Toward Metallized Pellets for Steelmaking by Hydrogen Cooling Reduction: Effect of Gas Flow Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Methods
2.2.1. Direct Reduction
2.2.2. Characterizations
3. Results and Discussion
3.1. Reduction Behavior
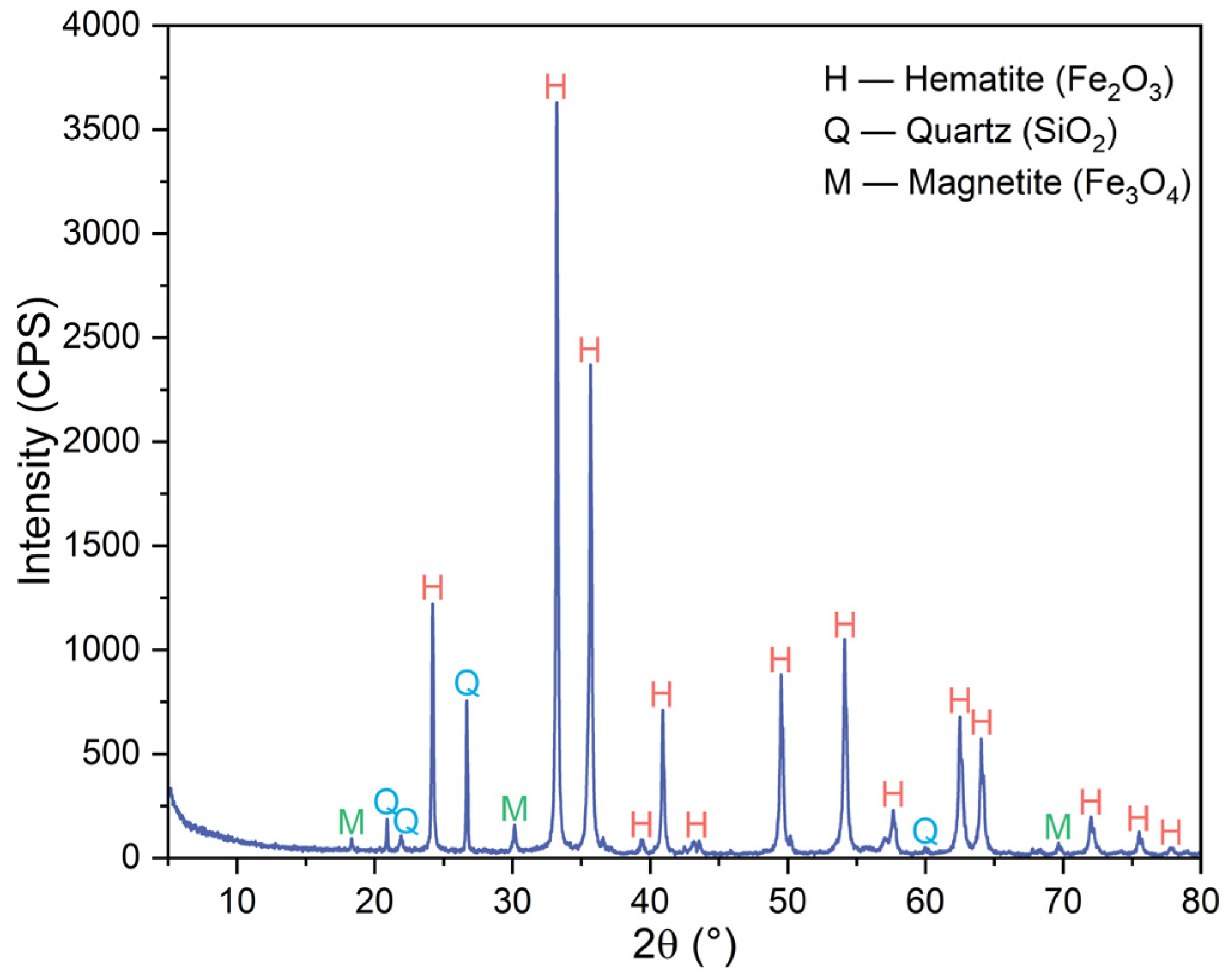
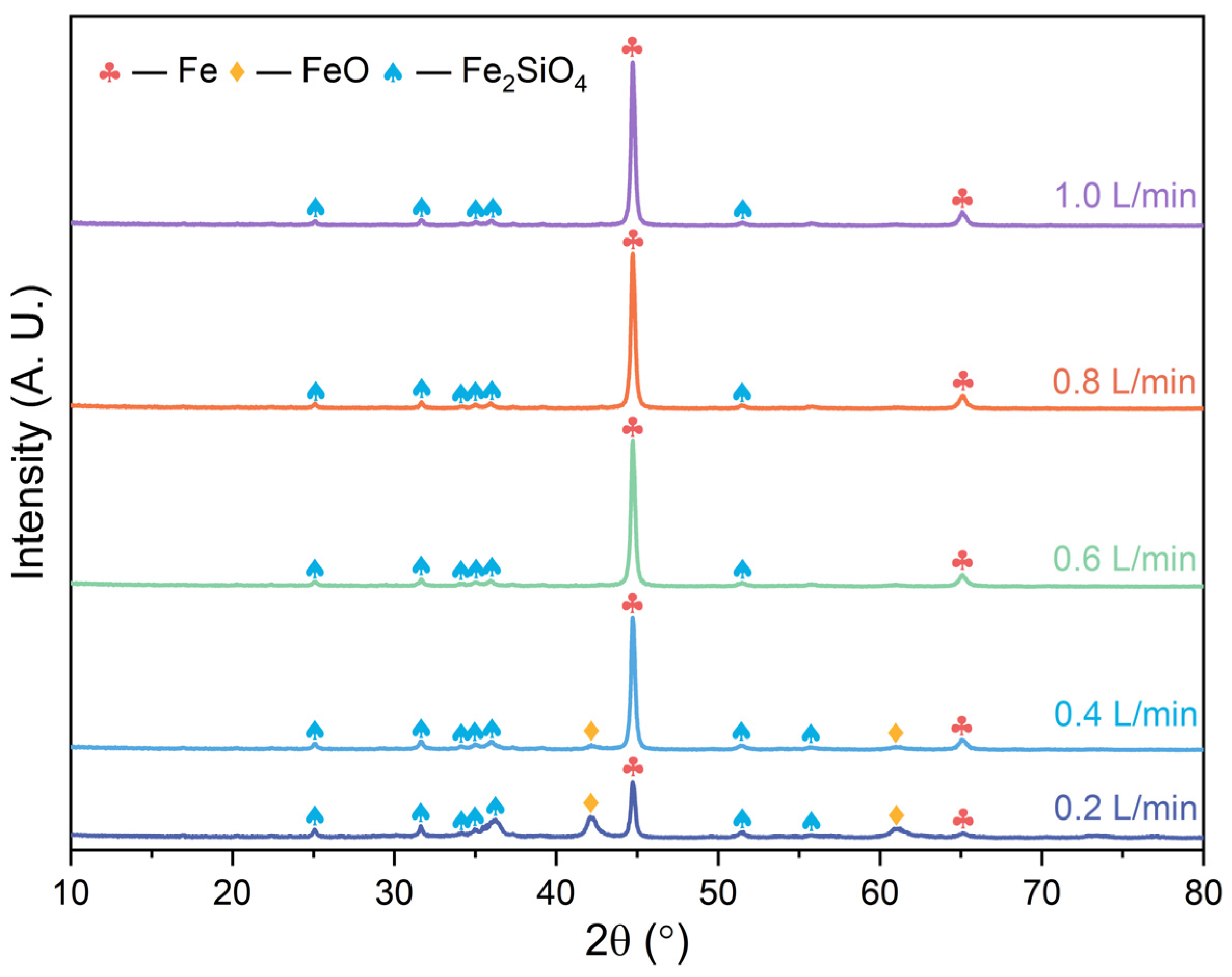
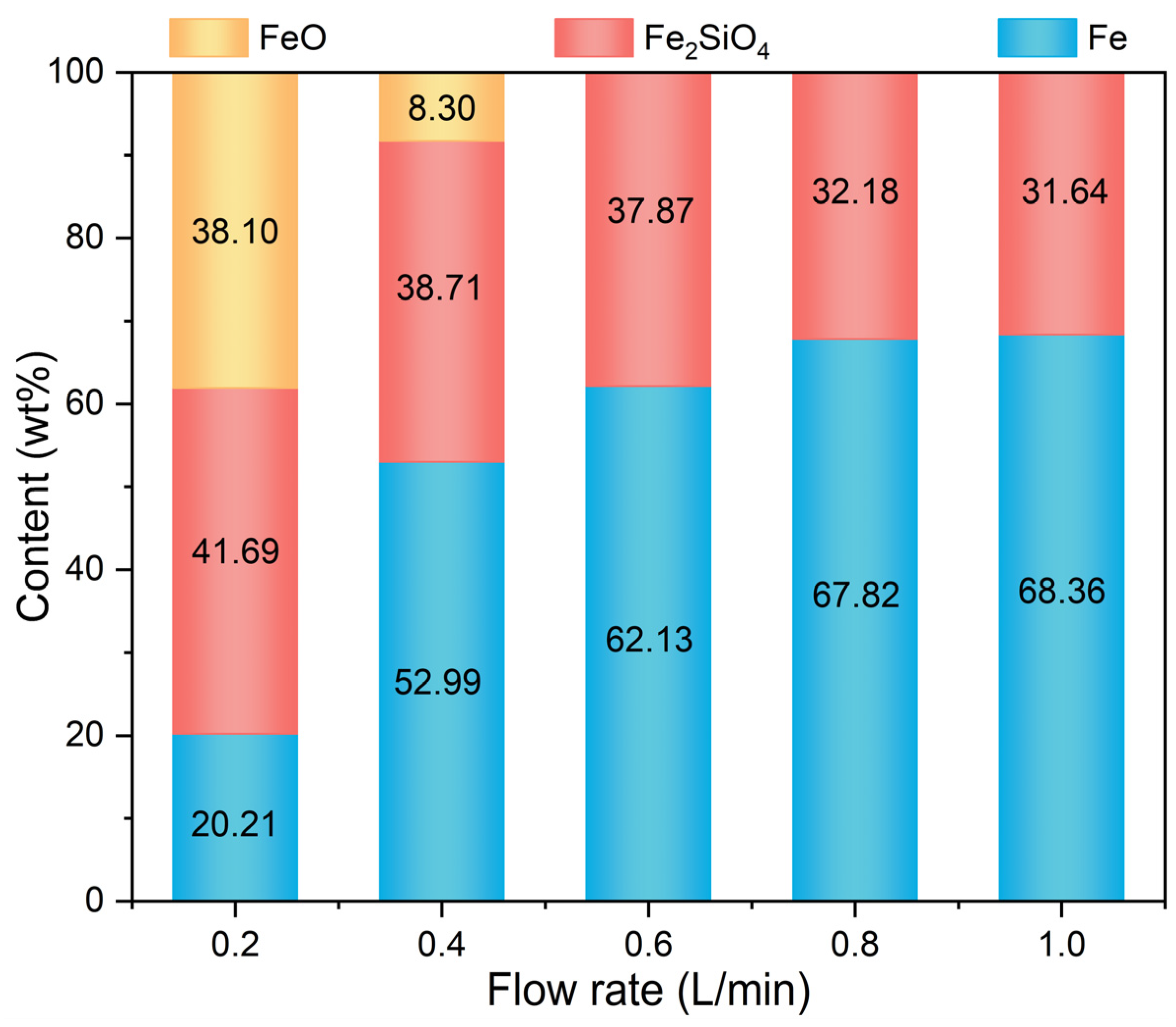
3.2. Phase Transformation
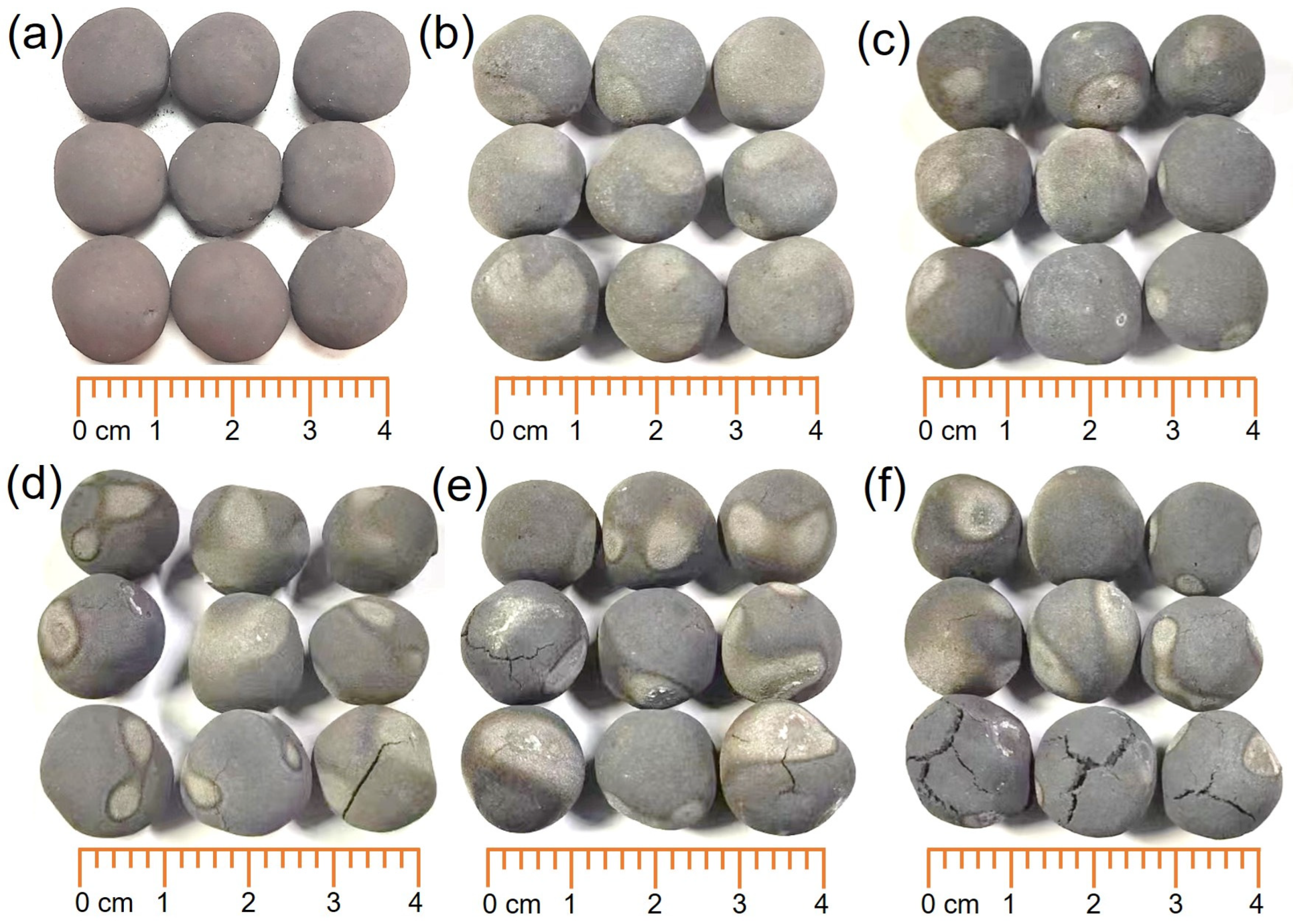
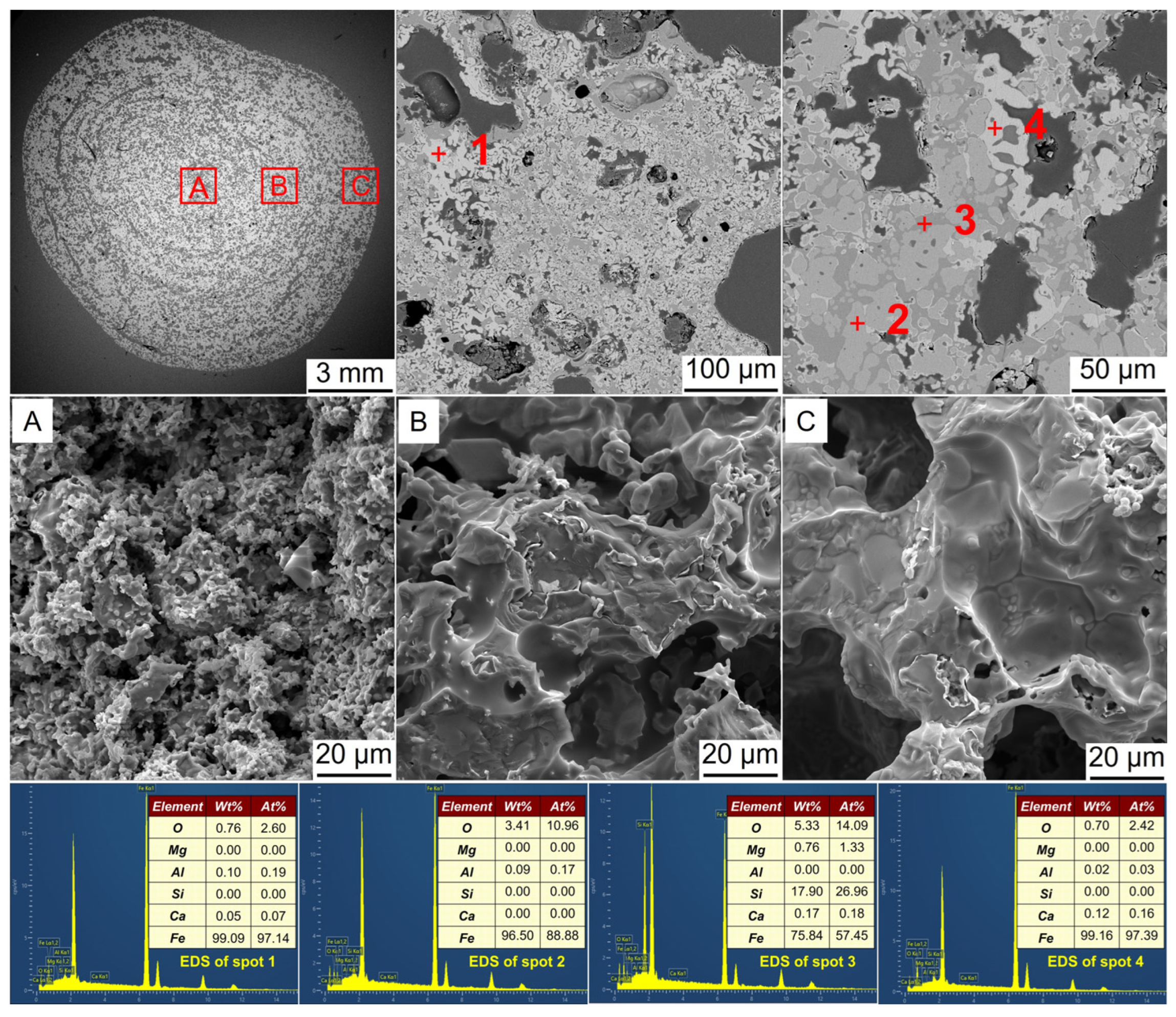
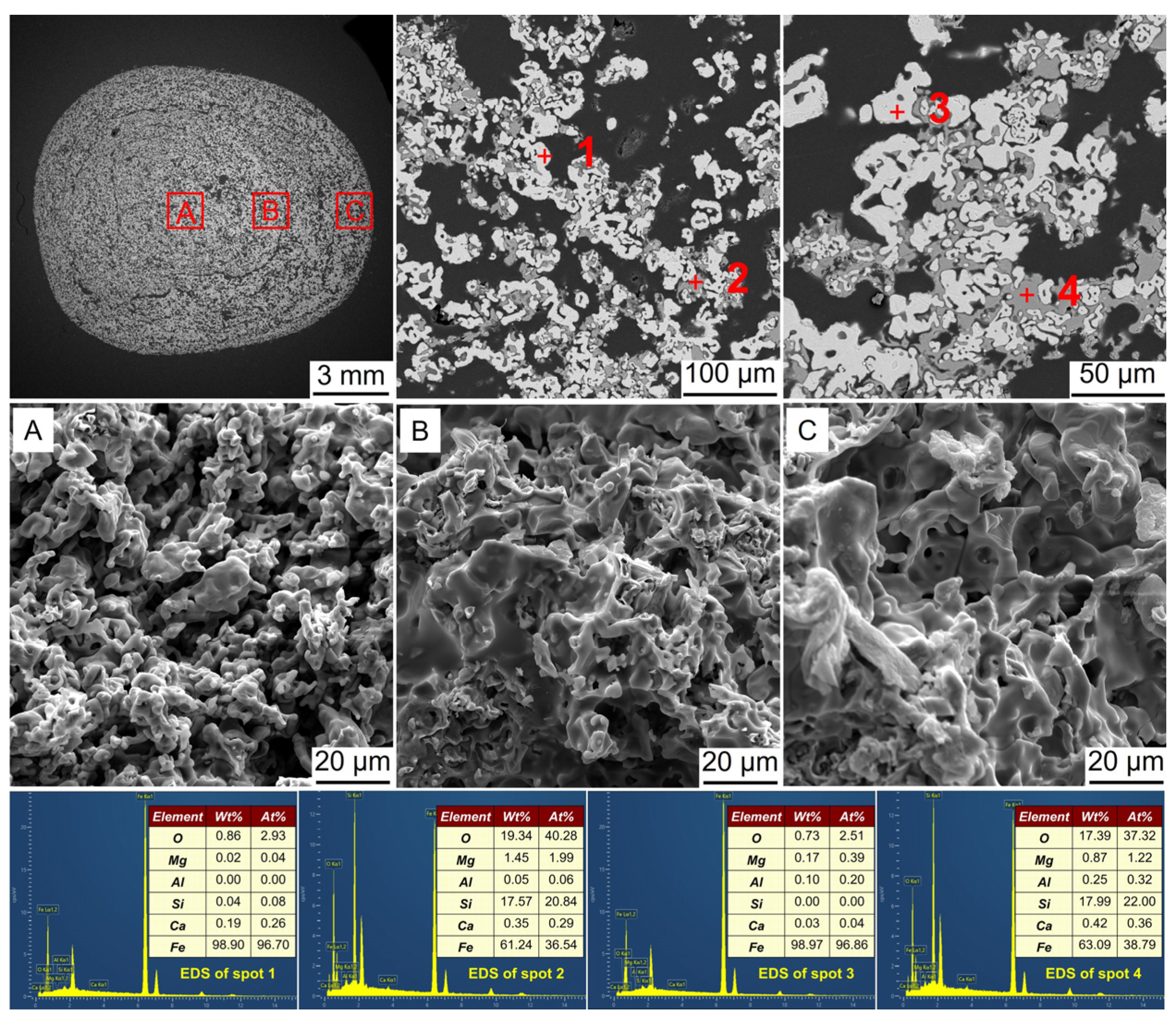
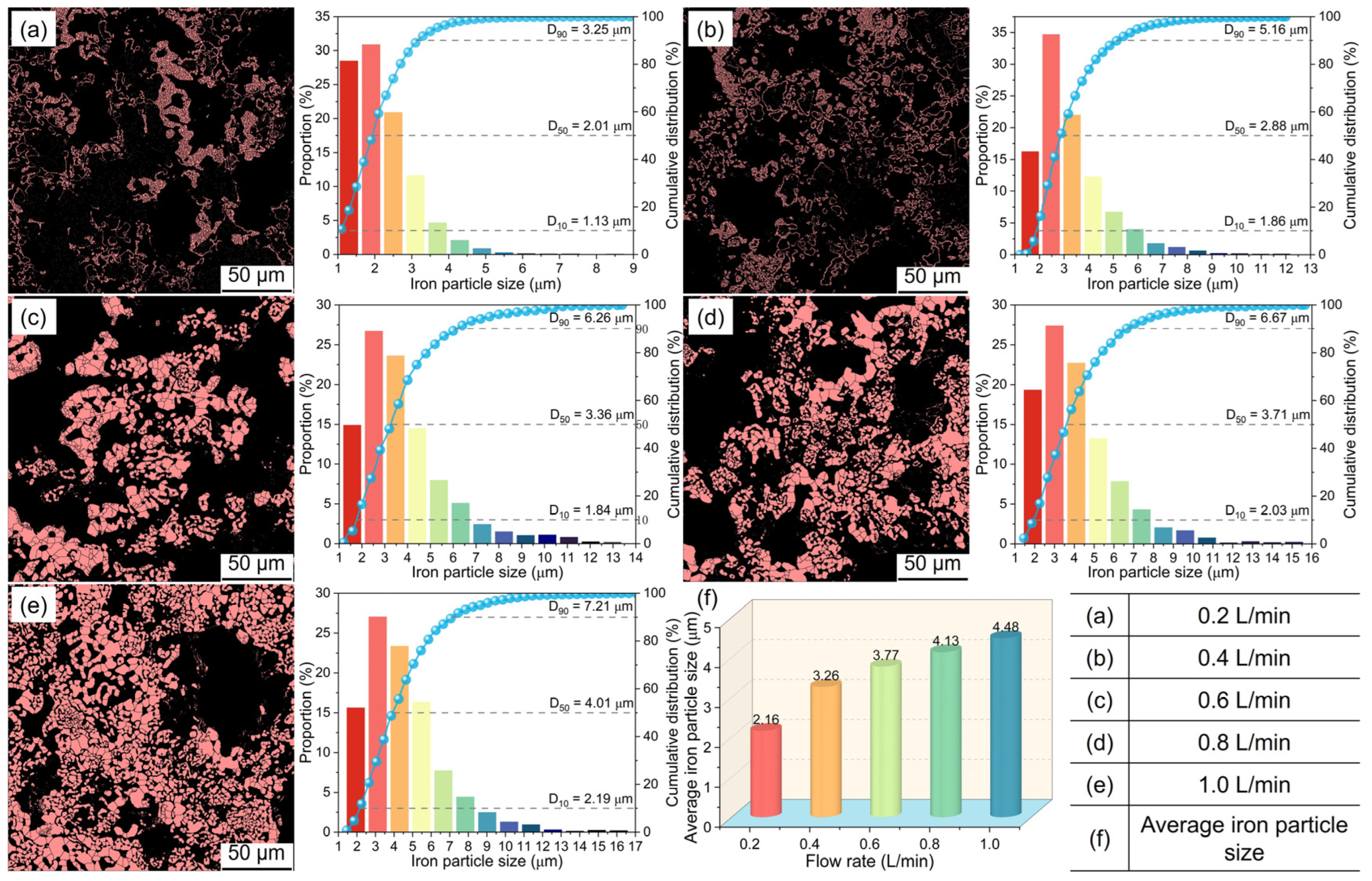
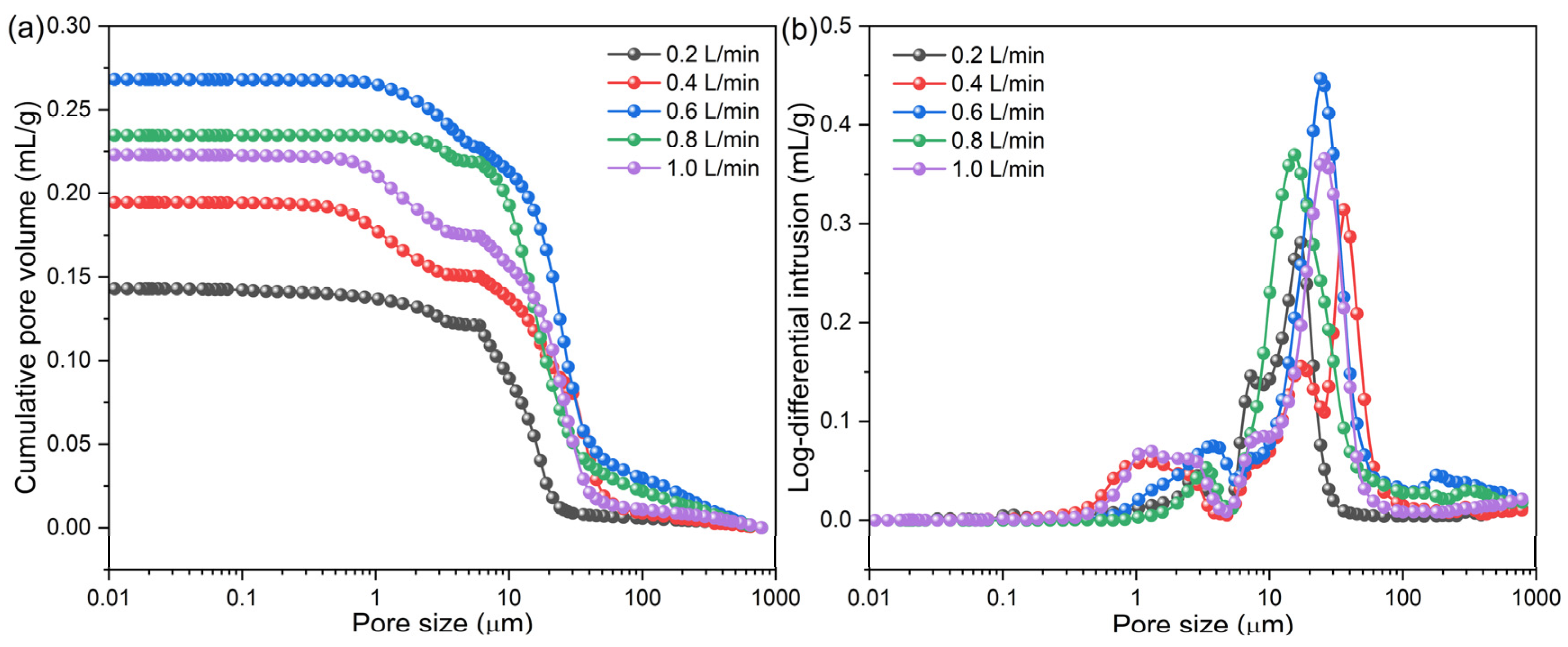
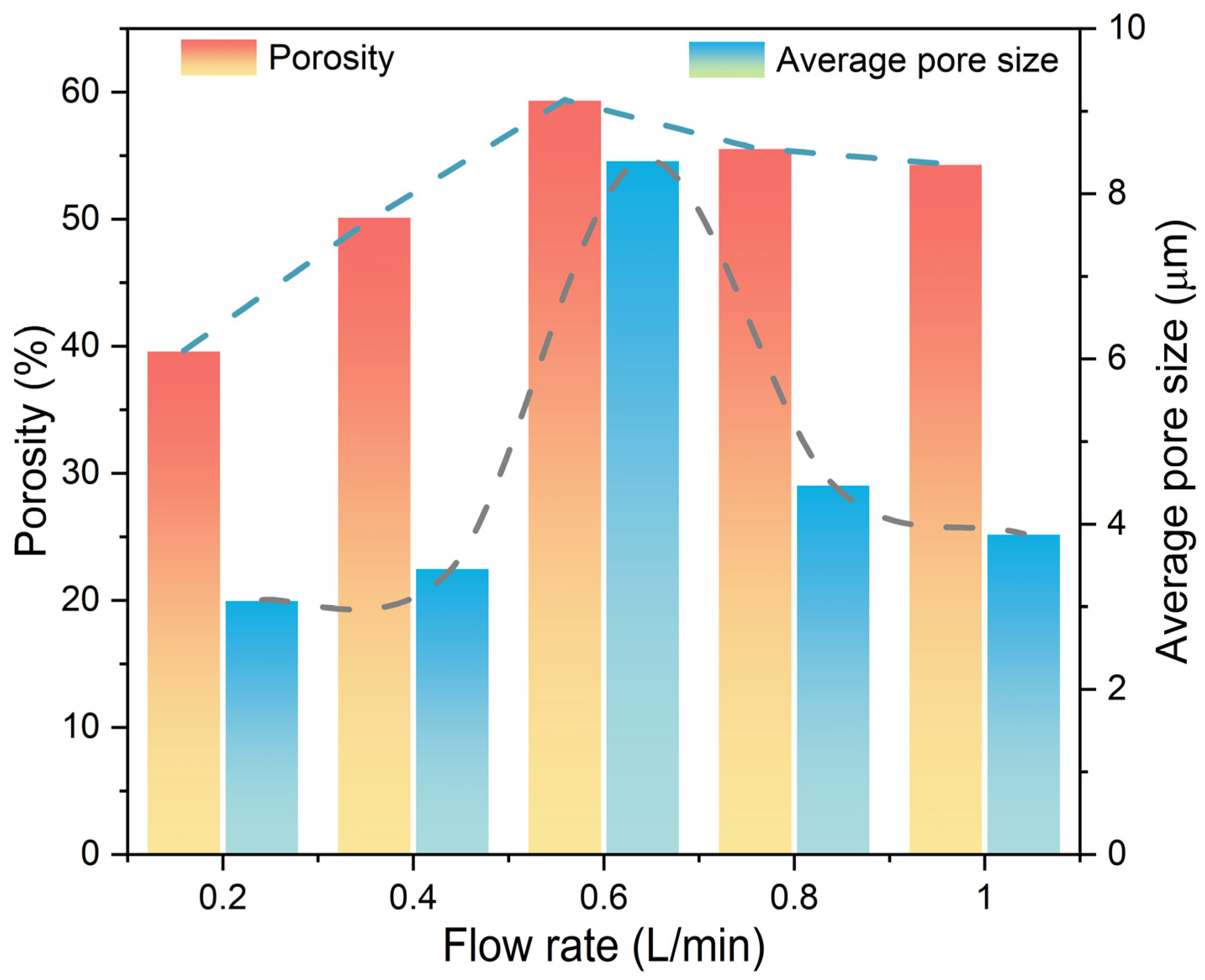
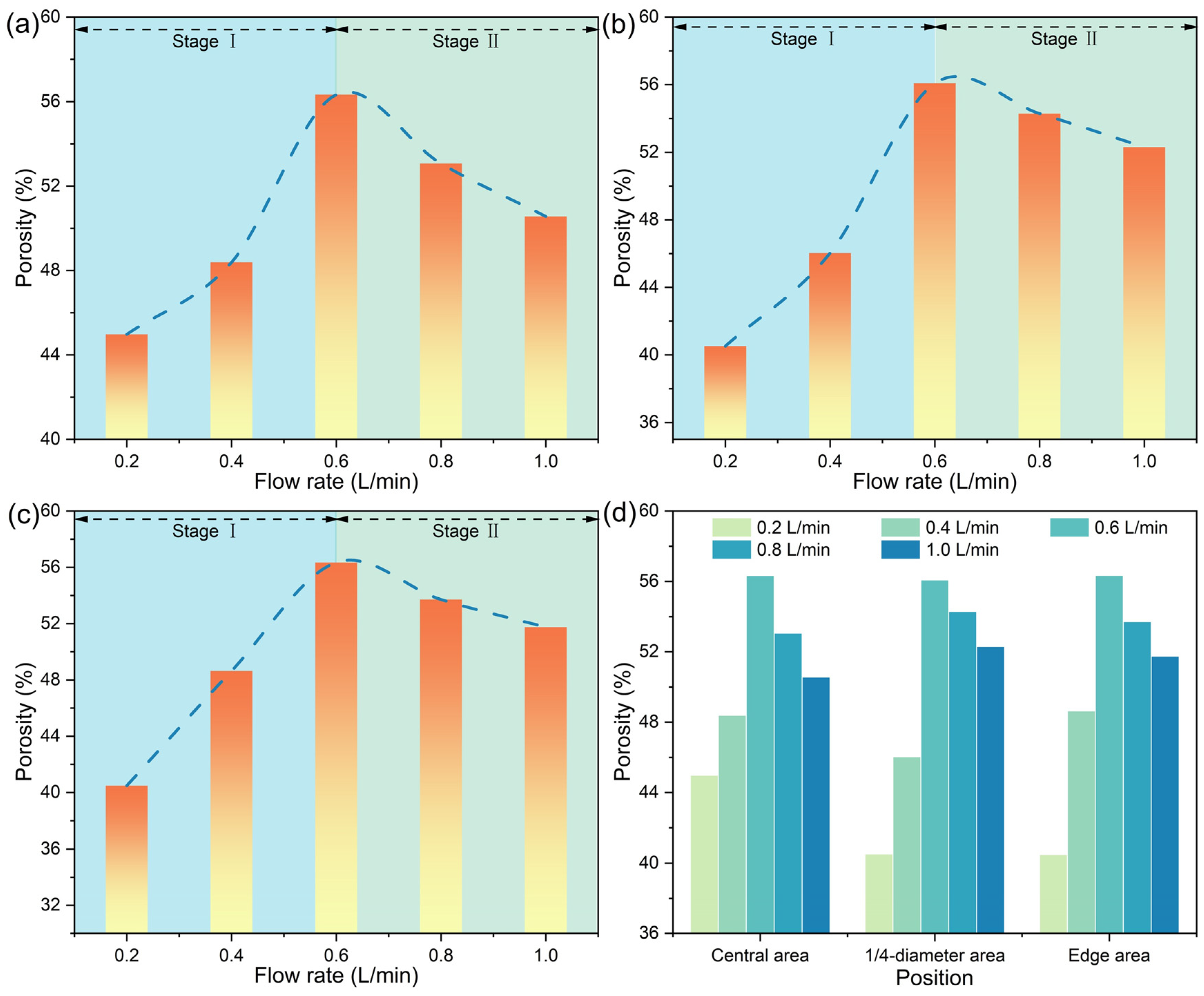
3.3. Microstructural Evolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, B.; Wu, R. Designing energy policy based on dynamic change in energy and carbon dioxide emission performance of China’s iron and steel industry. J. Clean. Prod. 2020, 256, 120412. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, B.; Guo, F.; Zhu, P. Exploring selected pathways to low and zero CO2 emissions in China’s iron and steel industry and their impacts on resources and energy. J. Clean. Prod. 2022, 340, 130813. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, H.; Ma, L.; Li, Z.; Ni, W. Low-carbon development for the iron and steel industry in China and the world: Status quo, future vision, and key actions. Sustainability 2021, 13, 12548. [Google Scholar] [CrossRef]
- Ren, M.; Lu, P.; Liu, X.; Hossain, M.S.; Fang, Y.; Hanaoka, T.; O’Gallachoir, B.; Glynn, J.; Dai, H. Decarbonizing China’s iron and steel industry from the supply and demand sides for carbon neutrality. Appl. Energy 2021, 298, 117209. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Tang, X.; Wang, Y.; An, H.; Yi, H. Decarbonization pathways of China’s iron and steel industry toward carbon neutrality, Resour. Conserv. Recycl. 2023, 194, 106994. [Google Scholar] [CrossRef]
- Zang, G.; Sun, P.; Elgowainy, A.; Bobba, P.; McMillan, C.; Ma, O.; Podkaminer, K.; Rustagi, N.; Melaina, M.; Koleva, M. Cost and life cycle analysis for deep CO2 emissions reduction of steelmaking: Blast furnace-basic oxygen furnace and electric arc furnace technologies. Int. J. Greenhouse Gas Control 2023, 128, 103958. [Google Scholar] [CrossRef]
- Barik, K.; Prusti, P.; Soren, S.; Meikap, B.C.; Biswal, S.K. Mineralogical investigation on preheating studies of high LOI iron ore pellet. Powder Technol. 2023, 418, 118315. [Google Scholar] [CrossRef]
- Palone, O.; Barberi, G.; Di Gruttola, F.; Gagliardi, G.G.; Cedola, L.; Borello, D. Assessment of a multistep revamping methodology for cleaner steel production. J. Clean. Prod. 2022, 381, 135146. [Google Scholar] [CrossRef]
- Burnett, J.W.H.; Li, J.; McCue, A.J.; Kechagiopoulos, P.N.; Howe, R.F.; Wang, X. Directing the H2-driven selective regeneration of NADH via Sn-doped Pt/SiO2. Green Chem. 2022, 24, 1451–1455. [Google Scholar] [CrossRef]
- Wang, R.R.; Zhao, Y.Q.; Babich, A.; Senk, D.; Fan, X.Y. Hydrogen direct reduction (H-DR) in steel industry—An overview of challenges and opportunities. J. Clean. Prod. 2021, 329, 129797. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.; Li, F.; Feng, C.; Liu, Z.; Zhou, Y. Development and progress on hydrogen metallurgy. Int. J. Miner. Metall. Mater. 2020, 27, 713–723. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.; Chu, M.; Zhu, M. Effect of porosity of Hongge vanadium titanomagnetite-oxidized pellet on its reduction swelling behavior and mechanism with hydrogen-rich gases. Powder Technol. 2019, 343, 194–203. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Liang, Z.; Peng, X.; Deng, X.; Li, J.; Yi, L.; Huang, B.; Chen, J. Direct reduction of iron ore pellets by H2-CO mixture: An in-situ investigation of the evolution and dynamics of swelling. Mater. Today Commun. 2023, 36, 106940. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, S.; Wang, Y.; Zhang, J.; Cheng, Q.; Ma, Y. Study on optimization of reduction temperature of hydrogen-based shaft furnace—Numerical simulation and multi-criteria evaluation. Int. J. Hydrogen Energy 2023, 48, 16132–16142. [Google Scholar] [CrossRef]
- Loder, A.; Siebenhofer, M.; Böhm, A.; Lux, S. Clean iron production through direct reduction of mineral iron carbonate with low-grade hydrogen sources; the effect of reduction feed gas composition on product and exit gas composition. Clean. Eng. Technol. 2021, 5, 100345. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, J.; Zuo, H.; Jiao, K.; Hu, Z.; Xing, X. Mechanisms of swelling of iron ore oxidized pellets in high reduction potential atmosphere. J. Iron Steel Res. Int. 2015, 22, 1–8. [Google Scholar] [CrossRef]
- Elsherbiny, A.A.; Qiu, D.; Wang, K.; Li, M.; Ahmed, M.; Hammam, A.; Zhu, Y.; Song, W.; Galal, A.M.; Chen, H.; et al. Parametric study on hematite pellet direct reduction by hydrogen. Powder Technol. 2024, 435, 119434. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, M.; Liu, Z.; Chen, S.; Xue, X. Effects of temperature and atmosphere on pellets reduction swelling index. J. Iron Steel Res. Int. 2012, 19, 7–19. [Google Scholar] [CrossRef]
- Patenthub Home Page. Available online: https://www.patenthub.cn/patent/CN118186208A.html (accessed on 29 July 2024).
- GB/T 24236–2009; Iron Ores for Shaft Direct-Reduction Feedstocks—Determination of the Reducibility Index, Final Degree of Reduction and Degree of Metallization. State General Administration of China for Quality Supervision and Inspection and Quarantine & Standardization Administration of China: Beijing, China, 2009.
- GB/T 6730.5–2022; Iron Ores—Determination of Total Iron Content—Titrimetric Methods after Titanium (III) Chloride Reduction. State Administration for Market Regulation and Standardization Administration of China & Standardization Administration of China: Beijing, China, 2022.
- GB/T 14201–2018; Iron Ore Pellets for Blast Furnace and Direct Reduction Feedstocks—Determination of the Crushing Strength. State Administration for Market Regulation and Standardization Administration of China & Standardization Administration of China: Beijing, China, 2018.
- Wang, P.; Wang, C.; Wang, H.; Long, H.; Zhou, T. Effects of SiO2, CaO and basicity on reduction behaviors and swelling properties of fluxed pellet at different stages. Powder Technol. 2022, 396, 477–489. [Google Scholar] [CrossRef]
- Peng, Z.; Fan, W.; Tang, H.; Xiang, C.; Ye, L.; Yin, T.; Rao, M. Direct conversion of blast furnace ferronickel slag to thermal insulation materials. Constr. Build. Mater. 2024, 412, 134499. [Google Scholar] [CrossRef]
- Feinman, J.; Smith, N.D.; Muskat, D.A. Effect of gas flow rate on kinetics of reduction of iron oxide pellets with hydrogen. Ind. Eng. Chem. Process Des. Dev. 1965, 4, 270–274. [Google Scholar] [CrossRef]
- Singh, A.K.; Mishra, B.; Sinha, O.P. Reduction kinetics of fluxed iron ore pellets made of coarse iron ore particles. Steel Res. Int. 2024, in press. [Google Scholar] [CrossRef]
- Sakurai, S.; Namai, A.; Hashimoto, K.; Ohkoshi, S.-I. First observation of phase transformation of all four Fe2O3 phases (γ → ε → β → α-phase). J. Am. Chem. Soc. 2009, 131, 18299–18303. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, D.; Huang, Y.; Jiang, T. Swelling behavior of hot preheated pellets in reduction roasting process. J. Cent. South Univ. 2016, 23, 2792–2799. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, Z.; Zhu, D.; Pan, J.; Lu, S. Isothermal reduction kinetics and microstructure evolution of various vanadium titanomagnetite pellets in direct reduction. J. Alloys Compd. 2023, 953, 170126. [Google Scholar] [CrossRef]
- Wang, H.; Shen, L.; Bao, H.; Zhang, W.; Zhang, X.; Luo, L.; Song, S. Investigation of solid-state carbothermal reduction of fayalite with and without added metallic iron. JOM 2021, 73, 703–711. [Google Scholar] [CrossRef]
- Ma, Y.; Filho, I.R.S.; Bai, Y.; Schenk, J.; Patisson, F.; Beck, A.; Van Bokhoven, J.A.; Willinger, M.G.; Li, K.; Xie, D.; et al. Hierarchical nature of hydrogen-based direct reduction of iron oxides. Scr. Mater. 2022, 213, 114571. [Google Scholar] [CrossRef]
- Abu Tahari, M.N.; Salleh, F.; Tengku Saharuddin, T.S.; Samsuri, A.; Samidin, S.; Yarmo, M.A. Influence of hydrogen and carbon monoxide on reduction behavior of iron oxide at high temperature: Effect on reduction gas concentrations. Int. J. Hydrogen Energy 2021, 46, 24791–24805. [Google Scholar] [CrossRef]
- Kazemi, M.; Glaser, B.; Sichen, D. Study on direct reduction of hematite pellets using a new TG setup. Steel Res. Int. 2014, 85, 718–728. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, J.; Chu, M.; Wang, X.; Zheng, A.; Wang, X.; Li, Y. Direct reduction swelling behavior of pellets in hydrogen-based shaft furnaces under typical atmospheres. Int. J. Miner. Metall. Mater. 2022, 29, 1891–1900. [Google Scholar] [CrossRef]
- Scharm, C.; Küster, F.; Laabs, M.; Huang, Q.; Volkova, O.; Reinmöller, M.; Guhl, S.; Meyer, B. Direct reduction of iron ore pellets by H2 and CO: In-situ investigation of the structural transformation and reduction progression caused by atmosphere and temperature. Miner. Eng. 2022, 180, 107459. [Google Scholar] [CrossRef]






| TFe | SiO2 | FeO | Al2O3 | CaO | Na2O | MgO | K2O | P | S |
|---|---|---|---|---|---|---|---|---|---|
| 64.00 | 8.51 | 1.48 | 0.42 | 0.18 | 0.18 | 0.15 | 0.10 | 0.045 | 0.0046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Peng, Z.; Tian, R.; Luo, G.; Yi, L.; Rao, M. Toward Metallized Pellets for Steelmaking by Hydrogen Cooling Reduction: Effect of Gas Flow Rate. Materials 2024, 17, 3896. https://doi.org/10.3390/ma17163896
Fan W, Peng Z, Tian R, Luo G, Yi L, Rao M. Toward Metallized Pellets for Steelmaking by Hydrogen Cooling Reduction: Effect of Gas Flow Rate. Materials. 2024; 17(16):3896. https://doi.org/10.3390/ma17163896
Chicago/Turabian StyleFan, Wanlong, Zhiwei Peng, Ran Tian, Guanwen Luo, Lingyun Yi, and Mingjun Rao. 2024. "Toward Metallized Pellets for Steelmaking by Hydrogen Cooling Reduction: Effect of Gas Flow Rate" Materials 17, no. 16: 3896. https://doi.org/10.3390/ma17163896
APA StyleFan, W., Peng, Z., Tian, R., Luo, G., Yi, L., & Rao, M. (2024). Toward Metallized Pellets for Steelmaking by Hydrogen Cooling Reduction: Effect of Gas Flow Rate. Materials, 17(16), 3896. https://doi.org/10.3390/ma17163896









