Influence of Ultraviolet Light and Alternating Wet–Dry Environments on the Corrosion Behavior of Weathering Steels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
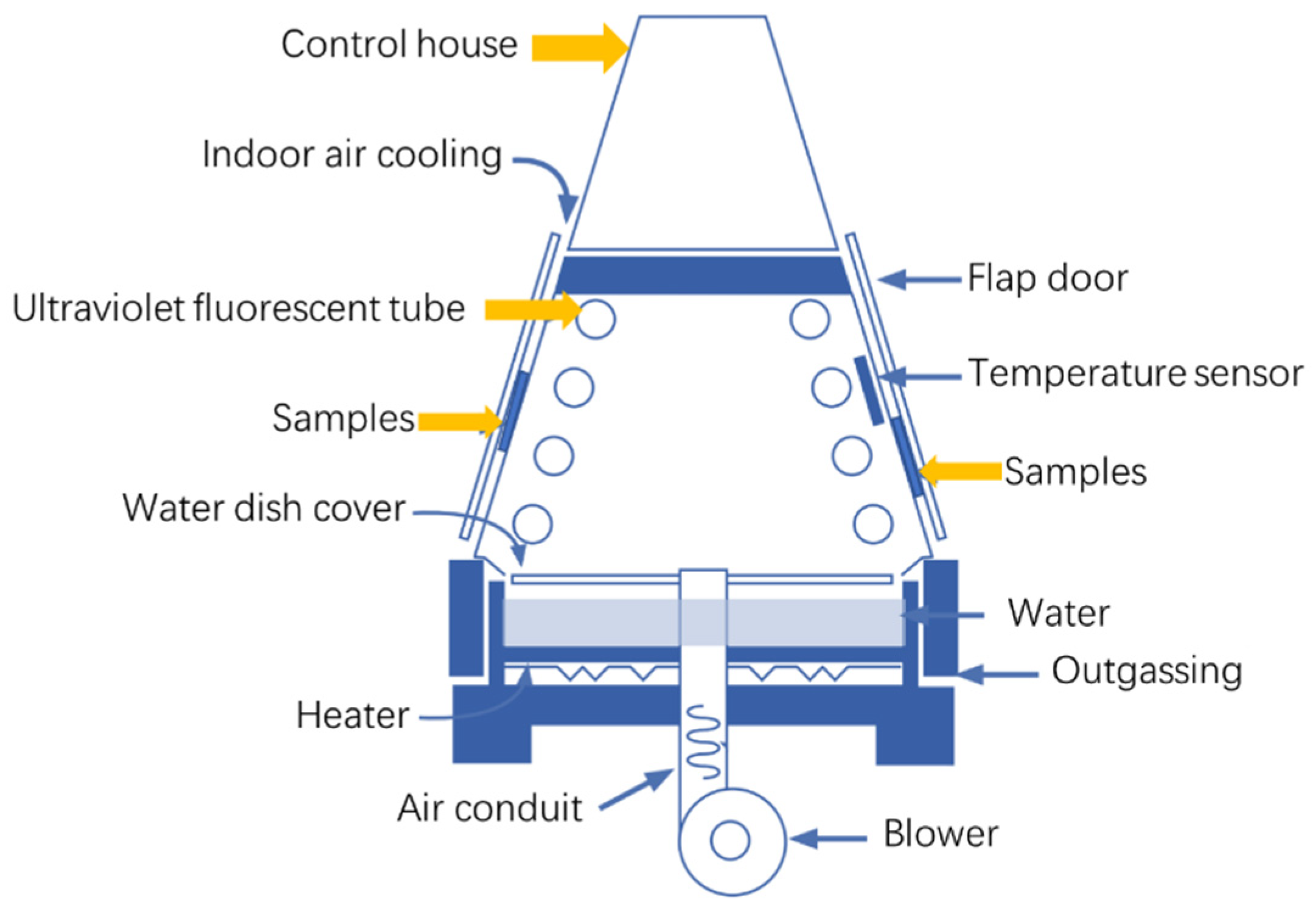
2.2. Cyclic Corrosion Acceleration Tests
2.3. Characterization of the Rust Layers
2.4. Electrochemical Test
3. Results and Discussion
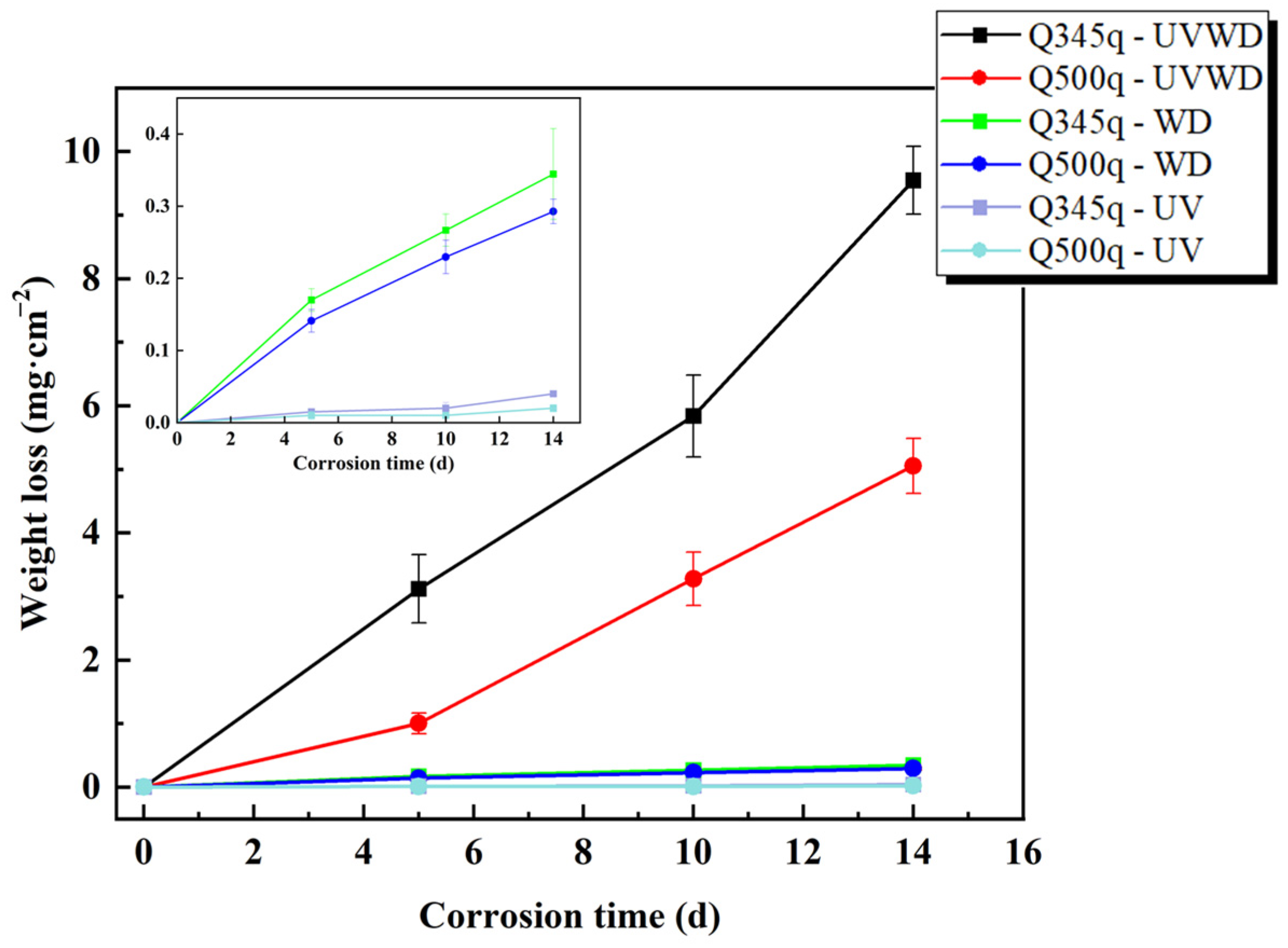
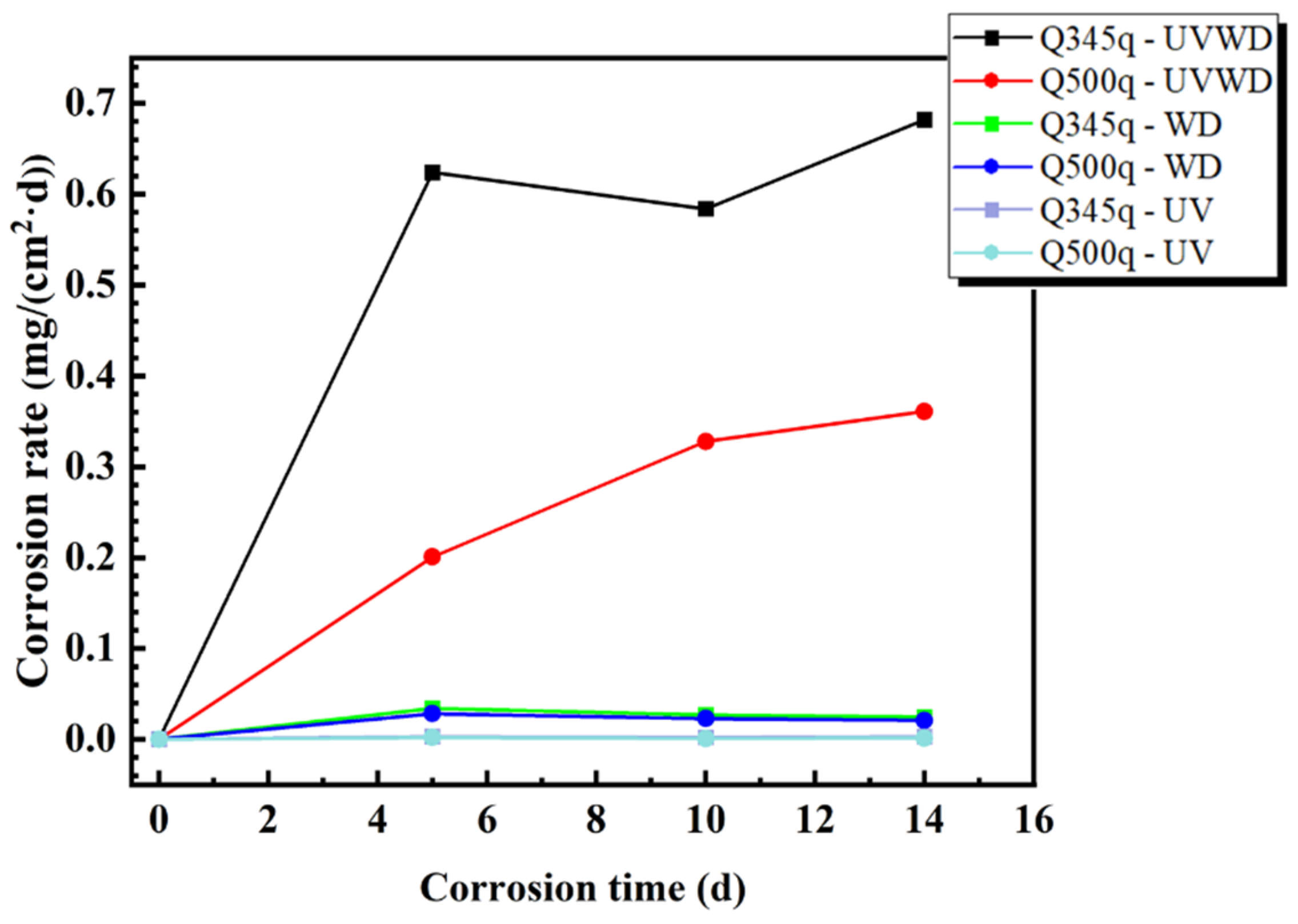
3.1. Corrosion Rate Analysis
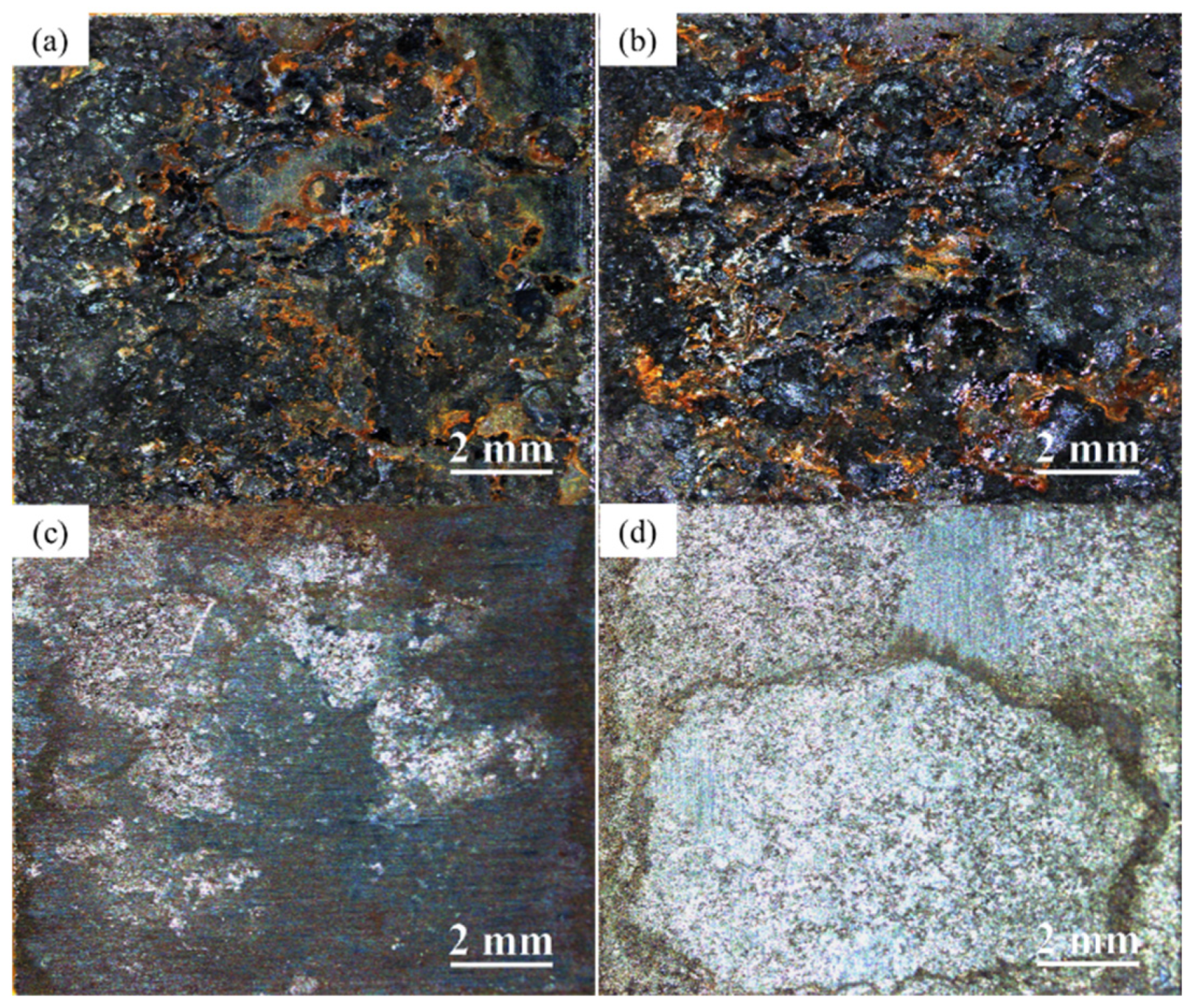
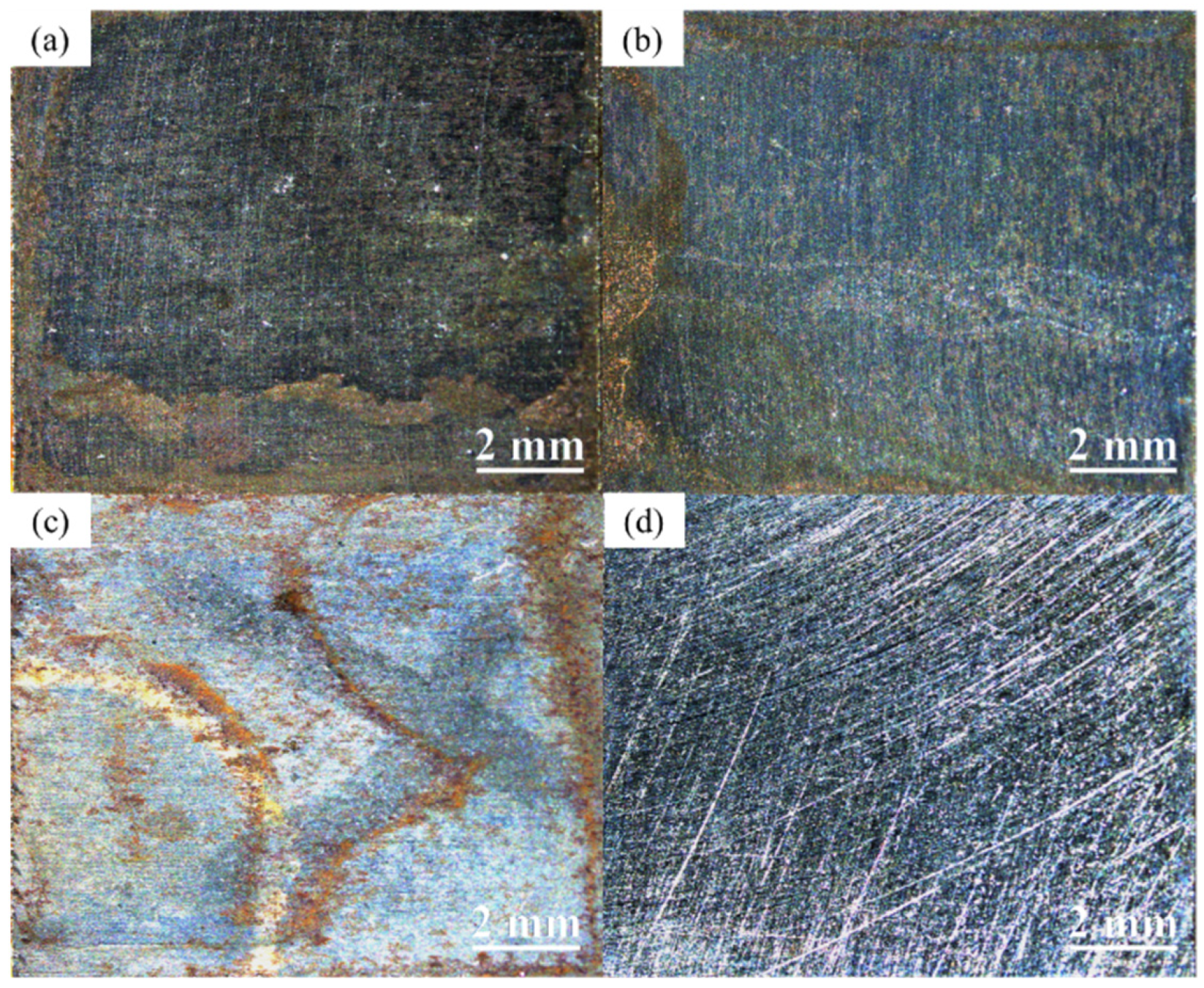
3.2. Macroscopic Corrosion Morphology
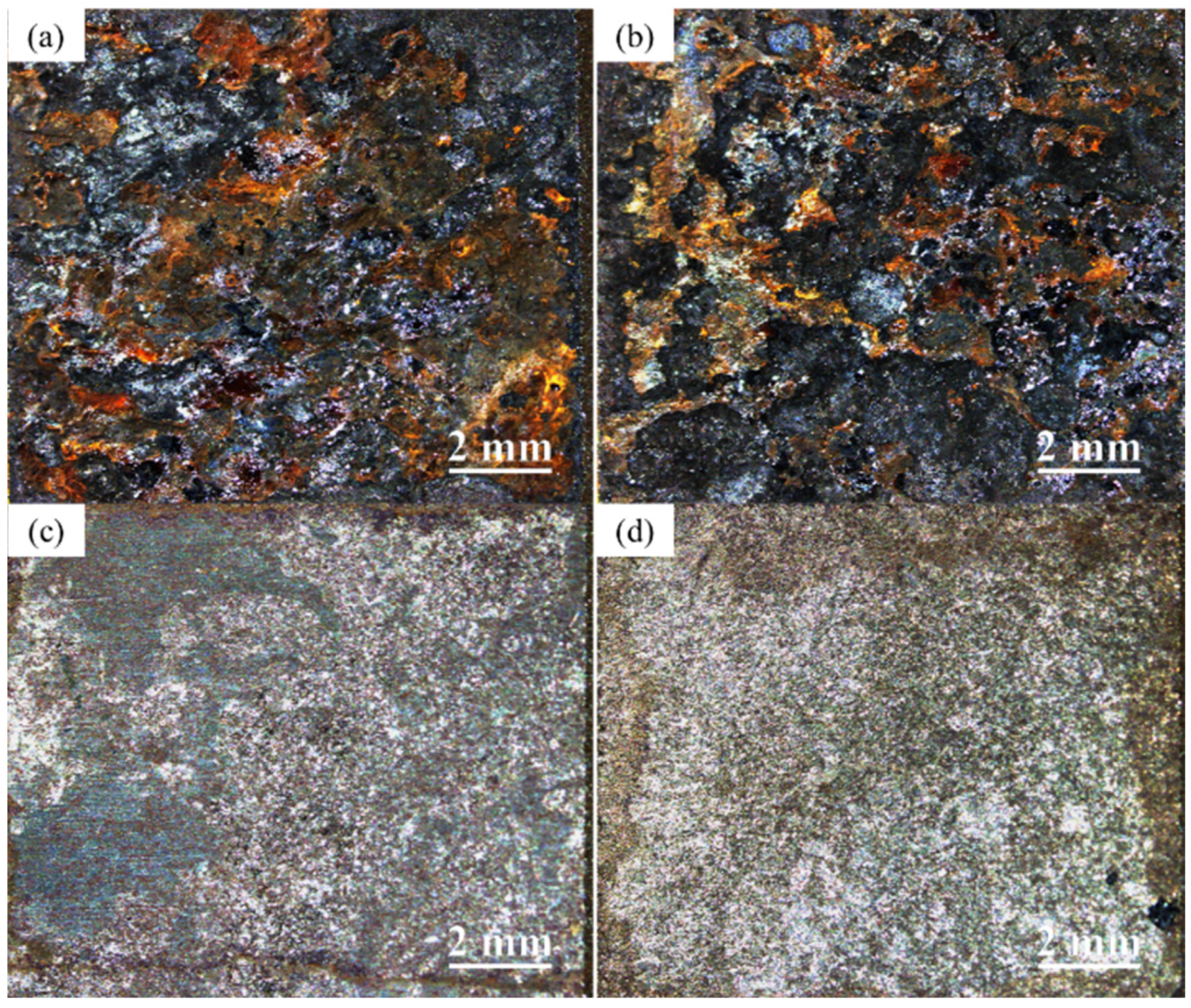
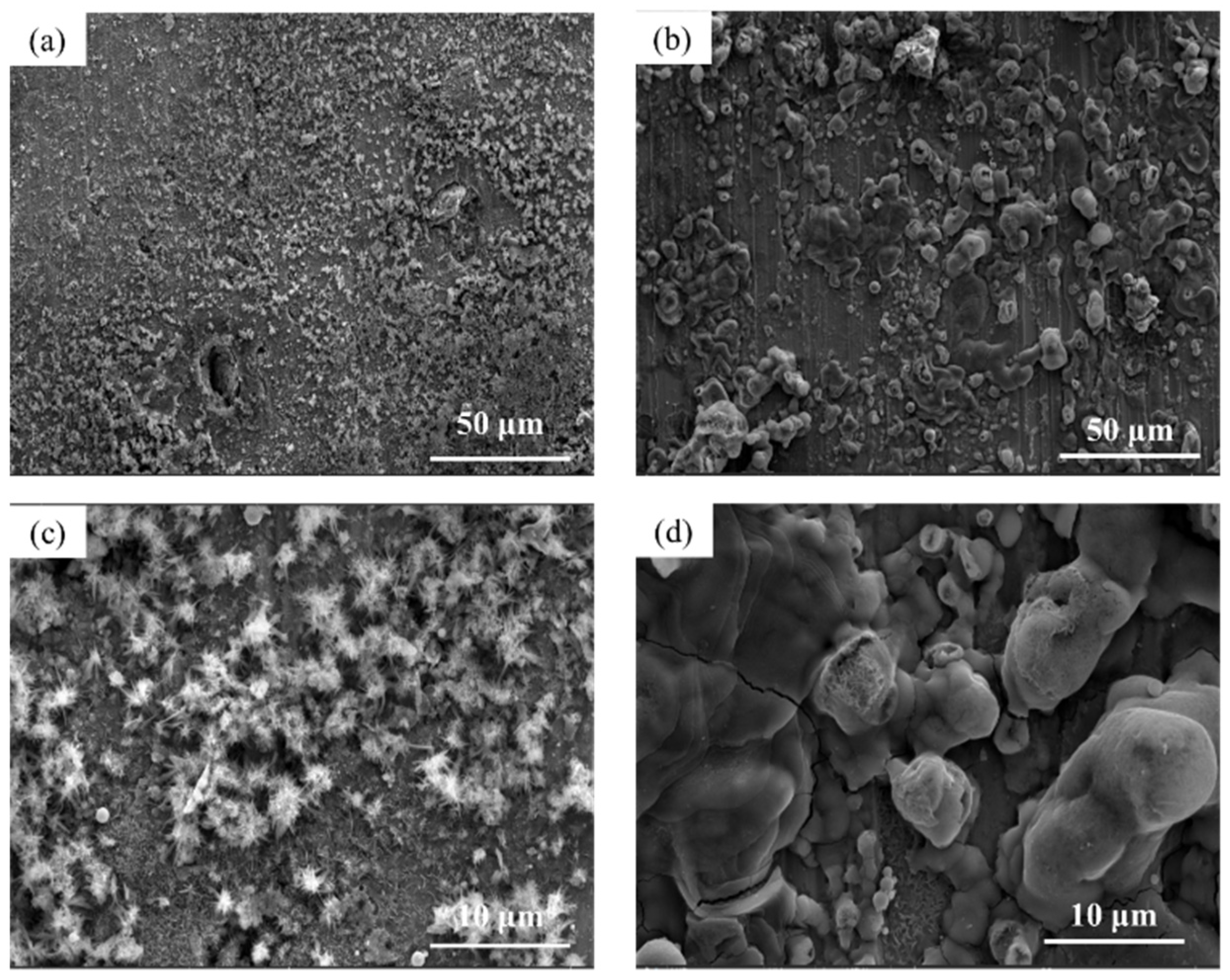
3.3. Microscopic Corrosion Morphology
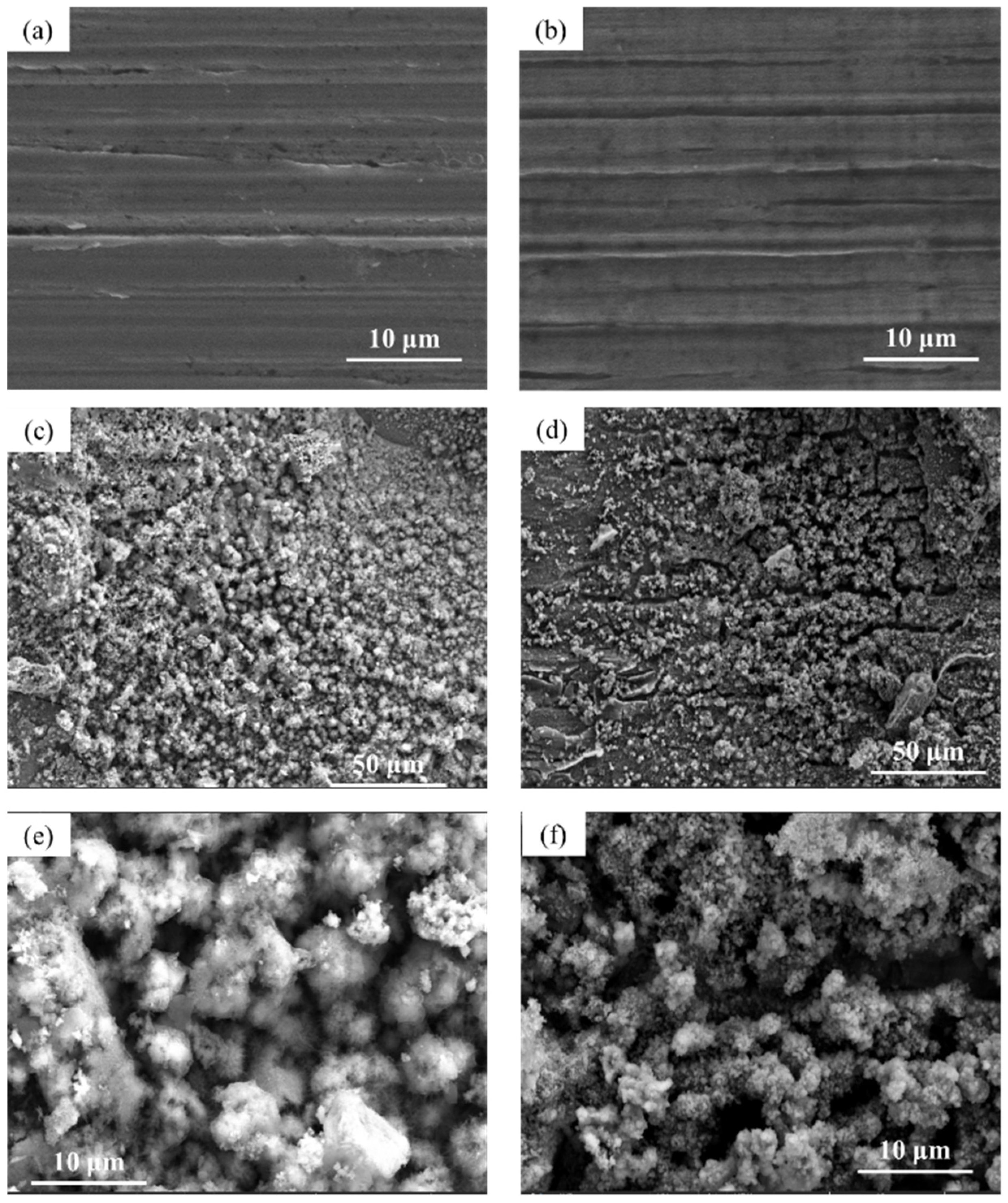
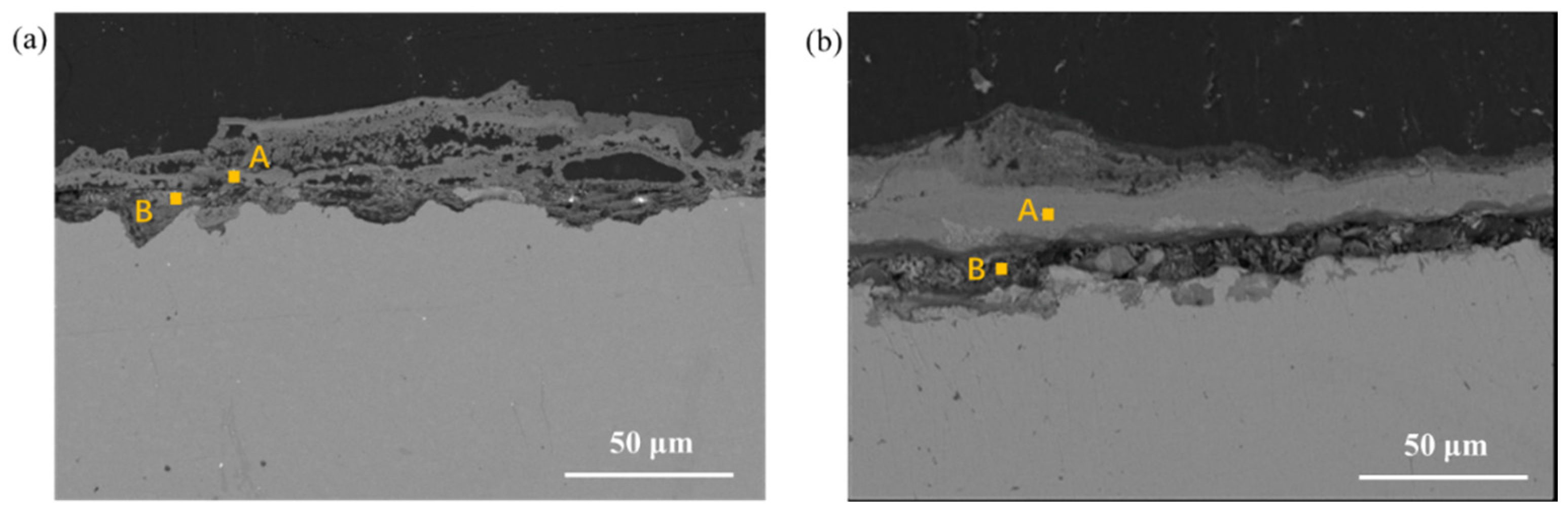
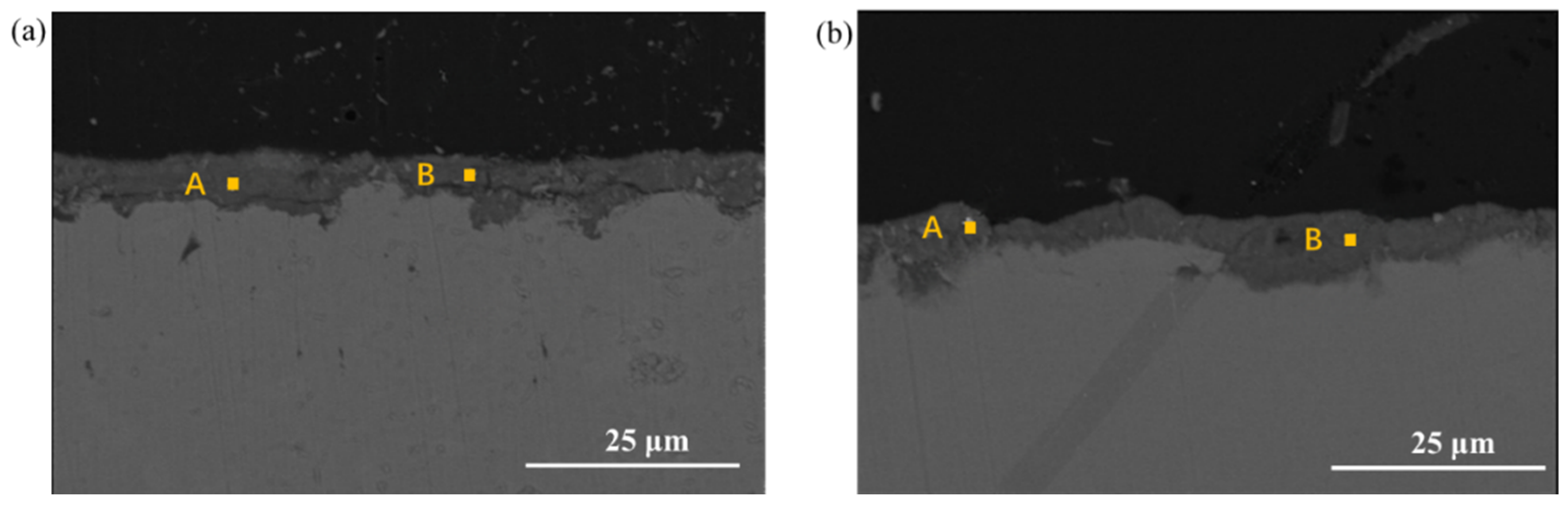
3.4. Cross-Section Observation of the Rust Layer
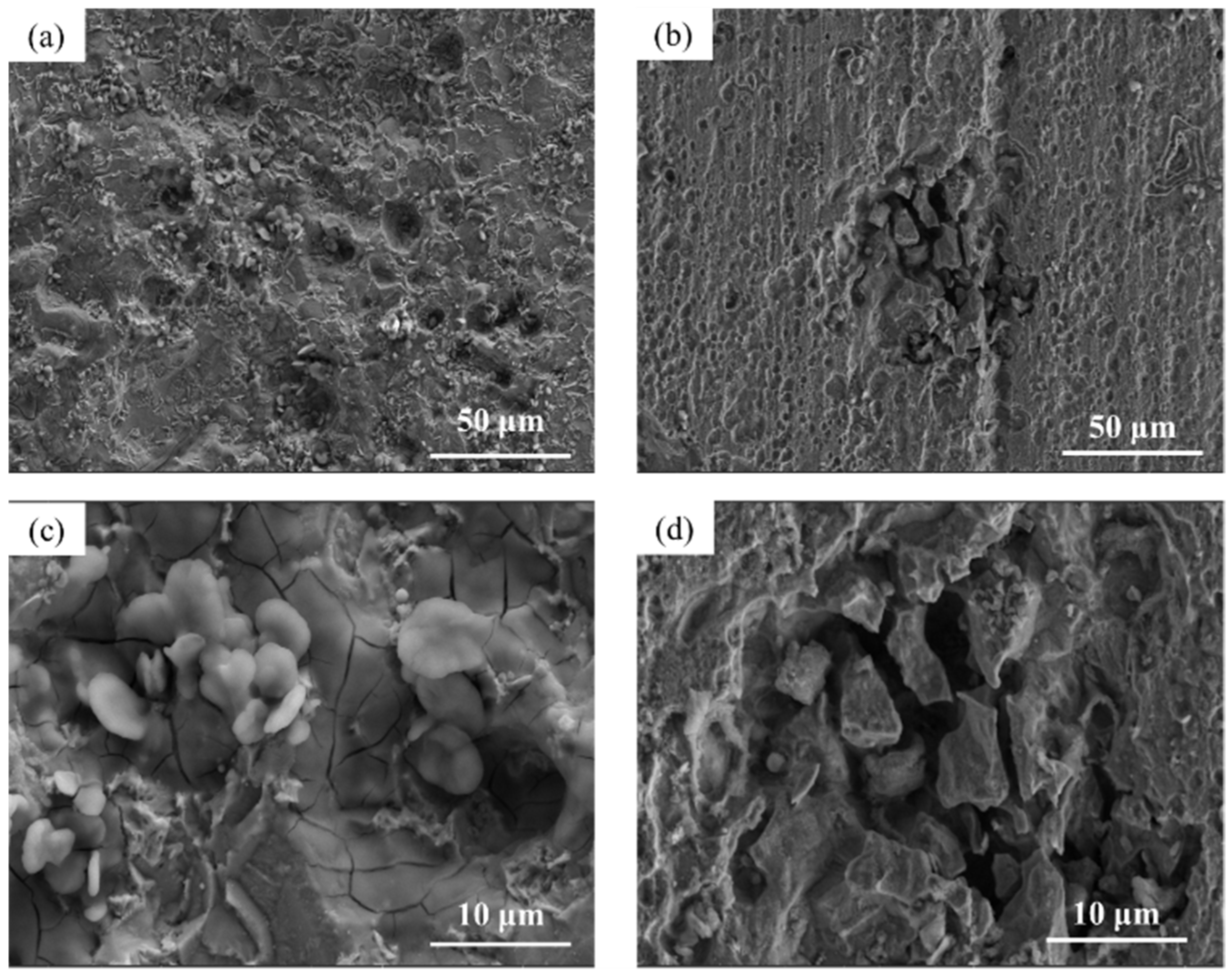
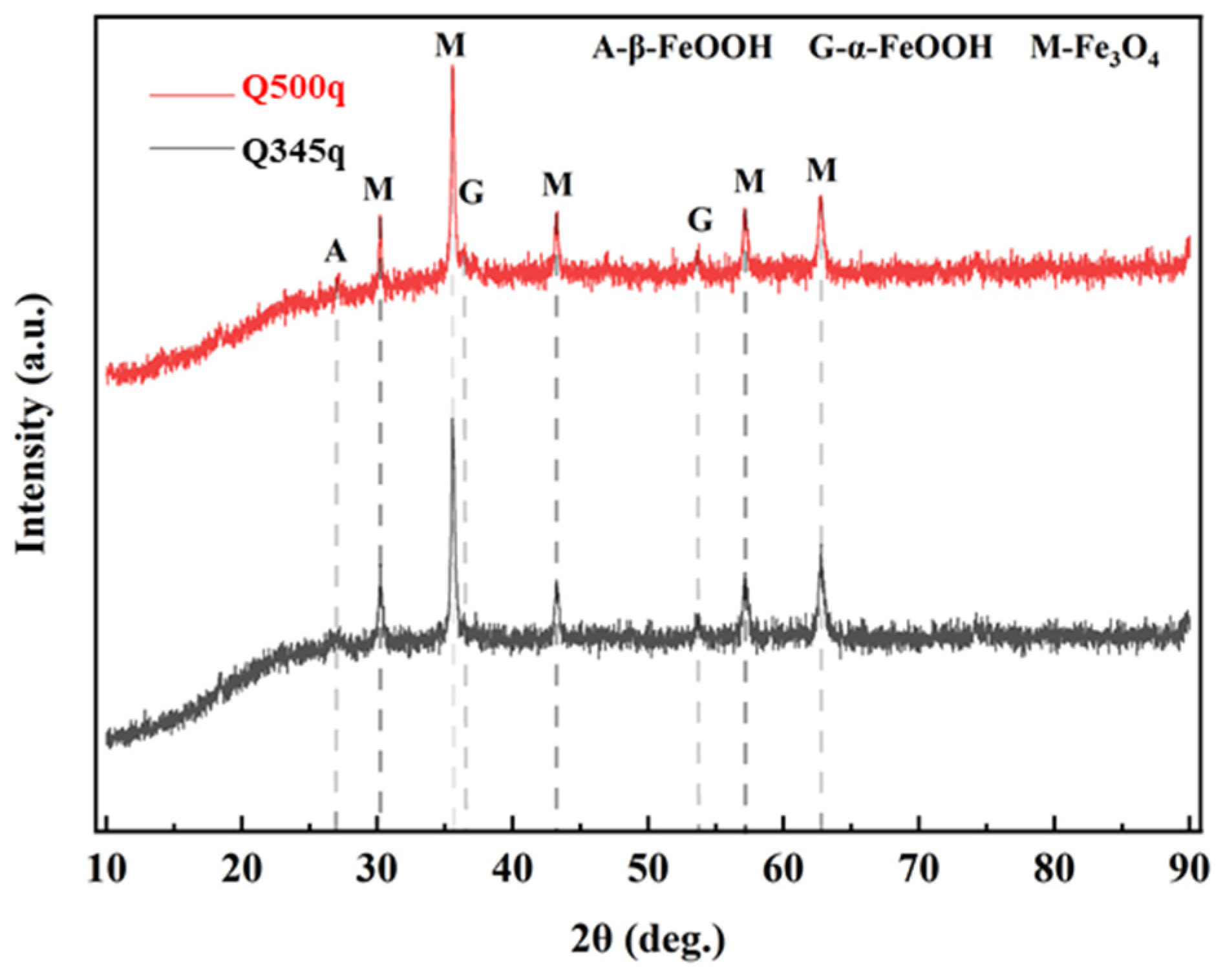
3.5. Composition of the Rust Layer
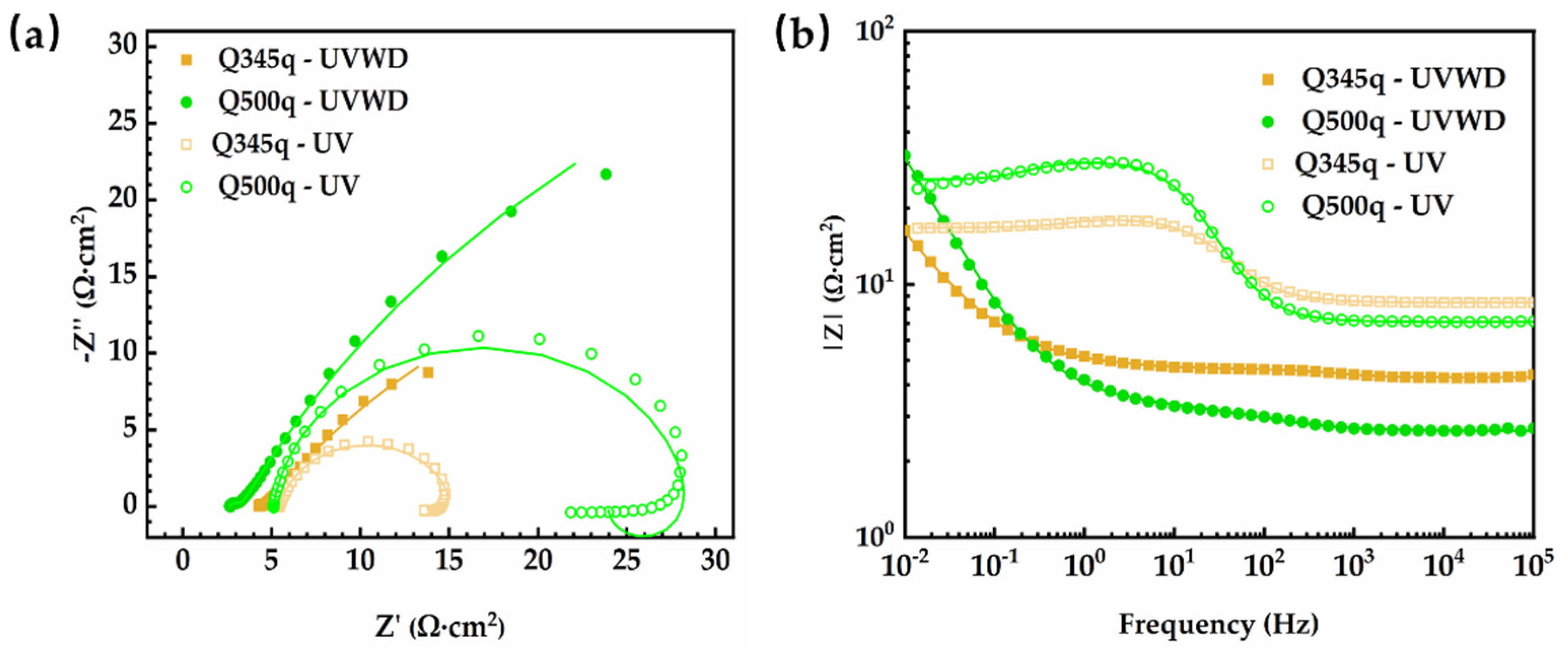
3.6. EIS Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morcillo, M.; Díaz, I.; Cano, H.; Chico, B.; de la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part II: Testing, inspection, maintenance. Constr. Build. Mater. 2019, 222, 750–765. [Google Scholar] [CrossRef]
- Santa, A.C.; Tamayo, J.A.; Correa, C.D.; Gómez, M.A.; Castaño, J.G.; Baena, L.M. Atmospheric corrosion maps as a tool for designing and maintaining building materials: A review. Heliyon 2022, 8, e10438. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, M.; Díaz, I.; Chico, B.; Cano, H.; de la Fuente, D. Weathering steels: From empirical development to scientific design. A review. Corros. Sci. 2014, 83, 6–31. [Google Scholar] [CrossRef]
- Sharun, V.; Rajasekaran, M.; Kumar, S.S.; Tripathi, V.; Sharma, R.; Puthilibai, G.; Sudhakar, M.; Negash, K. Study on Developments in Protection Coating Techniques for Steel. Adv. Mater. Sci. Eng. 2022, 2022, 843043. [Google Scholar] [CrossRef]
- Liu, Y.W.; Zhang, J.; Wei, Y.H.; Wang, Z.Y. Effect of different UV intensity on corrosion behavior of carbon steel exposed to simulated Nansha atmospheric environment. Mater. Chem. Phys. 2019, 237, 121855. [Google Scholar] [CrossRef]
- Zhang, N.; Lian, Z.; Zhang, W.; He, B.; Hu, X.; Zhu, T.; Jiang, B. Exploring the Corrosion Behavior of Low-Ni Cu-P-Cr-Ni Weathering Steel with Different P Contents in a Simulated Atmospheric Environment. J. Mater. Eng. Perform. 2023, 32, 44–54. [Google Scholar] [CrossRef]
- Zhang, W.H.; Yang, S.W.; Geng, W.T.; Hu, Q.; Zhou, L.J. Corrosion behavior of the low alloy weathering steels coupled with stainless steel in simulated open atmosphere. Mater. Chem. Phys. 2022, 288, 126409. [Google Scholar] [CrossRef]
- Miura, S.; Murase, M.; Okamoto, T.; Tung, D.D.; Iwasaki, E. Corrosion Behavior and Applicability of Weathering Steel in Vietnam. J. Mater. Civ. Eng. 2017, 29, 04016264. [Google Scholar] [CrossRef]
- Yoo, Y.R.; Choi, S.H.; Kim, Y.S. Atmospheric Corrosion Behavior of Weathering Steel Exposed to the Outdoors for 10 Years in Korea. Corros. Sci. Technol. 2022, 21, 258–272. [Google Scholar] [CrossRef]
- Feng, J.; Mikihito, H.; Kazuki, O.; Jia, L. An analysis on spatial properties of corroded surface of weathering steel by different environmental conditions. Corros. Eng. Sci. Technol. 2023, 58, 577–587. [Google Scholar] [CrossRef]
- Vuong, V.D.; Nguyen, D.B.; Pham, N.T.; Tran, A.T.; Kawai, M.; Vu, A.Q.; Thao, N.D.; Van Le, T. Five-Year Field Exposure for Visualized Corrosion of STK400 Graded Steel Pile in Brackish Environment of Phu My Industrial Port (Southern Vietnam). J. Mater. Eng. Perform. 2022, 31, 2801–2809. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Fouly, A.; Hashmi, F.H. Controlling Atmospheric Corrosion of Weathering Steel Using Anodic Polarization Protection Technique. Processes 2021, 9, 1469. [Google Scholar] [CrossRef]
- Gallardo-Castán, E.; Lugo-Islas, G.; García-Navarro, N.; Ibañez Cornejo, J.G.; Galicia-Aguilar, G.; Ramírez-Reyes, J.L. Weather Conditions, Corrosivity Indexes and Electrochemical Corrosion of Carbon Steel, Copper and Zinc in the Coastal Atmosphere of Tuxpan, Veracruz, Mexico. J. Mex. Chem. Soc. 2023, 67, 483–494. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Wan, H.; Deng, J.; Li, W.; Liu, F. Actual Xisha marine atmospheric corrosion behavior of 30CrMnSiA steel in different parts of the aircraft. Eng. Fail. Anal. 2023, 154, 107684. [Google Scholar] [CrossRef]
- Song, L.Y.; Ma, X.M.; Chen, Z.Y.; Hou, B.R. The role of UV illumination on the initial atmospheric corrosion of 09CuPCrNi weathering steel in the presence of NaCl particles. Corros. Sci. 2014, 87, 427–437. [Google Scholar] [CrossRef]
- Cole, I.S.; Ganther, W.D.; Sinclair, J.D.; Lau, D.; Paterson, D.A. A study of the wetting of metal surfaces in order to understand the processes controlling atmospheric corrosion. J. Electrochem. Soc. 2004, 151, B627–B635. [Google Scholar] [CrossRef]
- Zhao, T.L.; Liu, K.; Li, Q. Comparison of the rusting behaviors of S450EW weathering steel under continuous spray and wet/dry cycling. Constr. Build. Mater. 2021, 309, 125211. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.W.; Zhang, W.H.; Guo, H.; He, X.L. Influence of outer rust layers on corrosion of carbon steel and weathering steel during wet-dry cycles. Corros. Sci. 2014, 82, 165–172. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, G.I.; Son, G.H.; Yoo, Y.H.; Hong, S.; Kim, J.G. New Accelerated Corrosion Test Method Simulating Atmospheric Corrosion of Complex Phase Steel Combining Cyclic Corrosion Test and Electrochemically Accelerated Corrosion Test. Materials 2023, 16, 3132. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, W.; Sun, Y.; Yang, W.; Chen, L.; Xie, J.; Li, W. Corrosion behavior of the 3 wt.% Ni weathering steel with replacing 1 wt.% Cr in the simulated tropical marine atmospheric environment. J. Phys. Chem. Solids 2023, 175, 111221. [Google Scholar] [CrossRef]
- Qiu, P.; Chen, Z.; Yang, H.; Yang, L.; Luo, L.; Chen, C. The Transformation of Corrosion Products on Weathering Steel by Visible-Light Illumination under Simulated Marine Atmospheric Condition. Int. J. Electrochem. Sci. 2016, 11, 10498–10510. [Google Scholar] [CrossRef]
- Jaén, J.A.; Guzmán, K.; Iglesias, J.; Manrique, G.C. Ten years outdoor exposure of steel in an urban and coastal tropical atmosphere. Corros. Eng. Sci. Technol. 2021, 56, 522–529. [Google Scholar] [CrossRef]
- Alvarenga, E.D.A.; Lins, V.D.C. Atmospheric corrosion evaluation of electrogalvanized, hot-dip galvanized and galvannealed interstitial free steels using accelerated field and cyclic tests. Surf. Coat. Technol. 2016, 306, 428–438. [Google Scholar] [CrossRef]
- Assumpção, R.F.; Silva, A.P.; Lins VF, C.; Sicupira, D.C. Corrosion Performance of New-Type Si-Based Weathering Steel in Marine Environment. J. Mater. Eng. Perform. 2022, 32, 8541–8548. [Google Scholar] [CrossRef]
- Wen, C.; Tian, Y.W.; Wang, G.; Hu, J.Z.; Deng, P.C. The Influence of Nickel on Corrosion Behavior of Low Alloy Steel in a Cyclic Wet-dry Condition. Int. J. Electrochem. Sci. 2016, 11, 4161–4173. [Google Scholar] [CrossRef]
- Oyabu, M.; Nomura, K.; Koike, Y.; Okazawa, A. Mossbauer and XRD analysis of corrosion products on weathering steel treated by wet-dry cycles using various solutions. Hyperfine Interact. 2016, 237, 68. [Google Scholar] [CrossRef]
- Haruna, T.; Nakai, M.; Hirohata, Y. Mass Gain Rates of Various Steels during Atmospheric Corrosion under Cyclic Conditions of Dry and Controlled-humidity Air. Tetsu Hagane-J. Iron Steel Inst. Jpn. 2021, 107, 1036–1046. [Google Scholar] [CrossRef]
- ASTMG154-23; Standard Practice for Operating Fluorescent Ultraviolet (UV) Lamp Apparatus for Exposure of Materials. ASTM International: West Conshohocken, PA, USA, 2023.
- ISO8407-2021; Corrosion of Metals and Alloys-Removal of Corrosion Products from Corrosion Test Specimens. ISO: Geneva, Switzerland, 2021.
- Yu, Q.; Yang, X.; Dong, W.; Wang, Q.; Zhang, F.; Gu, X. Layer-by-layer investigation of the multilayer corrosion products for different Ni content weathering steel via a novel Pull-off testing. Corros. Sci. 2022, 195, 109988. [Google Scholar] [CrossRef]

| Element | C | Mn | Nb | V | Ti | Si | Cr |
|---|---|---|---|---|---|---|---|
| Q345q | 0.13 | 1.55 | 0.01 | — | 0.012 | 0.42 | — |
| Q500q | 0.09 | 1.40 | 0.02 | 0.06 | 0.027 | 0.45 | 0.32 |
| Element | Ni | Cu | Mo | N | P | S | |
| Q345q | — | — | — | 0.004 | 0.015 | 0.002 | |
| Q500q | 0.42 | 0.50 | 0.015 | 0.003 | 0.012 | 0.002 |
| A | n | R2 | |
|---|---|---|---|
| Q345q in UVWD | 0.4204 | 1.1751 | 0.986 |
| Q500q in UVWD | 0.1085 | 1.4602 | 0.994 |
| Q345q in WD | 0.0582 | 0.6645 | 0.997 |
| Q500q in WD | 0.0450 | 0.7088 | 0.998 |
| Q345q in UV | 0.0017 | 1.1859 | 0.862 |
| Q500q in UV | 0.0213 | 0.8105 | 0.663 |
| Sample | Position | Fe (wt.%) | O (wt.%) | Mn (wt.%) |
|---|---|---|---|---|
| Q345q | A | 77.1 | 22.9 | |
| B | 75.4 | 23.8 | 0.80 | |
| Q500q | A | 74.0 | 26.0 | - |
| B | 94.7 | 3.9 | 1.3 |
| Sample | Position | Fe (wt.%) | O (wt.%) | Mn (wt.%) |
|---|---|---|---|---|
| Q345q | A | 79.0 | 21.0 | - |
| B | 78.3 | 21.7 | - | |
| Q500q | A | 79.8 | 19.0 | 1.2 |
| B | 80.3 | 18.6 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, Y.; Du, X.; Lin, T.; Wang, H.; Meng, F.; Wang, F. Influence of Ultraviolet Light and Alternating Wet–Dry Environments on the Corrosion Behavior of Weathering Steels. Materials 2024, 17, 3870. https://doi.org/10.3390/ma17153870
Yang Y, Wang Y, Du X, Lin T, Wang H, Meng F, Wang F. Influence of Ultraviolet Light and Alternating Wet–Dry Environments on the Corrosion Behavior of Weathering Steels. Materials. 2024; 17(15):3870. https://doi.org/10.3390/ma17153870
Chicago/Turabian StyleYang, Ying, Yubo Wang, Xinyu Du, Tianzi Lin, Han Wang, Fandi Meng, and Fuhui Wang. 2024. "Influence of Ultraviolet Light and Alternating Wet–Dry Environments on the Corrosion Behavior of Weathering Steels" Materials 17, no. 15: 3870. https://doi.org/10.3390/ma17153870




