Composite Nanoarchitectonics of Electrospun Piezoelectric PVDF/AgNPs for Biomedical Applications, Including Breast Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
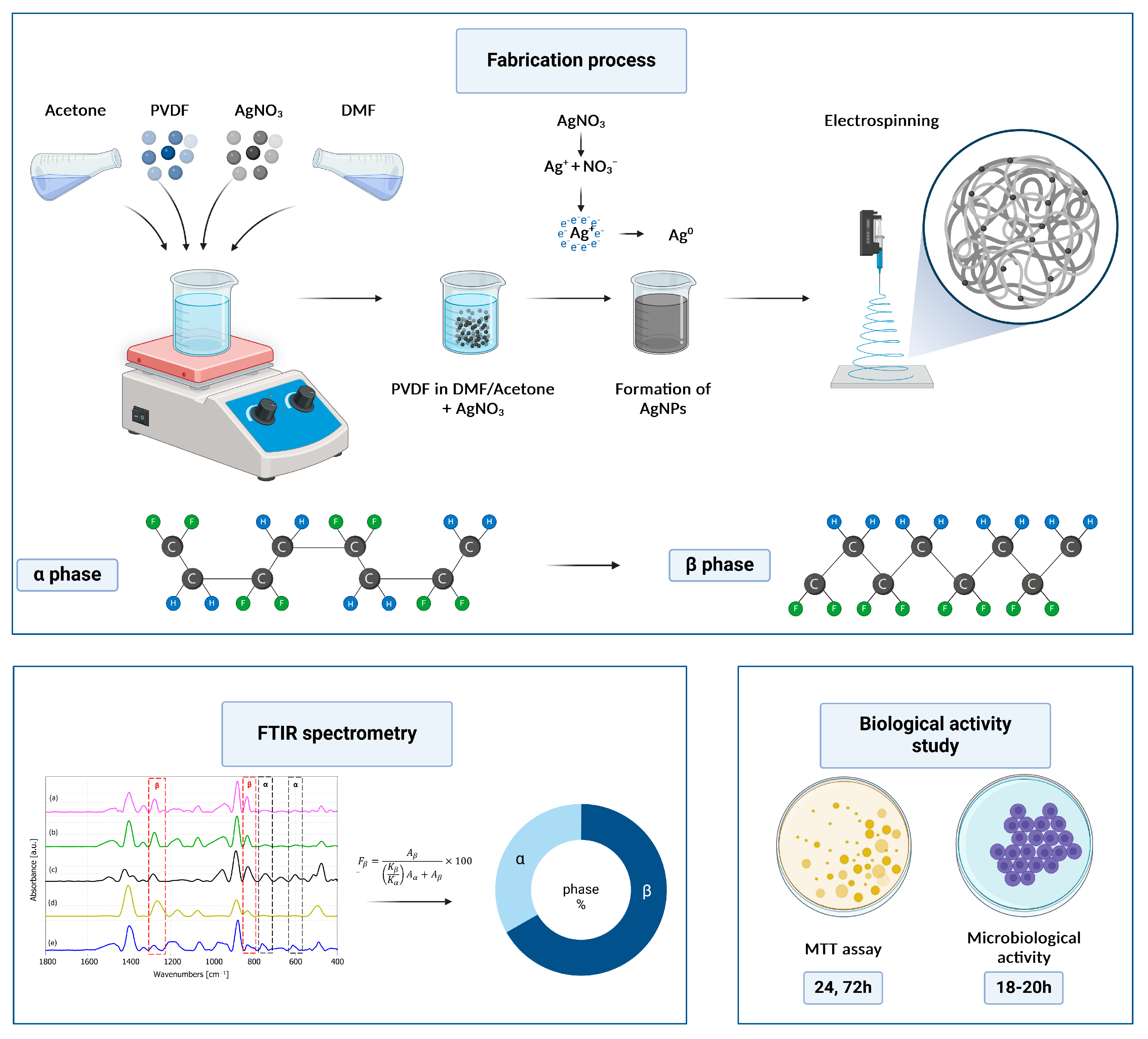
2.1. Fabrication of the PVDF Nanofibers
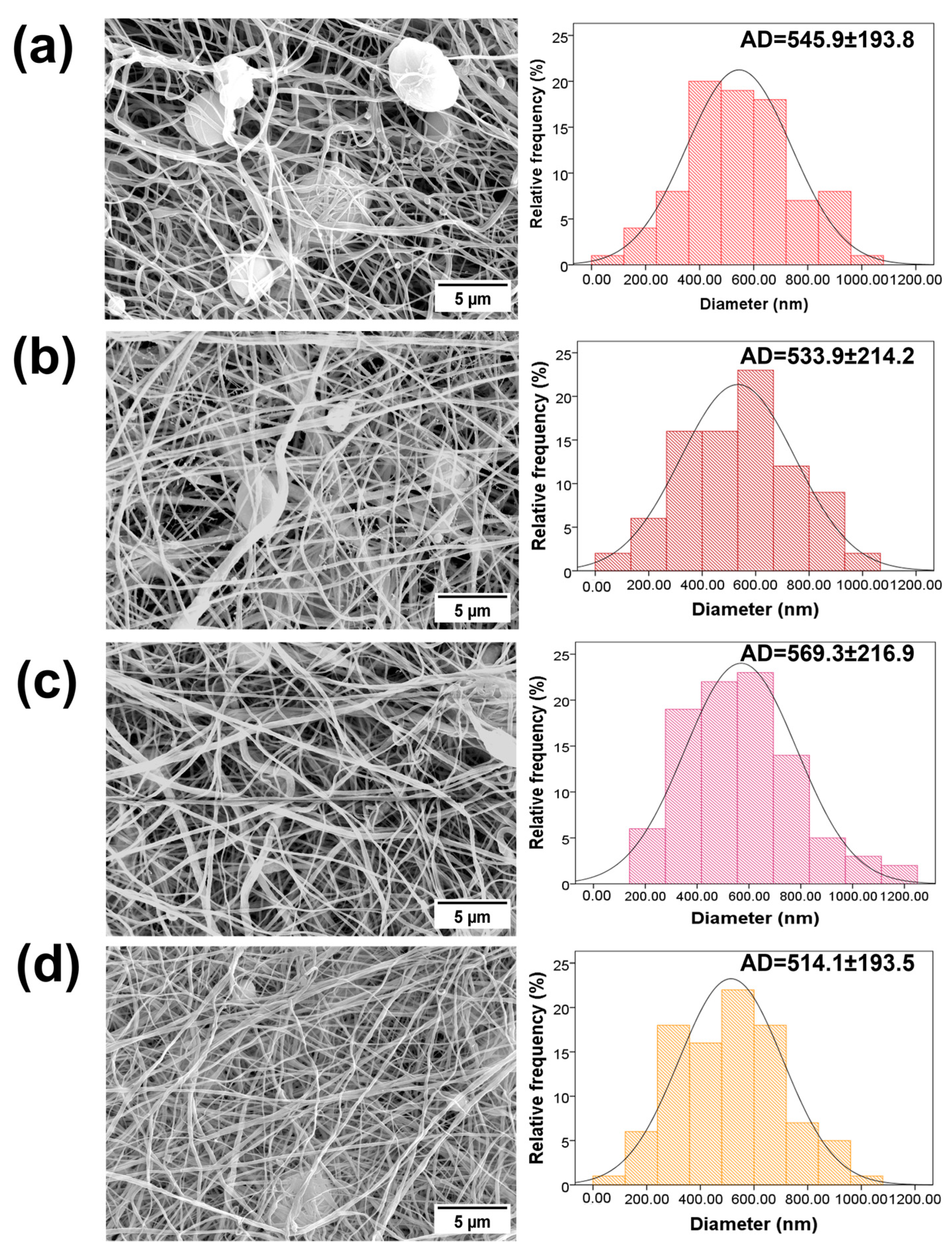
2.2. Scaffold Characterization and Morphology
2.3. SEM and EDS Analysis
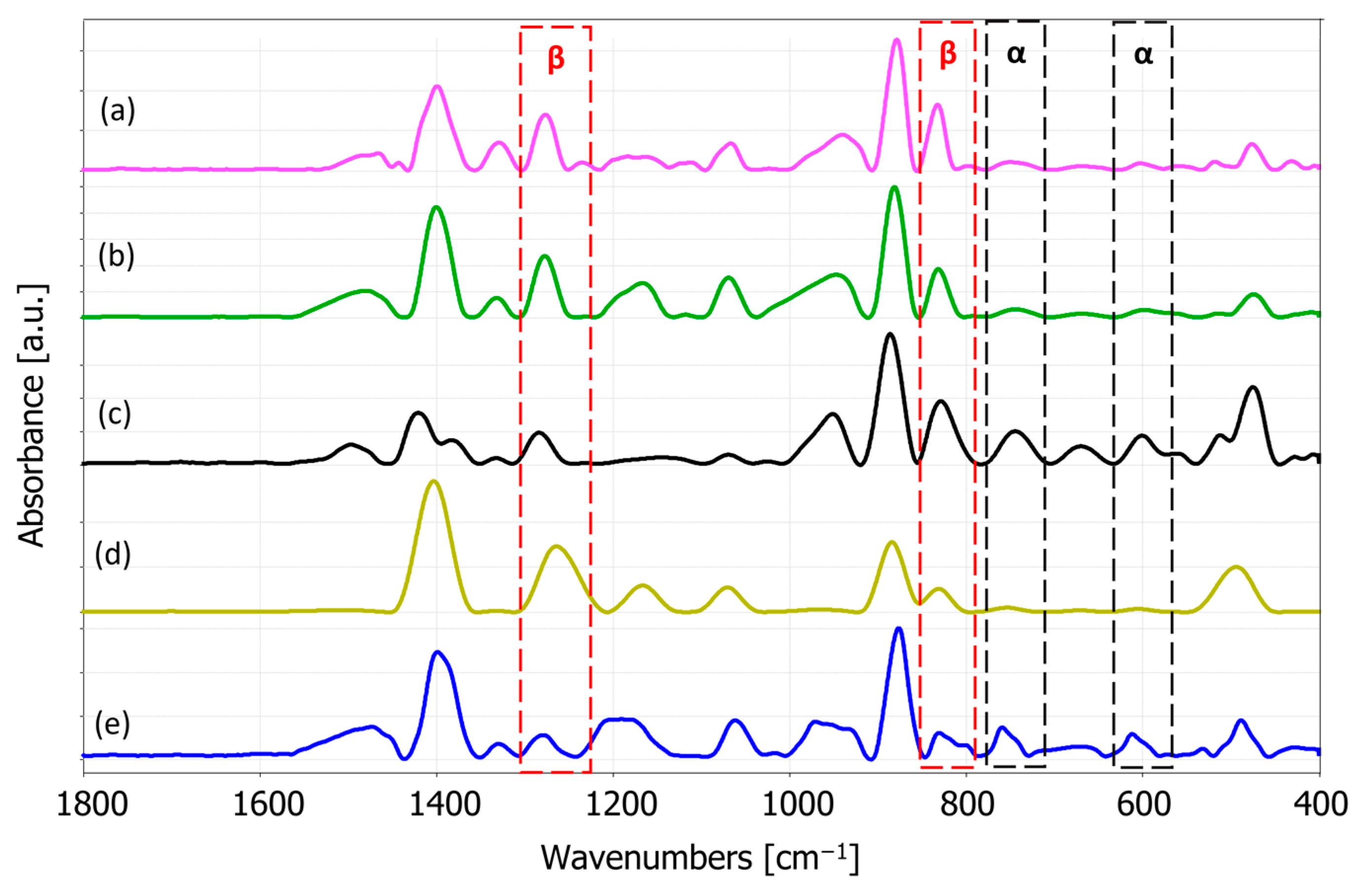
2.4. FTIR Spectroscopy Analysis
2.5. Biological Activity of the PVDF/Ag Electrospun Nanofibers
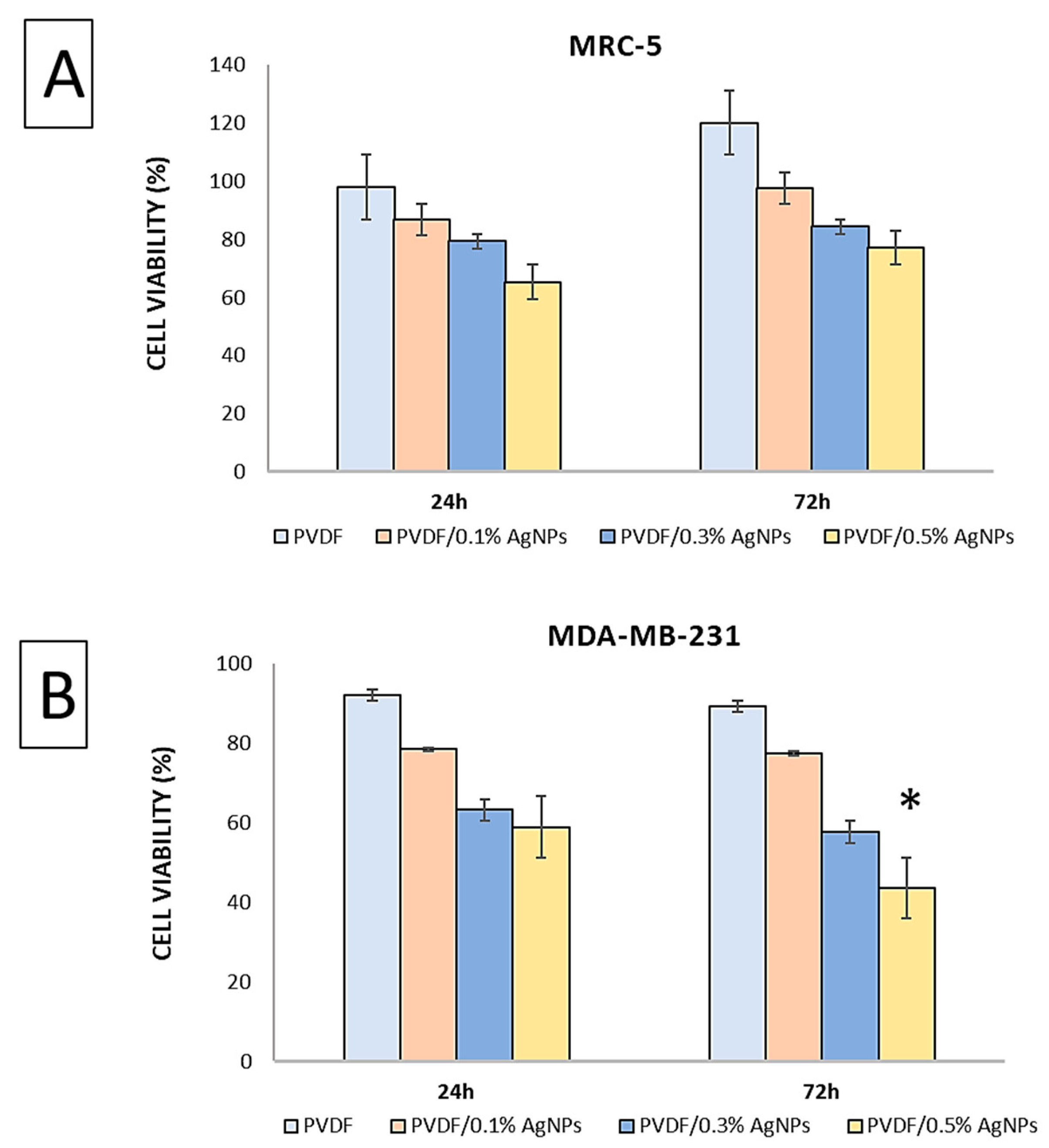
2.5.1. Cytotoxicity Assay (In Vitro Cell Culture Study)
2.5.2. Microbiological Assessment
2.5.3. Statistical Analysis
3. Results
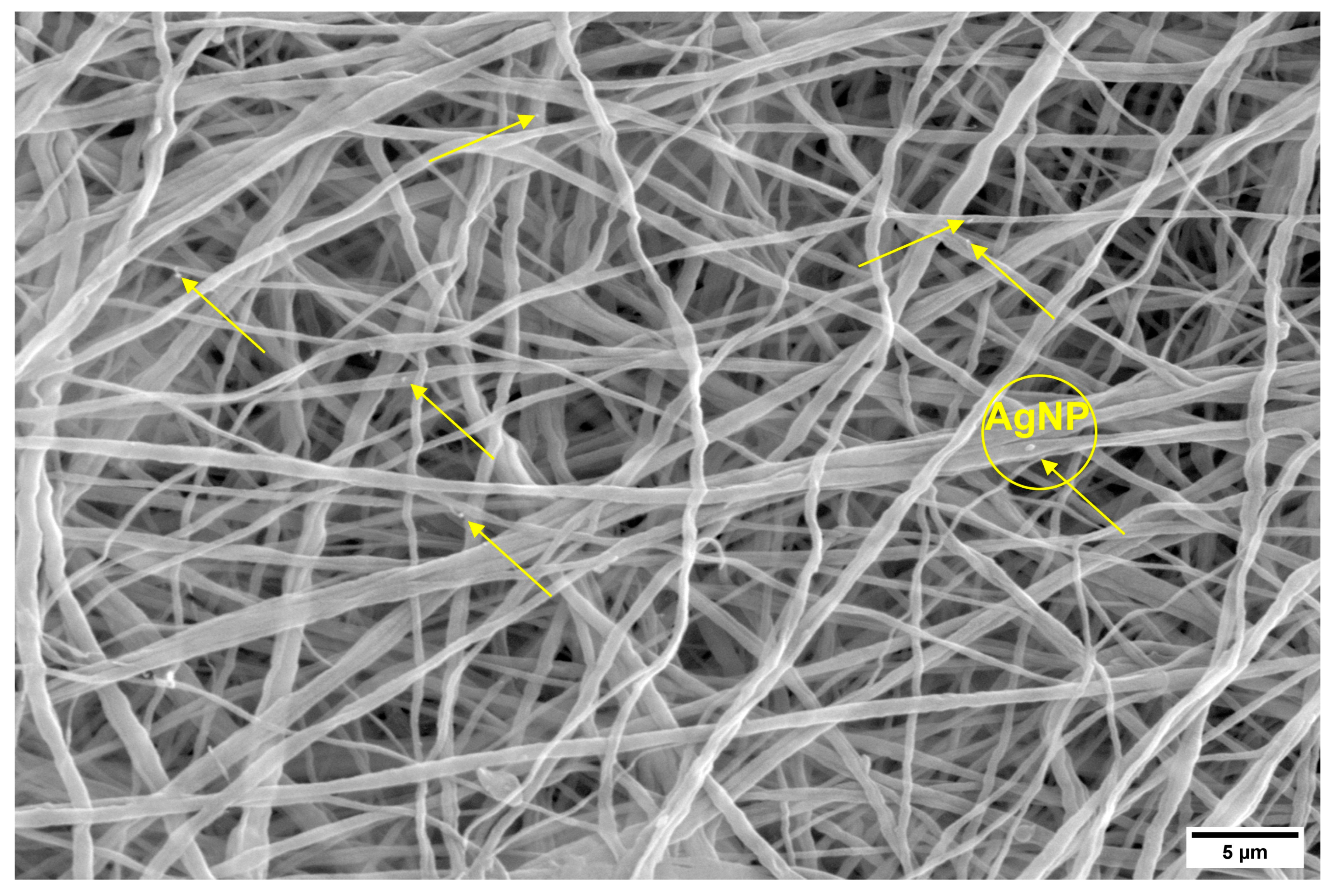
3.1. Characterization and Morphology of the PVDF and PVDF/AgNPs Nanofibers
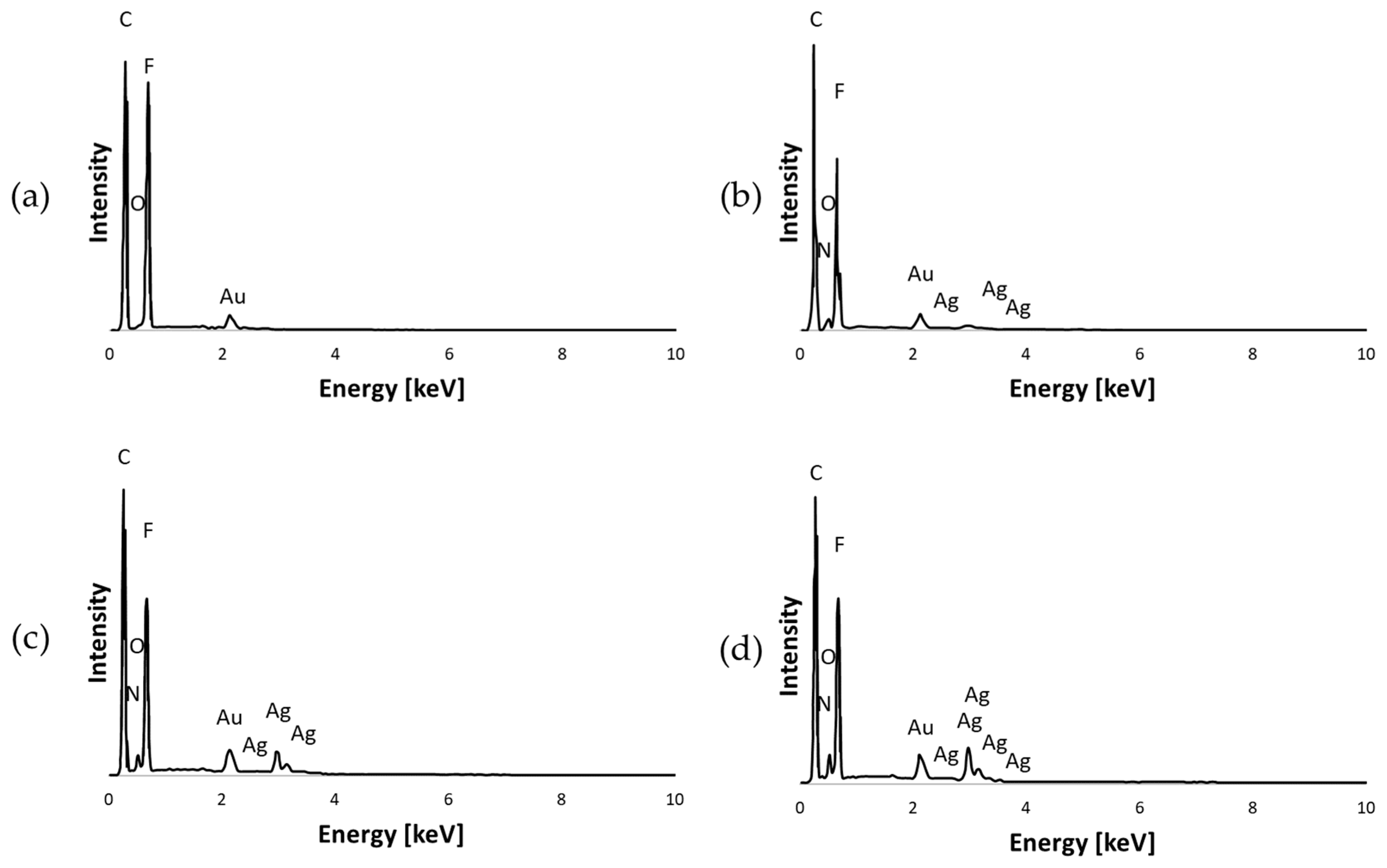
3.1.1. EDS Analysis
3.1.2. FTIR Spectroscopy Analysis
3.2. In Vitro Cytotoxicity Assay
Analysis of the Antibacterial Activity of PVDF/AgNPs Fiber Mats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Q.; Briscoe, J. Piezoelectric Energy Harvester Technologies: Synthesis, Mechanisms, and Multifunctional Applications. ACS Appl. Mater. Interfaces 2024, 16, 29491–29520. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Karan, S.K.; Chowdhury, R.; Ghosh, I.; Saha, S.; Das, K.; Mondal, A.; Nanda, A.; Khatua, B.B.; Reddy, C.M. Mechanically Flexible Piezoelectric Organic Single Crystals for Electrical Energy Harvesting. Chem 2024, 10, 1741–1754. [Google Scholar] [CrossRef]
- Pecunia, V.; Silva, S.R.P.; Phillips, J.D.; Artegiani, E.; Romeo, A.; Shim, H.; Park, J.; Kim, J.H.; Yun, J.S.; Welch, G.C.; et al. Roadmap on Energy Harvesting Materials. J. Phys. Mater. 2023, 6, 042501. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Orlova, T.; Chernozem, R.V.; Ivanova, A.A.; Bartasyte, A.; Mathur, S.; Surmeneva, M.A. Hybrid Lead-Free Polymer-Based Nanocomposites with Improved Piezoelectric Response for Biomedical Energy-Harvesting Applications: A Review. Nano Energy 2019, 62, 475–506. [Google Scholar] [CrossRef]
- Kim, H.; Rigo, B.; Wong, G.; Lee, Y.J.; Yeo, W.-H. Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring. Nano-Micro Lett. 2024, 16, 52. [Google Scholar] [CrossRef]
- Niu, Q.; Chen, J.; Fan, S.; Yao, X.; Gu, Y.; Hsiao, B.S.; Wei, H.; Zhang, Y. Silk Nanoribbon Films with Enriched Silk II Structure and Enhanced Piezoelectricity for Self-Powered Implantable and Wearable Devices. Nano Today 2024, 56, 102228. [Google Scholar] [CrossRef]
- Lee, M.; Chen, C.; Wang, S.; Cha, S.N.; Park, Y.J.; Kim, J.M.; Chou, L.; Wang, Z.L. A Hybrid Piezoelectric Structure for Wearable Nanogenerators. Adv. Mater. 2012, 24, 1759–1764. [Google Scholar] [CrossRef]
- Azarnoush, A.; Dambri, O.A.; Karatop, E.Ü.; Makrakis, D.; Cherkaoui, S. Simulation and Performance Evaluation of a Bio-Inspired Nanogenerator for Medical Applications. IEEE Trans. Biomed. Eng. 2023, 70, 2616–2623. [Google Scholar] [CrossRef]
- Bairagi, S.; Shahid-ul-Islam; Kumar, C.; Babu, A.; Aliyana, A.K.; Stylios, G.; Pillai, S.C.; Mulvihill, D.M. Wearable Nanocomposite Textile-Based Piezoelectric and Triboelectric Nanogenerators: Progress and Perspectives. Nano Energy 2023, 118, 108962. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Q.; Xiang, Y.; Wang, Z.; Hu, X. Stretchable and Self-Healing Electronic Skin Based on a Piezoelectric/Triboelectric Polyester Elastomer for Deep and Superficial Sensation. J. Mater. Chem. A 2023, 11, 20120–20128. [Google Scholar] [CrossRef]
- Owusu, F.; Venkatesan, T.R.; Nüesch, F.A.; Negri, R.M.; Opris, D.M. How to Make Elastomers Piezoelectric? Adv. Mater. Technol. 2023, 8, 2300099. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Yang, Y.; Wu, Y.; Shi, Q. Flexible Piezoelectric Sensor Based on Polyvinylidene Fluoride/Polyacrylonitrile/Carboxy-Terminated Multi-Walled Carbon Nanotube Composite Films for Human Motion Monitoring. Nanotechnology 2024, 35, 235501. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, H.; Han, Q.; Guan, W.; Sun, S.; Zheng, T.; Liu, Y.; Wang, X.; Huang, R.; Li, G. Piezoelectric Materials for Neuroregeneration: A Review. Biomater. Sci. 2023, 11, 7296–7310. [Google Scholar] [CrossRef] [PubMed]
- Casal, D.; Casimiro, M.; Ferreira, L.; Leal, J.; Rodrigues, G.; Lopes, R.; Moura, D.; Gonçalves, L.; Lago, J.; Pais, D.; et al. Review of Piezoelectrical Materials Potentially Useful for Peripheral Nerve Repair. Biomedicines 2023, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.; Chakraborty, S.; Barman, S.R.; Gavit, P.; Indrakumar, S.; Agrawal, A.; Lin, Z.-H.; Chatterjee, K. Progress in the Development of Piezoelectric Biomaterials for Tissue Remodeling. Biomaterials 2024, 307, 122528. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Basu, B.; Dubey, A.K. Electrical Stimulation and Piezoelectric Biomaterials for Bone Tissue Engineering Applications. Biomaterials 2020, 258, 120280. [Google Scholar] [CrossRef]
- Dai, J.; Shao, J.; Zhang, Y.; Hang, R.; Yao, X.; Bai, L.; Hang, R. Piezoelectric Dressings for Advanced Wound Healing. J. Mater. Chem. B 2024, 12, 1973–1990. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Pattnaik, S.; Rout, D.; Praharaj, S.; Mohanty, C. Emerging Self-Powered Piezoelectric Based Nanobiomaterials as a Platform for Accelerated Wound Healing: Recent Advances and Future Perspectives. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 1078–1100. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, Y.; Liu, W.; Li, S.; Wang, X.; Zhou, W.; Zhang, G.; Liu, K.; Zhang, H.; Zhao, Y.; et al. Untethered Artificial Muscles Powered by Wearable Sweat-Based Energy Generator. Nano Today 2023, 49, 101765. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zheng, J.M.; Ren, G.Y.; Zhang, P.H.; Xu, C. A Flexible Piezoelectric Force Sensor Based on PVDF Fabrics. Smart Mater. Struct. 2011, 20, 045009. [Google Scholar] [CrossRef]
- Kim, C.-H.; Yoo, Y.; Ayode Otitoju, T.; Kim, I.-C.; Nam, S.-E.; Park, Y.-I.; Cho, Y.H. Controlling the Polymorphs of PVDF Membranes: Effects of a Salt Additive on PVDF Polarization during Phase Separation Processes. Sep. Purif. Technol. 2024, 351, 128120. [Google Scholar] [CrossRef]
- Austria, H.F.M.; Sardome, R.P.; Setiawan, O.; Huang, T.-H.; Lei, W.-C.; Tian, X.-Y.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y.; Tayo, L.L.; et al. Efficient Membrane Fouling Mitigation in Self-Cleaning Piezoelectric PVDF-Graphene Loose Nanofiltration Membranes for Sustainable Textile Wastewater Treatment. Sep. Purif. Technol. 2024, 346, 127317. [Google Scholar] [CrossRef]
- Messer, D.K.; Örnek, M.; Nunes, C.T.; Paral, M.W.; Son, S.F. Characterization and Calibration of a Piezo-Energetic Composite Film as a Reactive Gauge. J. Appl. Phys. 2024, 135, 144902. [Google Scholar] [CrossRef]
- Ali, M.R.R.; Tigno, S.D.; Caldona, E.B. Piezoelectric Approaches to Organic Polymeric Materials. Polym. Int. 2024, 73, 176–190. [Google Scholar] [CrossRef]
- Kumar, Y.; Trivedi, A.; Shukla, S.K. Investigating the Influence of Frequency on Piezo-Dynamics of Polyvinylidene Fluoride (PVDF) Films Embedded in Confined Geomaterials. J. Vib. Eng. Technol. 2024. [Google Scholar] [CrossRef]
- Duraccio, D.; Capra, P.P.; Malucelli, G. UV-Curable Coatings for Energy Harvesting Applications: Current State-of-the-Art and Future Perspectives. Micro Nano Eng. 2024, 23, 100266. [Google Scholar] [CrossRef]
- Fukada, E. History and Recent Progress in Piezoelectric Polymers. IEEE Trans. Ultrason. Ferroelect. Freq. Contr. 2000, 47, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Mina, M.; Carbonneau, M.; Marchand, M.; Tran, S.D. Advancements in Wearable and Implantable Intraocular Pressure Biosensors for Ophthalmology: A Comprehensive Review. Micromachines 2023, 14, 1915. [Google Scholar] [CrossRef]
- Khan, A.A.; Kim, J.-H. Recent Advances in Materials and Manufacturing of Implantable Devices for Continuous Health Monitoring. Biosens. Bioelectron. 2024, 261, 116461. [Google Scholar] [CrossRef] [PubMed]
- Hanein, Y.; Goding, J. Guest Editorial: Implantable Bioelectronics. APL Bioeng. 2024, 8, 020401. [Google Scholar] [CrossRef]
- Zhao, B.; Kuo, N.-C.; Liu, B.; Li, Y.-A.; Iotti, L.; Niknejad, A.M. A Batteryless Padless Crystalless 16 μm × 116 μm “Dielet” Near-Field Radio With On-Chip Coil Antenna. IEEE J. Solid-State Circuits 2020, 55, 249–260. [Google Scholar] [CrossRef]
- Roy, M.; Bhattacharjee, S.; Neogi, B.; Saha, P. Design and Development of an Implantable Circuit for Adjusting Required Pressure inside of Respiratory System. Microsyst Technol 2024. [Google Scholar] [CrossRef]
- Ueberschlag, P. PVDF Piezoelectric Polymer. Sens. Rev. 2001, 21, 118–126. [Google Scholar] [CrossRef]
- Anic, M.; Prodanovic, M.; Milenkovic, S.; Filipovic, N.; Grujovic, N.; Zivic, F. The Review of Materials for Energy Harvesting. In Proceedings of the 2021 IEEE 21st International Conference on Bioinformatics and Bioengineering (BIBE), Kragujevac, Serbia, 25–27 October 2021; IEEE: Kragujevac, Serbia, 2021; pp. 1–6. [Google Scholar]
- Anic, M.; Prodanovic, M.; Milenkovic, S.; Filipovic, N.; Grujovic, N.; Zivic, F. Electrospinning as the fabrication technology for the energy harvesting composites. In Proceedings of the 38th International Conference on Production Engineering of Serbia ICPE-S 2021, Čačak, Serbia, 14–15 October 2021; Fakultet Tehničkih Nauka u Čačku, Univerziteta u Kragujevcu: Čačak, Serbia, 2021; pp. 167–178. [Google Scholar]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the β Phase Poly(Vinylidene Fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly(Vinylidene Fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Gomes, J.; Serrado Nunes, J.; Sencadas, V.; Lanceros-Mendez, S. Influence of the β-Phase Content and Degree of Crystallinity on the Piezo- and Ferroelectric Properties of Poly(Vinylidene Fluoride). Smart Mater. Struct. 2010, 19, 065010. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Zhu, Y. Study on the Effect of Stretching Behavior on the Properties of PVDF Films and Mechanism. Polymer 2024, 297, 126884. [Google Scholar] [CrossRef]
- Hartono, A.; Satira, S.; Djamal, M.; Ramli, R.; Bahar, H.; Sanjaya, E. Effect of Mechanical Treatment Temperature on Electrical Properties and Crystallite Size of PVDF Film. AMPC 2013, 03, 71–76. [Google Scholar] [CrossRef]
- Patil, Y.; Zhao, J.; Ameduri, B.; Rastogi, S. Tailoring Electroactive β- and γ-Phases via Synthesis in the Nascent Poly(Vinylidene Fluoride) Homopolymers. Macromolecules 2024, 57, 616–627. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dasgupta Ghosh, B.; Ghosh, A.; Roy, S. Polyvinylidene Fluoride Nanocomposites as Piezoelectric Nanogenerator: Properties, Fabrication and Market Applications. Adv. Eng. Mater. 2024, 26, 2400445. [Google Scholar] [CrossRef]
- Bindhu, A.; Arun, A.P.; Pathak, M. Review on Polyvinylidene Fluoride-Based Triboelectric Nanogenerators for Applications in Health Monitoring and Energy Harvesting. ACS Appl. Electron. Mater. 2024, 6, 47–72. [Google Scholar] [CrossRef]
- Purushothaman, S.M.; Tronco, M.F.; Kottathodi, B.; Royaud, I.; Ponçot, M.; Kalarikkal, N.; Thomas, S.; Rouxel, D. A Review on Electrospun PVDF-Based Nanocomposites: Recent Trends and Developments in Energy Harvesting and Sensing Applications. Polymer 2023, 283, 126179. [Google Scholar] [CrossRef]
- Chen, Z.; Guan, M.; Bian, Y.; Yin, X. Multifunctional Electrospun Nanofibers for Biosensing and Biomedical Engineering Applications. Biosensors 2023, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Jiang, B.; Zhang, Q.; Chen, H.; Shen, Y.; Wang, Z.; Yin, X. Process Investigation on Robust Electrospinning of Non-Aligned and Aligned Polyvinylidene Fluoride Nanofiber Mats for Flexible Piezoelectric Sensors. Polymers 2024, 16, 816. [Google Scholar] [CrossRef] [PubMed]
- Pusty, M.; Sinha, L.; Shirage, P.M. A Flexible Self-Poled Piezoelectric Nanogenerator Based on a rGO–Ag/PVDF Nanocomposite. New J. Chem. 2019, 43, 284–294. [Google Scholar] [CrossRef]
- El-Masry, M.M. Synthesize and Characterization of Ag-CuO/rGO Nanoparticles as a Filler of the PVDF Polymer to Improve Its Polar β Phase and Electrical Conductivity for Polymer Batteries Applications. J. Polym. Res. 2023, 30, 345. [Google Scholar] [CrossRef]
- Martins, P.; Brito-Pereira, R.; Ribeiro, S.; Lanceros-Mendez, S.; Ribeiro, C. Magnetoelectrics for Biomedical Applications: 130 Years Later, Bridging Materials, Energy, and Life. Nano Energy 2024, 126, 109569. [Google Scholar] [CrossRef]
- Nikitin, A.A.; Ivanova, A.V.; Semkina, A.S.; Lazareva, P.A.; Abakumov, M.A. Magneto-Mechanical Approach in Biomedicine: Benefits, Challenges, and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 11134. [Google Scholar] [CrossRef]
- Lu, Q.; Choi, K.; Nam, J.-D.; Choi, H.J. Magnetic Polymer Composite Particles: Design and Magnetorheology. Polymers 2021, 13, 512. [Google Scholar] [CrossRef]
- Pan, C.-T.; Dutt, K.; Yen, C.-K.; Kumar, A.; Kaushik, A.C.; Wei, D.-Q.; Kumar, A.; Wen, Z.-H.; Hsu, W.-H.; Shiue, Y.-L. Characterization of Piezoelectric Properties of Ag-NPs Doped PVDFNanocomposite Fibres Membrane Prepared by Near Field Electrospinning. Comb. Chem. High Throughput Screen. 2022, 25, 720–729. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhao, Q.; Wang, X.; Shi, C.; Hu, X.; Li, J. A Hydrophilic/Hydrophobic Janus Membrane Used as Wound Dressings with Enhanced Antibacterial Properties. Fibers Polym. 2022, 23, 2511–2516. [Google Scholar] [CrossRef]
- Strużyńska, L. Dual Implications of Nanosilver-Induced Autophagy: Nanotoxicity and Anti-Cancer Effects. Int. J. Mol. Sci. 2023, 24, 15386. [Google Scholar] [CrossRef] [PubMed]
- Khafaga, A.F.; Gaballa, M.M.S.; Karam, R.; Shoulah, S.A.; Shamma, R.N.; Khalifa, N.E.; Farrag, N.E.; Noreldin, A.E. Synergistic Therapeutic Strategies and Engineered Nanoparticles for Anti-Vascular Endothelial Growth Factor Therapy in Cancer. Life Sci. 2024, 341, 122499. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Saravanan, M. Biosynthesis of Silver Nanoparticles from Staphylococcus Aureus and Its Antimicrobial Activity against MRSA and MRSE. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Gómez, L.J.; Pérez-González, G.L.; Bogdanchikova, N.; Pestryakov, A.; Nimaev, V.; Soloveva, A.; Cornejo-Bravo, J.M.; Toledaño-Magaña, Y. Antimicrobial Effect of Electrospun Nanofibers Loaded with Silver Nanoparticles: Influence of Ag Incorporation Method. J. Nanomater. 2021, 2021, 9920755. [Google Scholar] [CrossRef]
- Mendoza Villicana, A.; Gochi Ponce, Y.; Grande, D.; José Manuel, C.B.; Zizumbo López, A.; González Joaquín, M.C.; Chávez Santoscoy, R.A.; Paz González, J.A.; Bogdanchikova, N.; Pérez González, G.L.; et al. Evaluation of Strategies to Incorporate Silver Nanoparticles into Electrospun Microfibers for the Preparation of Wound Dressings and Their Antimicrobial Activity. Polym.-Plast. Technol. Mater. 2023, 62, 1029–1056. [Google Scholar] [CrossRef]
- Dashtizad, S.; Alizadeh, P.; Yourdkhani, A. Improving Piezoelectric Properties of PVDF Fibers by Compositing with BaTiO3-Ag Particles Prepared by Sol-Gel Method and Photochemical Reaction. J. Alloys Compd. 2021, 883, 160810. [Google Scholar] [CrossRef]
- Naik, R.; Somasekhara Rao, T. Preparation and Characterization of Flexible PVDF Based Polymer Film for Energy Harvesting Applications. Mater. Today Proc. 2019, 18, 5107–5113. [Google Scholar] [CrossRef]
- Virijević, K.; Živanović, M.N.; Nikolić, D.; Milivojević, N.; Pavić, J.; Morić, I.; Šenerović, L.; Dragačević, L.; Thurner, P.J.; Rufin, M.; et al. AI-Driven Optimization of PCL/PEG Electrospun Scaffolds for Enhanced In Vivo Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 22989–23002. [Google Scholar] [CrossRef]
- Virijević, K.; Živanović, M.; Pavić, J.; Dragačević, L.; Ljujić, B.; Miletić Kovačević, M.; Papić, M.; Živanović, S.; Milenković, S.; Radojević, I.; et al. Electrospun Gelatin Scaffolds with Incorporated Antibiotics for Skin Wound Healing. Pharmaceuticals 2024, 17, 851. [Google Scholar] [CrossRef]
- Andrews, J.M. BSAC Standardized Disc Susceptibility Testing Method (Version 4). J. Antimicrob. Chemother. 2005, 56, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Gallus, I.J.; Boyraz, E.; Maryška, J. Antimicrobial Properties of Nanofiber Membrane and Commercial Micromembrane by Modification with Diethylenetriamine (DETA) and Attachment of Silver Nanoparticles. J. Nanomater. 2023, 2023, 8927774. [Google Scholar] [CrossRef]
- Ismail, A.M.; El-Newehy, M.H.; El-Naggar, M.E.; Meera Moydeen, A.; Menazea, A.A. Enhancement the Electrical Conductivity of the Synthesized Polyvinylidene Fluoride/Polyvinyl Chloride Composite Doped with Palladium Nanoparticles via Laser Ablation. J. Mater. Res. Technol. 2020, 9, 11178–11188. [Google Scholar] [CrossRef]
- Tommalieh, M.J.; Ismail, A.M.; Awwad, N.S.; Ibrahium, H.A.; Youssef, M.A.; Menazea, A.A. Investigation of Electrical Conductivity of Gold Nanoparticles Scattered in Polyvinylidene Fluoride/Polyvinyl Chloride via Laser Ablation for Electrical Applications. J. Electron. Mater. 2020, 49, 7603–7608. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Abdul-Majeed, M.A. Preparation and Characterization of AgNp/PVDF Cmposite Ultrafiltration Membrane. J. Eng. 2018, 24, 50–63. [Google Scholar] [CrossRef]
- Han, G.; Su, Y.; Feng, Y.; Lu, N. Approaches for Increasing the β-Phase Concentration of Electrospun Polyvinylidene Fluoride (PVDF) Nanofibers. ES Mater. Manuf. 2019, 6, 75–80. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Cestari, M. Effect of Crystallization Temperature on the Crystalline Phase Content and Morphology of Poly(Vinylidene Fluoride). J. Polym. Sci. B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Castkova, K.; Kastyl, J.; Sobola, D.; Petrus, J.; Stastna, E.; Riha, D.; Tofel, P. Structure–Properties Relationship of Electrospun PVDF Fibers. Nanomaterials 2020, 10, 1221. [Google Scholar] [CrossRef]
- Issa, A.; Al-Maadeed, M.; Luyt, A.; Ponnamma, D.; Hassan, M. Physico-Mechanical, Dielectric, and Piezoelectric Properties of PVDF Electrospun Mats Containing Silver Nanoparticles. C—J. Carbon Res. 2017, 3, 30. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of Biologically Synthesized Silver Nanoparticles in MDA-MB-231 Human Breast Cancer Cells. BioMed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef]
- Selvi, B.C.G.; Madhavan, J.; Santhanam, A. Cytotoxic Effect of Silver Nanoparticles Synthesized from Padina Tetrastromatica on Breast Cancer Cell Line. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035015. [Google Scholar] [CrossRef]
- Muhammad, N.; Zhao, H.; Song, W.; Gu, M.; Li, Q.; Liu, Y.; Li, C.; Wang, J.; Zhan, H. Silver Nanoparticles Functionalized Paclitaxel Nanocrystals Enhance Overall Anti-Cancer Effect on Human Cancer Cells. Nanotechnology 2021, 32, 085105. [Google Scholar] [CrossRef] [PubMed]
- Felix Swamidoss, V.; Bangaru, M.; Nalathambi, G.; Sangeetha, D.; Selvam, A.K. Silver-Incorporated Poly Vinylidene Fluoride Nanofibers for Bacterial Filtration. Aerosol Sci. Technol. 2019, 53, 196–206. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhou, J.; Chatterton, N.P.; Li, Y.; Huang, J.; Wang, X. Polyacrylonitrile Nanofibers Coated with Silver Nanoparticles Using a Modified Coaxial Electrospinning Process. Int. J. Nanomed. 2012, 7, 5725–5732. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, C.F.; Souza, P.R.; Jacinto, G.S.; Braga, T.L.; Ricken, Y.S.; Souza, G.K.; Caetano, W.; Radovanovic, E.; Arns, C.W.; Rai, M.; et al. Silver Nanoparticles In Situ Synthesized and Incorporated in Uniaxial and Core–Shell Electrospun Nanofibers to Inhibit Coronavirus. Pharmaceutics 2024, 16, 268. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, H.; Kumpikaitė, E. Anti-Bacterial Nanofibers and Their Biomedical Applications—A Review. J. Text. Inst. 2024, 1–19. [Google Scholar] [CrossRef]
- Kalimuldina, G.; Turdakyn, N.; Abay, I.; Medeubayev, A.; Nurpeissova, A.; Adair, D.; Bakenov, Z. A Review of Piezoelectric PVDF Film by Electrospinning and Its Applications. Sensors 2020, 20, 5214. [Google Scholar] [CrossRef]
- Pan, C.-T.; Dutt, K.; Kumar, A.; Kumar, R.; Chuang, C.-H.; Lo, Y.-T.; Wen, Z.-H.; Wang, C.-S.; Kuo, S.-W. PVDF/AgNP/MXene Composites-Based near-Field Electrospun Fiber with Enhanced Piezoelectric Performance for Self-Powered Wearable Sensors. Int. J. Bioprinting 2022, 9, 647. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Farahzadi, R.; Fathi, E.; Alizadeh, M.; Abdolalizadeh Amir, S.; Khodaei Ardakan, A.; Jafari, S. Investigation the Apoptotic Effect of Silver Nanoparticles (Ag-NPs) on MDA-MB 231 Breast Cancer Epithelial Cells via Signaling Pathways. Heliyon 2024, 10, e26959. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Murugesan, B.; Pandiyan, N.; Chinnalagu, D.K.; Rangasamy, G.; Mahalingam, S. Electrospinning Cellulose Acetate/Silk Fibroin/Au-Ag Hybrid Composite Nanofiber for Enhanced Biocidal Activity against MCF-7 Breast Cancer Cell. Mater. Sci. Eng. C 2021, 123, 112019. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Roy, B.; Shaw, P.; Mondal, P.; Mondal, M.K.; Chowdhury, P.; Bhattacharya, S.; Chattopadhyay, A. Cytotoxic Effect of Green Synthesized Silver Nanoparticles in MCF7 and MDA-MB-231 Human Breast Cancer Cells in Vitro. Nucleus 2020, 63, 191–202. [Google Scholar] [CrossRef]
- Yuan, J.; Geng, J.; Xing, Z.; Shen, J.; Kang, I.; Byun, H. Electrospinning of Antibacterial Poly(Vinylidene Fluoride) Nanofibers Containing Silver Nanoparticles. J. Appl. Polymer Sci. 2010, 116, 668–672. [Google Scholar] [CrossRef]
- Irfan, M.; Uddin, Z.; Ahmad, F.; Rasheed, A.; Qadir, M.B.; Ahmad, S.; Aykut, Y.; Nazir, A. Ecofriendly Development of Electrospun Antibacterial Membranes Loaded with Silver Nanoparticles. J. Ind. Text. 2022, 51, 2412S–2425S. [Google Scholar] [CrossRef]
- Medrano-Lopez, J.A.; Villalpando, I.; Salazar, M.I.; Torres-Torres, C. Hierarchical Nanobiosensors at the End of the SARS-CoV-2 Pandemic. Biosensors 2024, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K. Nanoarchitectonics: The Method for Everything in Materials Science. Bull. Chem. Soc. Jpn. 2024, 97, uoad001. [Google Scholar] [CrossRef]
- Heo, Y.; Alexe, M. Boosting Piezoelectricity under Illumination via the Bulk Photovoltaic Effect and the Schottky Barrier Effect in BiFeO3. Adv. Mater. 2022, 34, 2105845. [Google Scholar] [CrossRef]
- Kozielski, L.; Erhart, J.; Clemens, F. Light-Intensity-Induced Characterization of Elastic Constants and D33 Piezoelectric Coefficient of PLZT Single Fiber Based Transducers. Sensors 2013, 13, 2419–2429. [Google Scholar] [CrossRef]
- Frick, A.; Van Vliet, W.A.; Žukauskaitė, A.; Zywitzki, O.; Modes, T.; Kostenko, A.; Von Hauff, E. Volumetric 3D-Printed Piezoelectric Polymer Films. Adv. Mater. Technol. 2024, 9, 2301469. [Google Scholar] [CrossRef]
- Gruzdenko, A.; Mulder, D.J.; Schenning, A.P.H.J.; Den Toonder, J.M.J.; Debije, M.G. Dual-Wavelength Volumetric Microlithography for Rapid Production of 4D Microstructures. ACS Appl. Mater. Interfaces 2024, 16, 22696–22703. [Google Scholar] [CrossRef] [PubMed]
- Barnakov, Y.A.; Paul, O.; Joaquim, A.; Falconer, A.; Mu, R.; Barnakov, V.Y.; Dikin, D.; Petranovskii, V.P.; Zavalin, A.; Ueda, A.; et al. Light Intensity-Induced Phase Transitions in Graphene Oxide Doped Polyvinylidene Fluoride. Opt. Mater. Express 2018, 8, 2579. [Google Scholar] [CrossRef]
- Bao, R.; Hu, Y.; Yang, Q.; Pan, C. Piezo-Phototronic Effect on Optoelectronic Nanodevices. MRS Bull. 2018, 43, 952–958. [Google Scholar] [CrossRef]

| Solution | Electrospinning Parameters | |||||
|---|---|---|---|---|---|---|
| PVDF Concentration [%] | Solvent [v/v%] | Voltage [kV] | Needle [gauge] | Flow Rate [mL/h] | Tip to Collector Distance [cm] | Temperature, Humidity |
| 21% | 75% DMF:25% Ac | 30 | 18 | 0.5 | 15 | 30 °C, 45% |
| Sample | [%] |
|---|---|
| solution cast | 46% |
| Pure PVDF | 80% |
| PVDF/0.1% AgNPs | 91% |
| PVDF/0.3% AgNPs | 96% |
| PVDF/0.5% AgNPs | 60% |
| Tested Bacteria/Tested Material | Staphylococcus aureus ATCC 25923 [mm] * | Pseudomonas aeruginosa ATCC 27853 [mm] * |
|---|---|---|
| PVDF/AgNPs (0.1%) nanofiber | - | - |
| PVDF/AgNPs (0.3%) nanofiber | 7 ± 0.00 | 8 ± 0.47 |
| PVDF/AgNPs (0.5%) nanofiber | 10 ± 1.25 | 10 ± 0.47 |
| Gentamicin tests disc 10 μg | 21 ± 1.25 | 19 ± 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milenković, S.; Virijević, K.; Živić, F.; Radojević, I.; Grujović, N. Composite Nanoarchitectonics of Electrospun Piezoelectric PVDF/AgNPs for Biomedical Applications, Including Breast Cancer Treatment. Materials 2024, 17, 3872. https://doi.org/10.3390/ma17153872
Milenković S, Virijević K, Živić F, Radojević I, Grujović N. Composite Nanoarchitectonics of Electrospun Piezoelectric PVDF/AgNPs for Biomedical Applications, Including Breast Cancer Treatment. Materials. 2024; 17(15):3872. https://doi.org/10.3390/ma17153872
Chicago/Turabian StyleMilenković, Strahinja, Katarina Virijević, Fatima Živić, Ivana Radojević, and Nenad Grujović. 2024. "Composite Nanoarchitectonics of Electrospun Piezoelectric PVDF/AgNPs for Biomedical Applications, Including Breast Cancer Treatment" Materials 17, no. 15: 3872. https://doi.org/10.3390/ma17153872






