Transformation of Cu2O into Metallic Copper within Matrix of Carboxylic Cation Exchangers: Synthesis and Thermogravimetric Studies of Novel Composite Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CCEs with Metallic Copper Particles
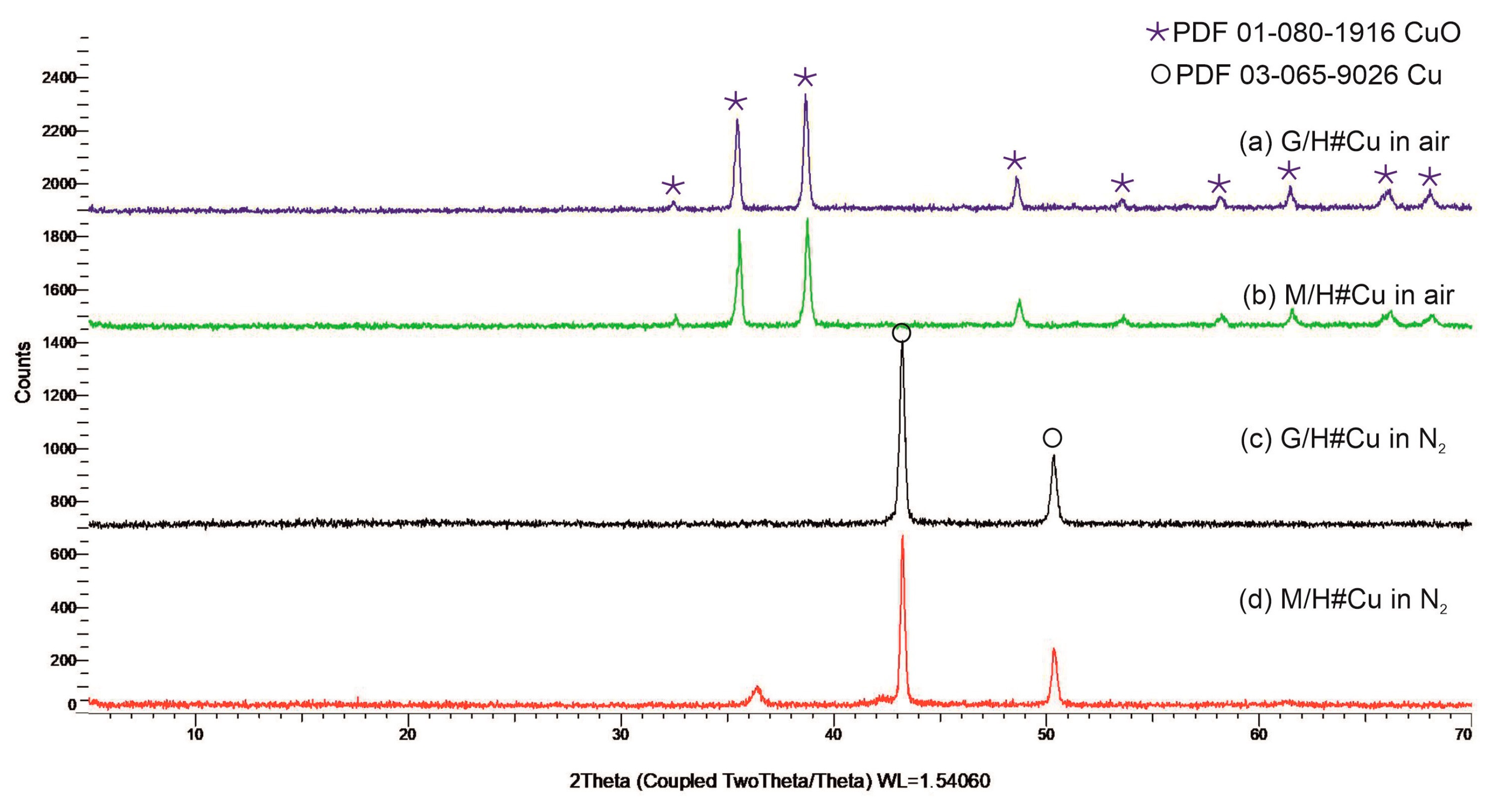
2.3. X-ray Powder Diffraction Analysis
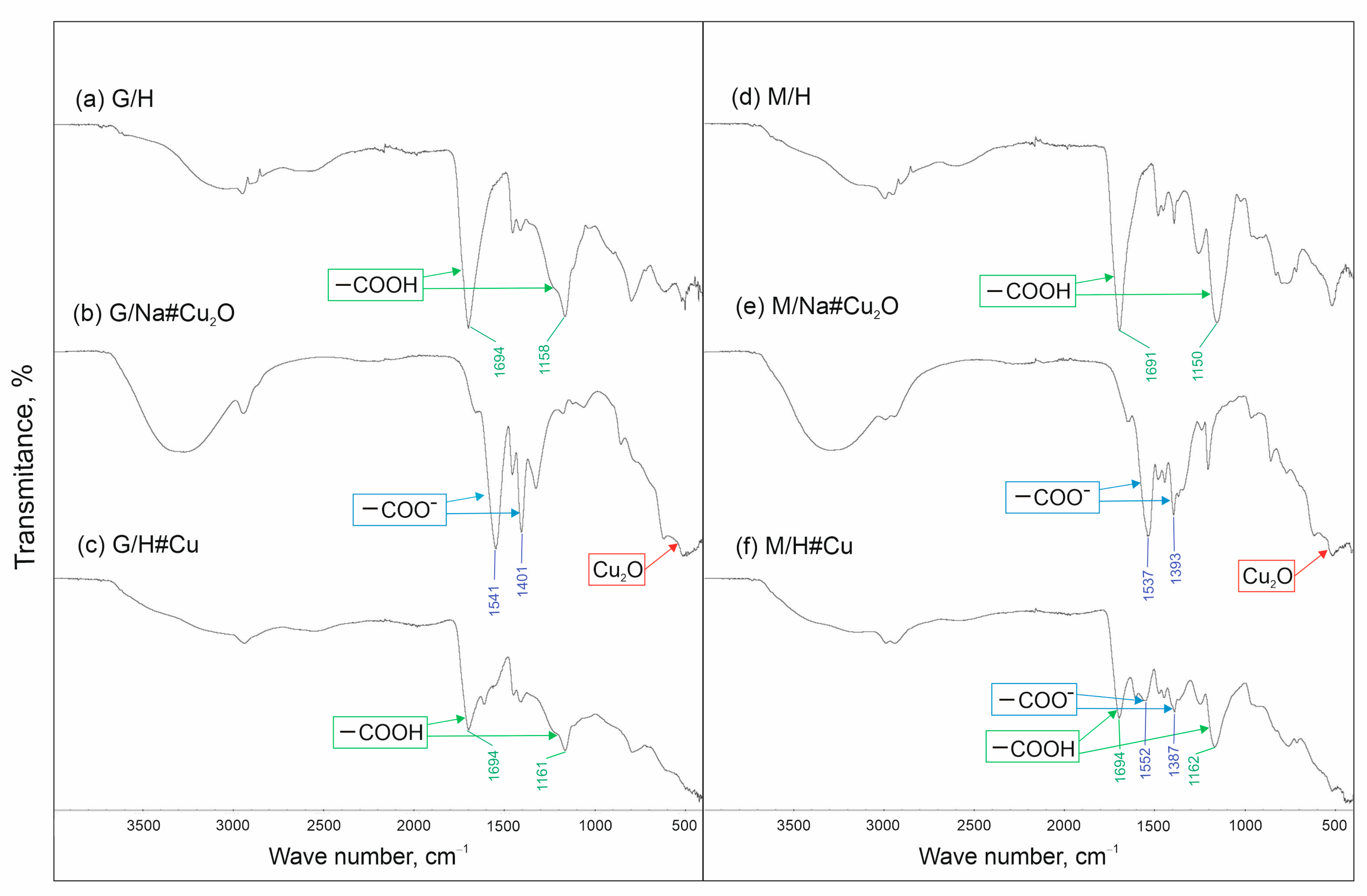
2.4. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
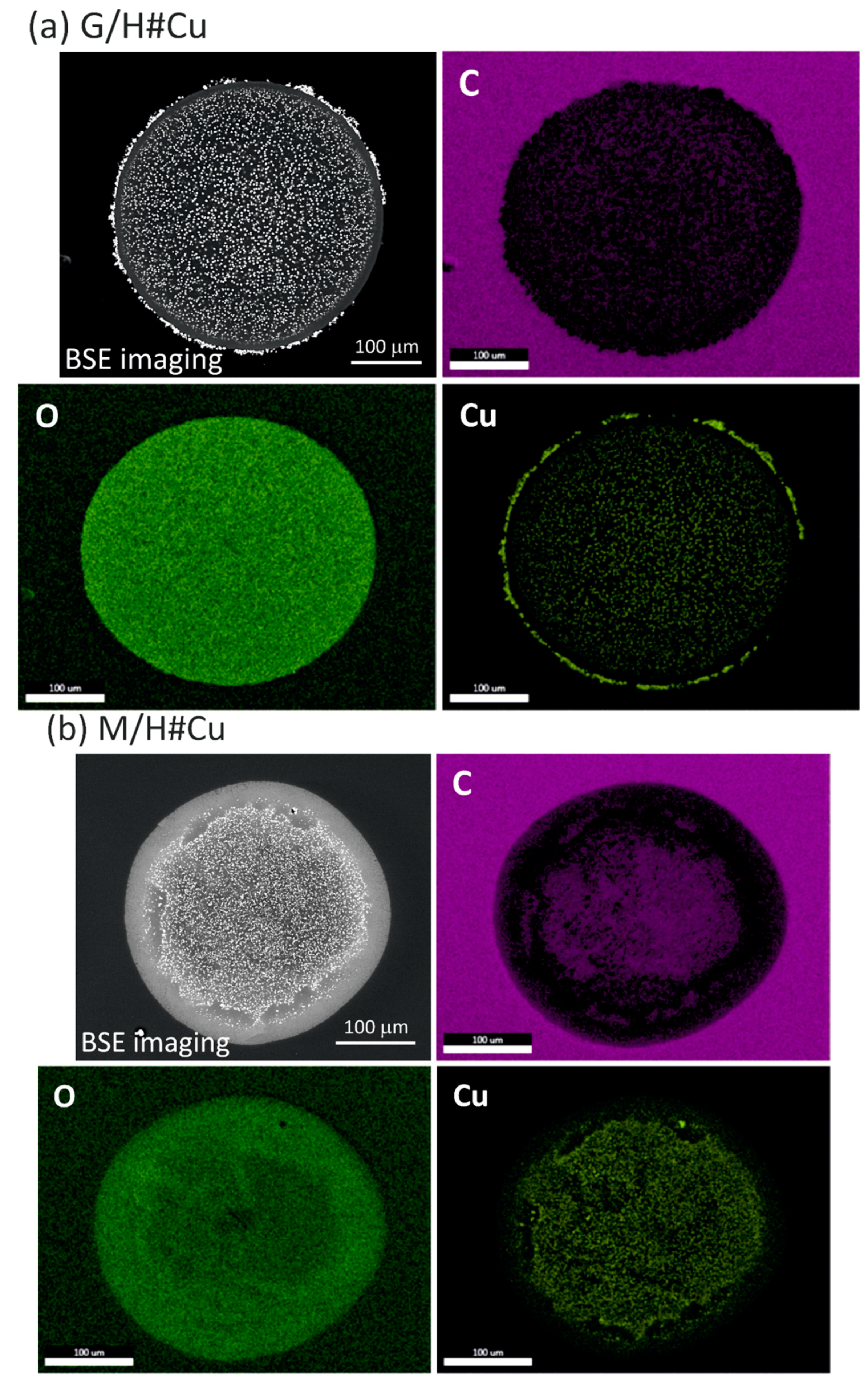
2.5. Scanning Electron Microscopy Analysis (SEM)
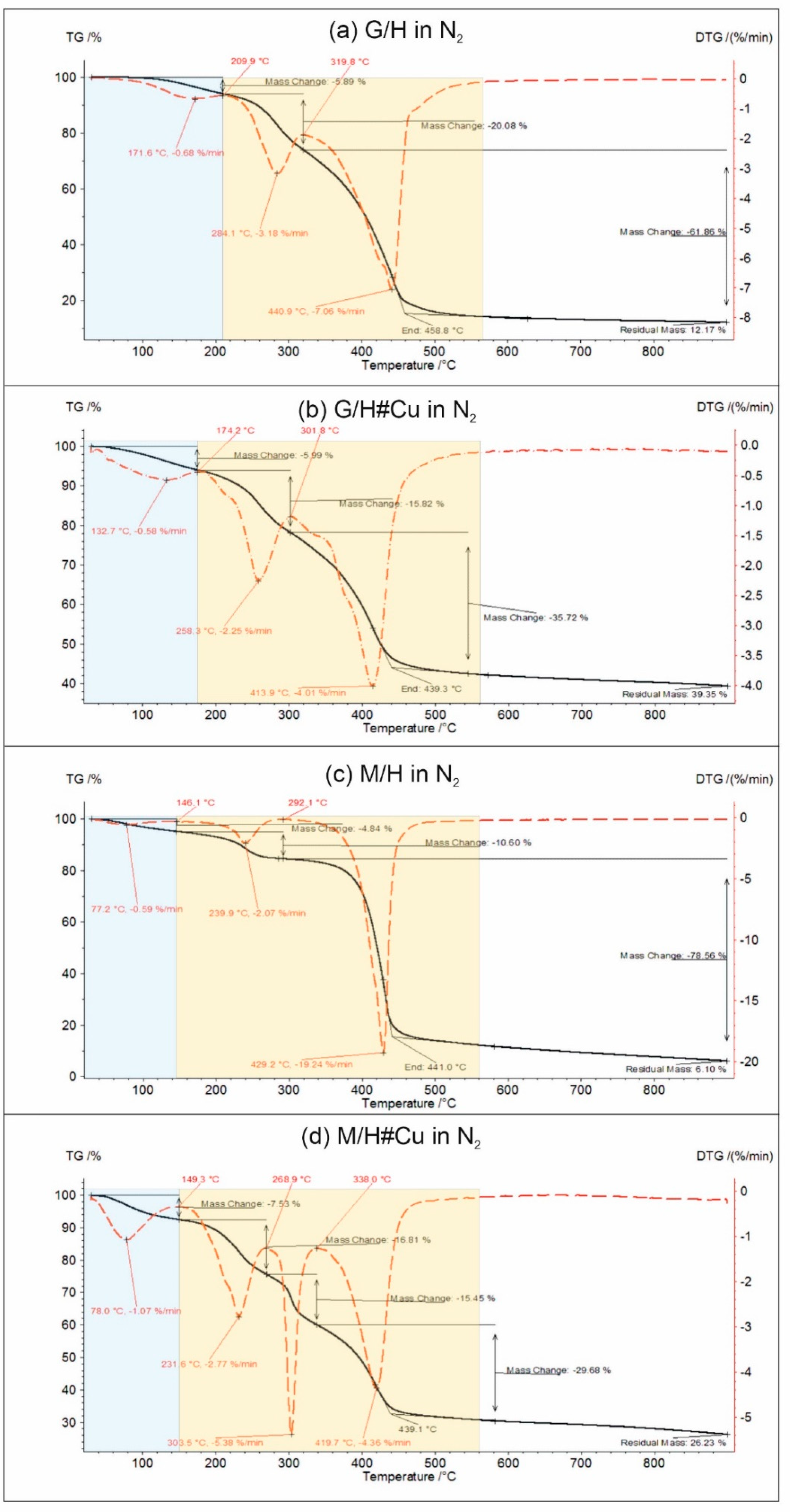
2.6. Thermogravimetric Analysis
3. Results and Discussion
3.1. Metallic Copper Deposition within G/H, M/H to Obtain G/H#Cu, M/H#Cu
3.2. Attempt to Obtain G/Na#Cu, M/Na#Cu
3.3. Thermogravimetric Studies, G/H vs. G/H#Cu, M/H vs. M/H#Cu
3.4. Thermal Analysis as a Useful Method for Evaluating CuO-, Cu2O-, Cu0-Doped Carboxylic Cation Exchangers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sudha, V.B.P.; Ganesan, S.; Pazhani, G.P.; Ramamurthy, T.; Nair, G.B.; Venkatasubramanian, P. Storing drinking water in copper pots kills contaminating diarrhoeagenic bacteria. J. Health Popul. Nutr. 2012, 30, 17–21. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, V.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2017, 124, 1032–1046. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Kohl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture, A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Abraham, J.; Dowling, K.; Florentine, S. Can copper products and surfaces reduces the spread of infectious microorganism and hospital acquired infections? Materials 2021, 14, 3444. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.T.; Neoh, K.G. Antimicrobial copper-based materials and coatings: Potential multifaceted biomedical applications. ACS Appl. Mater. Interfaces 2020, 12, 21159–21182. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, P.; Brar, R.S.; Babu, N.J. Nanoscale zerovalent copper (nZVC) catalyzed environmental remediation of organic and inorganic contaminants A review. Heliyon 2022, 8, e10140. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Naren, N.; Li, L.; Ma, L.Z.; Zhang, X.X. Experimental evolution of bacterial survival on metallic copper. Ecol. Evol. 2022, 2, e9225. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekkonen, T.H. Engineered nanomaterials for antimicrobial applications: A review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- Arora, P.; Tewary, S.; Krishnamurthi, S.; Kumari, N. Development of a low-cost copper device for inactivation of microorganism in drinking water for human consumption. J. Water Proc. Eng. 2022, 50, 103302. [Google Scholar] [CrossRef]
- Popescu, V.; Prodan, D.; Cuc, S.; Sarosi, C.; Furtos, G.; Moldovan, A.; Carpa, R.; Bombos, D. Antimicrobial poly(lactic acid)/copper nanocomposites for food packaging materials. Materials 2023, 16, 1415. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lin, Y.F.; Nain, A.; Huang, Y.F.; Chang, H.T. A critical review of copper nanoclusters for monitoring water quality. Sens. Actuators Rep. 2021, 3, 100026. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Xing, D.; Ren, Z.; Ye, L. Enhanced abatement of organic contaminants by zero-valent copper and sulfite. Environ. Chem. Lett. 2020, 8, 237–241. [Google Scholar] [CrossRef]
- Yang, W.; Dang, G.; Fujii, M.; Cai, C.; Wei, Y.; Zhang, Y. Heterogeneous activation of sulfite by a three-dimensional printed hierarchically porous copper catalyst for the degradation of tetracycline hydrochloride in water. ACS ES&T Water 2024, 4, 1730–1740. [Google Scholar]
- Yu, B.; Li, Z.; Zhang, S. Zero-valent copper-mediated peroxymonosulfate activation for efficient degradation of azo dye Orange G. Catalysts 2022, 12, 700. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Liu, W.; Ji, H.; Li, F.; Shen, C.; Fang, X.; Li, X.; Duan, X. A novel electrocatalytic filtration system with carbon nanotube supported nanoscale zerovalent copper toward ultrafast oxidation of organic pollutants. Water Res. 2021, 194, 116961. [Google Scholar] [CrossRef]
- Zhu, X.; Xiong, J.; Wang, Z.; Chen, R.; Cheng, G.; Wu, Y. Metallic copper-containing composite photocatalysts: Fundamental, materials design, and photoredox applications. Small Methods 2022, 6, 2101001. [Google Scholar] [CrossRef] [PubMed]
- Varga, G.; Szenti, I.; Kiss, J.; Baan, K.; Halasi, G.; Ovari, L.; Szamosvolgyi, A.; Mucsi, R.; Dodony, E.; Fogarassy, Z.; et al. Decisive role of Cu/Co interfaces in copper cobaltite derivatives for high performance CO2 methanation catalyst. J. CO2 Util. 2023, 75, 102582. [Google Scholar] [CrossRef]
- Bonthula, S.; Bonthula, S.R.; Pothu, R.; Srivastava, R.K.; Boddula, R.S.; Radwa, A.B.; Al-Qahtani, N. Recent advances in copper-based materials for sustainable environmental applications. Sustain. Chem. 2023, 4, 246–271. [Google Scholar] [CrossRef]
- AL-Thabaiti, S.A.; Obaid, Y.; Khan, Z.; Bashir, O.; Hussain, S. Cu nanoprticles: Synthesis, crystallographic characterization, and stability. Colloid Polym. Sci. 2015, 293, 2543–2554. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, K.; Shin, I.S.; Shin, K.S. Antioxidative metallic copper nanoparticles prepared by modified polyol methods and their catalytic activities. J. Nanopart. Res. 2020, 22, 8. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef]
- Prathna, T.C.; Sharma, S.K.; Kennedy, M. Nanoparticles in household level water treatment: An overview. Sep. Purif. Technol. 2018, 99, 260–270. [Google Scholar]
- Elmekawy, A.; Hegab, H.M.; Alsafar, H.; Yousef, A.F.; Banat, F.; Hasan, S.W. Bacterial nanotechnology: The intersection impact of bacteriology and nanotechnology on the wastewater sector. J. Environ. Chem. Eng. 2023, 11, 109212. [Google Scholar] [CrossRef]
- Malik, K.; Sharma, S.; Sharma, M.; Sonkar, S.M.; Mishra, A.; Kaur, L.; Ojha, H.; Pathak, M. A review on synthetic methods and applications of carbon supported copper nanomaterials. Mater. Today Commun. 2023, 37, 107169. [Google Scholar] [CrossRef]
- Chai, J.; Jiang, T.; Wang, Y.; Chen, Q.; Shi, J.; Du, Q. Simultaneous removal of ciprofloxacin and Cr (VI) from contaminated water by polymer-supported nanoscale zero-valent copper: Performance and mechanism. Colloids Surf. A 2024, 694, 134214. [Google Scholar] [CrossRef]
- Pranudta, A.; Chanthapon, N.; Kidkhunthod, P.; El-Moseley, M.M.; Nguyen, T.T.; Padungthon, S. Selective removal of Pb from lead-acid battery wastewater using hybrid gel cation exchanger loaded with hydrated iron oxide nanoparticles: Fabrication, characterization, and pilot-scale validation. J. Environ. Chem. Eng. 2021, 9, 106282. [Google Scholar] [CrossRef]
- Laiju, A.R.; Sarkar, S. A novel hybrid ferrous sulfide impregnated anion exchanger for trace removal of haxavalent chromium from contaminated water. Chemosphere 2022, 305, 135369. [Google Scholar] [CrossRef] [PubMed]
- Pranudta, A.; Patra, S.; Klysubun, W.; Amonpattaratkit, P.; Nguyen, T.T.; Nguyen, V.H.; El-Moselhy, M.M.; Padungthon, S. Insight into the molecular interaction of trace As (III) and As(V) onto the hybrid anion exchanger impregnated with Fe/Mn nanoparticles (HA502P-Fe/Mn). Chem. Eng. J. 2023, 54, 139991. [Google Scholar] [CrossRef]
- Belloni, C.; Korving, L.; Witkamp, G.J.; Bruck, E.; de Jager, P.P.; Dugulan, A.I. FeOOH and (Fe,Zn)OOH hybrid anion exchange adsorbents for phosphate recovery: A determination of Fe-phases and adsorption-desorption mechanism. Chem. Eng. J. 2023, 473, 145287. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Mucha, I.; Ociński, D.; Jacukowicz-Sobala, I. Multifunctional composite materials based on anion exchangers modified with copper compounds—A review of their synthesis methods, characteristics and applications. Polymers 2023, 15, 3606. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Mucha, I. Effect of the kind of cupric compound deposit on thermal decomposition of anion exchangers. Thermochim. Acta 2021, 695, 178812. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Mucha, I. Weakly hydrated anion exchangers doped with Cu2O and Cu0 particles—Thermogravimetric studies. Materials 2021, 14, 925. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I.; Jasiorski, M. Anomalous effect of Cu2O and CuO deposit on the porosity of a macroreticular anion exchanger. J. Nanopart. Res. 2021, 23, 126. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I.; Mucha, I. Copper rich composite materials based on carboxylic cation exchangers and their thermal transformation. Polymers 2021, 13, 3199. [Google Scholar] [CrossRef]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I.; Mucha, I. The influence of ionic form and Cu2O particles on thermal stability of carboxylic cation exchangers. Thermochim. Acta 2024, 733, 179678. [Google Scholar] [CrossRef]
- Kravchenko, T.A.; Chayka, M.Y.; Konev, D.V.; Polyanskiy, L.N.; Krysanov, V.A. The influence of the ion-exchange groups nature and the degree of chemical activation by silver on the process of copper electrodeposition into the ion exchanger. Electrochim. Acta 2007, 53, 330–336. [Google Scholar] [CrossRef]
- Kravchenko, T.A.; Sakardina, E.A.; Kalinichev, A.I.; Zolotukhina, E.V. Stabilization of copper nanoparticles with volume- and surface-distribution inside ion-exchange matrices. Russ. J. Phys. Chem. 2015, 89, 1648–1654. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S.; Imran, M. A green method of the synthesis of copper nanoparticles using l-ascorbic acid. Rev. Mater. 2014, 19, 197–203. [Google Scholar] [CrossRef]
- Wu, S. Preparation of fine copper powder using ascorbic acid as reducing agent and its application in MLCC. Mater. Lett. 2007, 61, 1125–1129. [Google Scholar] [CrossRef]
- Liu, Q.; Yasunami, T.; Kuruda, K.; Okido, M. Preparation of Cu nanoparticles with ascorbic acid by aqueous solution reduction method. Trans. Nonferrous Met. Soc. China 2012, 22, 2198–2203. [Google Scholar] [CrossRef]
- Ramos, A.R.; Tapia, A.K.G.; Pinol, C.M.N.; Lantican, N.B.; del Mundo, M.L.F.; Manalo, R.D.; Herrera, M.U. Effects of reaction temperature and reactant concentrations on the antimicrobial characteristics of copper precipitates synthesized using L-ascorbic acid as reducing agent. J. Sci. Adv. Mater. Devices 2019, 4, 66–71. [Google Scholar] [CrossRef]
- Jacukowicz-Sobala, I.; Stanisławska, E.; Baszczuk, A.; Jasiorski, M.; Kociołek-Balawejder, E. Size-controlled transformation of Cu2O into zero valent copper within the matrix of anion exchangers via green chemical reduction. Polymers 2020, 12, 2629. [Google Scholar] [CrossRef] [PubMed]
- Chambree, D.; Iditoiu, C.; Segal, E.; Cesaro, A. The study of non-isothermal degradation of acrylic ion-exchange resins. J. Therm. Anal. Calorim. 2005, 82, 803–811. [Google Scholar] [CrossRef]
- Chambree, D.; Iditoiu, C.; Segal, E. Non-isothermal dehydration kinetics of acrylic ion-exchange resins. J. Therm. Anal. Calorim. 2007, 88, 673–679. [Google Scholar] [CrossRef]
- Charmas, B.; Kucio, K.; Skubiszewska-Zięba, J.; Khalameida, S. Structural, thermal and energetic characteristics of synthetic active carbons prepared on the basis of ion-exchange resin Amberlite IRC 84. J. Thermal Anal. Calorim. 2019, 136, 1539–1549. [Google Scholar] [CrossRef]
- Charmas, B.; Sydorchuk, V.; Khalameida, S.; Kuśmierz, M.; Zięzio, M.; Kucio, K. Synthesis, physicochemical properties and photocatalytic activity of Cu-containing activated carbons prepared from sulfo-resins under visible irradiation. Appl. Surf. Sci. 2021, 568, 150865. [Google Scholar] [CrossRef]
- Bogoczek, R.; Pińkowska, H. Covalent reaction on carboxylic cation exchangers poly(acrylic acid-dvb/esters). React. Funct. Polym. 2003, 4, 117–130. [Google Scholar] [CrossRef]






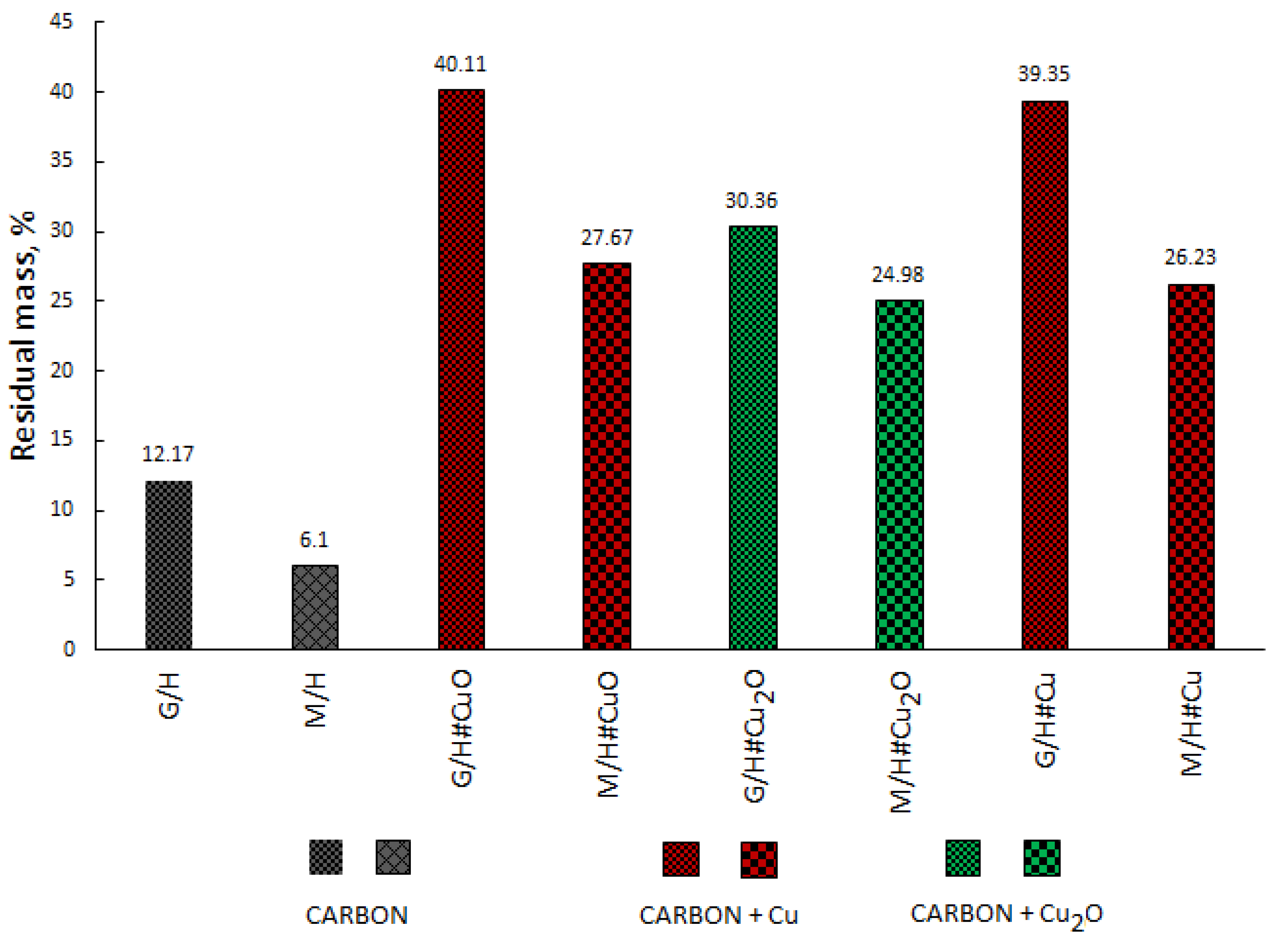
| Code | Cu, mg/g | Water, % * |
|---|---|---|
| G/Cu | 228.9 | 10.22 |
| M/Cu | 217.9 | 9.37 |
| G/Na#Cu2O | 199.2 | 15.96 |
| M/Na#Cu2O | 180.3 | 21.35 |
| Sample Code | Water Mass Loss, wt% | Peak Temperature of Polymer Decomposition, °C | End Temperature, °C | Residual Mass at 900 °C, wt% |
|---|---|---|---|---|
| (a) Decomposition in air | ||||
| G/H | 6.04 | 284.7, 426.9, 524.2 | 552.1 | 0.00 |
| G/H#Cu | 5.96 | 266.3, 344.2, 458.4 | 484.6 | 26.28 |
| M/H | 4.05 | 238.1, 416.3, 487.4 | 503.6 | 0.00 |
| M/H#Cu | 7.91 | 230.6, 312.4, 407.0 | 487.0 | 22.80 |
| (b) Decomposition in N2 | ||||
| G/H | 5.89 | 284.1, 440.9 | 458.8 | 12.17 |
| G/H#Cu | 5.99 | 258.3, 413.9 | 439.3 | 39.35 |
| M/H | 4.84 | 239.9, 429.2 | 441.0 | 6.10 |
| M/H#Cu | 7.53 | 231.6, 303.5, 417.7 | 439.1 | 26.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kociołek-Balawejder, E.; Winiarska, K.; Winiarski, J.; Mucha, I. Transformation of Cu2O into Metallic Copper within Matrix of Carboxylic Cation Exchangers: Synthesis and Thermogravimetric Studies of Novel Composite Materials. Materials 2024, 17, 3893. https://doi.org/10.3390/ma17163893
Kociołek-Balawejder E, Winiarska K, Winiarski J, Mucha I. Transformation of Cu2O into Metallic Copper within Matrix of Carboxylic Cation Exchangers: Synthesis and Thermogravimetric Studies of Novel Composite Materials. Materials. 2024; 17(16):3893. https://doi.org/10.3390/ma17163893
Chicago/Turabian StyleKociołek-Balawejder, Elżbieta, Katarzyna Winiarska, Juliusz Winiarski, and Igor Mucha. 2024. "Transformation of Cu2O into Metallic Copper within Matrix of Carboxylic Cation Exchangers: Synthesis and Thermogravimetric Studies of Novel Composite Materials" Materials 17, no. 16: 3893. https://doi.org/10.3390/ma17163893
APA StyleKociołek-Balawejder, E., Winiarska, K., Winiarski, J., & Mucha, I. (2024). Transformation of Cu2O into Metallic Copper within Matrix of Carboxylic Cation Exchangers: Synthesis and Thermogravimetric Studies of Novel Composite Materials. Materials, 17(16), 3893. https://doi.org/10.3390/ma17163893









