The Effect of Artificial Ageing on the Changes in Selected Properties of Organic Coated Sheets
Abstract
:1. Introduction
- Application of finished foils—lamination;
- Application of plastisol;
- Application by swirling finely ground plastic powder in fluidisation chambers;
- Flame spraying (with plastisols, powder materials);
- The individual layers naturally cooperate (substrate, pre-treatment, paints);
- The process is highly controlled, ensuring uniformity in cleaning, pre-treatment, and paint application.
- Mechanical
- Metal/metal: oxidative, thermal, abrasive (rubbing), pitting (surface treatment), and combined;
- Hard particles—abrasive/metal: abrasive bound/metal, abrasive powder/metal, abrasive in liquid-flowing medium—hydraulic erosion, and abrasive in gas-flowing medium/metal;
- Liquid-flowing medium/metal: turbulent flow of liquid/metal—hydraulic erosion, and flow of liquid and water vapour/metal—cavitation;
- Gas—flowing medium/metal: pneumatic erosion.
- Physical–chemical wear,
- Chemical wear:
- The nature of the surface—corrosion—can be chemical or electrochemical, namely: under tension, under fatigue, or under vibration;
- Method of failure—uniform corrosion or uneven corrosion—can be point, spot—local, intercrystalline, and extraction—selection, or by irradiation.
- Storage conditions;
- Conditioning conditions;
- Test conditions.
- Low (L)—up to 7 years;
- Medium (M)—from 7 years to 15 years;
- High (H)—from 15 years to 25 years;
- Very high (VH)—more than 25 years.
2. Materials and Methods
- RAL 9002 Grey–White (white), with a sheet thickness of 0.4 and a paint thickness on the top side of 10 + 5 μm;
- RAL 8017 Oxide Red (brown), with a sheet thickness of 0.5 and a paint thickness on the top side of 20 + 5 μm;
- RAL 9006 Standard Polyester (Silver), with a sheet thickness of 0.6 and a paint thickness on the top side of 20 + 5 μm.
- High resistance to UV radiation—RUV 2;
- Coating thickness (topcoat + primer [μm])—20 + 5 μm;
- Adhesion (grid test)—0–1.
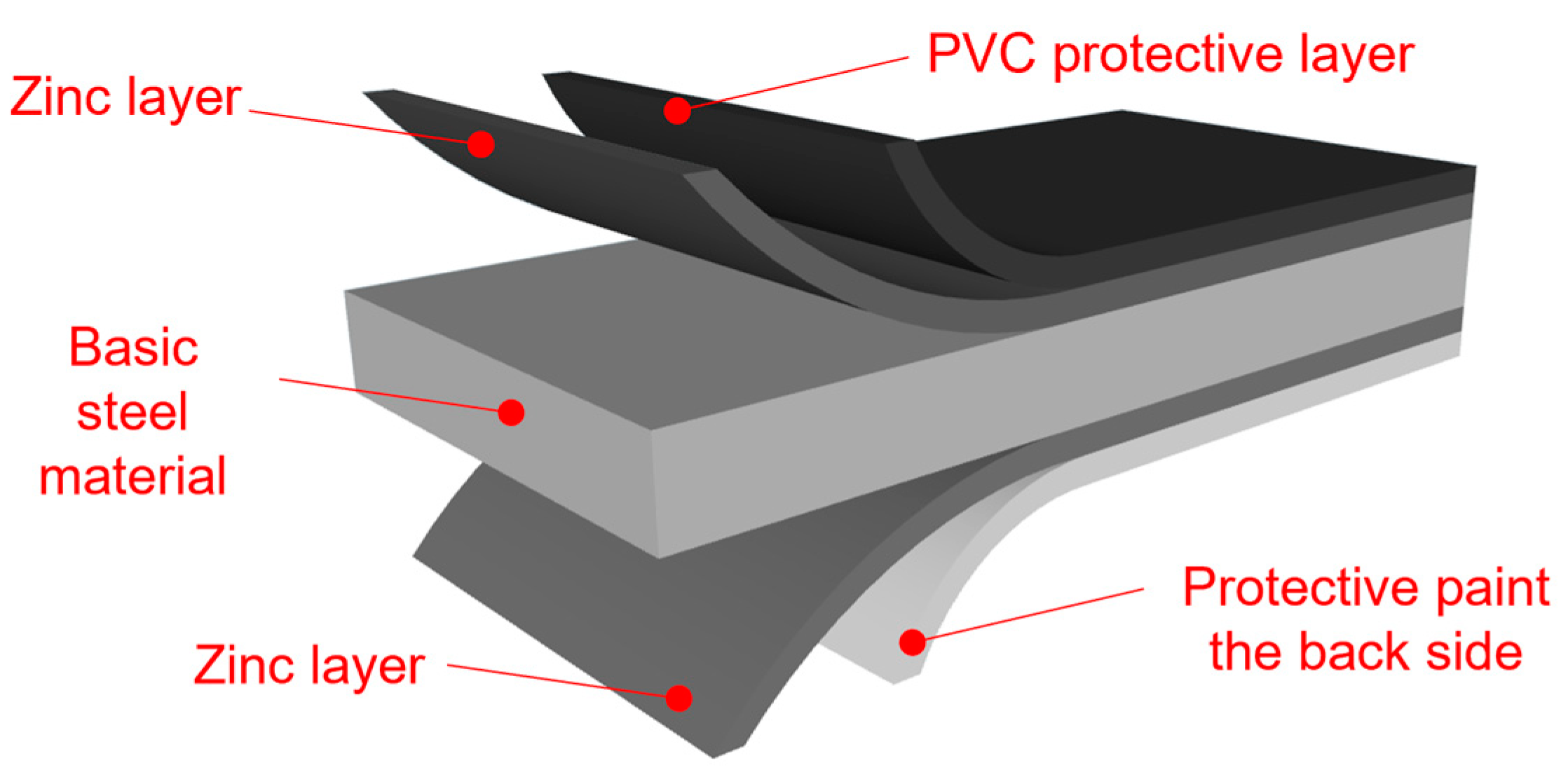
- Conversion intermediate layer (passivation) without chromium content (thus, Cr-free);
- Polyester-based primer with a thickness of 5 μm;
- Topcoat based on polyester with a thickness of 20 μm;
- Backcoat based on polyester with a thickness of 7 μm.
2.1. Experimental Methods
- Evaluation of chosen mechanical properties and evaluation of coating behaviour of tested plastic coated samples after tensile stress (where we evaluated surface changes, curling of the coatings after the tensile test, and artificial ageing) at normal temperature and after ageing at temperatures of 100 °C and 120 °C;
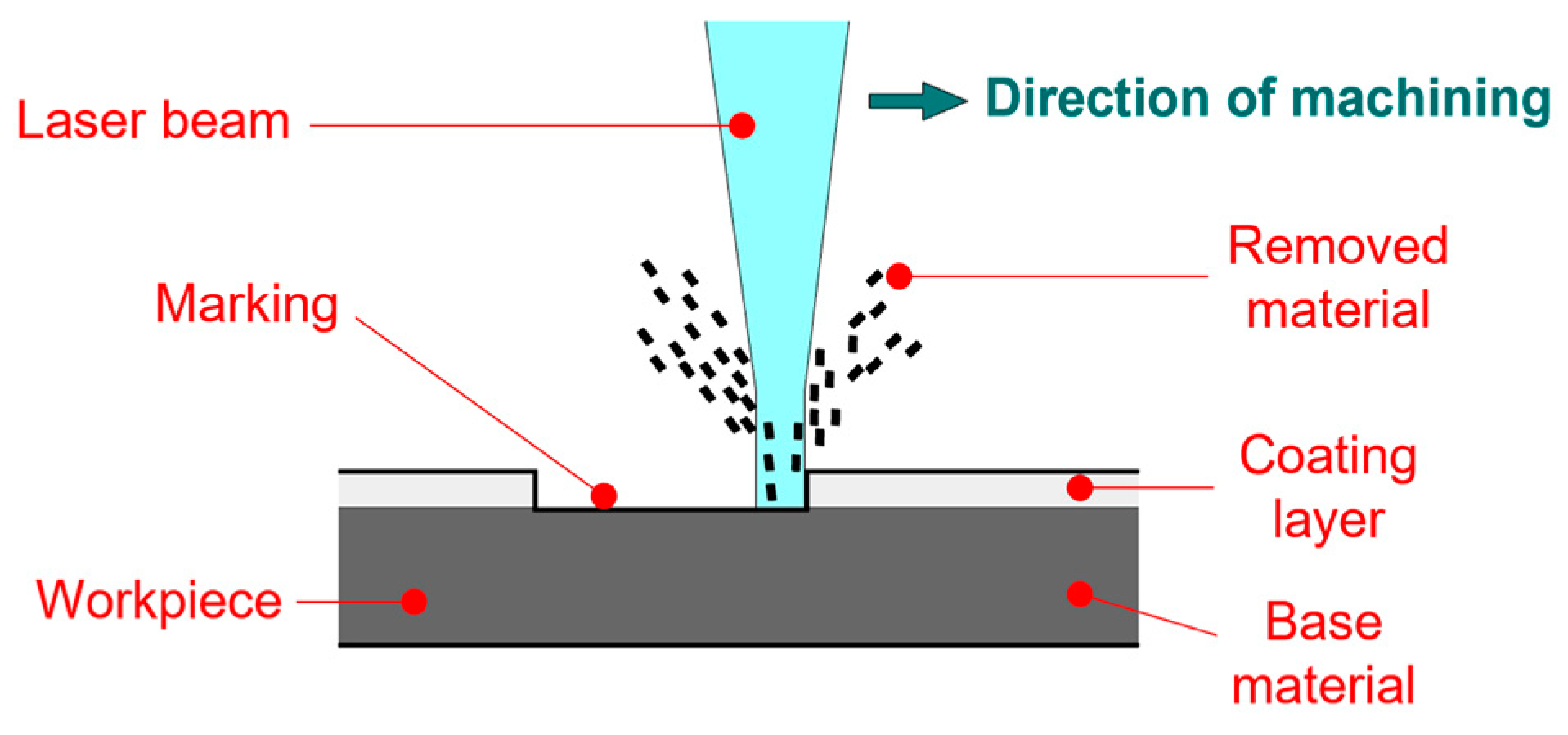
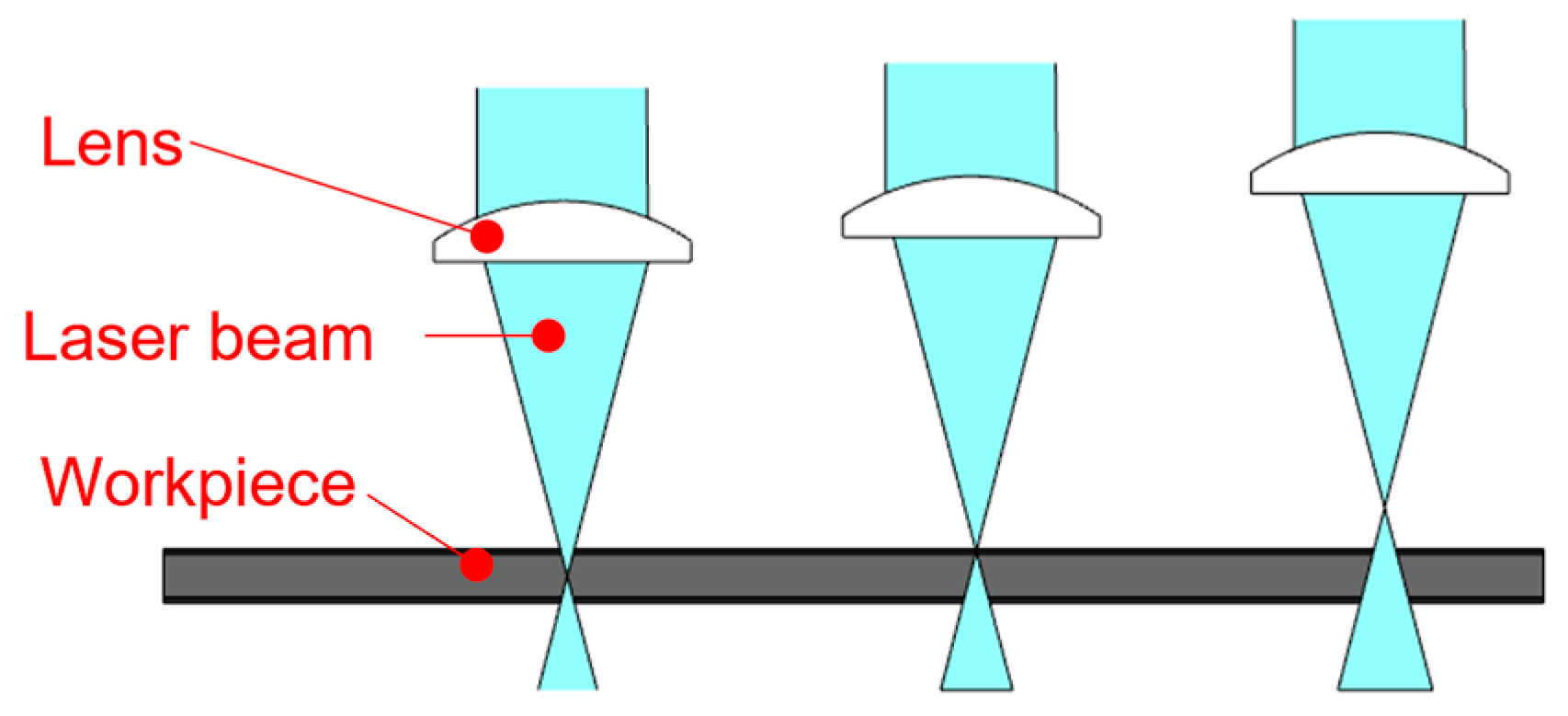
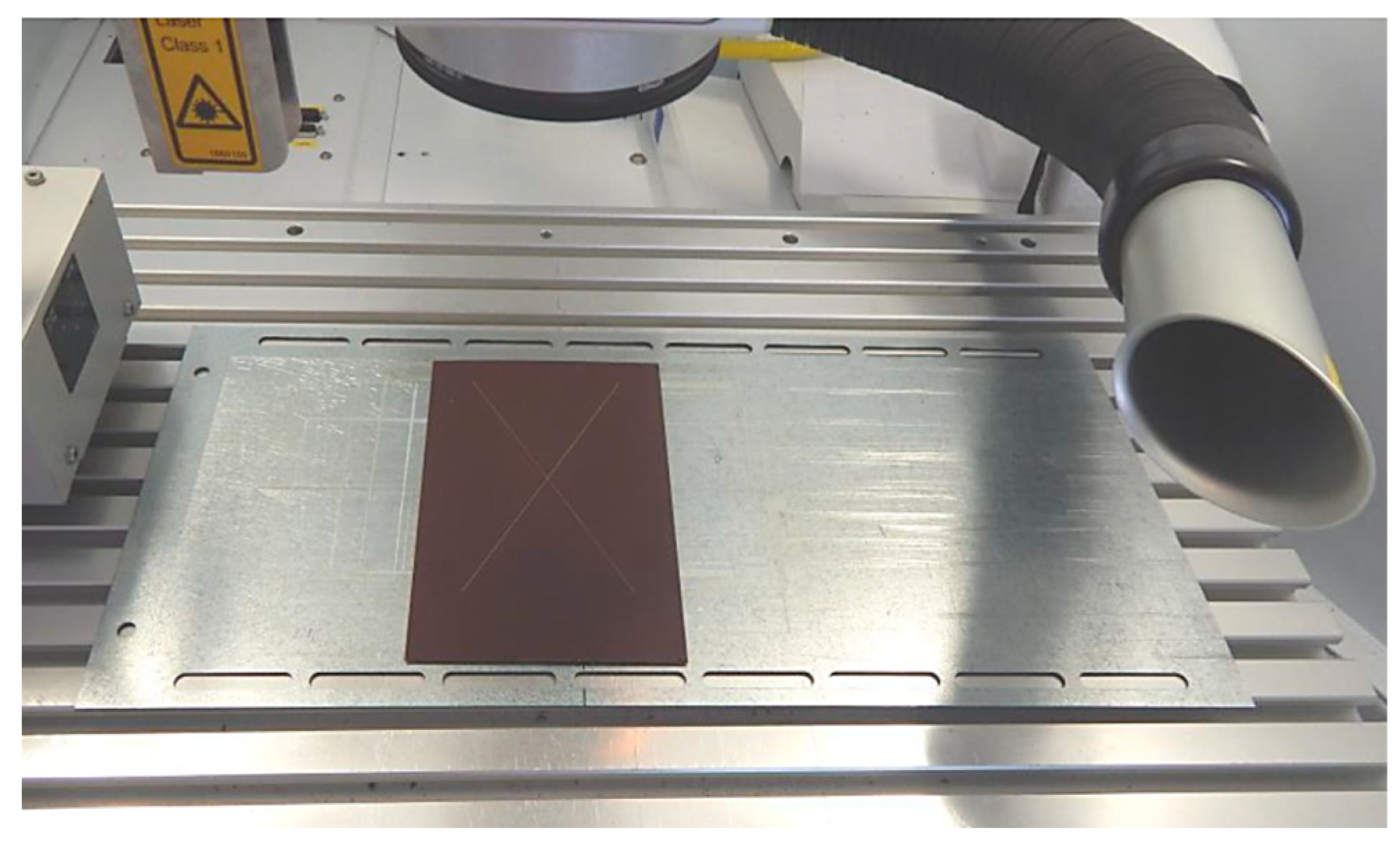
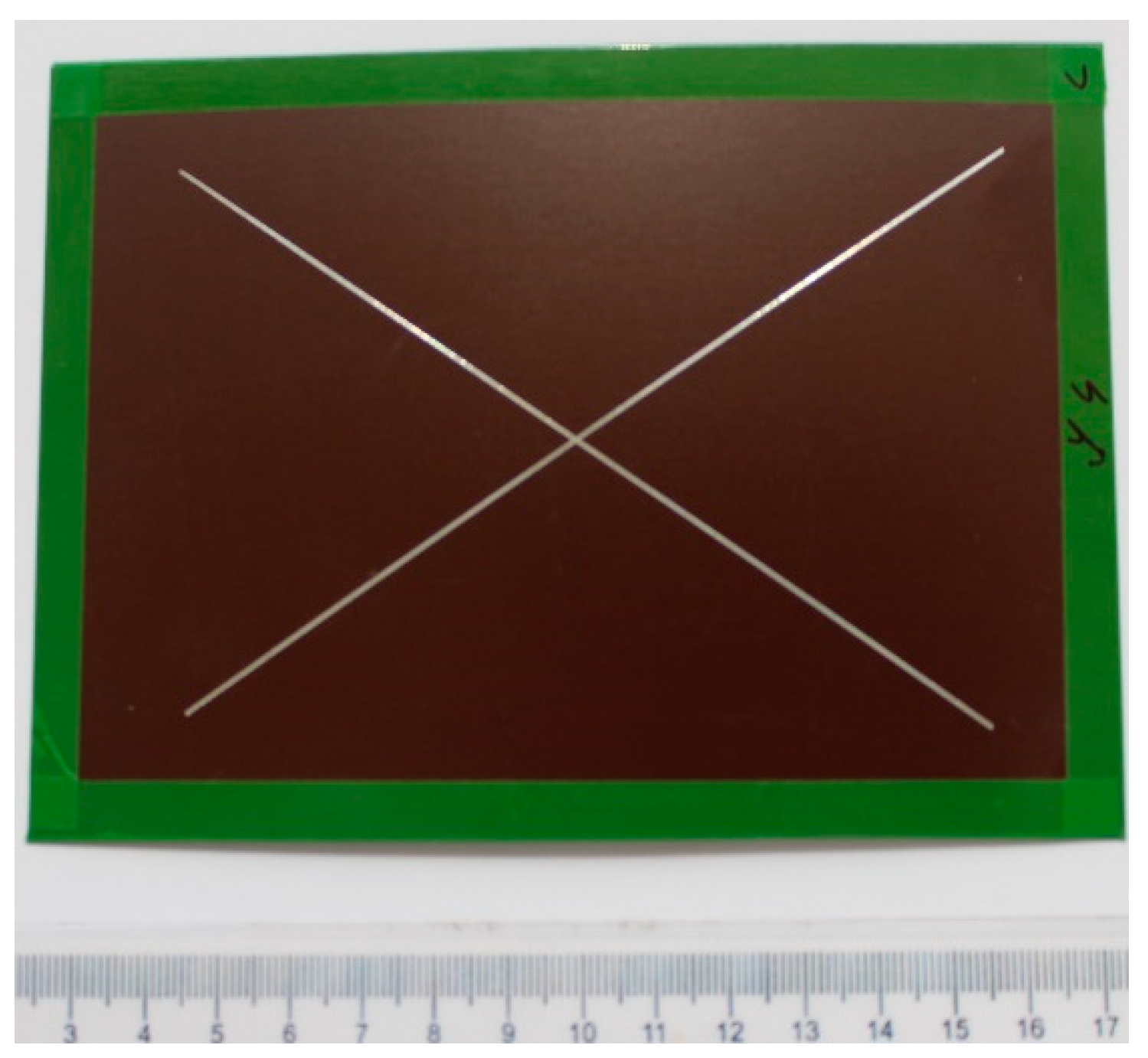
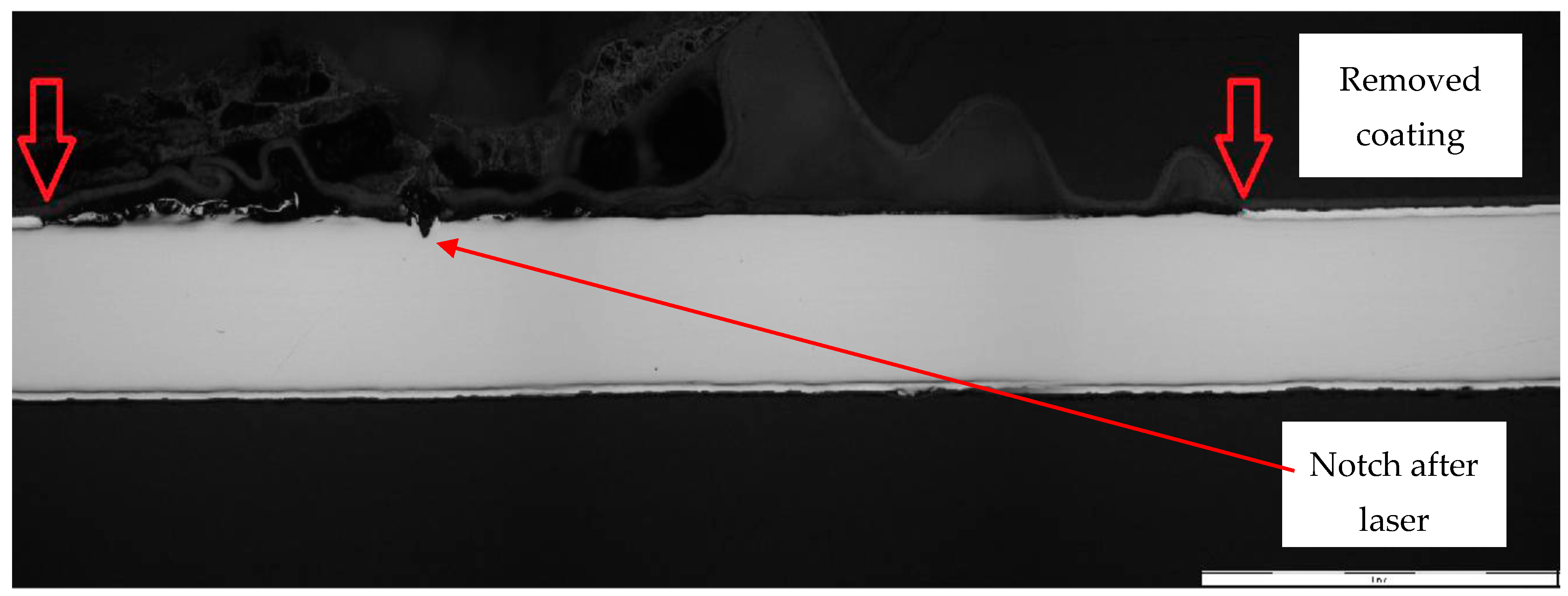
- Laser marking of the tested plastic-coated samples, creation of test cut, and evaluation of the plastic coating after laser marking (like burning of the coating and measurement of the distance of the affected zone and destroyed plastic layer). Mechanical test tools were used to create a test cut in practice; a laser beam was used to obtain a smooth test cut through the entire coating system up to the applied material;
- Evaluation of the tested samples after laser marking (plastic coating sheet samples after laser marking) by metallography and after the salt corrosion test.
2.1.1. Preparation of the Artificial Ageing of Tested Plastic-Coated Samples
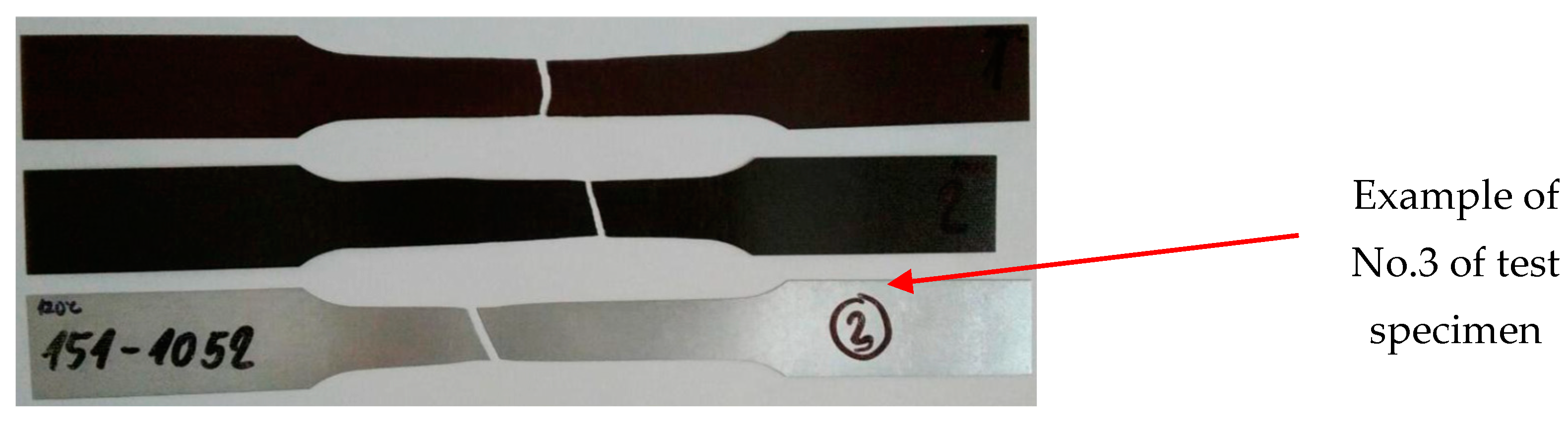
2.1.2. Static Tensile Testing
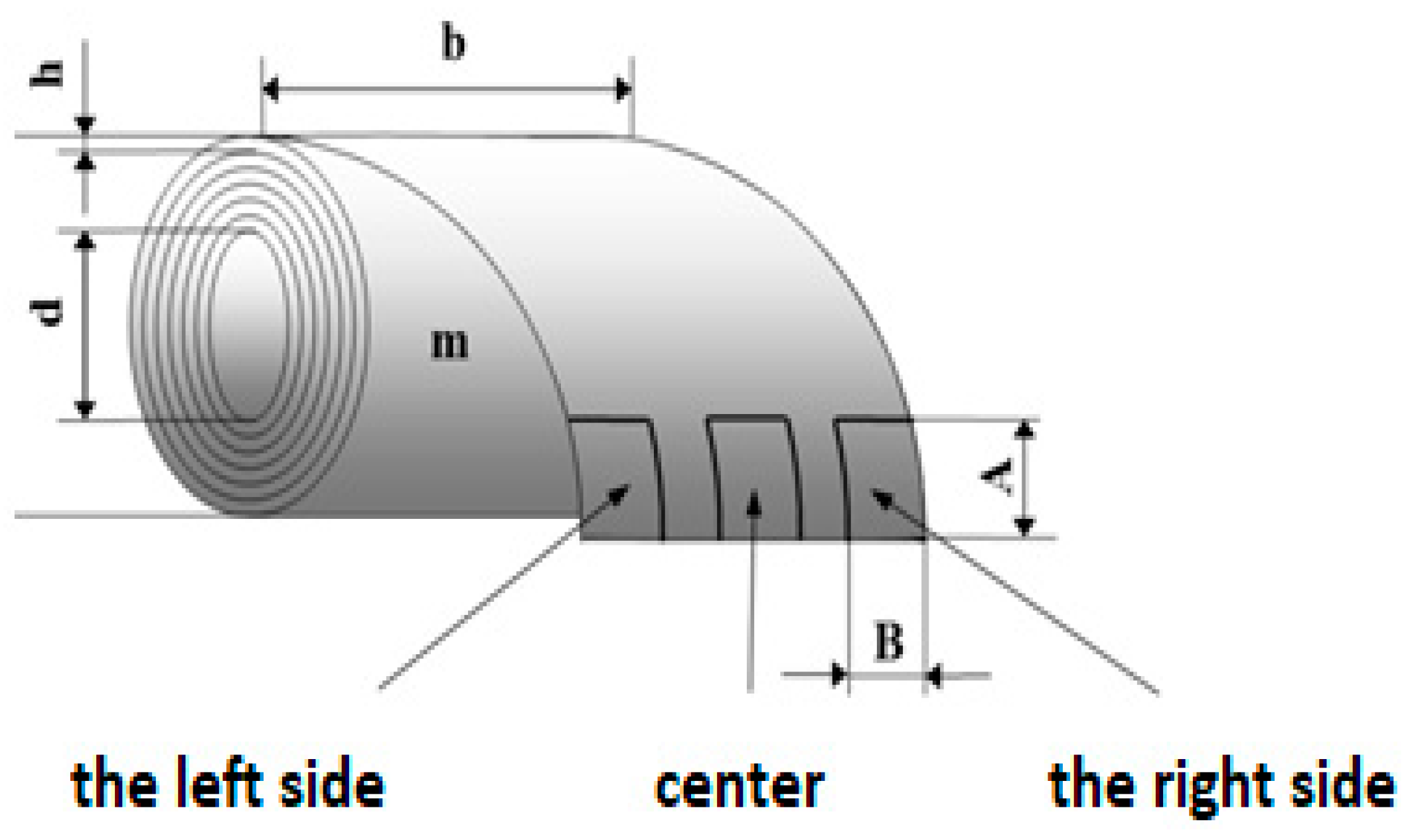
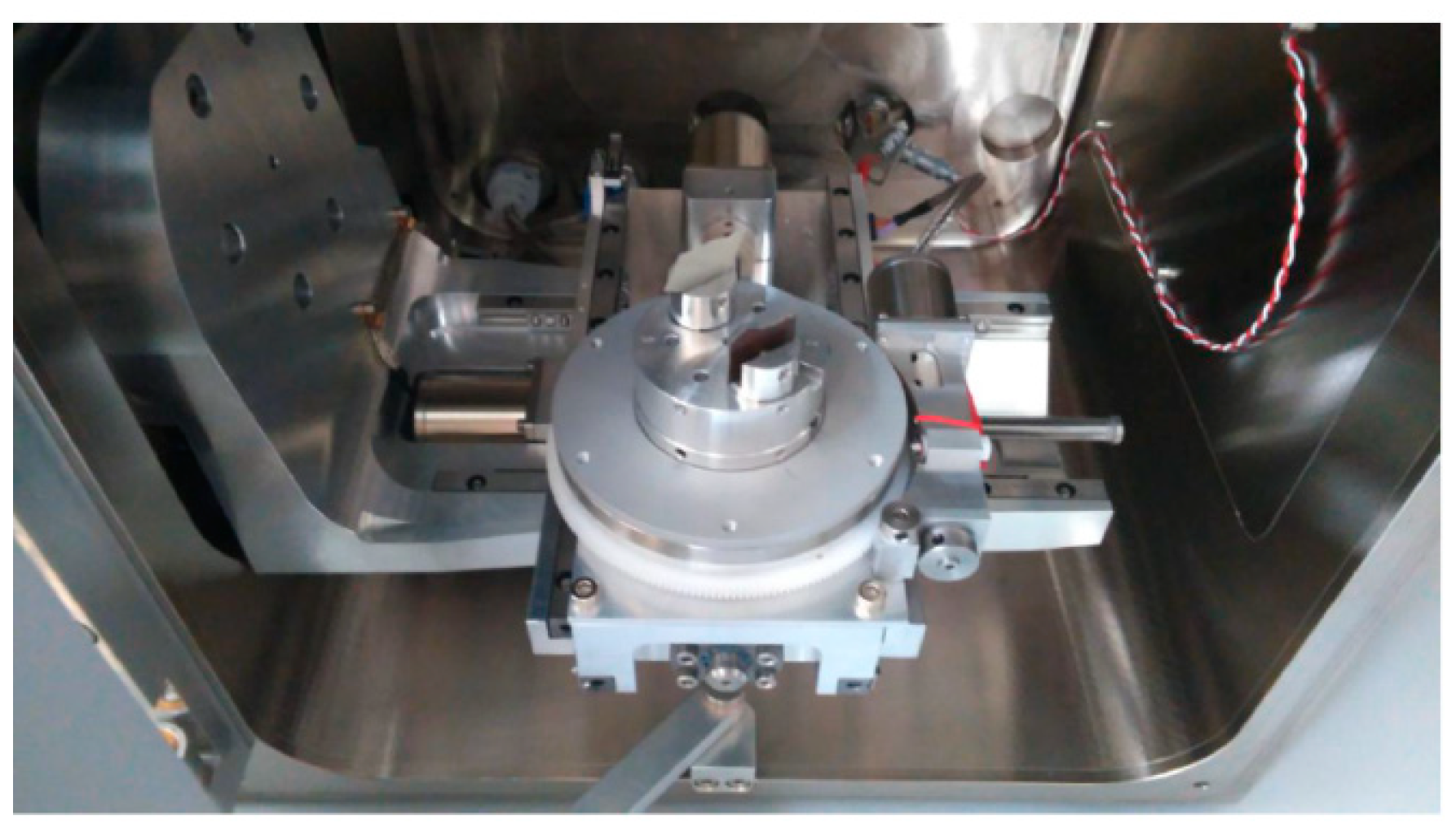
2.1.3. Preparation of the Tested Samples for the Laser Cut and for the Salt Corrosion Test
3. Results and Discussion
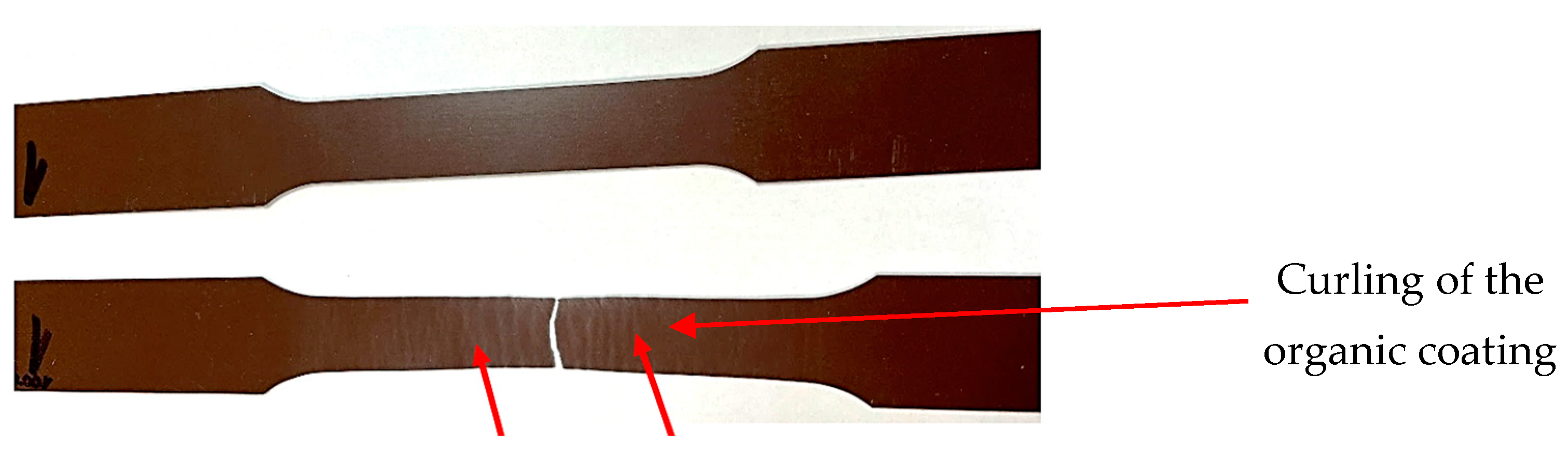
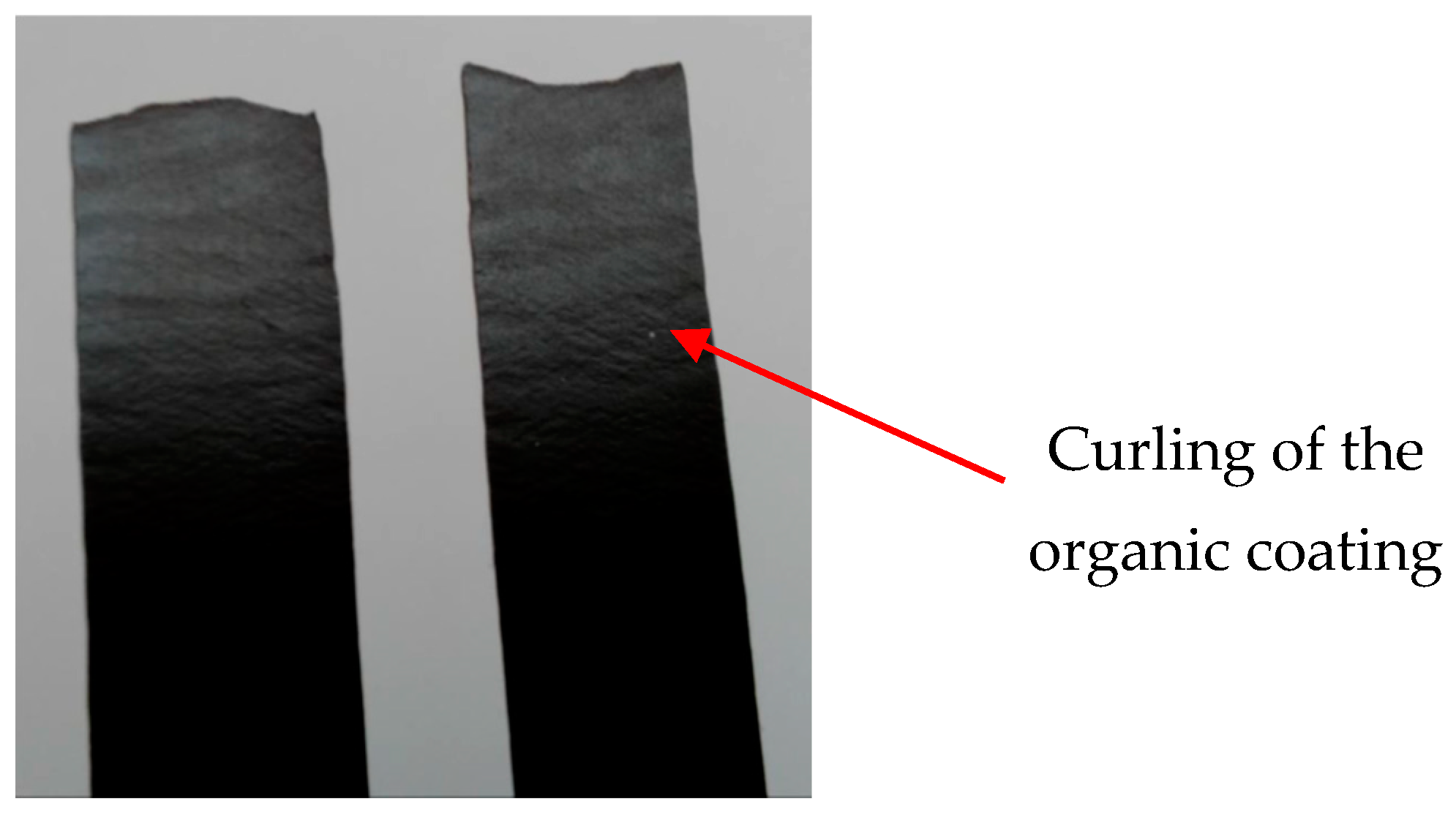
3.1. Visual Assessment of Samples after Artificial Ageing
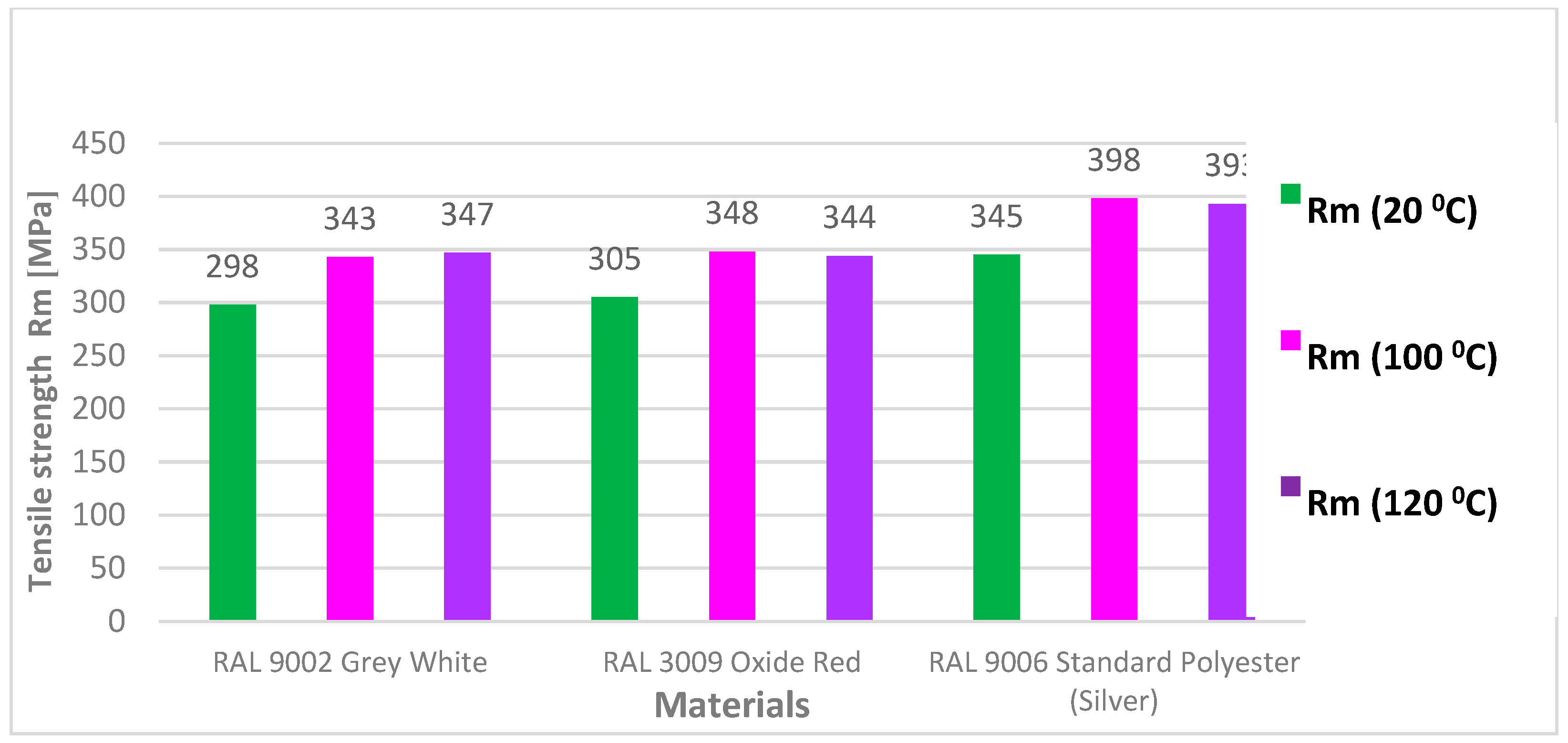
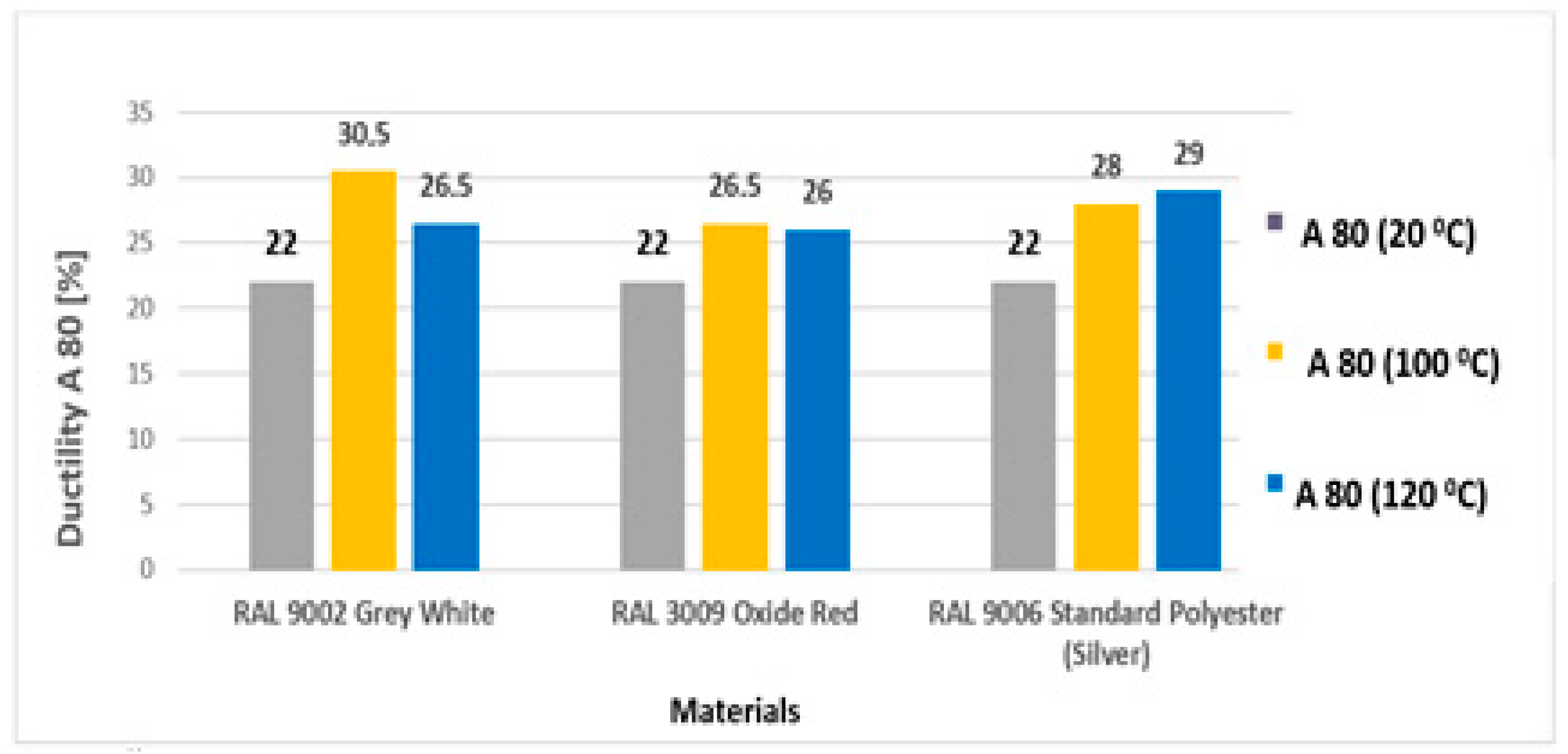
3.2. Tensile Test According to STN EN 10002-1
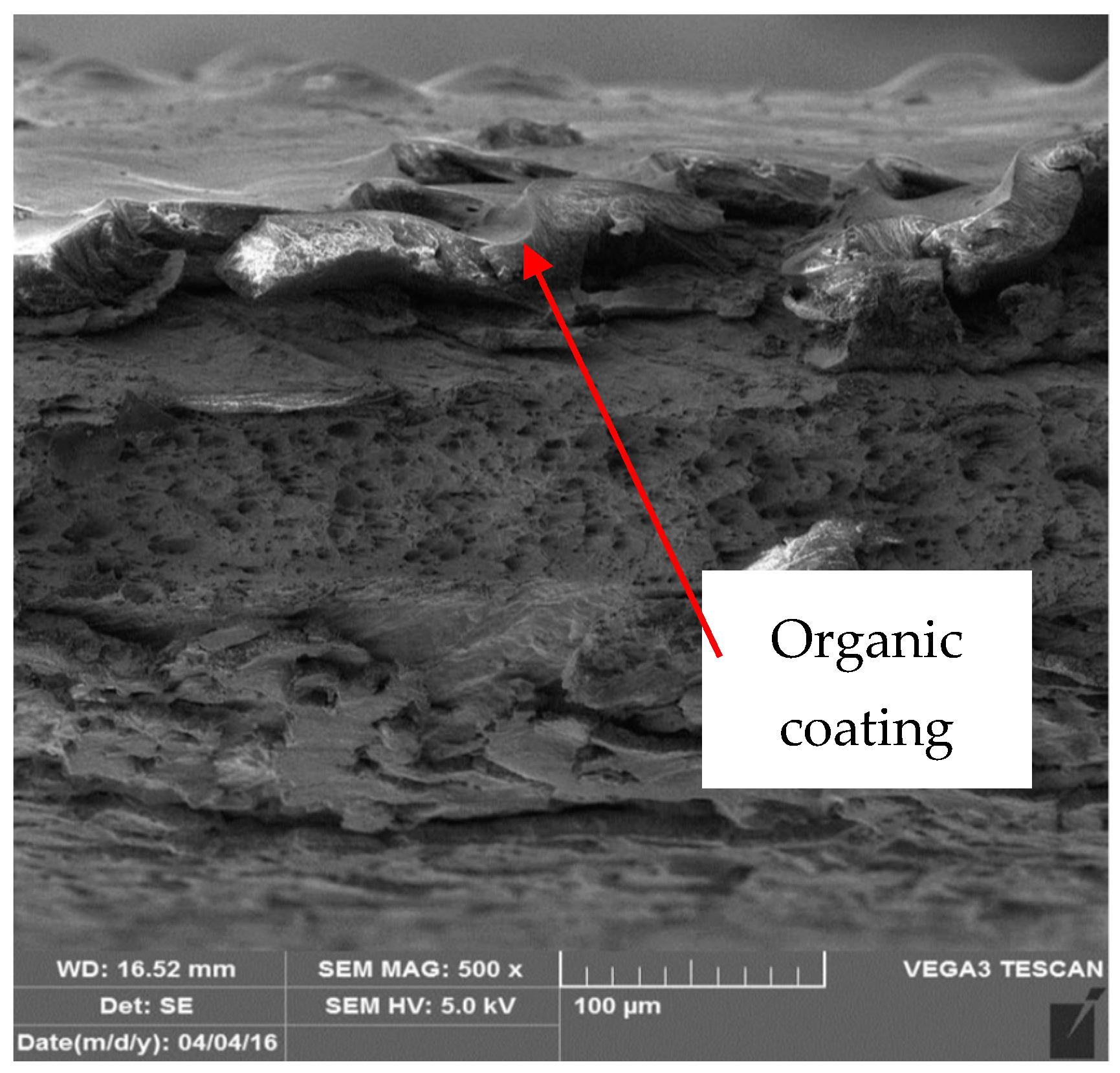
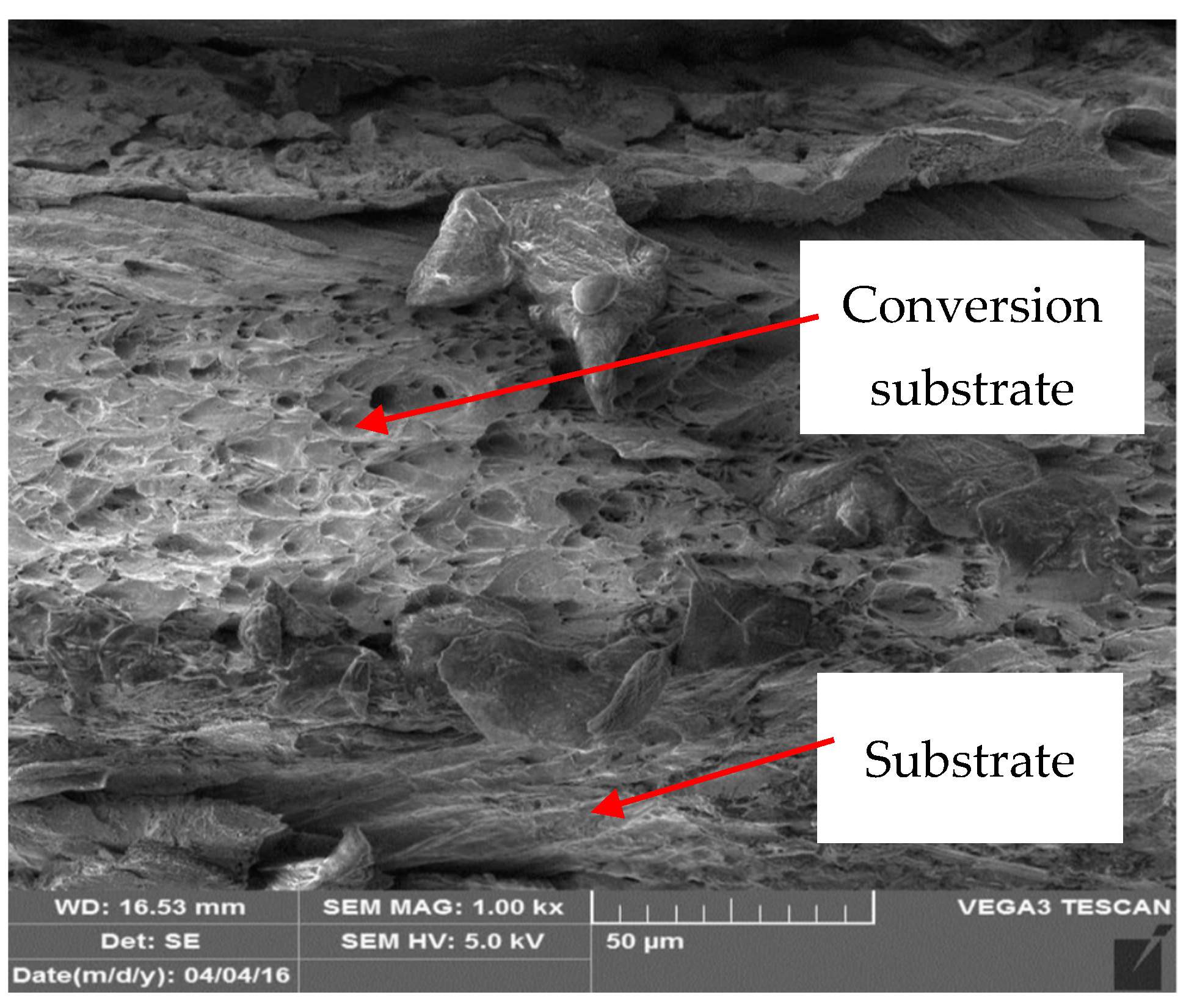
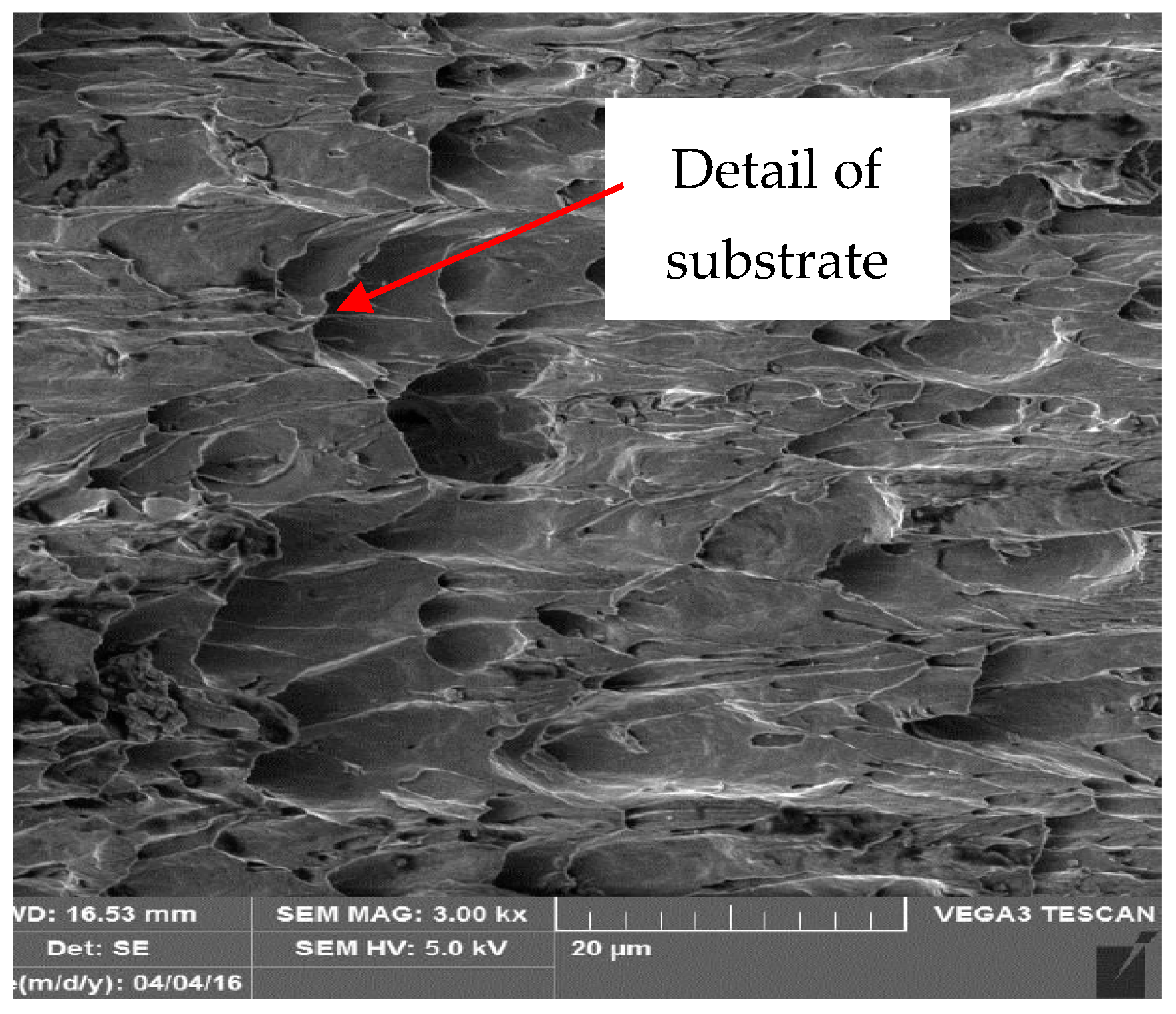
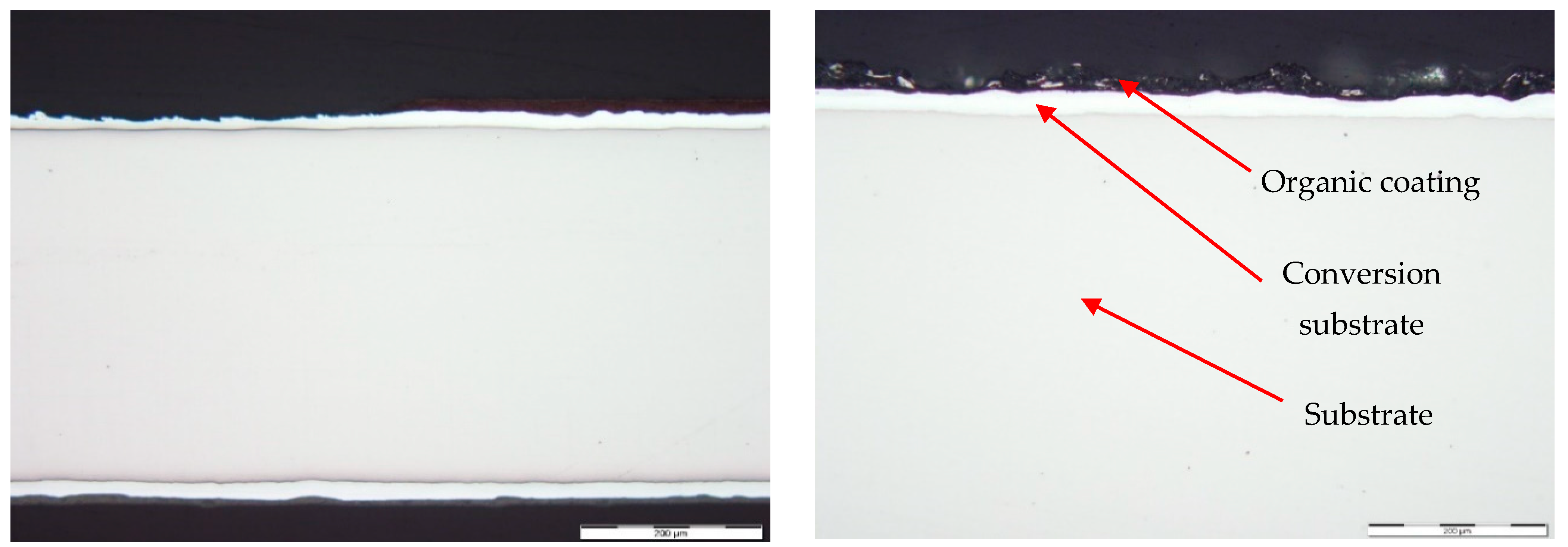
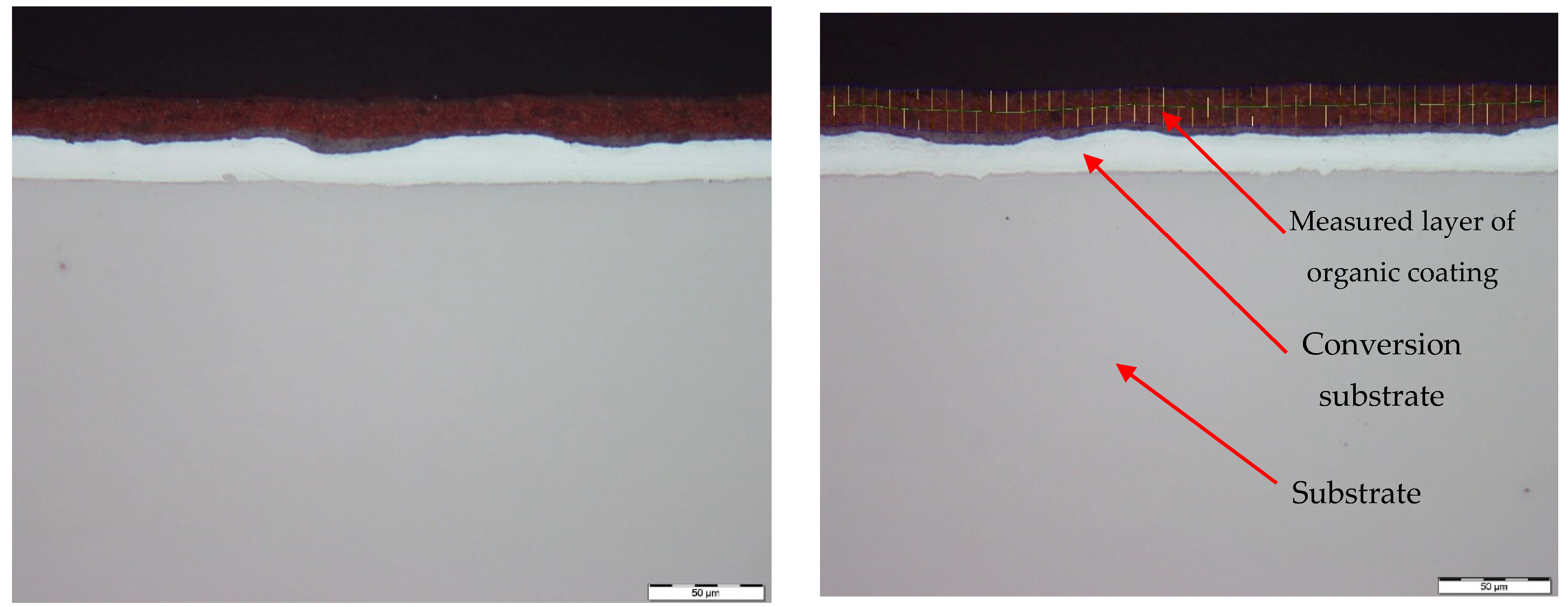
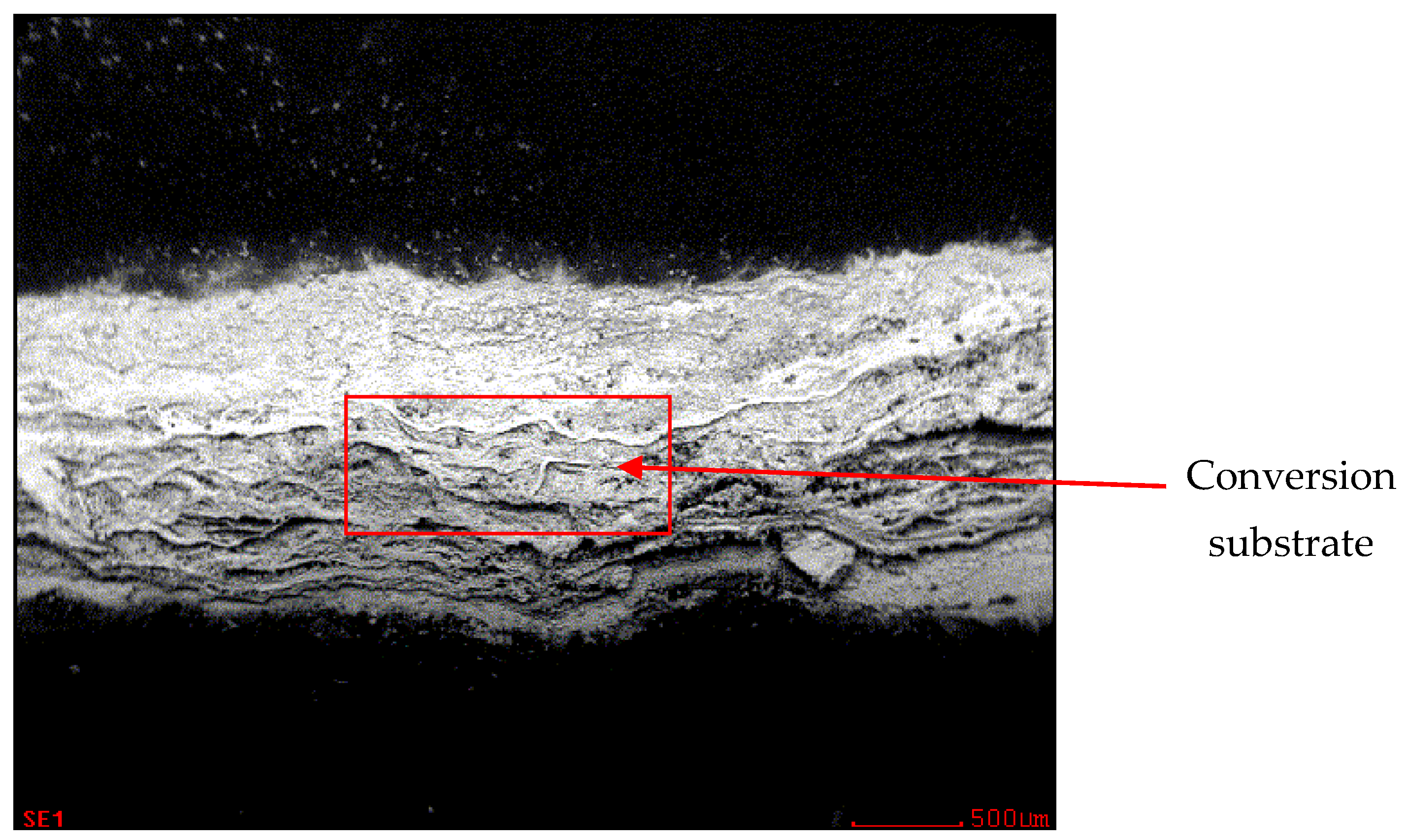
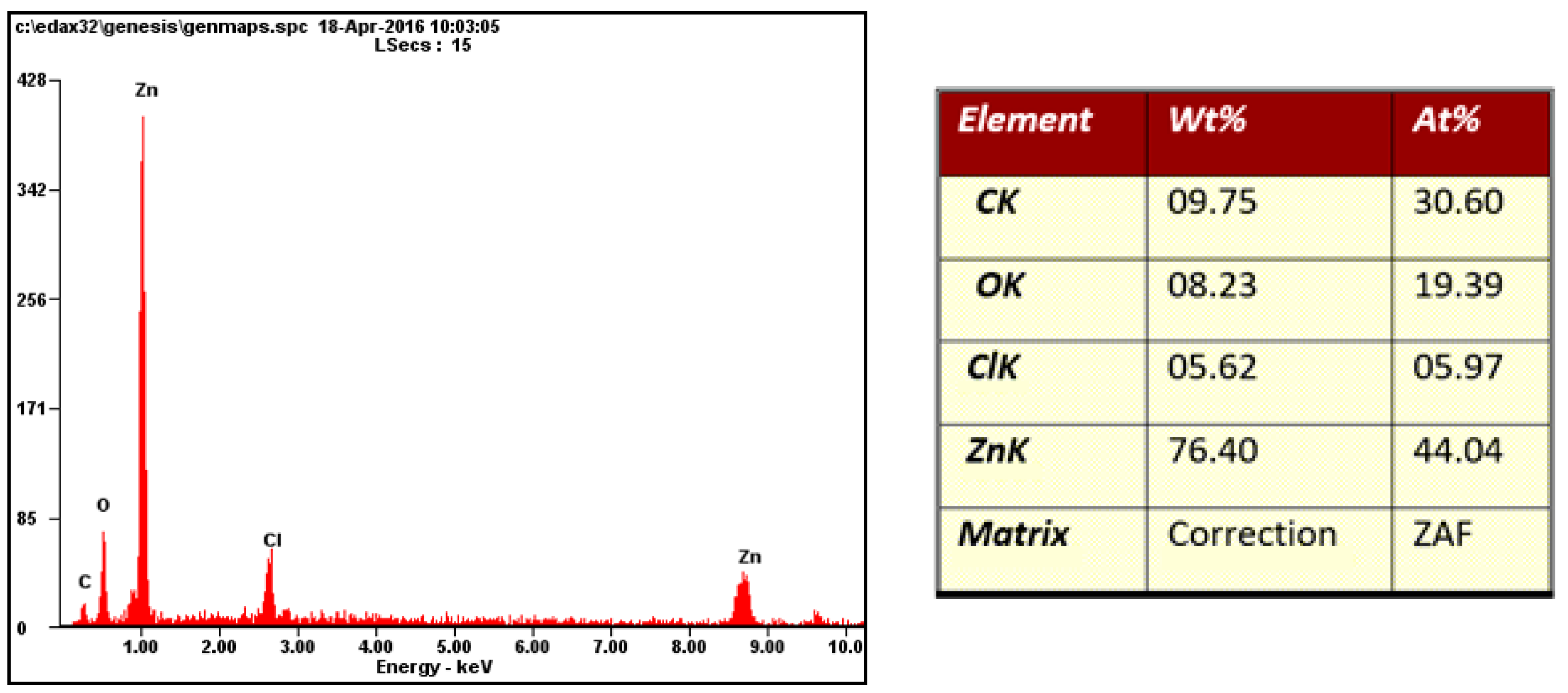
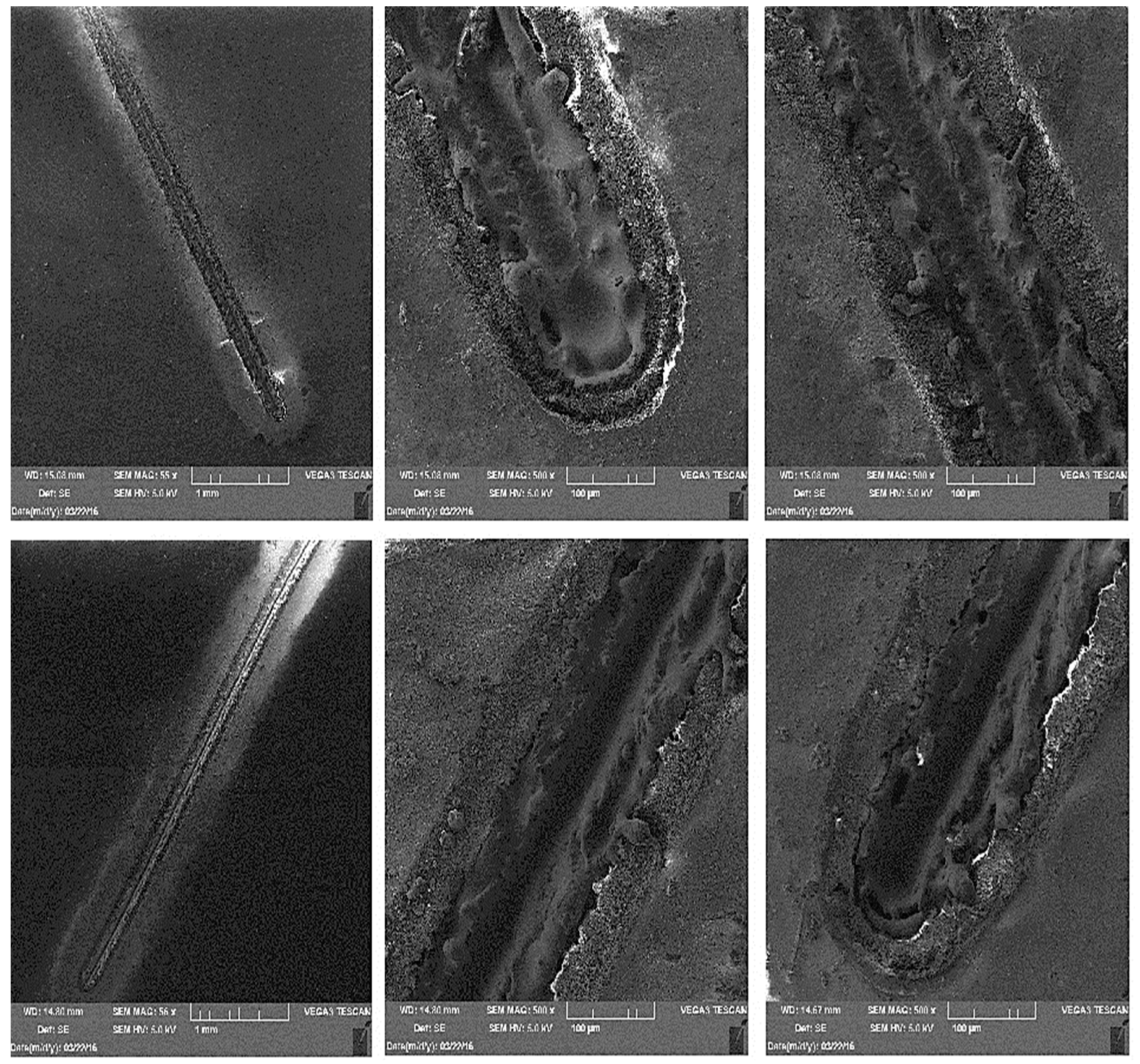
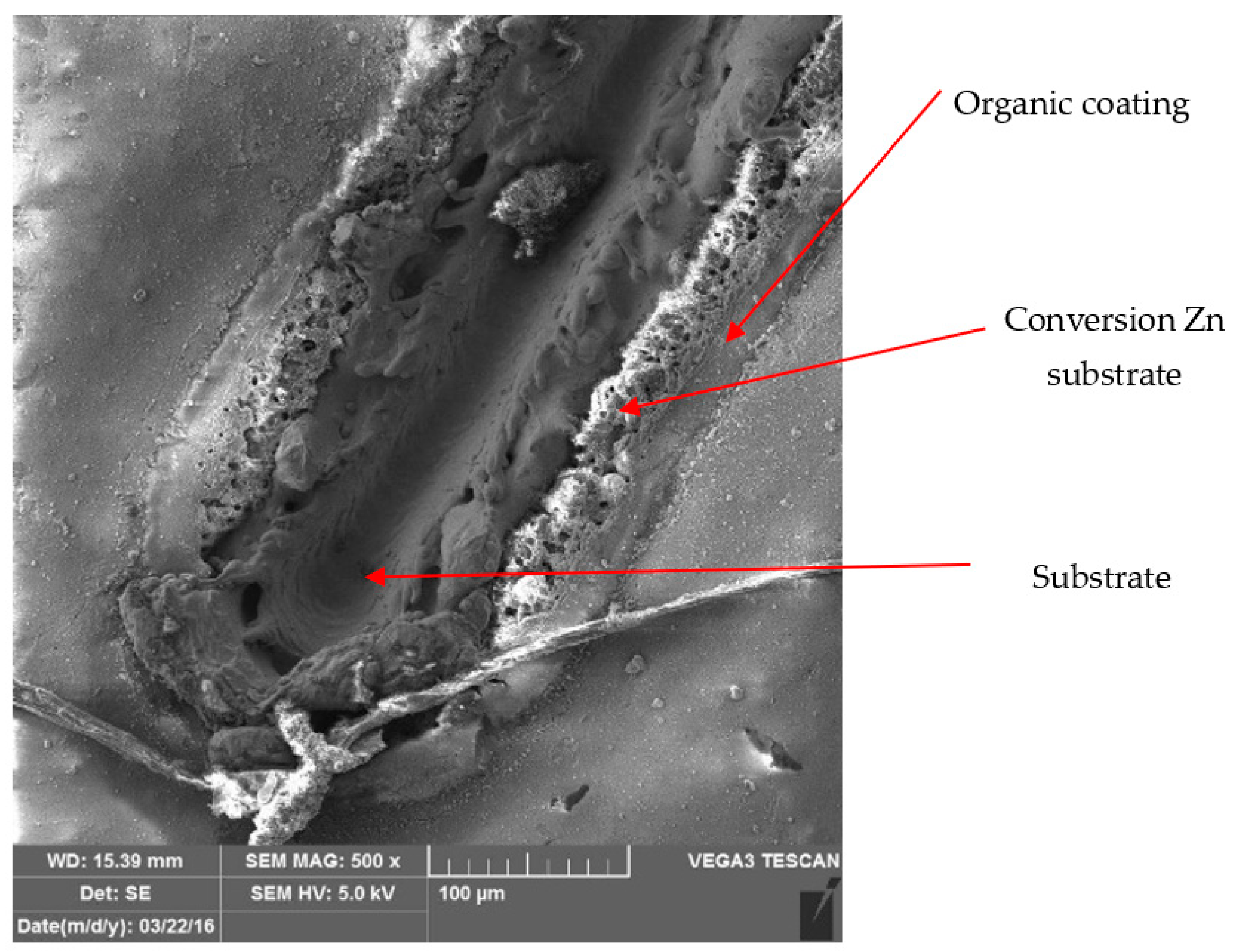
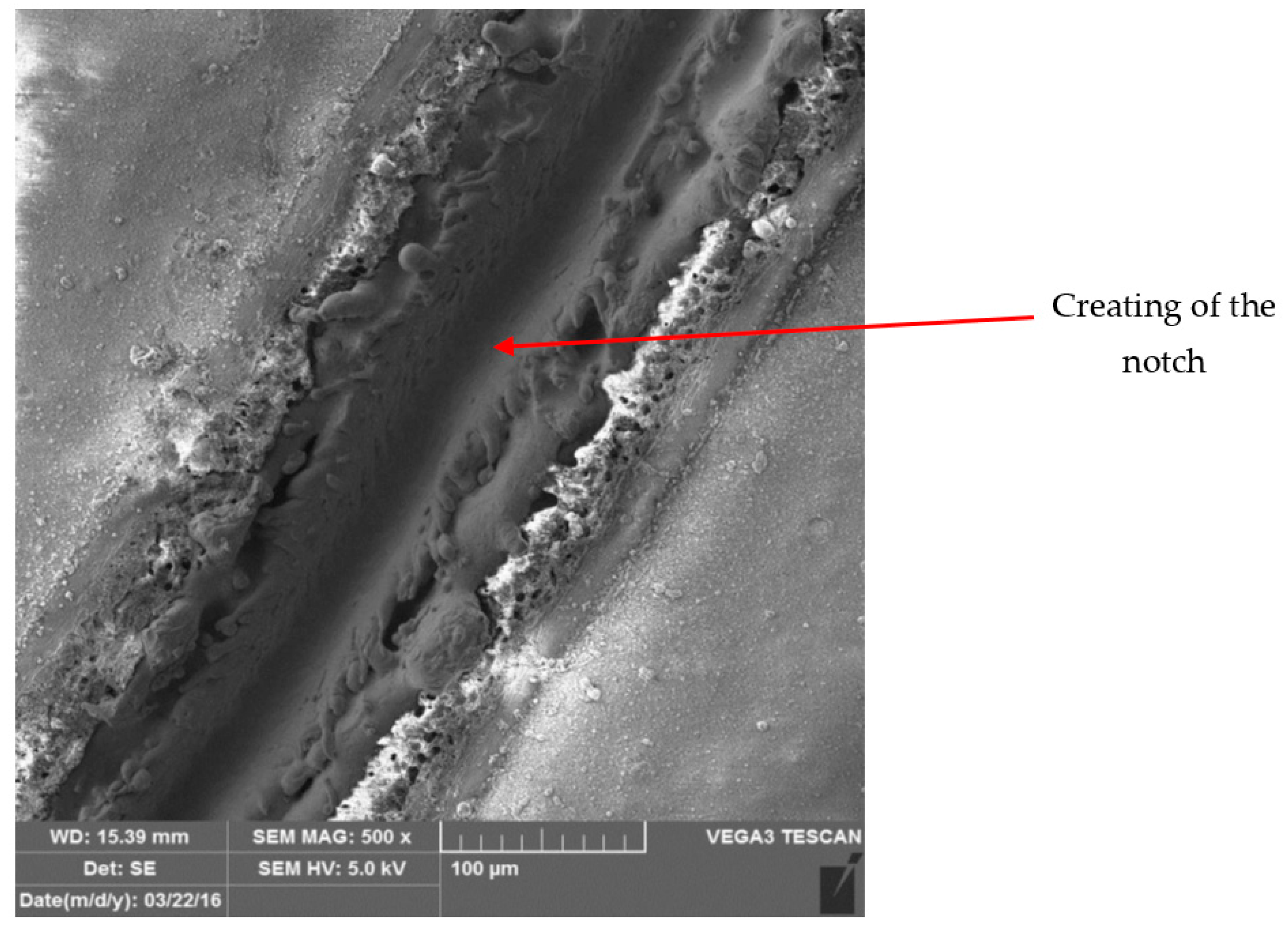
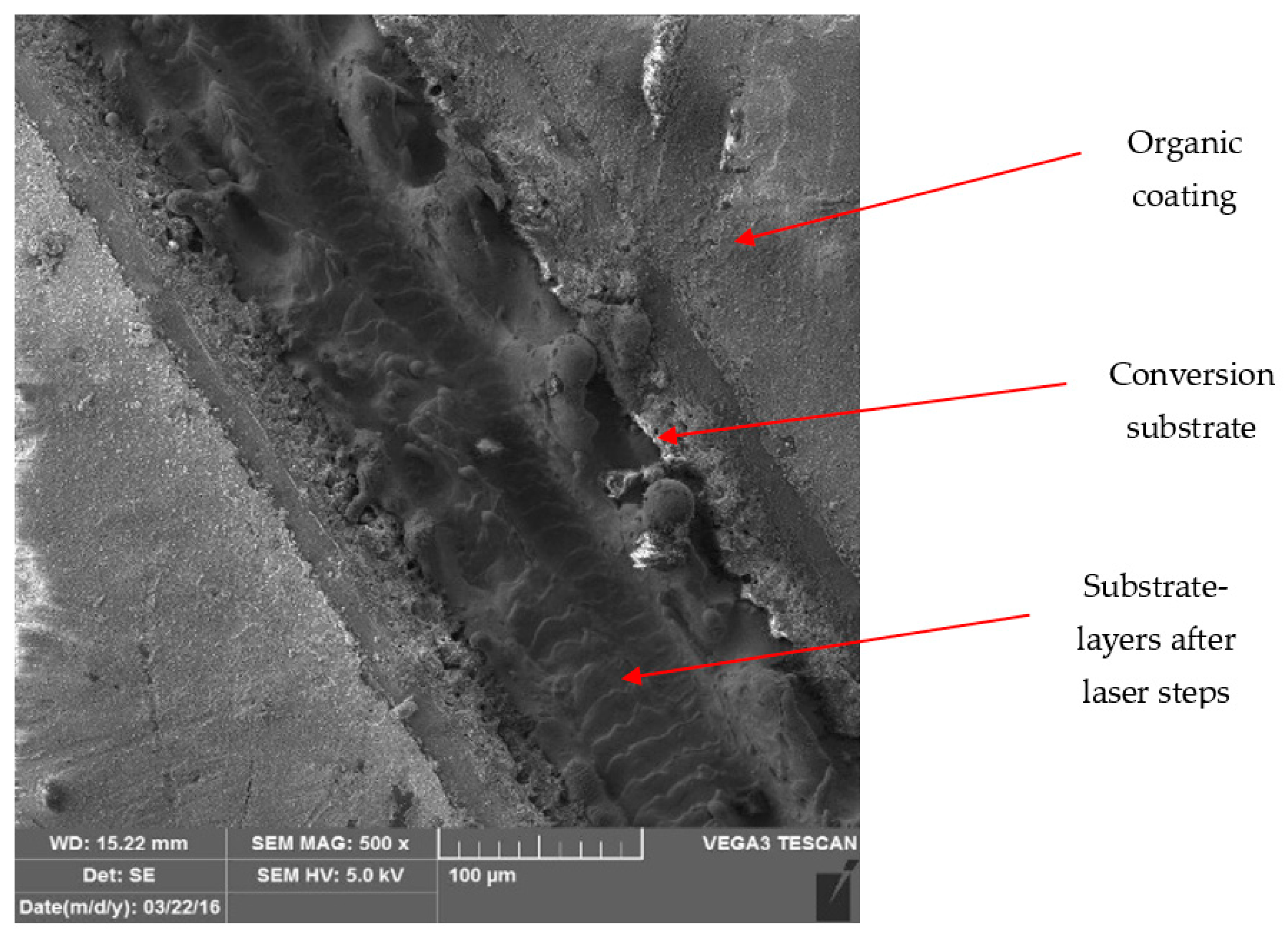
3.3. Metallographic and Microscopic Evaluation of Test Samples
- Up to a depth of 81.3 μm from the top surface of the sheet metal—from the primary paint to the steel;
- Up to a depth of 56.2 μm from the conversion intermediate layer to the steel;
- Reaching a depth of 40.0 μm into the steel.
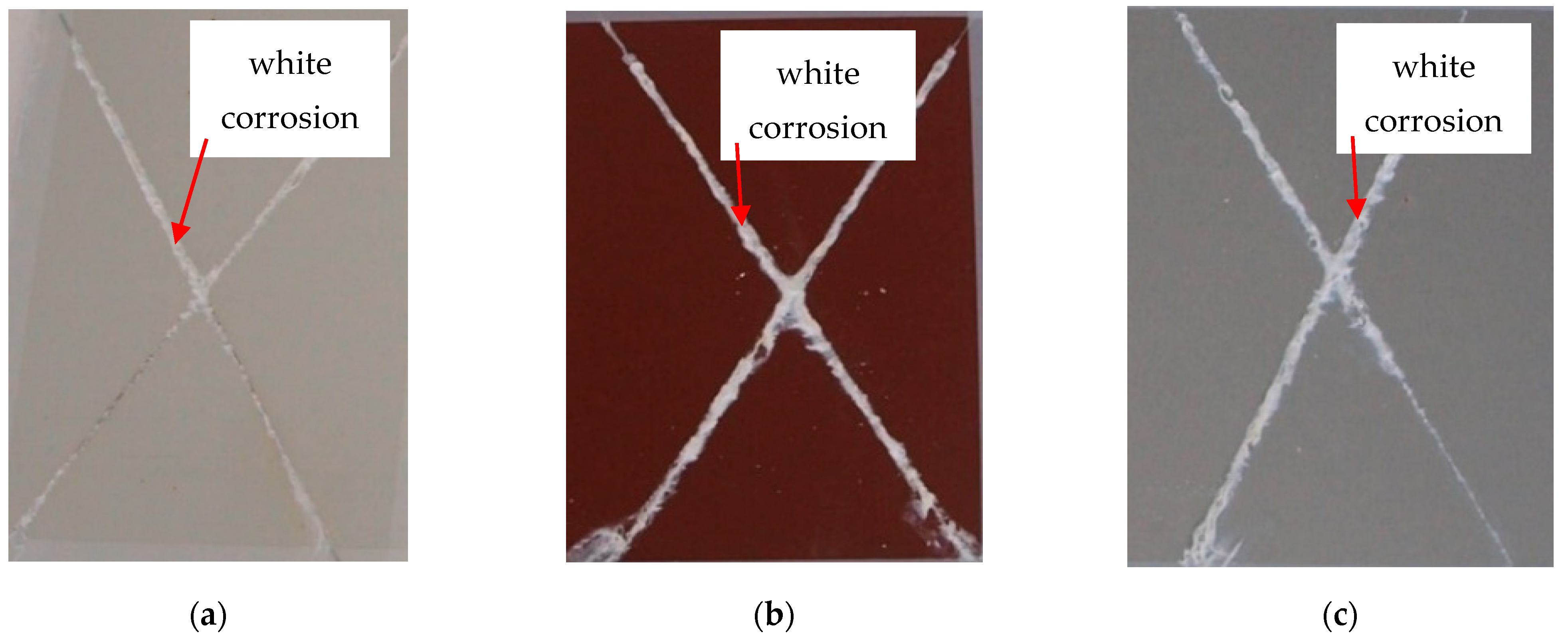
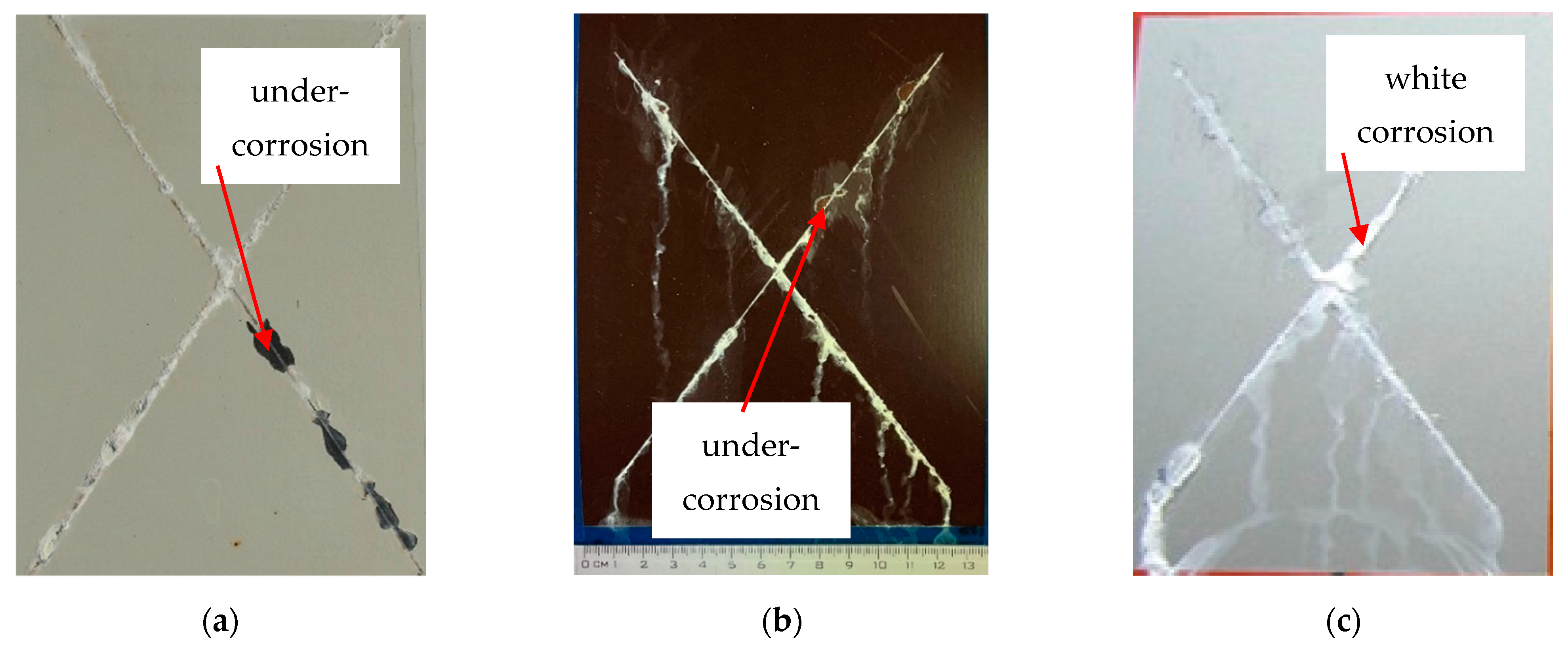
3.4. Evaluation of the Corrosion Test
- (a)
- Exposure after 240 h;
- (b)
- Exposure after 360 h;
- In the case of silver test samples, the volume of the corrosion products of zinc increased, but there was no corrosion of the base material. They were classified into group 2;
- In the case of brown test samples, there was peeling of the coating in the vicinity of the test section and the corrosion of the base material was recorded. The brown test samples were classified into group 3;
- In the case of white test samples, there was peeling of the coating around the test cut, but no corrosion of the base material was recorded. The white test samples were classified into group 3.
4. Conclusions
- After artificial ageing in laboratory conditions, no damage was visible on the surface of the organic coated samples and the colour of the surface of the samples remained unchanged. After the uniaxial tensile test, the Re and Rm values of the ageing samples increased as it was suggested. Due to the influence of testing, the mechanical properties of the test samples did not change significantly; the difference was in the range of 40–50 MPa. By visual evaluation of the tensile test samples, the degradation of the coatings was observed, which was manifested by the curling of the coating after tearing the sample;
- The corrosion resistance of the evaluated coating systems made of organic coating materials and a Zn layer was sufficient after the corrosion tests; cathodic protection was also confirmed in the place of the artificially created test section. The corrosion resistance of the coatings in the place of the artificially created test cut after 240 h was evaluated in a salt mist environment. After 240 h, the formation of corrosion products—white corrosion of the Zn layer—on the base material was observed. After 360 h, corrosion of the underlying base material also appeared in the RAL 8017 Oxide Red (brown) samples. The cathode protection on the test samples was sufficient for the colours RAL 9002 Grey–White and RAL 9006 Standard Polyester (Silver);
- For the creation of artificial cuts on the coating, it is possible to recommend the use of a laser; the penetration was up to the base material through the entire coating system.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, F.N.; Nichols, M.E.; Pappas, S.P. Organic Coatings: Science and Technology, 4th ed.; WILEY: Hoboken, NJ, USA, 2017; p. 512. [Google Scholar]
- Dplast Catalogue. Organic Coated Sheets (Lakoplastované Plechy). 2023. Available online: https://www.dplast.cz/index.php/en/en-products/plastic-coated-sheet-metals (accessed on 17 July 2024).
- Chemolak: Catalogue Anti Corrosion Protection. 2023. Available online: www.chemolak.sk (accessed on 17 July 2024).
- Mtcomax Catalogue: Organic Coating Line. 2023. Available online: https://www.mtcomax.cz/dokumenty/ (accessed on 17 July 2024).
- U.S. Steel Catalogue: Organic Coated Sheets. 2023. Available online: https://www.usske.sk/sk/produkty/ocel-s-organickymi-povlakmi/index (accessed on 17 July 2024).
- Výber Správneho Lakoplastovaného Výrobku (Selection of the Right Coated Product). 2015. Available online: https://docplayer.gr/44654561-Oktober-2015-technicky-list-vyber-spravneho-lakoplastovaneho-vyrobku-ecca-cee-narodna-skupina-slovensko.html (accessed on 17 July 2024).
- Lamina Catalogue: Coated Sheets. 2023. Available online: https://www.lamina.sk/plechy/tabulove-zvitkove-plechy/lakoplastovane-plechy/ (accessed on 17 July 2024).
- ECCA—European Coil Coating Association. 2024. Available online: https://prepaintedmetal.eu (accessed on 17 July 2024).
- Russell, L.; Steven, F.M.; Myneni, S.C.B. Mapping organic coatings on atmospheric particles. Geophys. Res. Lett. 2002, 29, 26-1–26-4. [Google Scholar] [CrossRef]
- Batenburg, A.M. Effects of organic coatings on the reactivity of aerosol particles. Atmos. Aerosols 2013, 1–3. Available online: https://www.academia.edu/8746647/effects_of_organic_coatings_on_the_reactivity_of_aerosol_particles (accessed on 17 July 2024).
- Clifford, K. Schoff: Organic coatings: The paradoxical materials. Prog. Org. Coat. 2005, 52, 21–27. [Google Scholar]
- Deflorian, F.; Fedrizzi, L.; Rossi, S.; F Buratti, P.; Bonora, L. Electrochemical characterisation of organic coatings for the automotive industry. Prog. Org. Coat. 2000, 39, 9–13. [Google Scholar] [CrossRef]
- Muralidhar, Y.; Saha, J.K.; Ghosh, S.K. Surface, Chemical, and Mechanical Properties of Polyurethane-Coated Galvanized Steel Sheets. J. Mater. Eng. Perform. 2023, 1059–9495. [Google Scholar]
- Przystupa, K. Quality Evaluation of Selected Organic Coatings Used on Roofing Sheets. Materials 2022, 15, 1310. [Google Scholar] [CrossRef] [PubMed]
- Cervová, J.; Hagarová, M. Vplyv inhibítorov korózie na účinnpsť ochrany kovových materiálov. Koroze A Ochr. Mater. 2013, 57, 56–60. [Google Scholar]
- Kalendova, A.; Veselý, D.; Kohl, M.; Stejskal, J. Anticorrosion efficiency of zinc-filled epoxy coatings containing conducting polymers and pigments. Prog. Org. Coat. 2015, 78, 1–20. [Google Scholar] [CrossRef]
- Kalendova, A. Metody Testovani Vlastnosti Organickych Povlaku, Part I. Korozne a Inhibicni Ucinnost Organickych Povlaku (Methods of Testing Properties of Organic Coatings Part I. Corrosion Inhibition Efficiency of Organic Coatings); UP: Pardubice, Czech Republic, 2001; p. 248. [Google Scholar]
- Sevcikova, J.; Hagarova, M. Povrchové Inžinierstvo (Surface Engineering); Kosice, Hutnicka fakulta, TU in Kosice: Kosice, Slovakia, 2007; p. 110. [Google Scholar]
- Brezinová, J.; Liptáková, T.; Guzanová, A.; Draganovská, D.; Kottfer, D. Korózia Materiálov a Špeciálne Technológie Povrchových Úprav. (Material Corrosion and Special Surface Finishing Technologies), 1st ed.; TU in Kosice, SjF: Kosice, Slovakia, 2020; p. 308 s. [Google Scholar]
- Bajat, J.B.; Mišković-Stanković, V.B.; Popić, J.P.; Dražić, D.M. Adhesion characteristics and corrosion stability of epoxy coatings electrodeposited on phosphated hot-dip galvanized steel. Prog. Org. Coat. 2008, 63, 201–208. [Google Scholar] [CrossRef]
- Bhadu, M.K.; Guin, A.K.; Singh, V.; Choudhary, S.K. Corrosion study of power-coated galvanised steel. ISRN Corros. 2013, 1, 1–9. [Google Scholar]
- Bajat, J.B.; Popić, J.; Mišković-Stanković, V. The influence of aluminium surface pretreatment on the corrosion stability and adhesion of powder polyester coating. Prog. Org. Coat. 2010, 4, 316–321. [Google Scholar] [CrossRef]
- Janosko, E.; Guzanová, A.; Szelag, P.; Draganovská, D.; Moro, R. Influence of conversion coatings on the resistance of adhesive joints to under corrosion. Koroze a Ochrana Materiálu 2021, 65, 70–78. [Google Scholar]
- Barroux, A.; Ducommun, N.; Nivet, E.; Laffont, L.; Blanc, C. Pitting corrosion of 17-4PH stainless steel manufactured by laser beam melting. Corros. Sci. 2020, 169, 108594. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 1–18. [Google Scholar] [CrossRef]
- Standard EN ISO 12 944; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems—Part 5: Protective Paint Systems. ISO: Geneva, Switzerland, 2018.
- Weikang, L.; Yitian, Z.; Grant, E.E.; Qiang, G.; Tianzhen, C.; Shuning, S.; Heitzmann, M.; Darren, M.; Grøndahl, L.; Mingyuan, L.; et al. Mechanical properties and scratch recovery of nanoclay/polyester composite coatings for pre-coated metal (PCM) sheets. Compos. Part B Eng. 2024, 273, 111217. [Google Scholar]
- Sobotova, L.; Badida, M.; Wessely, E. Research of laser cleaning of materials and environmental requirements. In Proceedings of the DAAAM 2020: 31th DAAAM International Symposium on Intelligent Manufacturing and Automation, Mostar, Bosnia and Herzegovina, 21–24 October 2020; pp. 176–183. [Google Scholar]
- Sobotova, L.; Badida, M. Laser marking as environmental technology. Open Eng. 2017, 7, 303–316. [Google Scholar]
- Ciecinska, B.; Sobotova, L.; Gagala, I. Surface laser treatment as an innovative and ecological technology for adhesive bonding. In SGEM 2017; STEF92 Technology Ltd.: Sofia, Bulgaria, 2017; pp. 915–922. [Google Scholar]
- Duchac, A.; Kejzlar, P. Optimization of raster point deposition methodology for deformation analyses. Mater. Sci. Forum 2023, 1081, 155–160. [Google Scholar] [CrossRef]
- Leone, C.; Bassoli, E.; Genna, S.; Gatto, A. Experimental investigation and optimisation of laser direct part marking of Inconel 718. Opt. Lasers Eng. 2018, 111, 154–166. [Google Scholar]
- Dasaev, M.R.; Kalakuckaya, O.V.; Trushin, E.S.; Grigoriev, S.V.; Ryzhenkov, O.V. Effect of laser treatment on zinced steel surface wettability and corrosion resistance. J. Phys. Conf. Ser. 2022, 2388, 1–6. [Google Scholar] [CrossRef]
- Iakovlev, A.; Ruzankina, J.; Kascheev, S.; Vasilyev, O.; Parfenov, J.; Grishkanich, A. Laser anti-corrosion treatment of metal surfaces. In Proceedings of the High-Power Laser Materials Processing: Applications, Diagnostics, and Systems VI, San Francisco, CA, USA, 31 January–2 February 2017; Volume 10097, p. 100970S. [Google Scholar]
- Zhuang, S.; Kainuma, S.; Yang, M.; Haraguchi, M.; Asano, T. Surface Observation of Laser-Treated Carbon Steel and Application of Laser Cleaning for Corroded Steel Member; IOP Conf. Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1165, p. 012001. [Google Scholar]
- Madhukar, Y.K.; Mullick, S.; Shukla, D.K.; Kumar, S.; Nath, A.K. Effect of laser operating mode in paint removal with a fiber laser. Appl. Surf. Sci. 2013, 264, 892–901. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Lu, G.; Lia, J.; Zhang, Y.; Sokol, D.W. A metal marking method based on laser shock processing. Mater. Manuf. Process. 2019, 34, 598–603. [Google Scholar] [CrossRef]
- Seubert, C.; Nichols, M.; Henderson, K.; Mechtel, M.; Klimmasch, T.; Pohl, T. The effect of weathering and thermal treatment on the scratch recovery characteristics of clearcoats. J. Coat. Technol. Res. 2010, 7, 159–166. [Google Scholar] [CrossRef]
- Yankov, E.; Firov, M.; Nikolova, M. Changes in the structure during laser treatment of austenitic steel. In Proceedings of the 55th Science Conference of Ruse University, Ruse, Bulgaria, 28–29 October 2016; pp. 1–14. [Google Scholar]
- Kusimski, J.; Przybylowicz, J.; Woldan, S.K.A. Structure Properties Change in Case of Laser Remelting of Surface Layers and Coatings; Hutnik, 1999; pp. 14–20. [Google Scholar]
- Labisz, K. Thermal Fatigue Influence of Laser Treated Tool Steel Surface. Procedia Eng. 2014, 74, 429–442. [Google Scholar]
- Tarasova, T.V. Prospects of the use of laser radiation for raising the wear resistance of corrosion - resistant steels. Met. Sci. Heat Treat. 2010, 52, 284–288. [Google Scholar] [CrossRef]
- Steen, W.M. Laser material processing-an overview. J. Opt. A Pure Appl. Opt. 2003, 5, S3–S7. [Google Scholar]
- Bartkowska, A. Effect of laser modification on structure and selected properties of tool steel. J. Res. Appl. Agric. Eng. 2015, 60, 1–7. [Google Scholar]
- Laakso, P.; Pantsar, H.; Leinonen, H.; Helle, A. Preliminary study of corrosion and wear properties of laser color marked stainless steel. In Conference Proceedings ICALEO 2008; LIA: Orlando, FL, USA, 2008; pp. 212–221. [Google Scholar]
- Khalfallah, I.Y.; Rahoma, M.N.; Abboud, J.H.; Benyounis, K.Y. Microstructure and corrosion behavior of austenitic stainless steel treated with laser. J. Opt. Laser Technol. 2011, 43, 806–813. [Google Scholar] [CrossRef]
- EN STN 10346:2009; Continuously Hot-Dip Coated Steel Flat Products—Technical Delivery Conditions. ISO: Geneva, Switzerland, 2009.
- ASTM D 1654-92 (Reapproved 2000); Corrosion Attack Around the Cut. Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments. ISO: Geneva, Switzerland, 2000.
- ISO EN STN 1110:2019; Plastics—Polyamides—Accelerated Conditioning of Test Specimens. ISO: Geneva, Switzerland, 2019.
- ISO EN STN 558:1980(en); Conditioning and Testing—Standard Atmospheres—Definitions. ISO: Geneva, Switzerland, 1980.
- JIS G3302:2019; Hot-Dip Zinc-Coated Steel Sheet and Strip. Japanese Industrial Standard: Tokyo, Japan, 2019.
- TN USSK 10346-4/13; Surface Quality: Coated Surface A According to EN 10346:2015. Available online: https://www.usske.sk/en/products/hot-dip-galvanized-steel (accessed on 17 July 2024).
- EN STN 10002-1:2001; Metallic Materials—Tensile Testing—Part 1: Method of Test at Ambient Temperature. ISO: Geneva, Switzerland, 2001.
- Trumpf SLOVAKIA, a.s., Cataloques: Laser Marking (Popisovanie Laserom). 2018. Available online: https://www.trumpf.com/sk_SK/ (accessed on 17 July 2024).
- Polymérne Konštrukčné Materiály (Polymer Design Materials), 2012. [cit. 2015-02-12]. Available online: http://kmi2.uniza.sk/wp-content/uploads/2009/10/POLYMERY-Po-RECENZII.pdf (accessed on 17 July 2024).
- ISO EN STN 12944-6:2018; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems Part 6: Laboratory Performance Test Methods. ISO: Geneva, Switzerland, 2018.
- ISO 2409:2020; Paints and Varnishes—Cross-Cut Test. ISO: Geneva, Switzerland, 2020.
- ISO EN STN 9227:2022; Corrosion Tests in Artificial Atmospheres; Salt Spray Tests. ISO: Geneva, Switzerland, 2022.
- ISO 9227:2022/Amd 1:2024; Corrosion Tests in Artificial Atmospheres—Salt Spray Tests. Amendment 1: Footnote of Warning. ISO: Geneva, Switzerland, 2024.













| European Union EN 10346/09 | Japan JIS G3302 [52] | USA EN 10346/09 ASTM | U.S. Steel Kosice TN USSK 10346-4/13 [53] ZINKOMAGTM |
|---|---|---|---|
| DX51D +Z + ZF | SGC C SGC D1 | A 526, A 527, A 528 A 653 CS Type A, B, C | DX51D + Z + ZF |
| Grade According to EN 10346/09 U.S. Steel Kosice | Chemical Composition (wt. %) | |||||
|---|---|---|---|---|---|---|
| CMAX | MnMAX | PMAX | SMAX | SiMAX | TiMAX | |
| DX51D + Z + ZF | 0.18 | 1.20 | 0.12 | 0.045 | 0.50 | 0.30 |
| Grades According to EN 10346/09 U.S. Steel Košice | Rp0.2 (MPa) | Rm (MPa) | A MIN (%) Lo = 80 mm | r90 min. | n90 min. |
|---|---|---|---|---|---|
| DX51D + Z + ZF | - | 270–500 | 22 | - | - |
| Body Type | Marking | Prepared by Mechanical Processing |
|---|---|---|
| Total length (mm) | l3 | ≥150 (200) |
| Length of narrowed parallel part (mm) | l1 | 80 ± 2 |
| Radius (mm) | r | 20–25 |
| Spacing between wide parallel parts (mm) | l2 | 104–113 |
| Width of the clamping part (mm) | b2 | 30 + 0.2 |
| Width of narrowed parallel part (mm) | b1 | 18 + 0.2 |
| Recommended thickness (mm) | h | 1 + 0.2 |
| Initial measured length (mm) | L0 | 60 + 0.5 |
| Initial distance of jaws (mm) | L | 115 + 1 |
| Degree | Corrosion Area |
|---|---|
| 1 very small |  |
| 2 small |  |
| 3 a middle |  |
| 3 b middle |  |
| 4 a significant |  |
| 4 b significant |  |
| 5 very significant |  |
| Samples Number | Ageing Temperature (°C) | Sample Dimensions (mm) | ReH (MPa) | Rp0.2 (MPa) | ReL (MPa) | Rm (MPa) | A80 (%) | Ag (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| a0 | b0 | c0 | ||||||||
| 1 | at 100 °C | 0.496 | 20 | 80 | 356 | 332 | 323 | 343 | 30.5 | 18.5 |
| 2 | at 100 °C | 0.493 | 20 | 80 | 364 | 328 | 323 | 348 | 26.5 | 7.9 |
| 3 | at 100 °C | 0.413 | 20 | 80 | 326 | 324 | 323 | 398 | 28.0 | 17.2 |
| Samples Number | Ageing Temperature (°C) | Sample Dimension (mm) | ReH (MPa) | Rp0.2 (MPa) | ReL (MPa) | Rm (MPa) | A80 (%) | Ag (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| a0 | b0 | c0 | ||||||||
| 1 | at 120 °C | 0.496 | 20 | 80 | 358 | 334 | 330 | 347 | 26.5 | 9.0 |
| 2 | at 120 °C | 0.498 | 20 | 80 | 371 | 338 | 327 | 344 | 26.0 | 8.9 |
| 3 | at 120 °C | 0.417 | 20 | 80 | 339 | 336 | 325 | 393 | 29.0 | 17.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobotova, L.; Brezinova, J.; Badida, M.; Badidova, M.; Ciecinska, B.; Maslejova, A. The Effect of Artificial Ageing on the Changes in Selected Properties of Organic Coated Sheets. Materials 2024, 17, 3891. https://doi.org/10.3390/ma17163891
Sobotova L, Brezinova J, Badida M, Badidova M, Ciecinska B, Maslejova A. The Effect of Artificial Ageing on the Changes in Selected Properties of Organic Coated Sheets. Materials. 2024; 17(16):3891. https://doi.org/10.3390/ma17163891
Chicago/Turabian StyleSobotova, Lydia, Janette Brezinova, Miroslav Badida, Miroslava Badidova, Barbara Ciecinska, and Alica Maslejova. 2024. "The Effect of Artificial Ageing on the Changes in Selected Properties of Organic Coated Sheets" Materials 17, no. 16: 3891. https://doi.org/10.3390/ma17163891







