Characterization of Wear Resistance and Corrosion Resistance of Plasma Paste Borided Layers Produced on Pure Titanium
Abstract
:1. Introduction
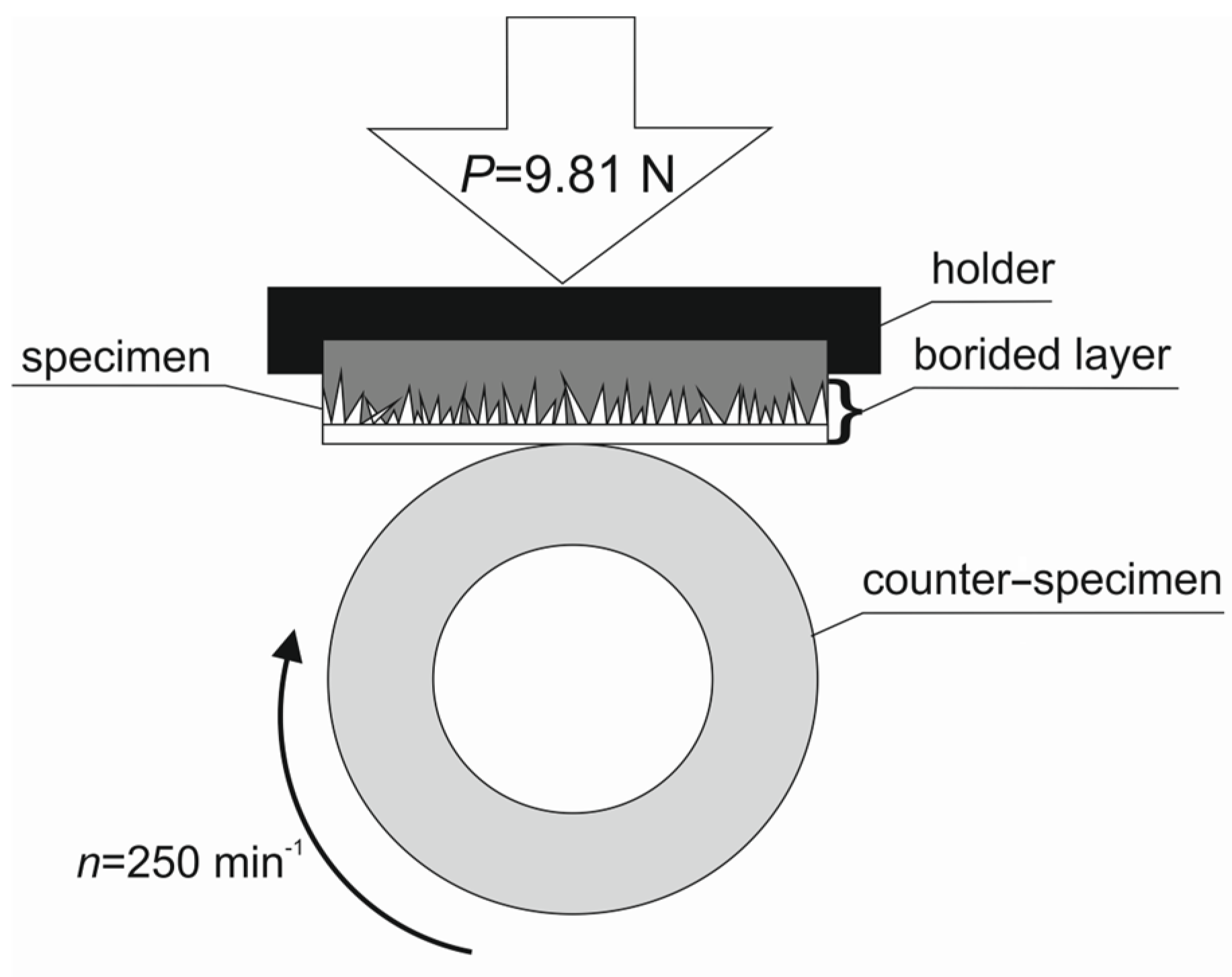
2. Materials and Methods
3. Results and Discussion
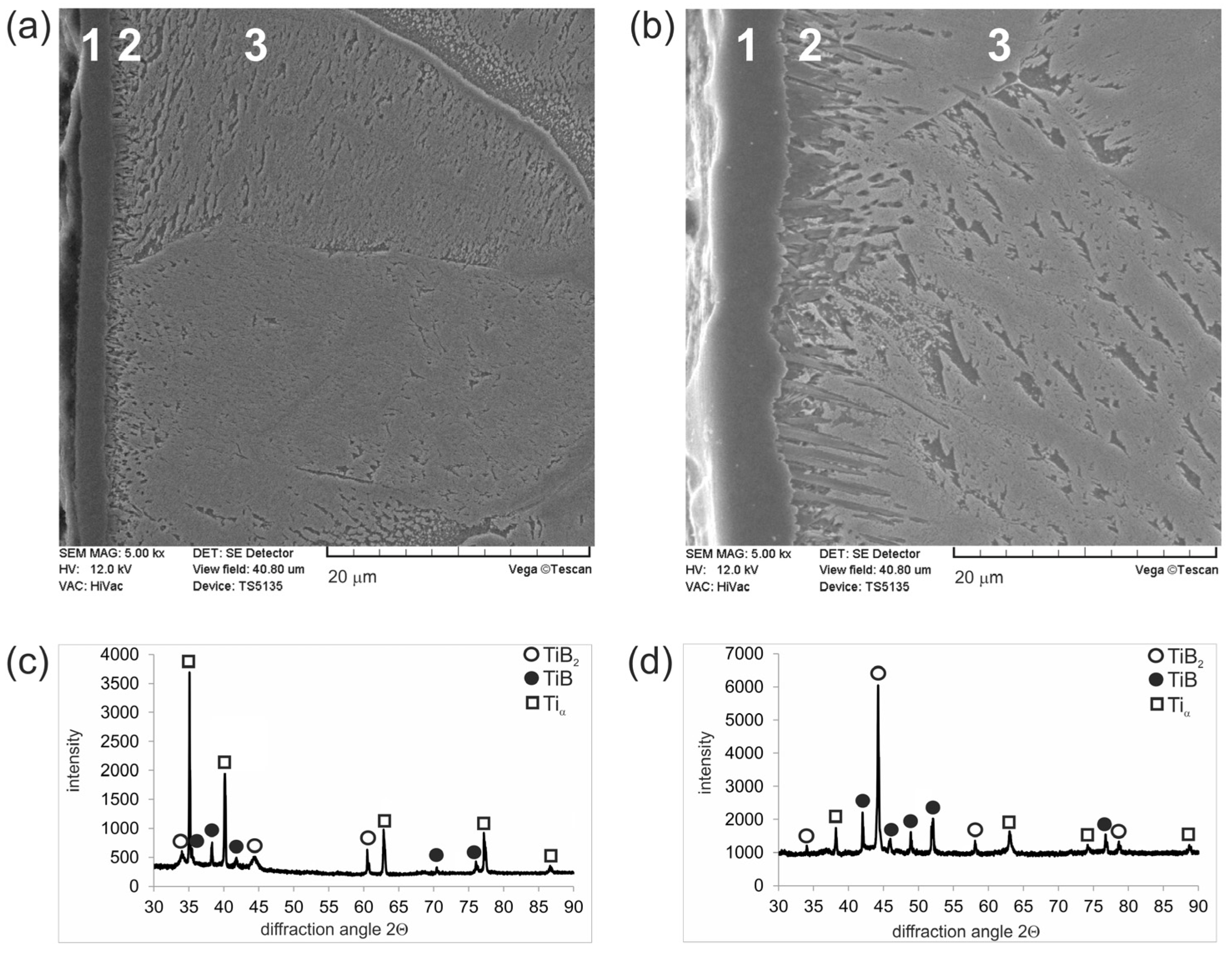
3.1. Microstructure
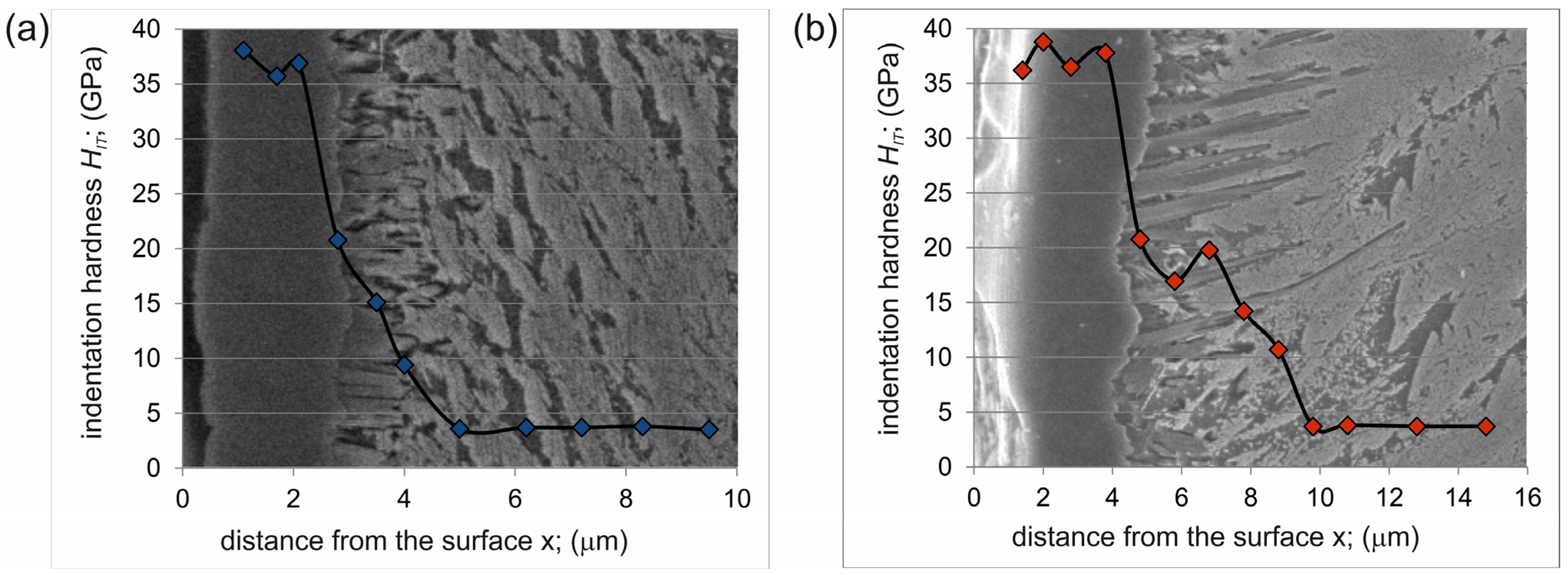
3.2. Indentation Hardness
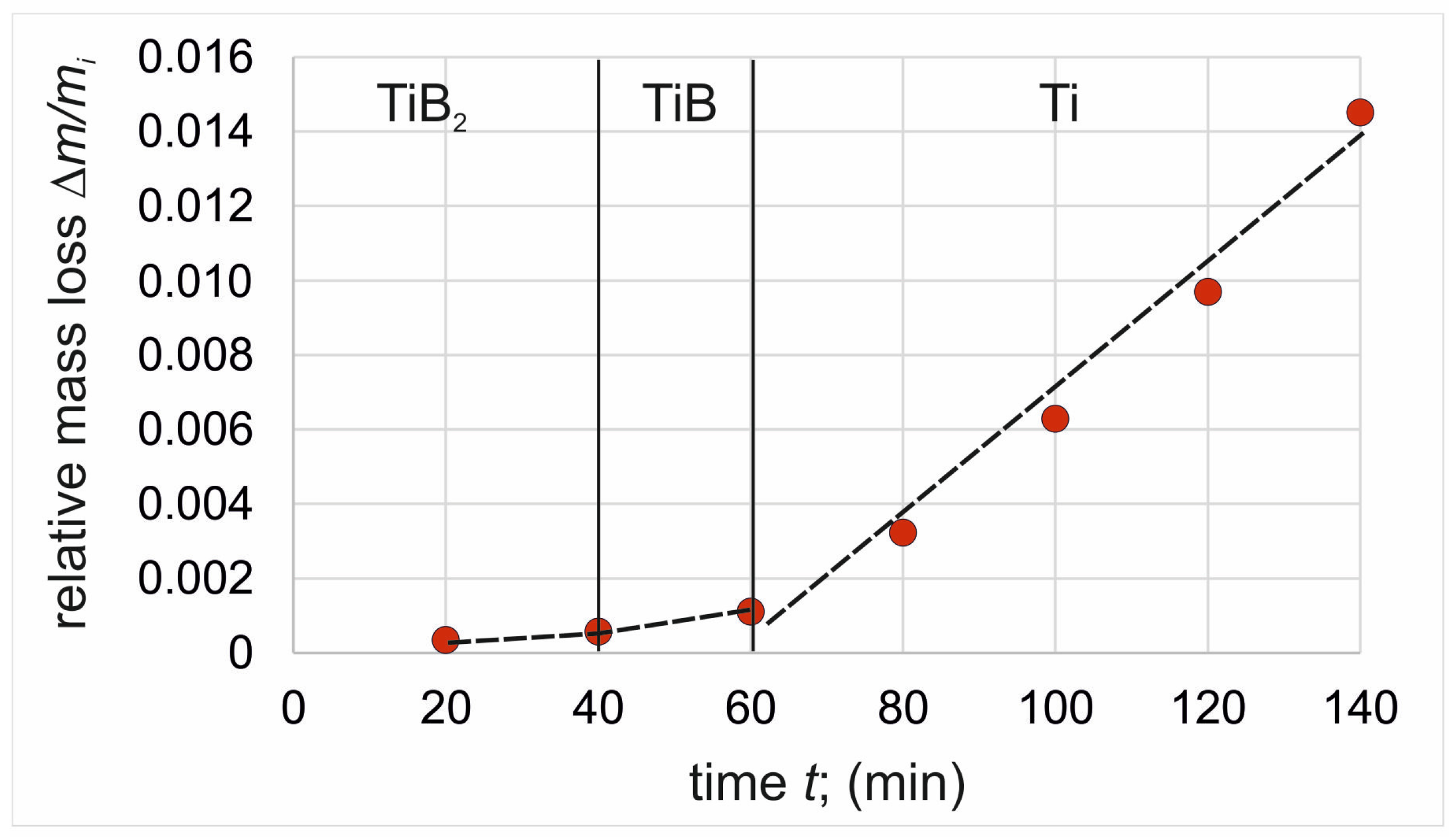
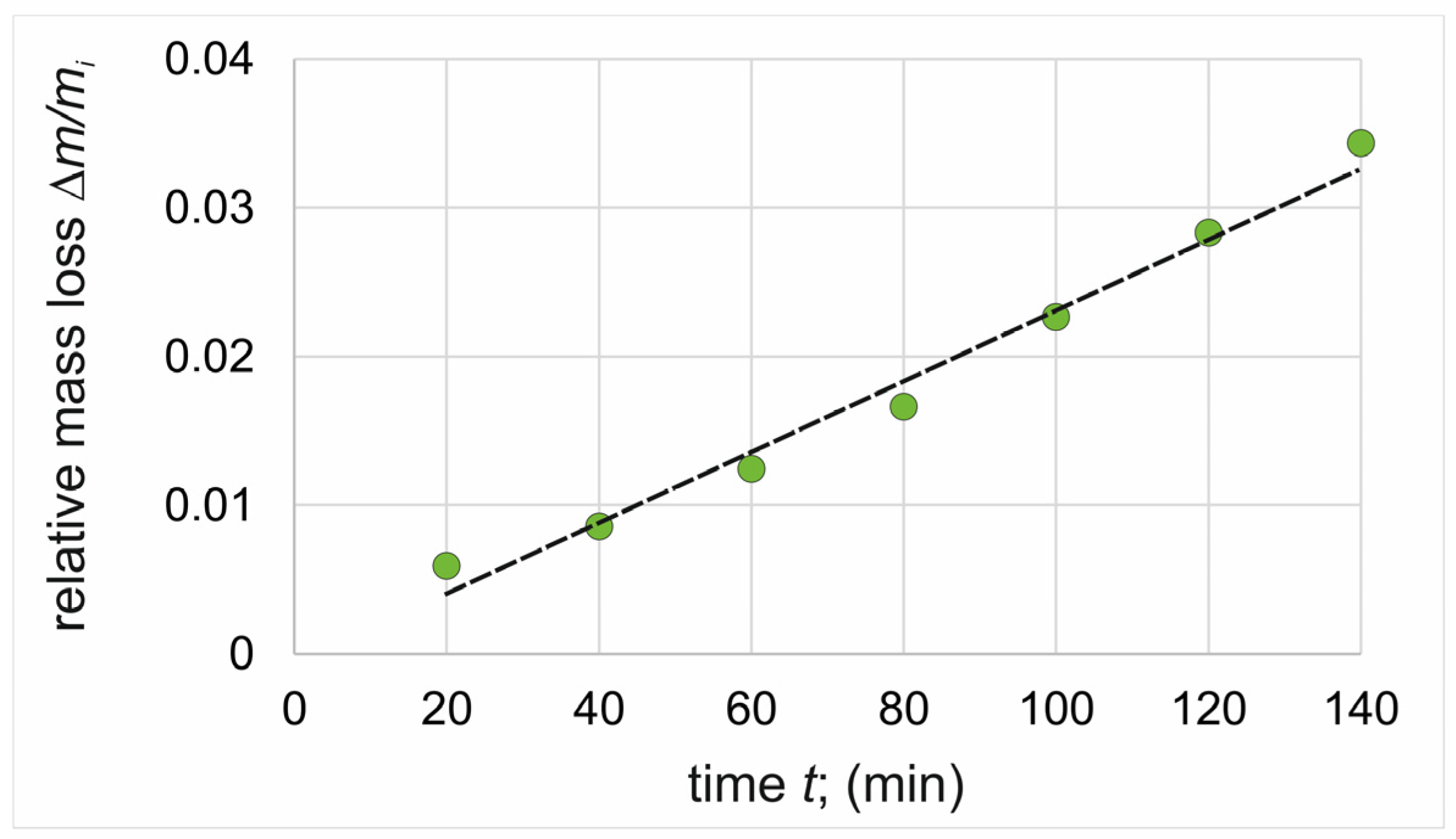
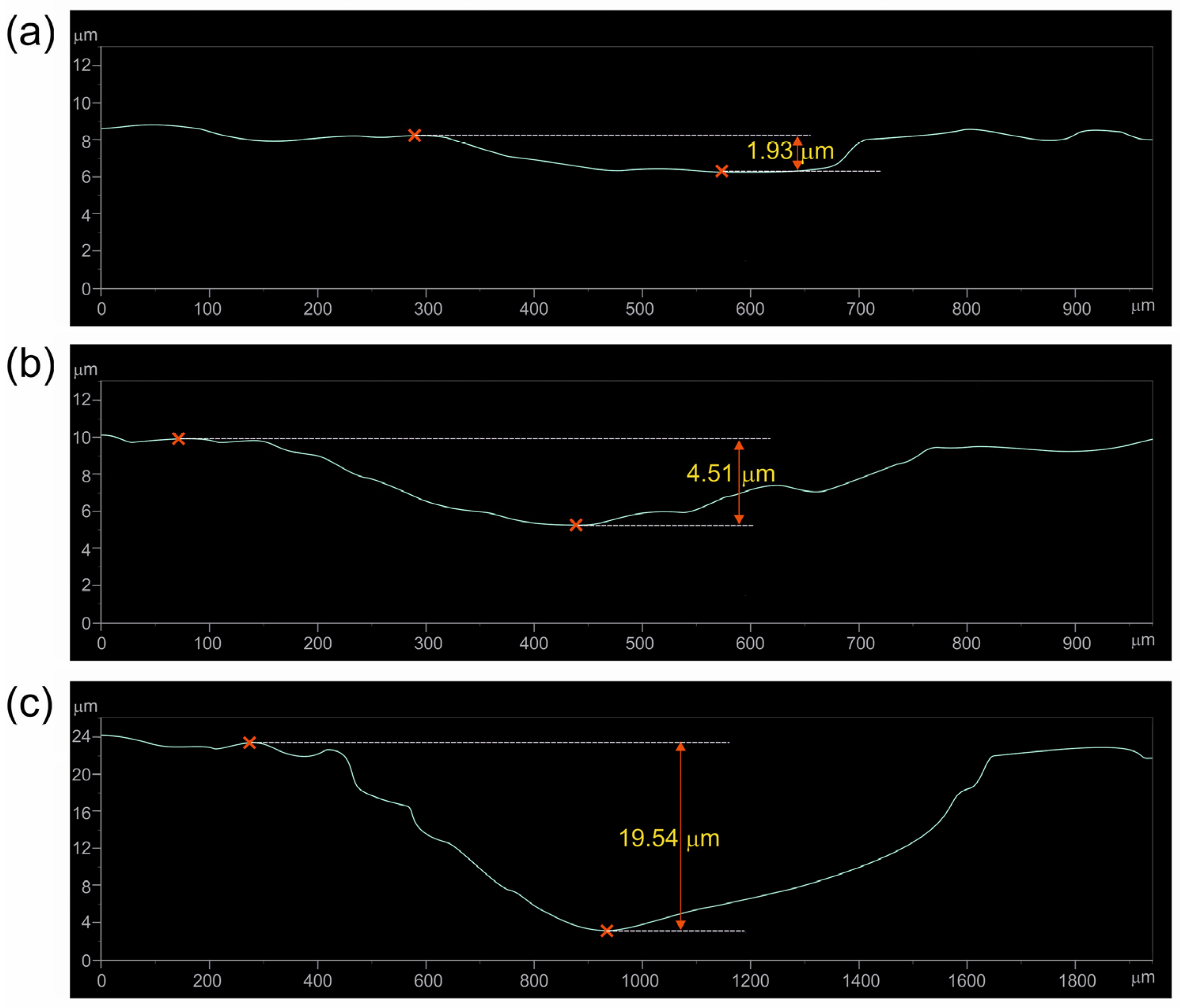
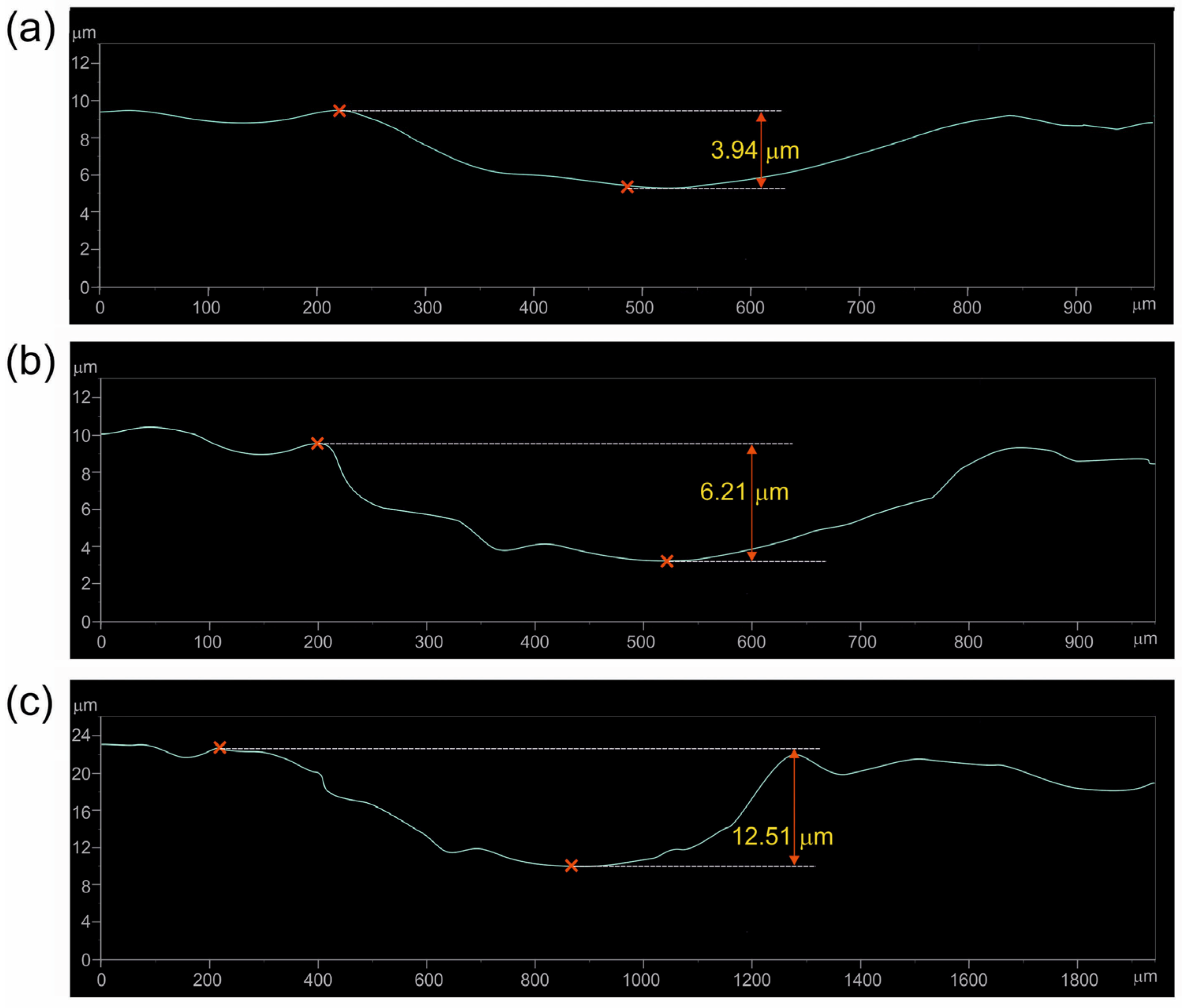
3.3. Wear Resistance
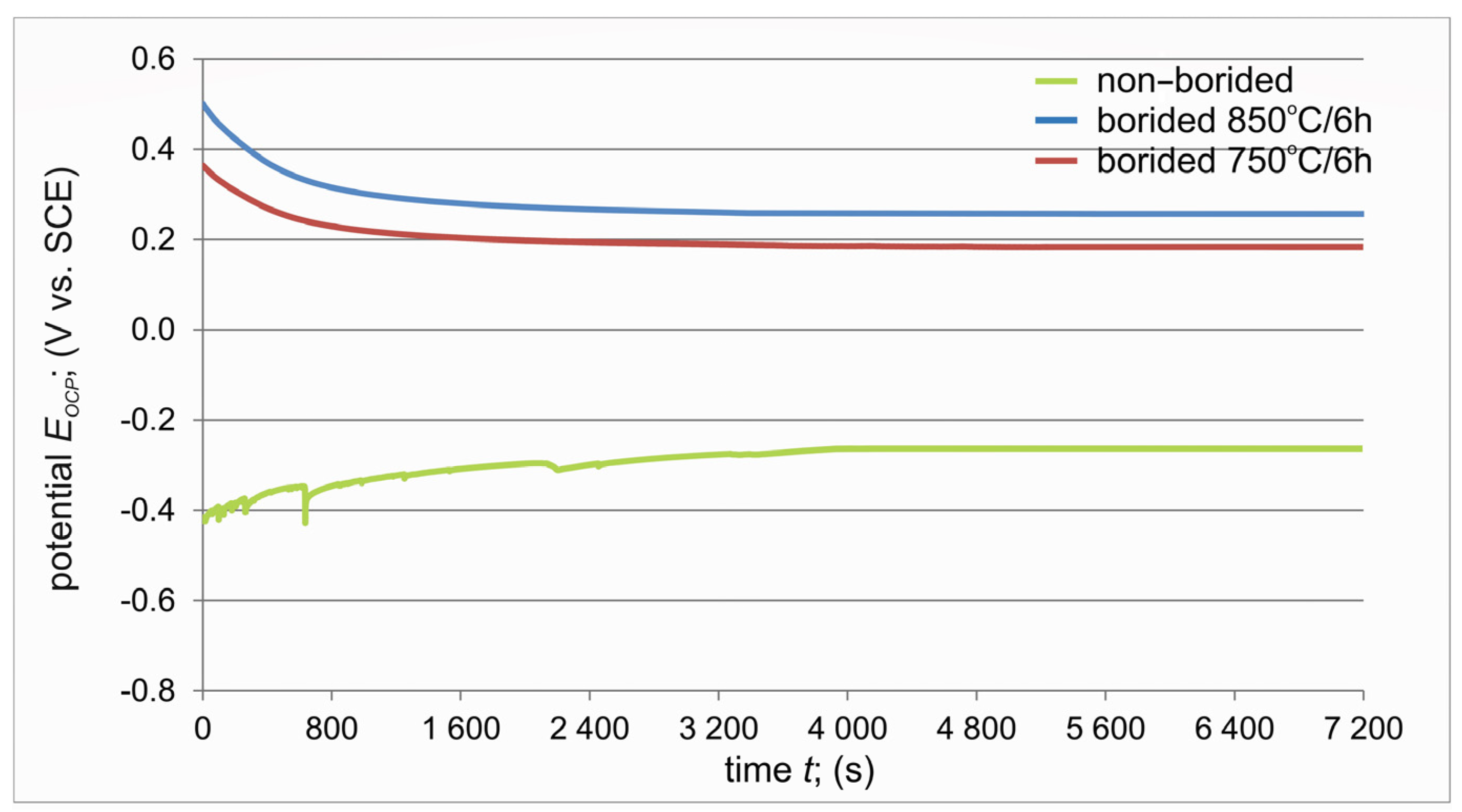
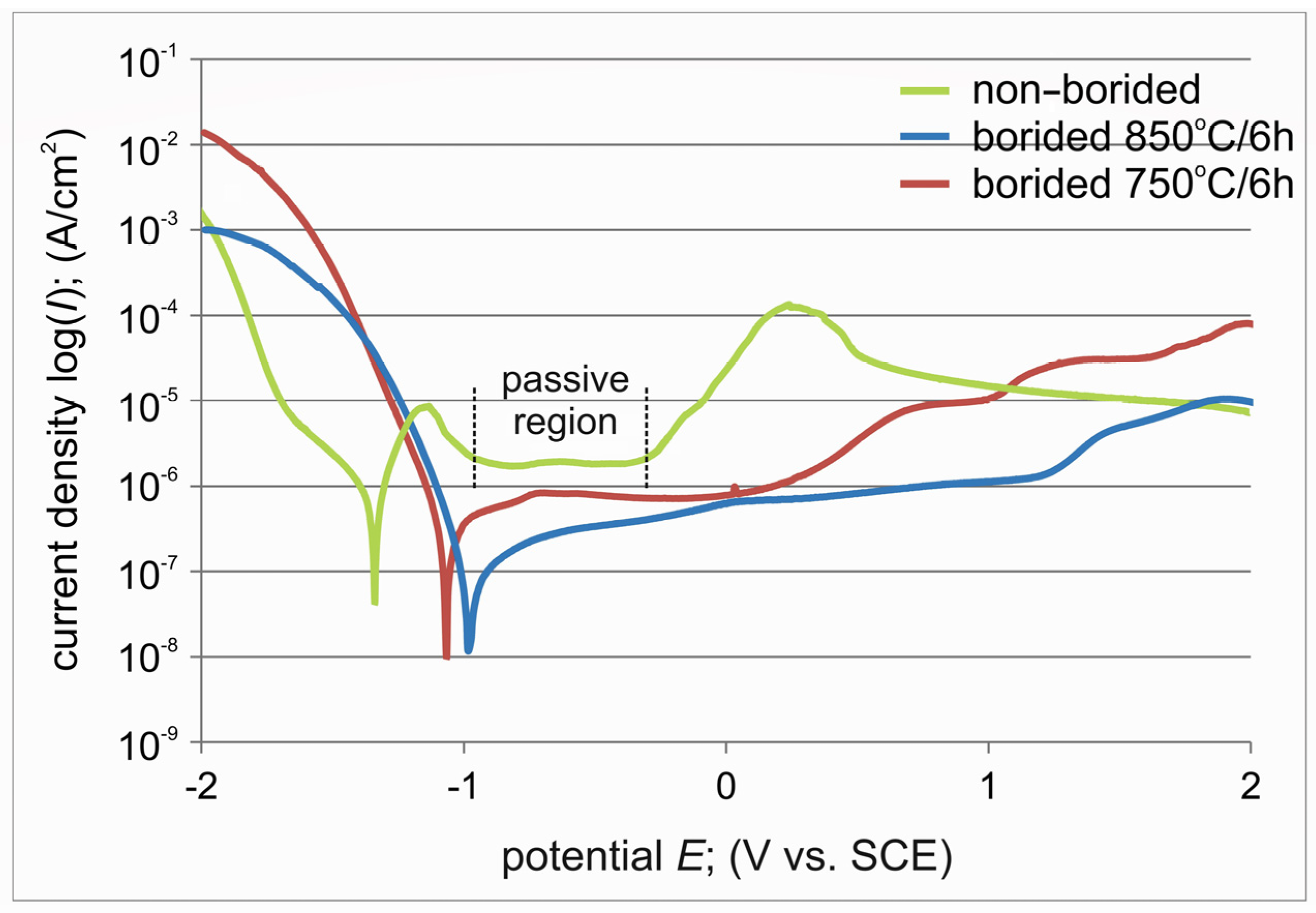
3.4. Corrosion Resistance
4. Conclusions
- Higher wear resistance was characteristic of the TiB2 layer due to its compact character and higher hardness (EIT = 35 ÷ 38 GPa).
- The specific morphology of TiB whiskers and the presence of substrate material between them resulted in their lower wear resistance compared to the outer TiB2 layer.
- Considering the time required for the complete wear of the borided layer, it is more advantageous to boride at a higher temperature, which is, obviously, related to the higher thickness of the layer produced.
- The more noble open circuit potential EOCP was measured for borided specimens.
- The shape of the open circuit potential curve registered for non-borided titanium indicated the tendency of this material to form a protective passive layer.
- The electrochemical parameters (Ecorr and Icorr) estimated from the potentiodynamic polarization curves indicated a higher corrosion resistance of both plasma paste borided specimens compared to the non-borided titanium.
- The thickness of the outer TiB2 zone determined the corrosion resistance of the borided specimens. In the case of a TiB2 layer of low thickness, pitting, which occurs quickly during the test, breaks through this zone easily, and corrosion of the TiB whisker zone occurs. The higher thickness of the TiB2 zone hindered the penetration of pits into the TiB whisker zone.
- Since the TiB whisker zone is not a continuous zone, but there is substrate material between the whiskers, the TiB whisker zone is not an effective barrier against further corrosion.
- The shape of the potentiodynamic polarization curve indicated that only non-borided titanium had a tendency toward passivation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boumaali, B.; Keddam, M.; Taktak, S. Growth kinetics of two-phase boride layers formed by boriding in molten salts on Cp-Ti substrate. Int. J. Mater. Eng. Innov. 2018, 9, 240–255. [Google Scholar] [CrossRef]
- Kartal, G.; Timur, S.; Urgen, M.; Erdemir, A. Electrochemical boriding of titanium for improved mechanical properties. Surf. Coat. Technol. 2010, 204, 3935–3939. [Google Scholar] [CrossRef]
- Kaestner, P.; Olfe, J.; Rie, K.T. Plasma-assisted boriding of pure titanium and TiAl6V4. Surf. Coat. Technol. 2001, 142–144, 248–252. [Google Scholar] [CrossRef]
- Munirathinam, B.; Narayanan, R.; Neelakantan, L. Electrochemical and semiconducting properties of thin passive film formed on titanium in chloride medium at various pH conditions. Thin Solid Film. 2016, 598, 260–270. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, S.; Fu, X.Z.; Wang, X.Z.; Luo, J.L. Transition of self-passivation and semiconductor property of titanium in the simulated environments of proton exchange membrane fuel cells. Appl. Surf. Sci. 2023, 612, 155930-1–155930-14. [Google Scholar] [CrossRef]
- Bloyce, A. Surface engineering of titanium alloys for wear protection. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 1998, 212, 467–476. [Google Scholar] [CrossRef]
- Coulon, P.A.; Alsthom, G. Resistance to wear of nitriding on titanium alloy using the laser-irradiation technique. J. Mater. Process. Technol. 1993, 38, 247–263. [Google Scholar] [CrossRef]
- Fleszar, A.; Wierzchon, T.; Kim, S.K.; Sobiecki, J.R. Properties of surface layers produced on the Ti6Al3Mo2Cr titanium alloy under glow discharge conditions. Surf. Coat. Technol. 2000, 131, 62–65. [Google Scholar] [CrossRef]
- Yilbaş, B.S.; Şahin, A.Z.; Al-Garni, A.Z.; Said, S.A.M.; Ahmed, Z.; Abdulaleem, B.J.; Sami, M. Plasma nitriding of Ti-6Al-4V alloy to improve some tribological properties. Surf. Coat. Technol. 1996, 80, 287–292. [Google Scholar] [CrossRef]
- Man, H.C.; Bai, M.; Cheng, F.T. Laser diffusion nitriding of Ti–6Al–4V for improving hardness and wear resistance. Appl. Surf. Sci. 2011, 258, 436–441. [Google Scholar] [CrossRef]
- Makuch, N.; Kulka, M.; Keddam, M.; Taktak, S.; Ataibis, V.; Dziarski, P. Growth kinetics and some mechanical properties of two-phase boride layers produced on commercially pure titanium during plasma paste boriding. Thin Solid Film. 2017, 626, 25–37. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, X.; Liu, D.; Bao, W.; Li, P.; Peng, M. Characteristics, wear and corrosion properties of borided pure titanium by pack boriding near α→β phase transition temperature. Ceram. Int. 2020, 46, 16380–16387. [Google Scholar] [CrossRef]
- Keddam, M.; Makuch, N.; Boumaali, B.; Piasecki, A.; Miklaszewski, A.; Kulka, M. Liquid boriding of Cp-Ti and Ti6Al4V alloy: Characterization of boride layers and tribological properties. Surf. Eng. Appl. Electrochem. 2020, 56, 348–357. [Google Scholar] [CrossRef]
- Kim, T.S.; Park, Y.G.; Wey, M.Y. Characterization of Ti–6Al–4V alloy modified by plasma carburizing process. Mater. Sci. Eng. A 2003, 361, 275–280. [Google Scholar] [CrossRef]
- Feng, Z.; Duan, Y.; Peng, M. Growth kinetics, and wear and corrosion properties of TiC coatings on pure titanium by carburizing. Metall. Mater. Trans. A 2023, 54, 2947–2959. [Google Scholar] [CrossRef]
- Atar, E.; Kayali, E.S.; Cimenoglu, H. Characteristics and wear performance of borided Ti6Al4V alloy. Surf. Coat. Technol. 2008, 202, 4583–4590. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Dziarski, P.; Piasecki, A.; Miklaszewski, A. Microstructure and properties of laser-borided composite layers formed on commercially pure titanium. Opt. Laser Technol. 2014, 56, 409–424. [Google Scholar] [CrossRef]
- Qu, D.Y.; Dan, L.I.U.; Wang, X.Y.; Duan, Y.H.; Peng, M.J. Corrosion and wear properties of TB2 titanium alloy borided by pack boriding with La2O3. Trans. Nonferrous Met. Soc. China 2022, 32, 868–881. [Google Scholar] [CrossRef]
- Xing, Z.; Feng, Z.; Wu, Y.; Lu, Y.; Duan, Y.; Zheng, S.; Peng, M.; Li, M.; Wu, Y. Growth kinetics and corrosion properties of pack-borided Ti-5Al-2.5Sn alloy. Surf. Coat. Technol. 2023, 473, 130003-1–130003-14. [Google Scholar] [CrossRef]
- Bose, S.; Pathak, L.C.; Singh, R. Response of boride coating on the Ti-6Al-4V alloy to corrosion and fretting corrosion behavior in Ringer’s solution for bio-implant application. Appl. Surf. Sci. 2018, 433, 1158–1174. [Google Scholar] [CrossRef]
- Rie, K.T. Recent advances in plasma diffusion processes. Surf. Coat. Technol. 1999, 112, 56–62. [Google Scholar] [CrossRef]
- Ataibis, V.; Taktak, S. Characteristics and growth kinetics of plasma paste borided Cp–Ti and Ti6Al4V alloy. Surf. Coat. Technol. 2015, 279, 65–71. [Google Scholar] [CrossRef]
- Keddam, M.; Taktak, S.; Tasgetiren, S. A diffusion model for the titanium borides on pure titanium. Surf. Eng. 2016, 32, 802–808. [Google Scholar] [CrossRef]
- Kunst, H.; Schaaber, O. Beobachtungen beim Oberflaechenborieren von Stahl II. Haerterei Tech. Mitteilungen 1967, 22, 1–25. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic 1modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Dziarski, P.; Piasecki, A. A study of nanoindentation for mechanical characterization of chromium and nickel borides’ mixtures formed by laser boriding. Ceram. Int. 2014, 40, 6083–6094. [Google Scholar] [CrossRef]
- Makuch, N.; Kulka, M.; Paczkowska, M. Nanomechanical properties of gas-borided layer produced on Nimonic 80A-alloy. Ceram. Int. 2017, 43, 8255–8261. [Google Scholar] [CrossRef]
- Perez, N. Electrochemistry and Corrosion Science; Kluwer Academic Publishers: Boston, MA, USA, 2004. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Majed, R.A. Effect of Molybdate Anions on Corrosion Behavior of Stainless Steel 304 In 0.1M NaCl Solution. Eng. Technol. J. 2009, 27, 2931–2945. [Google Scholar] [CrossRef]
- Al-Shama, L.M.; Saleh, J.M.; Hikmat, N.A. Potentiostatic studies of the corrosion of grey cast iron in sulphuric acid and sodium hydroxide solutions. Corros. Sci. 1987, 27, 221–228. [Google Scholar] [CrossRef]
- Lee, C.; Sanders, A.; Tikekar, N.; Ravi Chandran, K.S. Tribology of titanium boride-coated titanium balls against alumina ceramic: Wear, friction, and micromechanisms. Wear 2008, 265, 375–386. [Google Scholar] [CrossRef]
- Kara, G.; Purcek, G.; Yanar, H. Improvement of wear behaviour of titanium by boriding. Ind. Lubr. Tribol. 2017, 69, 65–70. [Google Scholar] [CrossRef]
- Canary, S.; Hersek, N.; Culha, A.; Bilgic, S. Evaluation of titanium in oral conditions and its electrochemical corrosion behavior. J. Oral Rehabil. 1998, 25, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Santana Jiménez, Y.; Tejera Gil, M.; Torrado Guerra, M.; Baltes, L.S.; Mirza Rosca, J.C. Interpretation of open circuit potential of two titanium alloys for a long time immersion in physiological fluid. Bull. Transilv. Univ. Braşov 2009, 2, 197–204. [Google Scholar]
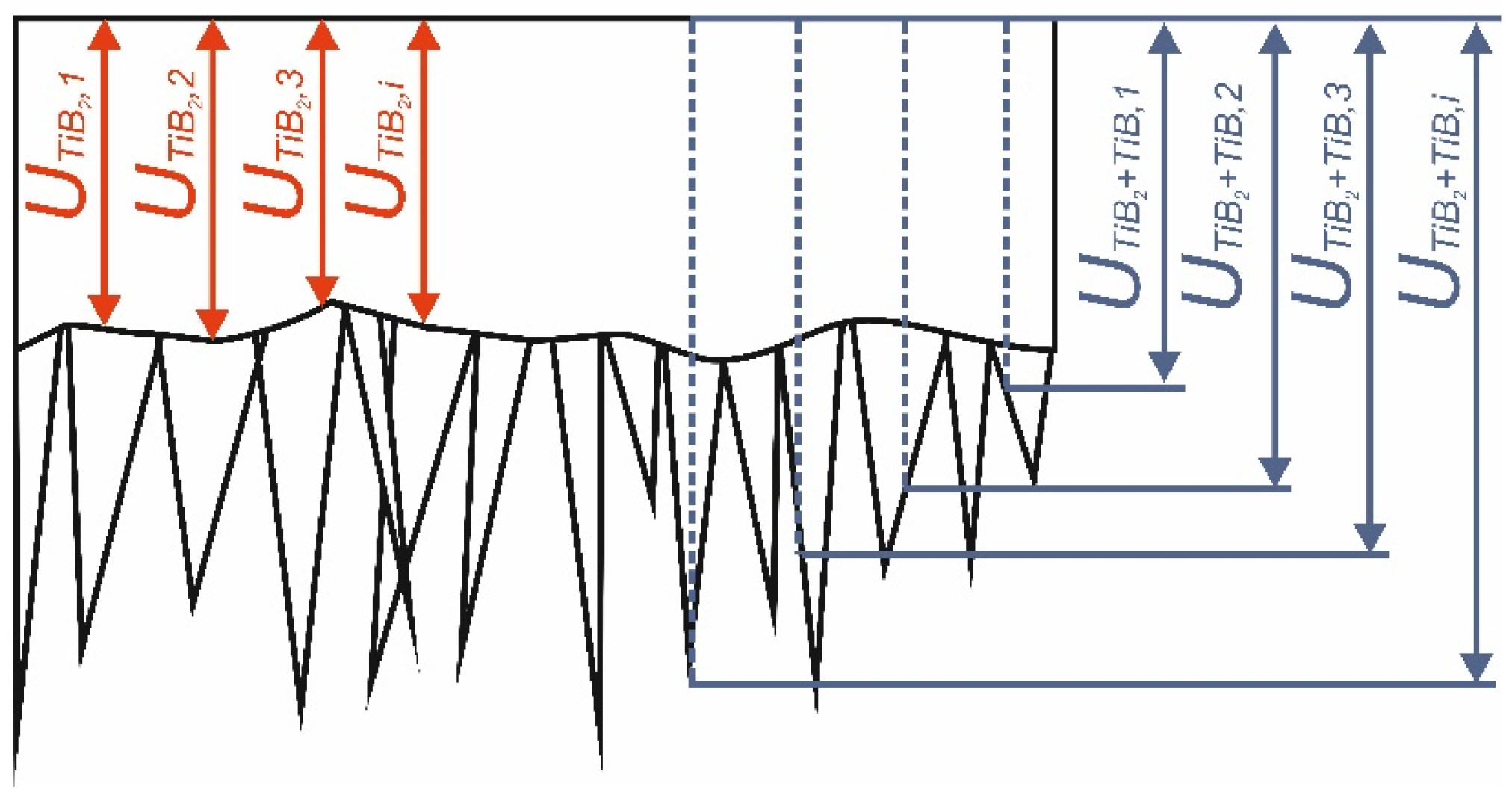


| Zone | Specimen Borided at 750 °C for 6 h | Specimen Borided at 850 °C for 6 h | Non-Borided Specimen | |||
|---|---|---|---|---|---|---|
| slope | R2 | slope | R2 | slope | R2 | |
| TiB2 | 0.000013 min−1 | 1 | 0.000011 min−1 | 0.993 | - | |
| TiB | 0.000064 min−1 | 1 | 0.000051 min−1 | 0.993 | - | |
| Ti (substrate) | 0.000202 min−1 | 0.978 | 0.000169 min−1 | 0.995 | 0.000231 min−1 | 0.985 |
| Wear Stage | Specimen Borided at 750 °C for 6 h | Specimen Borided at 850 °C for 6 h | |
|---|---|---|---|
| First | Depth of the worn tracks (µm) | 1.93 | 3.94 |
| Thickness of TiB2 layer UTiB2 (µm) | 2.42 | 4.14 | |
| Second | Depth of the worn tracks (µm) | 4.51 | 6.21 |
| Total thickness of layer TiB2 + TiB UTiB2+TiB (µm) | 3.61 | 7.30 | |
| Zone | Borided Specimen at 750 °C for 6 h | Borided Specimen at 850°C for 6 h | Non-Borided Specimen |
|---|---|---|---|
| TiB2 | 0.0712 mg/min | 0.0617 mg/min | - |
| TiB | 0.2233 mg/min | 0.2101 mg/min | - |
| Ti (substrate) | 0.8832 mg/min | 0.7285 mg/min | 1.1204 mg/min |
| Ref. | Substrate Material/Boriding Method | Boriding Parameters | TiB2 Layer Thickness (µm) | Total Layer Thickness (µm) | Results of Wear Test | Conditions of Wear Test |
|---|---|---|---|---|---|---|
| [11] | Cp-Ti/plasma paste boriding | non-borided 800 °C/3 h 800 °C/6 h 850 °C/3 h 850 °C/6 h | - 2.44 3.51 3.32 4.04 | - 3.97 5.11 5.46 7.30 | Δm/mi = 0.000965 Δm/mi = 0.000175 Δm/mi = 0.000087 Δm/mi = 0.000123 Δm/mi = 0.000105 | Load: 19.6 N; Counter-specimen: low-temperature tempered 100CrMnSi6-4 steel Test duration: 30 min. |
| [12] | Cp-Ti/powder boriding | non-borided 880 °C/20 h 920 °C/20 h | - 5 10 | - 70 40 | COF = 0.432; Δm/mi = 0.00048 COF = 0.353; Δm/mi = 0.00032 COF = 0.284; Δm/mi = 0.00011 | Load: 10 N; Counter-specimen: Al2O3 Test duration: 30 min. |
| [13] | Cp-Ti/liquid boriding | non-borided 1000 °C/12 h | - 9.4 | - 42.4 | Δm/mi = 0.00396 Δm/mi = 0.00074 | Load: 9.81 N; Counter-specimen: low-temperature tempered 100CrMnSi6-4 steel Test duration: 60 min. |
| [13] | Ti6Al4V/liquid boriding | non-borided 1000 °C/12 h | - 7.8 | - 25.7 | Δm/mi = 0.00381 Δm/mi = 0.00073 | Load: 9.81 N; Counter-specimen: low-temperature tempered 100CrMnSi6-4 steel Test duration: 30 min. |
| [16] | Ti6Al4V/powder boriding | non-borided 1100 °C/2.5 h | - 10 | - 60 | COF = 0.772; RWR = 100% COF = 0.381; RWR = 1% | Load: 12 N Counter-specimen: sapphire ball Test duration: 150 min. |
| [18] | TB2/ powder boriding | non-borided 1100 °C/20 h | - 7.01 | - 21.84 | Δm = 1.52 mg; Δm/mi = 0.00034 Δm = 0.61 mg; Δm/mi = 0.00014 | Load: 10 N; Counter-specimen: Al2O3 Test duration: 30 min. |
| [this work] | Cp-Ti/ plasma paste boriding | non-borided 750 °C/6 h 750 °C/6 h 850 °C/6 h 850 °C/6 h | - 2.4 2.4 4.1 4.1 | - 3.6 3.6 7.3 7.3 | Δm/ti = 1.1204 mg/min. TiB2: Δm/t= 0.0712 mg/min. TiB: Δm/t= 0.2233 mg/min. TiB2: Δm/t= 0.0617 mg/min. TiB: Δm/t = 0.2101 mg/min. | Load: 9.81 N; Counter-specimen: low-temperature tempered 100CrMnSi6-4 steel Test duration: 140 min. |
| Parameter | Borided Specimen at 750 °C for 6 h | Borided Specimen at 850 °C for 6 h | Non-Borided Specimen |
|---|---|---|---|
| Ecorr (V) | −1.091 | −0.985 | −1.341 |
| Icorr (A/cm2) | 1.56 × 10−7 | 7.78 × 10−8 | 8.73 × 10−7 |
| Ipass (A/cm2) | - | - | 1.96 × 10−6 |
| CR (µm/year) | 25.679 | 12.522 | 138.450 |
| Ref. | Substrate Material/Boriding Method | Boriding Parameters | TiB2 Layer Thickness (µm) | Total Layer Thickness (µm) | Corrosive Solution | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|---|---|---|---|---|
| [12] | Cp-Ti/powder boriding | non-borided 880 °C/20 h 920 °C/20 h non-borided 880 °C/20 h 920 °C/20 h | - 5 10 - 5 10 | - 70 40 - 70 40 | 3.5% NaCl 3.5% NaCl 3.5% NaCl 5% H2SO4 5% H2SO4 5% H2SO4 | −1.229 −0.897 −0.562 −0.468 −0.438 −0.429 | 1.414 × 10−6 0.933 × 10−6 0.465 × 10−11 1.101 × 10−6 0.802 × 10−6 0.157 × 10−6 |
| [19] | Ti-5Al-2.5Sn/ powder boriding | non-borided 975 °C/12 h 1000 °C/12 h 1025 °C/12 h 1050 °C/12 h 1075 °C/12 h non-borided 975 °C/12 h 1000 °C/12 h 1025 °C/12 h 1050 °C/12 h 1075 °C/12 h | - 13 15 16 16 17 - 13 15 16 16 17 | - 22 27 33 43 46 - 22 27 33 43 46 | 3.5% NaCl 3.5% NaCl 3.5% NaCl 3.5% NaCl 3.5% NaCl 3.5% NaCl 5% H2SO4 5% H2SO4 5% H2SO4 5% H2SO4 5% H2SO4 5% H2SO4 | −0.655 −0.566 −0.533 −0.494 −0.424 −0.813 −0.757 −0.757 −0.666 −0.590 −0.506 −0.489 | 0.231 × 10−8 0.129 × 10−8 0.071 × 10−8 0.059 × 10−8 0.044 × 10−8 0.032 × 10−8 0.370 × 10−5 0.135 × 10−5 0.228 × 10−6 0.079 × 10−6 0.044 × 10−6 0.028 × 10−8 |
| [this work] | Cp-Ti/ plasma paste boriding | non-borided 750 °C/6 h 850 °C/6 h | - 2.4 4.1 | - 3.6 7.3 | 3.5% NaCl 3.5% NaCl 3.5% NaCl | −1.341 −1.091 −0.985 | 0.773 × 10−6 0.156 × 10−6 0.778 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziarski, P.; Makuch, N. Characterization of Wear Resistance and Corrosion Resistance of Plasma Paste Borided Layers Produced on Pure Titanium. Materials 2024, 17, 3922. https://doi.org/10.3390/ma17163922
Dziarski P, Makuch N. Characterization of Wear Resistance and Corrosion Resistance of Plasma Paste Borided Layers Produced on Pure Titanium. Materials. 2024; 17(16):3922. https://doi.org/10.3390/ma17163922
Chicago/Turabian StyleDziarski, Piotr, and Natalia Makuch. 2024. "Characterization of Wear Resistance and Corrosion Resistance of Plasma Paste Borided Layers Produced on Pure Titanium" Materials 17, no. 16: 3922. https://doi.org/10.3390/ma17163922






