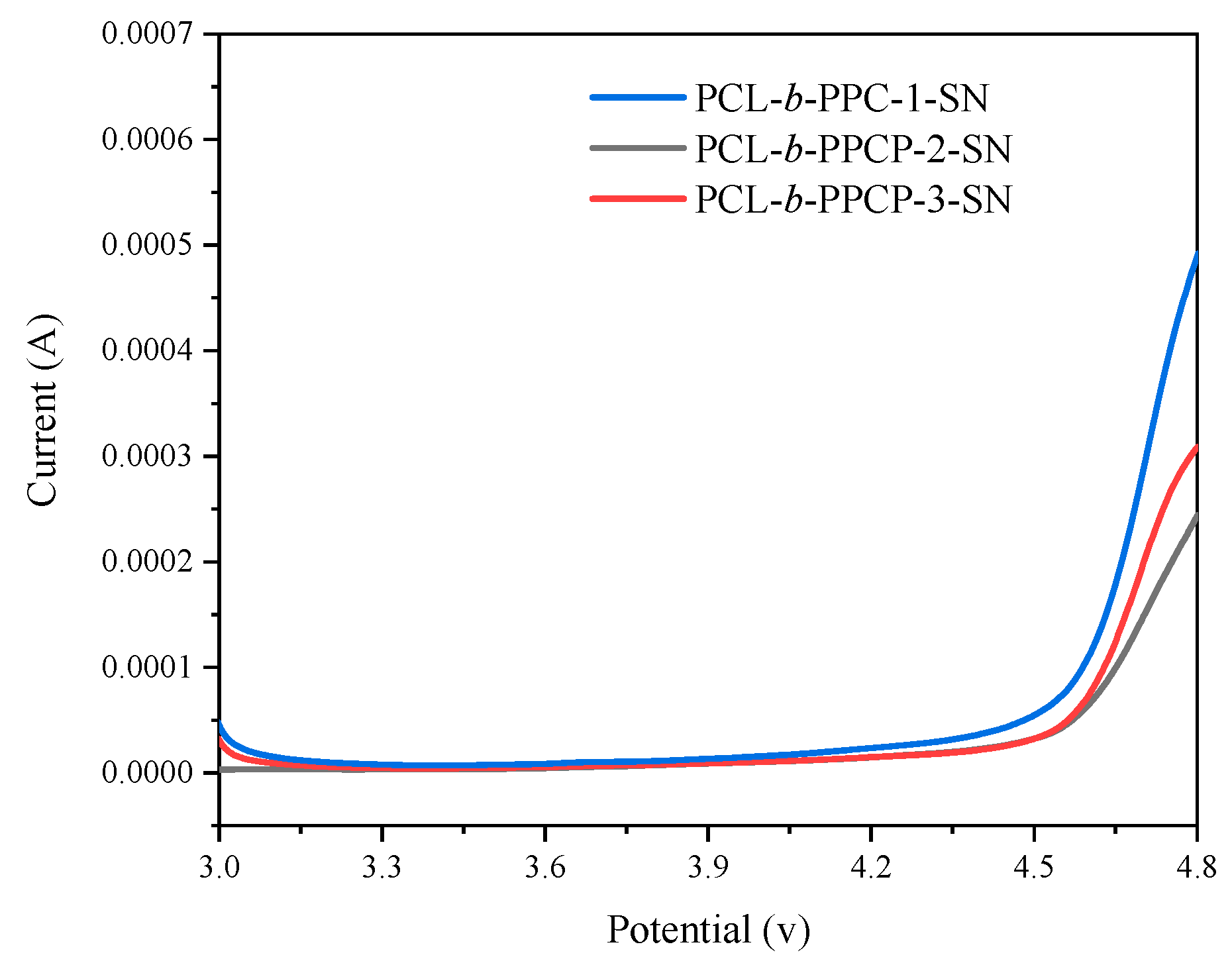
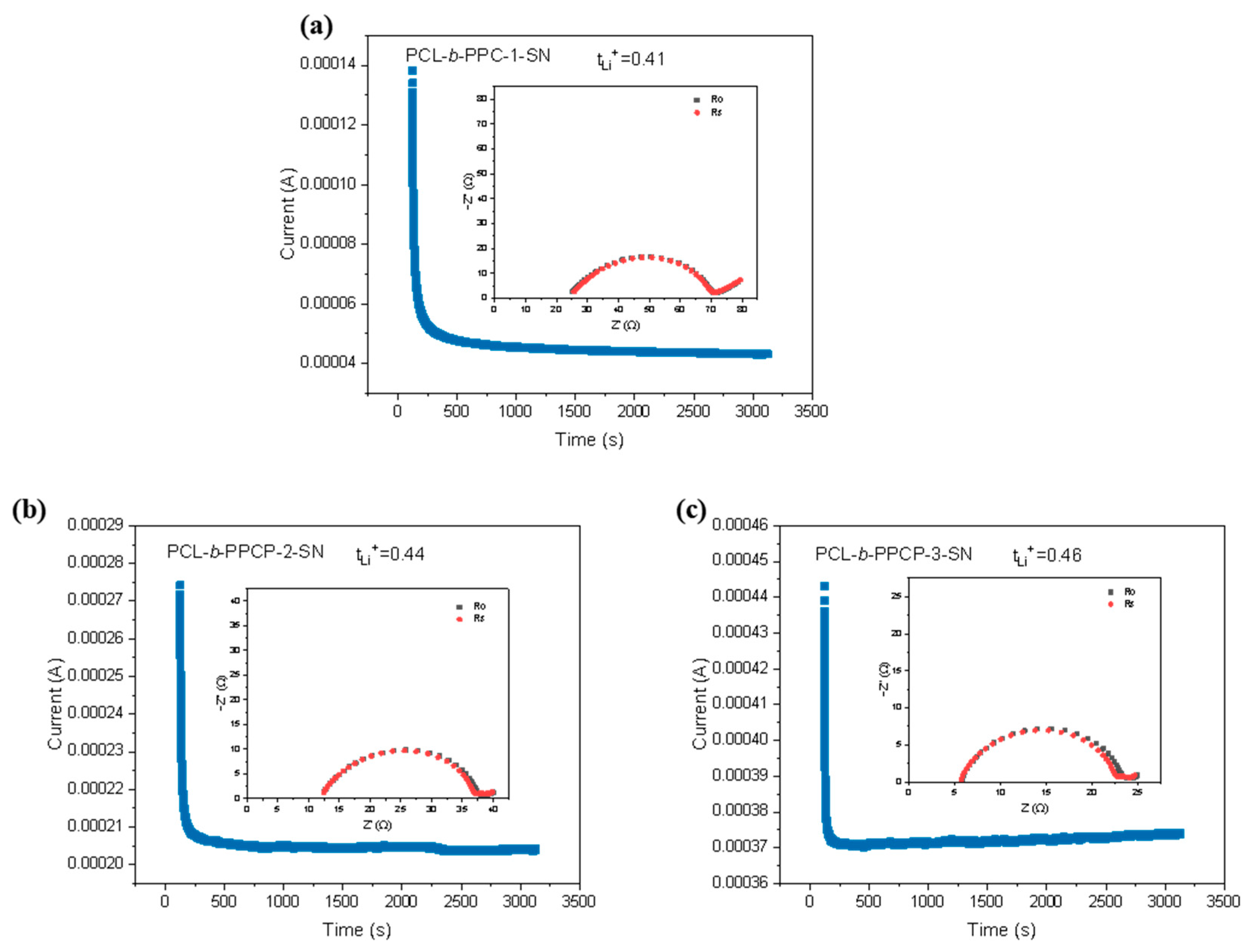
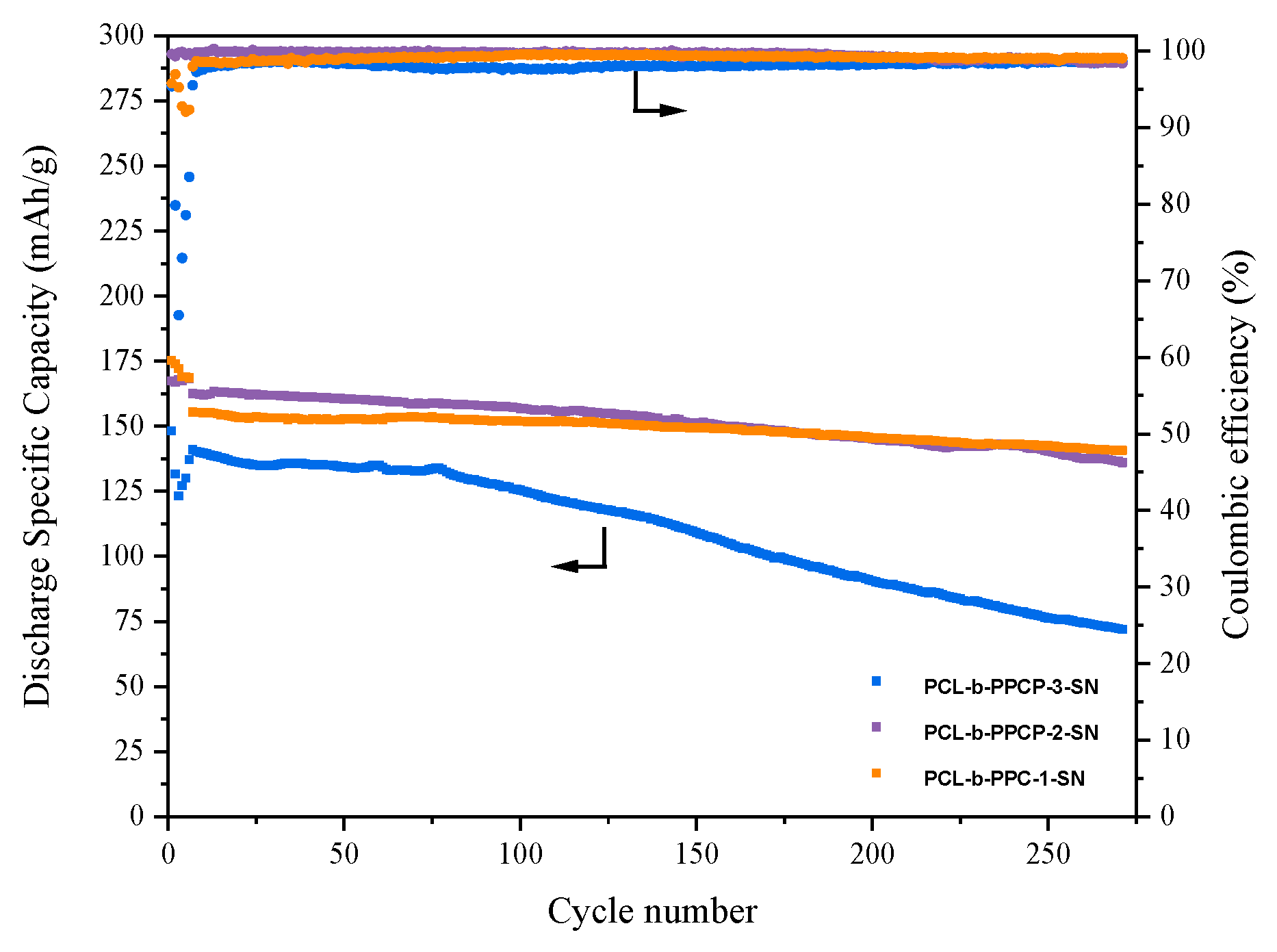
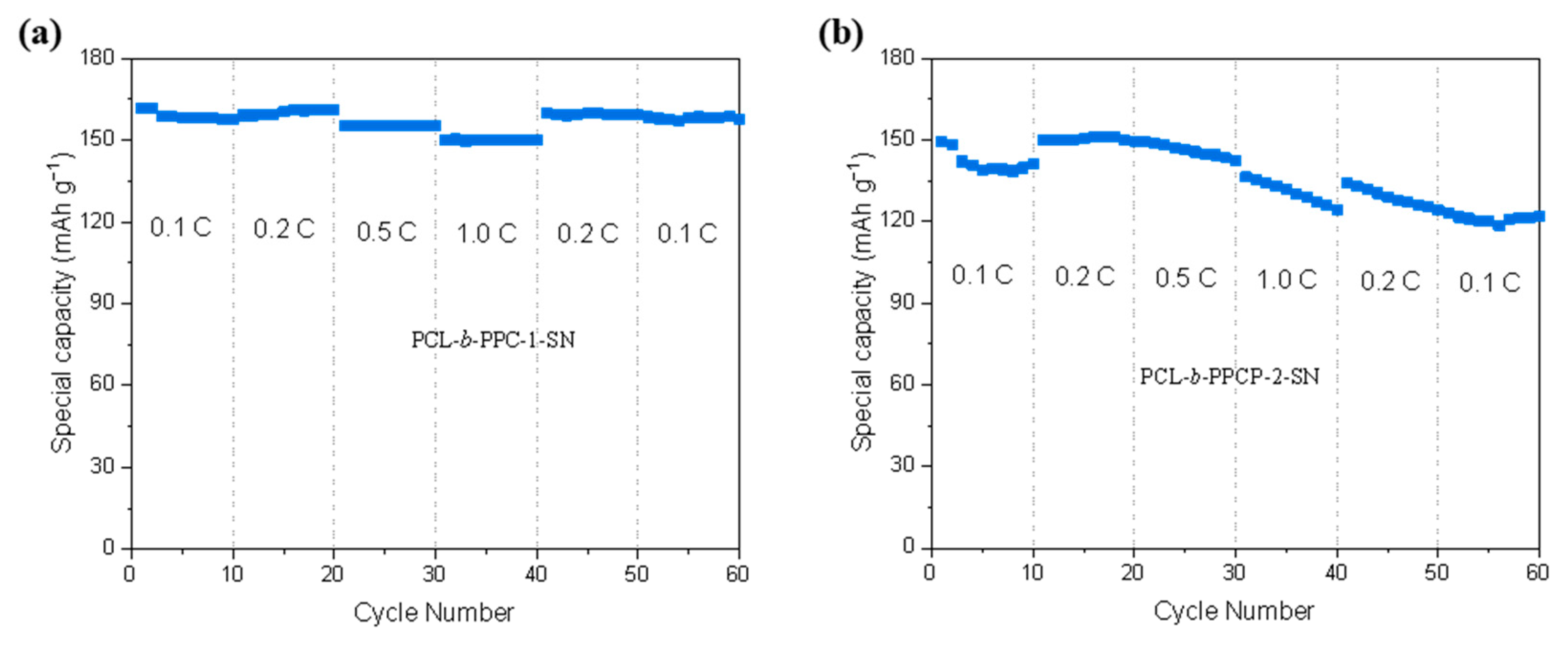
3.2. Electrochemical Performance Characterization of Electrolytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, Z.; Zhao, Q.; Tang, J.; Zhang, Q.; Liu, J.; Jin, Y.; Wang, H. Functional separators towards the suppression of lithium dendrites for rechargeable high-energy batteries. Mater. Horizons 2021, 8, 12–32. [Google Scholar] [CrossRef]
- Wang, H.; Song, J.; Zhang, K.; Fang, Q.; Zuo, Y.; Yang, T.; Yang, Y.; Gao, C.; Wang, X.; Pang, Q.; et al. A strongly complexed solid polymer electrolyte enables a stable solid state high-voltage lithium metal battery. Energy Environ. Sci. 2022, 15, 5149–5158. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, Y.; Cui, Y. Lithium Metal Anode Materials Design: Interphase and Host. Electrochem. Energy Rev. 2019, 2, 509–517. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Li, H.; Wu, F. Solid-state lithium batteries-from fundamental research to industrial progress. Prog. Mater. Sci. 2023, 139, 101182. [Google Scholar] [CrossRef]
- Lin, Z.; Sheng, O.; Cai, X.; Duan, D.; Yue, K.; Nai, J.; Wang, Y.; Liu, T.; Tao, X.; Liu, Y. Solid polymer electrolytes in all-solid-state lithium metal batteries: From microstructures to properties. J. Energy Chem. 2023, 81, 358–378. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Sun, X.; Chen, Y.; Zheng, J.; Zhang, S.; Li, S. Solid Polymer Electrolytes with Fragment-Separated Microphase for Advanced Li Ion Transport and Highly Stable Lithium Metal Batteries. Energy Storage Mater. 2024, 70, 103526. [Google Scholar] [CrossRef]
- Minakshi, M.; Sharma, N.; Ralph, D.; Appadoo, D.; Nallathamby, K. Synthesis and Characterization of Li(Co0.5Ni0.5)PO4 Cathode for Li-Ion Aqueous Battery Applications. Electrochem. Solid-State Lett. 2011, 14, A86–A89. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, M.; Zhou, T.; Raijmakers, L.; Vezhlev, E.; Wu, B.; Schülli, T.U.; Danilov, D.L.; Wei, Y.; Eichel, R.; et al. Interface Aspects in All-Solid-State Li-Based Batteries Reviewed. Adv. Energy Mater. 2021, 11, 2003939. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. All-Solid-State Batteries: Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries (Adv. Mater. 17/2018). Adv. Mater. 2018, 30, 1870122. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, P.; Li, X.; Wang, Z.; Qin, X.; Shao, M.; Zhang, L.; Zhou, W. Rational Design of F-Modified Polyester Electrolytes for Sustainable All-Solid-State Lithium Metal Batteries. J. Am. Chem. Soc. 2024, 146, 5940–5951. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Huang, B.; Huang, C.; Pei, D.; Liu, Z.; Yuan, S.; Hou, S.; Cao, G.; Jin, H. A high power density solid electrolyte based on polycaprolactone for high-performance all-solid-state flexible lithium batteries. Electrochim. Acta 2022, 424, 140624. [Google Scholar] [CrossRef]
- Mirsakiyeva, A.; Ebadi, M.; Araujo, C.M.; Brandell, D.; Broqvist, P.; Kullgren, J. Initial Steps in PEO Decomposition on a Li Metal Electrode. J. Phys. Chem. C 2019, 123, 22851–22857. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, R.; Sun, J.; Wu, M.; Zhao, T. Polyoxyethylene (PEO)|PEO–Perovskite|PEO Composite Electrolyte for All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 46930–46937. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Xiang, J.; Yuan, L.; Liao, Y.; Zhang, Y.; Xu, X.; Ji, H.; Huang, Y. Multifunctional Additive Enables a “5H” PEO Solid Electrolyte for High-Performance Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2024, 16, 21924–21931. [Google Scholar] [CrossRef] [PubMed]
- Sheng, O.; Jin, C.; Yang, T.; Ju, Z.; Luo, J.; Tao, X. Designing biomass-integrated solid polymer electrolytes for safe and energy-dense lithium metal batteries. Energy Environ. Sci. 2023, 16, 2804–2824. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Lu, T.; Guan, L.; Hou, L.; Du, H.; Wei, H.; Liu, X.; Wei, Y.; Zhou, H. Ultrathin Solid Polymer Electrolyte Design for High-Performance Li Metal Batteries: A Perspective of Synthetic Chemistry. Adv. Sci. 2023, 10, 2205233. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jamal, H.; Jeon, I.; Khan, F.; Chun, S.; Kim, J.H. Functionality of 1-Butyl-2,3-Dimethylimidazolium Bromide (BMI-Br) as a Solid Plasticizer in PEO-Based Polymer Electrolyte for Highly Reliable Lithium Metal Batteries. Adv. Energy Mater. 2023, 13, 2301674. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, N.; Dong, F.; Xie, H.; Liu, J. Semi-interpenetrating-network PEO-based polymer electrolytes with fast Li-ion conduction for solid Li-metal batteries. J. Energy Storage 2023, 73, 108809. [Google Scholar] [CrossRef]
- He, Z.-J.; Fan, L.-Z. Poly(ethylene carbonate)-based electrolytes with high concentration Li salt for all-solid-state lithium batteries. Rare Met. 2018, 37, 488–496. [Google Scholar] [CrossRef]
- Cui, Z.; Marangon, V.; Hassoun, J.; Tominaga, Y. Polycarbonate-based composite polymer electrolytes with Al2O3 enhanced by in situ polymerized electrolyte Interlayers for all-solid-state lithium-metal batteries. J. Power Sources 2024, 611, 234760. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, D.; Xiao, M.; Wang, S.; Feng, Y.; Huang, S.; Meng, Y. New potential substitute of PVDF binder: Poly(propylene carbonate) for solvent-free manufacturing high-loading cathodes of LiFePO4|Li batteries. Ionics 2023, 29, 3895–3906. [Google Scholar] [CrossRef]
- Jia, Z.; Jia, M.; Sun, Q.; Wang, N.; Bi, Z.; Guo, X. Configuration design toward sustainably-released polymer electrolytes for enhancing ionic transport and cycle stability of solid lithium batteries. Energy Storage Mater. 2024, 68, 103325. [Google Scholar] [CrossRef]
- Li, T.; Panda, P.K.; Hsieh, C.-T.; Gandomi, Y.A.; Yang, P.-C. Lithium iron phosphate cathode supported solid lithium batteries with dual composite solid electrolytes enabling high energy density and stable cyclability. J. Energy Storage 2024, 81, 110444. [Google Scholar] [CrossRef]
- Geng, M.; Su, G.; Huang, S.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. Practical challenges and future perspectives of solid polymer electrolyte: Microscopic structure and interface design. Mater. Chem. Front. 2023, 7, 5963–5988. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Dong, T.; Zhang, M.; Chai, J.; Dong, S.; Wu, T.; Zhou, X.; Cui, G. Aliphatic Polycarbonate-Based Solid-State Polymer Electrolytes for Advanced Lithium Batteries: Advances and Perspective. Small 2018, 14, e1800821. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xiao, M.; Wang, S.; Han, D.; Meng, Y. Gradient terpolymers with long ε-caprolactone rich sequence derived from propylene oxide, CO2, and ε-caprolactone catalyzed by zinc glutarate. Eur. Polym. J. 2016, 84, 245–255. [Google Scholar] [CrossRef]
- Fonseca, C.P.; Neves, S. Electrochemical properties of a biodegradable polymer electrolyte applied to a rechargeable lithium battery. J. Power Sources 2006, 159, 712–716. [Google Scholar] [CrossRef]
- Fonseca, C.P.; Rosa, D.S.; Gaboardi, F.; Neves, S. Development of a biodegradable polymer electrolyte for rechargeable batteries. J. Power Sources 2006, 155, 381–384. [Google Scholar] [CrossRef]
- Sownthari, K.; Suthanthiraraj, S.A. Preparation and properties of a gel polymer electrolyte system based on poly-ε-caprolactone containing 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Phys. Chem. Solids 2014, 75, 746–751. [Google Scholar] [CrossRef]
- Chen, Y.; Hsieh, Y.; Liu, K.L.; Wichmann, L.; Thienenkamp, J.H.; Choudhary, A.; Bedrov, D.; Winter, M.; Brunklaus, G. Green Polymer Electrolytes Based on Polycaprolactones for Solid-State High-Voltage Lithium Metal Batteries. Macromol. Rapid Commun. 2022, 43, 2200335. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, Z.; Wang, H.; Wang, J.; Xue, Z. Shape-memory and self-healing polyurethane-based solid polymer electrolytes constructed from polycaprolactone segment and disulfide metathesis. Energy Storage Mater. 2022, 51, 1–10. [Google Scholar] [CrossRef]
- Gicheha, D.; Cisse, A.N.; Bhuiyan, A.; Shamim, N. Non-Isothermal Crystallization Kinetics of Poly (ε-Caprolactone) (PCL) and MgO Incorporated PCL Nanofibers. Polymers 2023, 15, 3013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Duan, S.; Liu, Z.; Hou, S.; Tian, X.; Cao, G.; Jin, H. A High-Voltage Hybrid Solid Electrolyte Based on Polycaprolactone for High-Performance all-Solid-State Flexible Lithium Batteries. ACS Appl. Energy Mater. 2021, 4, 2318–2326. [Google Scholar] [CrossRef]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Synthesis and Characterization of Solid Polymer Electrolyte based on Activated Carbon for Solid State Capacitor. Electrochim. Acta 2014, 137, 497–503. [Google Scholar] [CrossRef]
- Jalbert, P.-M.; Commarieu, B.; Daigle, J.-C.; Claverie, J.P.; Zaghib, K. A 3D Network Based on Poly(ε-caprolactone) Macromonomers as Polymer Electrolyte for Solid State Lithium Metal Batteries. J. Electrochem. Soc. 2020, 167, 080527. [Google Scholar] [CrossRef]
- Li, S.; Guo, K.; Chen, G.; Wang, J.; Wang, Y.; Zhou, X.; Xue, Z. A self-catalyzed strategy towards facile fabrication of bottlebrush polyester-based solid polymer electrolytes. Energy Storage Mater. 2022, 46, 461–471. [Google Scholar] [CrossRef]



| Entry | Sample | Temperature/°C | Thickness/μm | R/Ω | σ/S cm−1 |
|---|---|---|---|---|---|
| 1 a | PCL-b-PPC-1 | 25 | 45 | 7600 | 3.10 × 10−7 |
| 2 a | PCL-b-PPC-1 | 60 | 45 | 645 | 3.65 × 10−6 |
| 3 b | PCL-b-PPC-1-EMC | 25 | 36 | 165 | 1.14 × 10−5 |
| 4 b | PCL-b-PPC-1-EMC | 60 | 36 | 55 | 3.45 × 10−5 |
| 5 a | PCL-b-PPCP-2 | 25 | 51 | 66,000 | 4.04 × 10−8 |
| 6 a | PCL-b-PPCP-2 | 60 | 51 | 6700 | 3.98 × 10−7 |
| 7 b | PCL-b-PPCP-2-EMC | 25 | 35 | 810 | 2.26 × 10−6 |
| 8 b | PCL-b-PPCP-2-EMC | 60 | 35 | 134 | 1.37 × 10−5 |
| 9 a | PCL-b-PPCP-3 | 25 | 50 | 49,000 | 5.34 × 10−8 |
| 10 a | PCL-b-PPCP-3 | 60 | 50 | 1640 | 1.60 × 10−6 |
| 11 b | PCL-b-PPCP-3-EMC | 25 | 39 | 1260 | 1.62 × 10−6 |
| 12 b | PCL-b-PPCP-3-EMC | 60 | 39 | 194 | 1.05 × 10−5 |
| Entry | Sample | Temperature/°C | Thickness/μm | R/Ω | σ/S cm−1 |
|---|---|---|---|---|---|
| 1 | PCL-b-PPC-1-EC/DMC | 25 | 34 | 98 | 1.82 × 10−5 |
| 2 | PCL-b-PPC-1-EC/DMC | 60 | 34 | 45 | 3.96 × 10−5 |
| 3 | PCL-b-PPCP-2-EC/DMC | 25 | 46 | 167 | 1.44 × 10−5 |
| 4 | PCL-b-PPCP-2-EC/DMC | 60 | 46 | 79 | 3.05 × 10−5 |
| 5 | PCL-b-PPCP-3-EC/DMC | 25 | 41 | 688 | 3.12 × 10−6 |
| 6 | PCL-b-PPCP-3-EC/DMC | 60 | 41 | 128 | 1.68 × 10−5 |
| Entry | Sample | Temperature/°C | Thickness/μm | R/Ω | σ/S cm−1 |
|---|---|---|---|---|---|
| 1 | PCL-b-PPC-1-SN | 25 | 40 | 40 | 5.24 × 10−5 |
| 2 | PCL-b-PPC-1-SN | 60 | 40 | 11 | 1.99 × 10−4 |
| 3 | PCL-b-PPCP-2-SN | 25 | 31 | 47 | 3.42 × 10−5 |
| 4 | PCL-b-PPCP-2-SN | 60 | 31 | 13 | 1.25 × 10−4 |
| 5 | PCL-b-PPCP-3-SN | 25 | 32 | 73 | 2.29 × 10−5 |
| 6 | PCL-b-PPCP-3-SN | 60 | 32 | 15 | 1.14 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Ma, B.; Huang, S.; Xiao, M.; Wang, S.; Han, D.; Meng, Y. Block Copoly (Ester-Carbonate) Electrolytes for LiFePO4|Li Batteries with Stable Cycling Performance. Materials 2024, 17, 3855. https://doi.org/10.3390/ma17153855
Su Y, Ma B, Huang S, Xiao M, Wang S, Han D, Meng Y. Block Copoly (Ester-Carbonate) Electrolytes for LiFePO4|Li Batteries with Stable Cycling Performance. Materials. 2024; 17(15):3855. https://doi.org/10.3390/ma17153855
Chicago/Turabian StyleSu, Yongjin, Bingyi Ma, Sheng Huang, Min Xiao, Shuanjin Wang, Dongmei Han, and Yuezhong Meng. 2024. "Block Copoly (Ester-Carbonate) Electrolytes for LiFePO4|Li Batteries with Stable Cycling Performance" Materials 17, no. 15: 3855. https://doi.org/10.3390/ma17153855
APA StyleSu, Y., Ma, B., Huang, S., Xiao, M., Wang, S., Han, D., & Meng, Y. (2024). Block Copoly (Ester-Carbonate) Electrolytes for LiFePO4|Li Batteries with Stable Cycling Performance. Materials, 17(15), 3855. https://doi.org/10.3390/ma17153855







