Pistia stratiotes L. Biochar for Sorptive Removal of Aqueous Inorganic Nitrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Biochar Preparation
2.3. Biochar Characterization and Analysis
2.4. Batch Sorption Experiments
3. Results and Discussion
3.1. Biochar Characterization
3.1.1. Physiochemical Characteristics
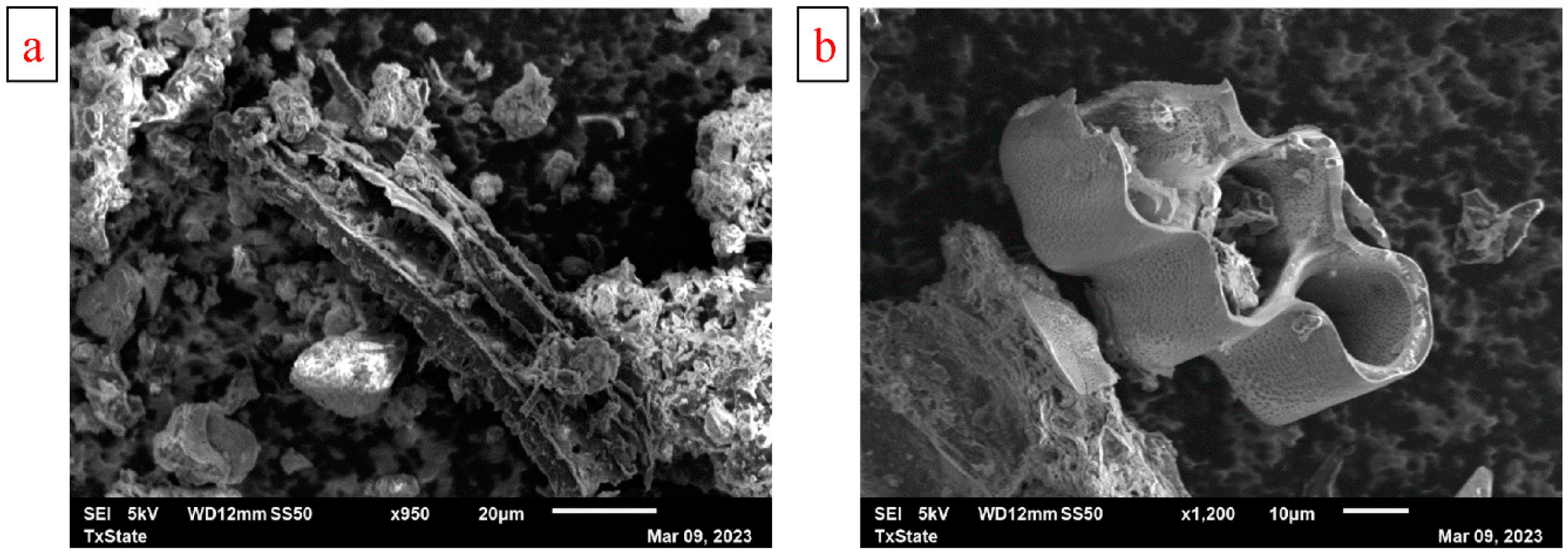
3.1.2. Surface Morphology and Elemental Composition
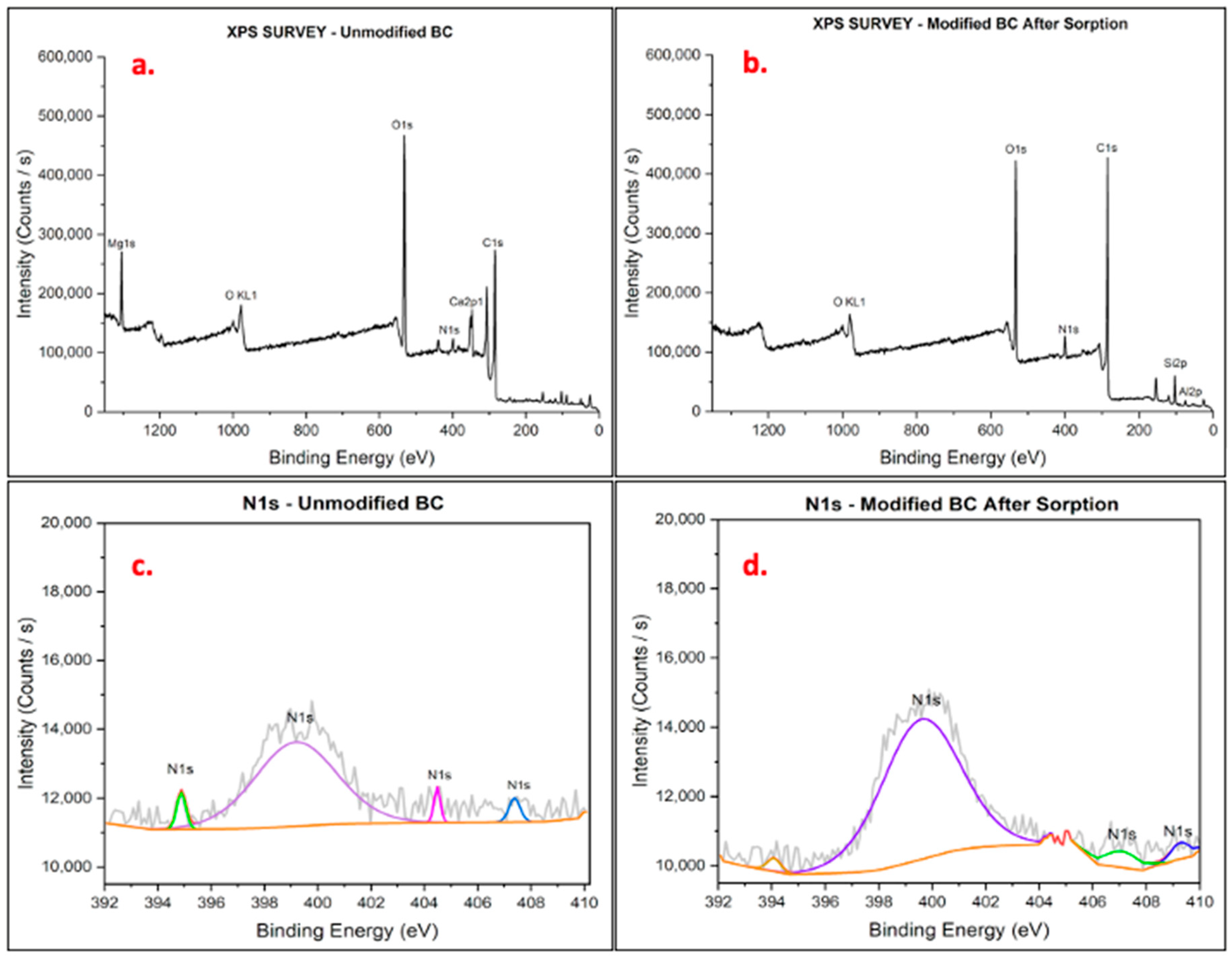
3.1.3. Chemical State Identification Using XPS
3.1.4. Surface Functional Groups
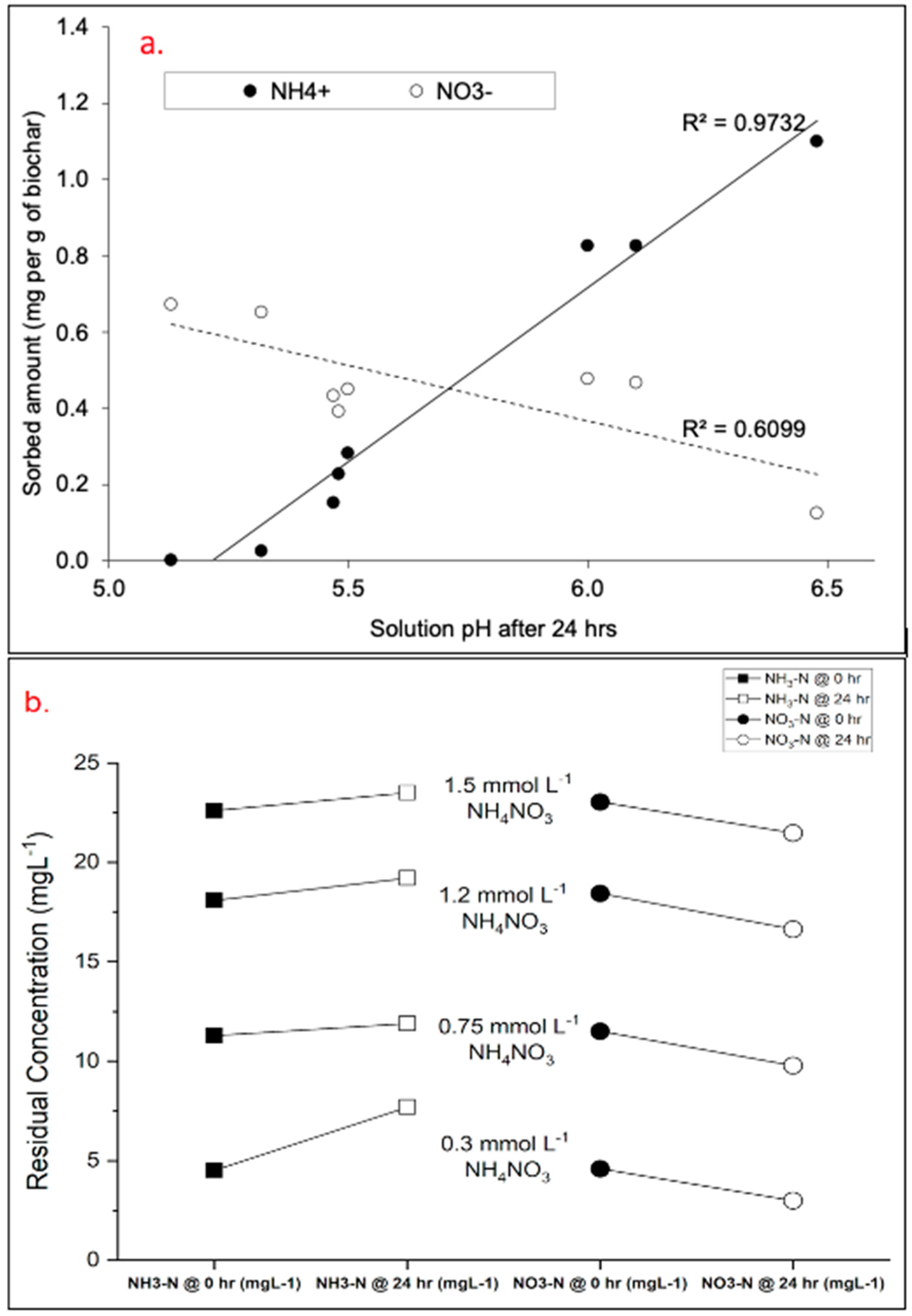
3.2. Batch Sorption Tests and Effect of Reaction pH
3.3. Potential Oxidative Transformation of NH4+ to NO3−
4. Conclusions
- The acid modification of biochar reduced the pH and electrical conductivity, making modified biochar more agreeable for environmental applications. It also decreased the point of zero charge, making it more plausible for NO3− adsorption.
- SEM revealed that the modified biochar had a smoother and more hollow porous structure than that unmodified biochar. Energy-dispersive X-ray analysis showed that modified biochar had higher percentages of carbon and nitrogen but a lower percentage of oxygen.
- Modified biochar performed better for NH4+ sorption at a near-neutral pH, showing >1.0 mg NH4+ sorbed per g of modified biochar and <0.2 mg NO3− sorbed per g of modified biochar. The opposite phenomenon was observed at the lowest pH (~5.1), where >0.6 mg NO3− was sorbed per g of modified biochar, while NH4+ sorption was negligible.
- However, the calculated sorbed amount of NH4+ based on the decreased NH4+ in the aqueous phase may not be solely ascribed to the sorption of NH4+ by biochar. It could have been associated with the oxidative transformation of NH4+ to NO3− by biochar.
- Additionally, the reduction in NO3− recorded when the concentration of NH4NO3 was varied may not be directly related to adsorption by modified biochar but rather to the transformation of NO3− to NH4+.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagadeesh, N.; Sundaram, B. Adsorption of Pollutants from Wastewater by Biochar: A Review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Babatunde, E.O.; Gurav, R.; Hwang, S. Recent Advances in Invasive Aquatic Plant Biomass Pretreatments for Value Addition. Waste Biomass Valor. 2023, 14, 3503–3527. [Google Scholar] [CrossRef]
- Florida Fish and Wildlife Conservation Commission Water Lettuce. Available online: https://myfwc.com/wildlifehabitats/habitat/invasive-plants/weed-alerts/water-lettuce/ (accessed on 6 December 2023).
- Gusti Wibowo, Y.; Tyaz Nugraha, A.; Rohman, A. Phytoremediation of Several Wastewater Sources Using Pistia Stratiotes and Eichhornia Crassipes in Indonesia. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100781. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.-H.; Liu, C.-X.; Sun, P.; Zeng, Y.-P.; Gao, Y.-Y.; Wang, H.-F.; Zeng, R.J. Enhancing Waste Management and Nutrient Recovery: Preparation of Adsorption-Type Sludge-Biochar Value-Added Fertilizer from Sewage Sludge and Pistia Stratiotes. J. Clean. Prod. 2023, 429, 139642. [Google Scholar] [CrossRef]
- Ajmal, Z.; Muhmood, A.; Dong, R.; Wu, S. Probing the Efficiency of Magnetically Modified Biomass-Derived Biochar for Effective Phosphate Removal. J. Environ. Manag. 2020, 253, 109730. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, V.M.; de Morais, E.R.C.; Faustino, S.J.B.; Hernandez, M.C.R.; da Silva Câmara Gaudêncio, H.R.; de Melo, R.R.; Bessa Junior, A.P. Floating Aquatic Macrophytes for the Treatment of Aquaculture Effluents. Environ. Sci. Pollut. Res. 2021, 28, 2600–2607. [Google Scholar] [CrossRef] [PubMed]
- Mosa, A.; El-Ghamry, A.; Tolba, M. Functionalized Biochar Derived from Heavy Metal Rich Feedstock: Phosphate Recovery and Reusing the Exhausted Biochar as an Enriched Soil Amendment. Chemosphere 2018, 198, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Zhang, G.; Zhi, Y.; Yang, D.; Lai, X.; Ren, T. Characterization of Acid-Aged Biochar and Its Ammonium Adsorption in an Aqueous Solution. Materials 2020, 13, 2270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating Biochar and Its Modifications for the Removal of Ammonium, Nitrate, and Phosphate in Water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Verma, M.; Singh, P.; Dhanorkar, M. Remediation of Emerging Pollutants Using Biochar Derived from Aquatic Biomass for Sustainable Waste and Pollution Management: A Review. J. Chem. Technol. Biotechnol. 2024, 99, 330–342. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of Ammonium and Nitrate to Biochars Is Electrostatic and pH-Dependent. Sci. Rep. 2018, 8, 17627. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Kim, H.J.; Choi, Y.-K.; Lee, H.-J.; Ham, S.; Cho, J.Y.; Kim, S.H.; Lee, S.H.; et al. Adsorptive Removal of Synthetic Plastic Components Bisphenol-A and Solvent Black-3 Dye from Single and Binary Solutions Using Pristine Pinecone Biochar. Chemosphere 2022, 296, 134034. [Google Scholar] [CrossRef]
- Gurav, R.G.; Bhatia, S.K.; Jagtap, U.B.; Yang, Y.-H.; Choi, Y.-K.; Tang, J.; Bhatnagar, A. Utilization of Invasive Weed Biomass for Biochar Production and Its Application in Agriculture and Environmental Clean-Up. In Bioremediation Using Weeds; Pant, D., Bhatia, S.K., Patel, A.K., Giri, A., Eds.; Springer: Singapore, 2021; pp. 207–224. ISBN 978-981-336-552-0. [Google Scholar]
- Suryawanshi, S.S.; Kamble, P.P.; Gurav, R.; Yang, Y.-H.; Jadhav, J.P. Statistical Comparison of Various Agricultural and Non-Agricultural Waste Biomass-Derived Biochar for Methylene Blue Dye Sorption. Biomass Conv. Bioref. 2023, 13, 5353–5366. [Google Scholar] [CrossRef]
- Gurav, R.; Mandal, S.; Smith, L.M.; Shi, S.Q.; Hwang, S. The Potential of Self-Activated Carbon for Adsorptive Removal of Toxic Phenoxyacetic Acid Herbicide from Water. Chemosphere 2023, 339, 139715. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Choi, Y.-K.; Vyavahare, G.; Bhatia, S.K.; Lyu, H.; Aware, C.; Kalyani, D.C.; Kan, E.; Jadhav, J.P.; Yang, Y.-H. 7-Production, Characterization, and Application of Biochar for Remediation of Dyes from Textile Industry Wastewater. In Current Developments in Bioengineering and Biotechnology; Govindwar, S.P., Kurade, M.B., Jeon, B.-H., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 231–251. ISBN 978-0-323-91235-8. [Google Scholar]
- Patil, P.M.; Parthasarathy, A.K.; Matkar, A.R.; Mahamuni-Badiger, P.; Hwang, S.; Gurav, R.; Dhanavade, M.J. Nitrite-Oxidizing Bacteria: Cultivation, Growth Physiology, and Chemotaxonomy. In Ammonia Oxidizing Bacteria; Shah, M.P., Ed.; Royal Society of Chemistry: London, UK, 2023; pp. 174–197. ISBN 978-1-83916-687-7. [Google Scholar]
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the Environment: A Critical Review of Their Distribution, Sensing Techniques, Ecological Effects and Remediation. Chemosphere 2022, 287, 131996. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, T.; Liu, R.; Luo, L. Performance of Five Plant Species in Removal of Nitrogen and Phosphorus from an Experimental Phytoremediation System in the Ningxia Irrigation Area. Environ. Monit. Assess 2017, 189, 497. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, J.; Hu, M. Adsorption of Ammonium from Aqueous Solutions on Environmentally Friendly Barbecue Bamboo Charcoal: Characteristics and Kinetic and Thermodynamic Studies. Environ. Prog. Sustain. Energy 2015, 34, 655–662. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J. Enhanced Removal of Nitrate from Water Using Surface Modification of Adsorbents—A Review. J. Environ. Manag. 2013, 131, 363–374. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen Losses from the Soil/Plant System: A Review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Phillips, R.L.; Song, B.; McMillan, A.M.S.; Grelet, G.; Weir, B.S.; Palmada, T.; Tobias, C. Chemical Formation of Hybrid Di-Nitrogen Calls Fungal Codenitrification into Question. Sci. Rep. 2016, 6, 39077. [Google Scholar] [CrossRef] [PubMed]
- Başer, B.; Yousaf, B.; Yetis, U.; Abbas, Q.; Kwon, E.E.; Wang, S.; Bolan, N.S.; Rinklebe, J. Formation of Nitrogen Functionalities in Biochar Materials and Their Role in the Mitigation of Hazardous Emerging Organic Pollutants from Wastewater. J. Hazard. Mater. 2021, 416, 126131. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, M.; Meng, X.; Zhou, J.L.; Zhang, H.; Shen, X. The Role of Biochar on Alleviating Ammonia Toxicity in Anaerobic Digestion of Nitrogen-Rich Wastes: A Review. Bioresour. Technol. 2022, 351, 126924. [Google Scholar] [CrossRef]
- Jellali, S.; El-Bassi, L.; Charabi, Y.; Usman, M.; Khiari, B.; Al-Wardy, M.; Jeguirim, M. Recent Advancements on Biochars Enrichment with Ammonium and Nitrates from Wastewaters: A Critical Review on Benefits for Environment and Agriculture. J. Environ. Manag. 2022, 305, 114368. [Google Scholar] [CrossRef] [PubMed]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Papiernik, S.K.; Malo, D.D.; Clay, D.E.; Kumar, S.; Gulbrandson, D.W. Nitrate Sorption and Desorption in Biochars from Fast Pyrolysis. Microporous Mesoporous Mater. 2013, 179, 250–257. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent Advances in Biochar Application for Water and Wastewater Treatment: A Review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of Ammonium in Aqueous Solutions by Pine Sawdust and Wheat Straw Biochars. Environ. Sci. Pollut. Res. 2018, 25, 25638–25647. [Google Scholar] [CrossRef]
- Akinbile, C.O.; Epebinu, E.M.; Olanrewaju, O.O.; Abolude, A.T. Performance Analysis of Bamboo-Based (Bambusa vulgaris) Activated Carbon in Aquaculture Wastewater Treatment. Sustain. Water Resour. Manag. 2022, 8, 178. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The Sorption and Desorption of Phosphate-P, Ammonium-N and Nitrate-N in Cacao Shell and Corn Cob Biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Wang, X.; Wang, K.; Zhu, L.; Wang, S. Nitrogen-Doped Hierarchical Porous Biochar Derived from Corn Stalks for Phenol-Enhanced Adsorption. Energy Fuels 2019, 33, 12459–12468. [Google Scholar] [CrossRef]
- Mizuta, K.; Matsumoto, T.; Hatate, Y.; Nishihara, K.; Nakanishi, T. Removal of Nitrate-Nitrogen from Drinking Water Using Bamboo Powder Charcoal. Bioresour. Technol. 2004, 95, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, H.; Liu, J.; Wang, X.; Li, J.; Shi, E.; Wang, C.; Yang, J.; Zhang, Z. A Study on and Adsorption Mechanism of Ammonium Nitrogen by Modified Corn Straw Biochar. R. Soc. Open Sci. 2023, 10, 221535. [Google Scholar] [CrossRef] [PubMed]
- Stüber, F.; Font, J.; Fortuny, A.; Bengoa, C.; Eftaxias, A.; Fabregat, A. Carbon Materials and Catalytic Wet Air Oxidation of Organic Pollutants in Wastewater. Top. Catal. 2005, 33, 3–50. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into Activated Carbon from Different Kinds of Chemical Activating Agents: A Review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, Y.; Lu, H.; Li, Z.; Li, X.; Han, J.; Ji, R.; Cheng, H.; Song, Y.; Xue, J.; et al. Aquatic Invasive Plant Biomass-Derived Magnetic Porous Biochar Prepared by Sequential Carbonization and Coprecipitation for Diethyl Phthalate Removal from Water. Sep. Purif. Technol. 2024, 349, 127829. [Google Scholar] [CrossRef]
- Šoštarić, T.D.; Petrović, M.S.; Pastor, F.T.; Lončarević, D.R.; Petrović, J.T.; Milojković, J.V.; Stojanović, M.D. Study of Heavy Metals Biosorption on Native and Alkali-Treated Apricot Shells and Its Application in Wastewater Treatment. J. Mol. Liq. 2018, 259, 340–349. [Google Scholar] [CrossRef]
- Siaka, M.; Owens, C.M.; Birch, G.F. Evaluation of Some Digestion Methods for the Determination of Heavy Metals in Sediment Samples by Flame-AAS. Anal. Lett. 1998, 31, 703–718. [Google Scholar] [CrossRef]
- Henry-Silva, G.G.; Camargo, A.F.M.; Pezzato, M.M. Growth of Free-Floating Aquatic Macrophytes in Different Concentrations of Nutrients. Hydrobiologia 2008, 610, 153–160. [Google Scholar] [CrossRef]
- Hussner, A.; Heidbuechel, P.; Heiligtag, S. Vegetative Overwintering and Viable Seed Production Explain the Establishment of Invasive Pistia Stratiotes in the Thermally Abnormal Erft River (North Rhine-Westphalia, Germany). Aquat. Bot. 2014, 119, 28–32. [Google Scholar] [CrossRef]
- Meri, N.H.; Alisa, A.B.; Talib, N.; Rashid, Z.A.; Ghani, W.A.W.A.K. Effect of Washing Pre-Treatment of Empty Fruit Bunch Hydrogel Biochar Composite Properties as Potential Adsorbent. Chem. Eng. Trans. 2017, 56, 1255–1260. [Google Scholar] [CrossRef]
- Bartoli, M.; Troiano, M.; Giudicianni, P.; Amato, D.; Giorcelli, M.; Solimene, R.; Tagliaferro, A. Effect of Heating Rate and Feedstock Nature on Electrical Conductivity of Biochar and Biochar-Based Composites. Appl. Energy Combust. Sci. 2022, 12, 100089. [Google Scholar] [CrossRef]
- Gabhi, R.; Basile, L.; Kirk, D.W.; Giorcelli, M.; Tagliaferro, A.; Jia, C.Q. Electrical Conductivity of Wood Biochar Monoliths and Its Dependence on Pyrolysis Temperature. Biochar 2020, 2, 369–378. [Google Scholar] [CrossRef]
- Kane, S.; Ulrich, R.; Harrington, A.; Stadie, N.P.; Ryan, C. Physical and Chemical Mechanisms That Influence the Electrical Conductivity of Lignin-Derived Biochar. Carbon Trends 2021, 5, 100088. [Google Scholar] [CrossRef]
- Kim, J.E.; Bhatia, S.K.; Song, H.J.; Yoo, E.; Jeon, H.J.; Yoon, J.-Y.; Yang, Y.; Gurav, R.; Yang, Y.-H.; Kim, H.J.; et al. Adsorptive Removal of Tetracycline from Aqueous Solution by Maple Leaf-Derived Biochar. Bioresour. Technol. 2020, 306, 123092. [Google Scholar] [CrossRef] [PubMed]
- Krauskopf, K.B. Dissolution and Precipitation of Silica at Low Temperatures. Geochim. Et Cosmochim. Acta 1956, 10, 1–26. [Google Scholar] [CrossRef]
- Akpasi, S.O.; Anekwe, I.M.S.; Adedeji, J.; Kiambi, S.L. Biochar Development as a Catalyst and Its Application. In Biochar-Productive Technologies, Properties and Applications; IntechOpen: London, UK, 2022; ISBN 978-1-80356-252-0. [Google Scholar]
- Kumar, M.; Xiong, X.; Sun, Y.; Yu, I.K.M.; Tsang, D.C.W.; Hou, D.; Gupta, J.; Bhaskar, T.; Pandey, A. Critical Review on Biochar-Supported Catalysts for Pollutant Degradation and Sustainable Biorefinery. Adv. Sustain. Syst. 2020, 4, 1900149. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Cao, L.; Tsang, D.C.W.; Zhang, S.; Ok, Y.S. A Review of Biochar-Based Catalysts for Chemical Synthesis, Biofuel Production, and Pollution Control. Bioresour. Technol. 2017, 246, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A Review on Modification Methods to Cellulose-Based Adsorbents to Improve Adsorption Capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- NIST National Institue of Standards and Technology X-ray Photoelectron Spectroscopy Database (SRD 20), Version 5.0. Available online: https://srdata.nist.gov/xps/SpectraIdentifier (accessed on 21 February 2024).
- Liao, S.; Pan, B.; Li, H.; Zhang, D.; Xing, B. Detecting Free Radicals in Biochars and Determining Their Ability to Inhibit the Germination and Growth of Corn, Wheat and Rice Seedlings. Environ. Sci. Technol. 2014, 48, 8581–8587. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of Persistent Free Radicals in Biochar to Activate Persulfate for Contaminant Degradation. Environ. Sci. Technol. 2015, 49, 5645–5653. [Google Scholar] [CrossRef]

| EC (µS/cm) | pH in Deionized Water | PZC | Total N (mg L−1 N) | |
|---|---|---|---|---|
| Unmodified BC | 69.97 | 10.08 | 9.0 | 0.04 |
| Modified BC | 6.19 | 4.92 | 6.0 | 0.0 |
| Element | Unmodified BC | Modified BC before Sorption | Modified BC after Sorption | |||
|---|---|---|---|---|---|---|
| Mass% | Atom% | Mass% | Atom% | Mass% | Atom% | |
| C | 41.43 | 50.25 | 49.30 | 56.70 | 48.86 | 56.23 |
| N | 12.47 | 12.97 | 15.05 | 14.84 | 16.02 | 15.81 |
| O | 34.61 | 31.51 | 29.59 | 25.55 | 28.88 | 24.95 |
| Mg | 2.31 | 1.38 | 0.72 | 0.41 | 0.87 | 0.50 |
| Al | 0.73 | 0.39 | 0.91 | 0.47 | 0.90 | 0.46 |
| Si | 2.64 | 1.37 | 3.50 | 1.72 | 3.52 | 1.73 |
| P | 0.18 | 0.09 | 0.06 | 0.02 | 0.07 | 0.03 |
| S | 0.11 | 0.05 | 0.07 | 0.03 | 0.09 | 0.04 |
| Cl | 0.09 | 0.04 | 0.11 | 0.04 | *n.d | *n.d |
| K | 0.42 | 0.16 | 0.21 | 0.08 | 0.21 | 0.08 |
| Ca | 4.78 | 1.73 | 0.22 | 0.08 | 0.30 | 0.10 |
| Fe | 0.23 | 0.06 | 0.26 | 0.06 | 0.28 | 0.07 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Test Method: EPA 200.7 | ||
|---|---|---|
| Element | Concentration per g of BC (mg L−1) | Practical Quantitation Limit |
| Cadmium | <0.005 | 0.005 |
| Calcium | 7.850 | 1.000 |
| Cobalt | <0.010 | 0.010 |
| Copper | 0.027 | 0.010 |
| Iron | 4.280 | 0.050 |
| Magnesium | 5.930 | 0.050 |
| Manganese | 0.270 | 0.010 |
| Nickel | 0.034 | 0.010 |
| Zinc | 0.156 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babatunde, E.O.; Gurav, R.; Hwang, S.S. Pistia stratiotes L. Biochar for Sorptive Removal of Aqueous Inorganic Nitrogen. Materials 2024, 17, 3858. https://doi.org/10.3390/ma17153858
Babatunde EO, Gurav R, Hwang SS. Pistia stratiotes L. Biochar for Sorptive Removal of Aqueous Inorganic Nitrogen. Materials. 2024; 17(15):3858. https://doi.org/10.3390/ma17153858
Chicago/Turabian StyleBabatunde, Eunice O., Ranjit Gurav, and Sangchul S. Hwang. 2024. "Pistia stratiotes L. Biochar for Sorptive Removal of Aqueous Inorganic Nitrogen" Materials 17, no. 15: 3858. https://doi.org/10.3390/ma17153858
APA StyleBabatunde, E. O., Gurav, R., & Hwang, S. S. (2024). Pistia stratiotes L. Biochar for Sorptive Removal of Aqueous Inorganic Nitrogen. Materials, 17(15), 3858. https://doi.org/10.3390/ma17153858







