Simulated Body Fluid-Assisted Stress Corrosion Cracking of a Rapidly Solidified Magnesium Alloy RS66
Abstract
1. Introduction
2. Experimental Procedure
2.1. Test Materials and Test Environment
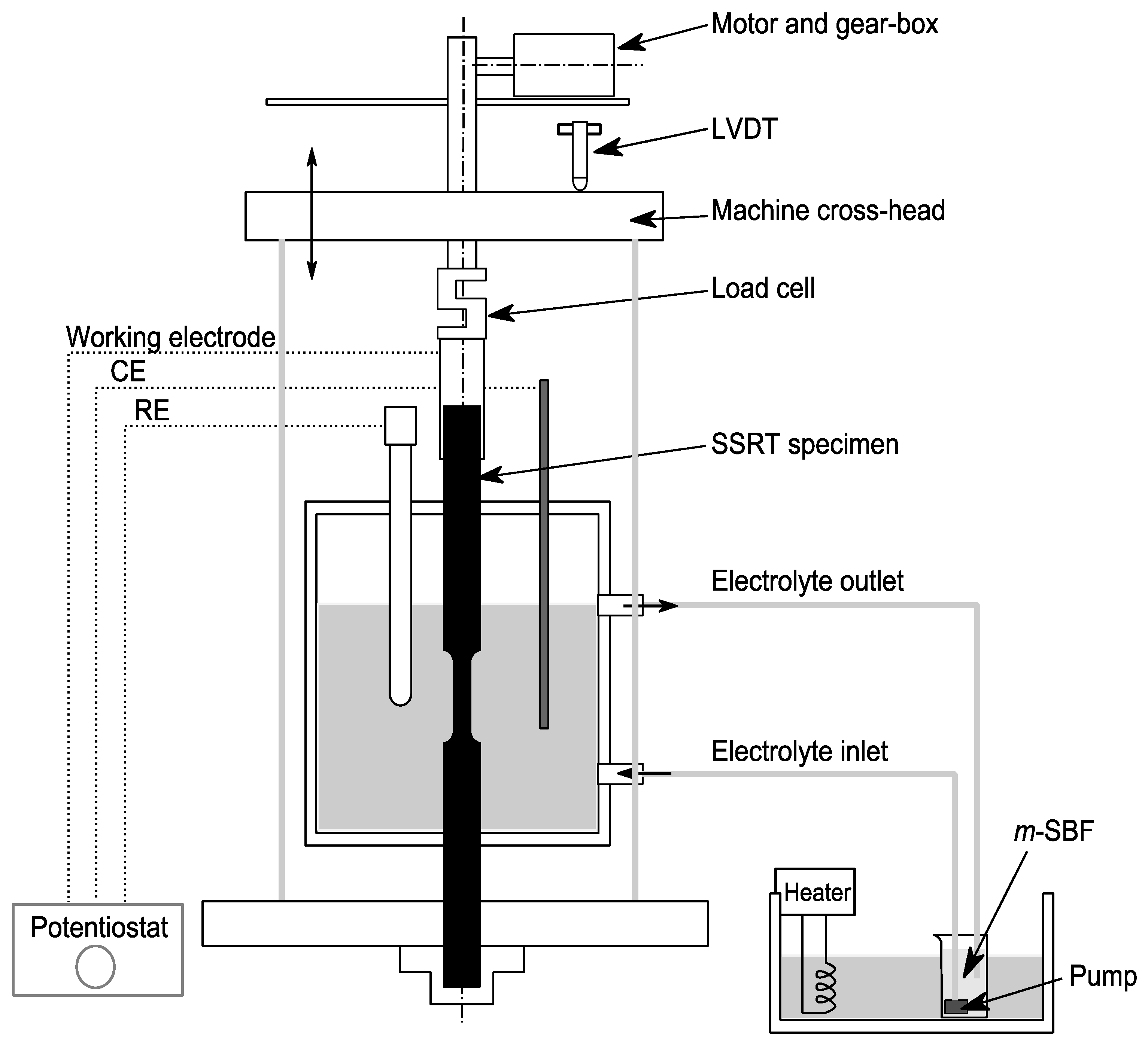
2.2. SCC Assessment by SSRT
2.3. Fractography
3. Results and Discussion
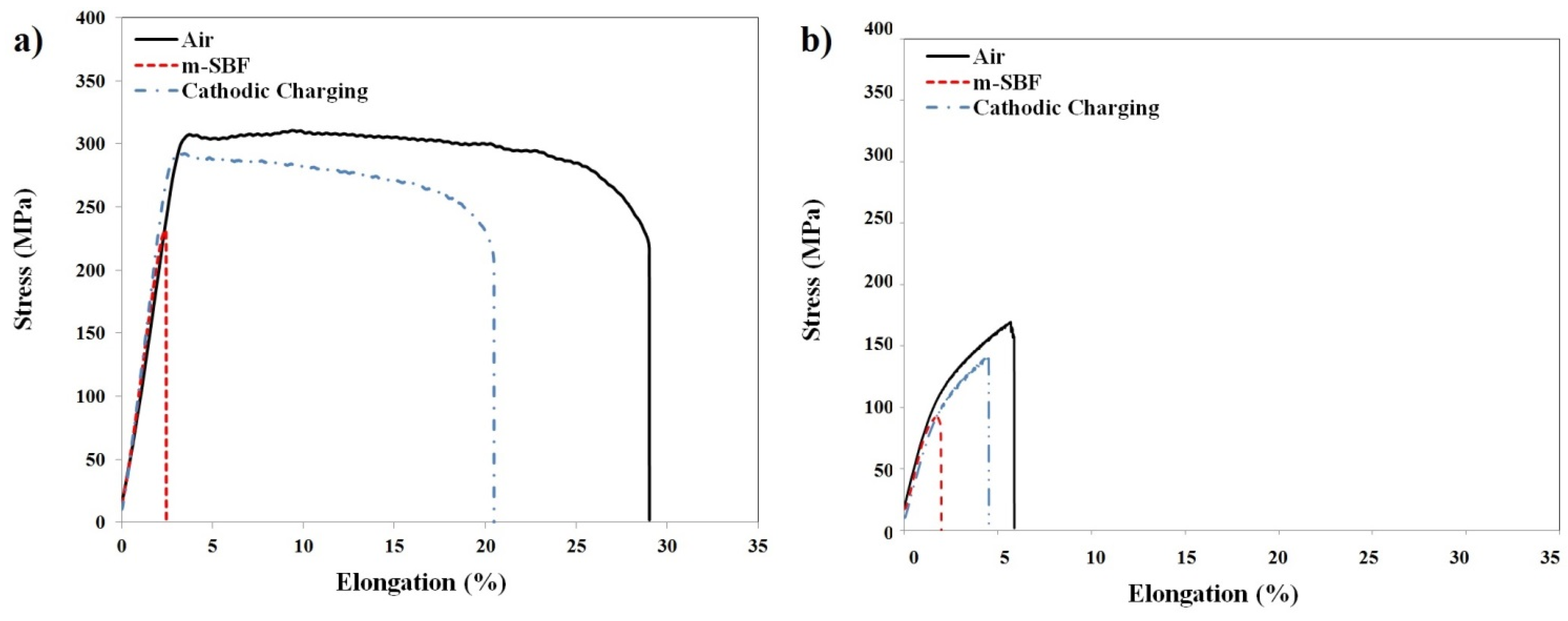
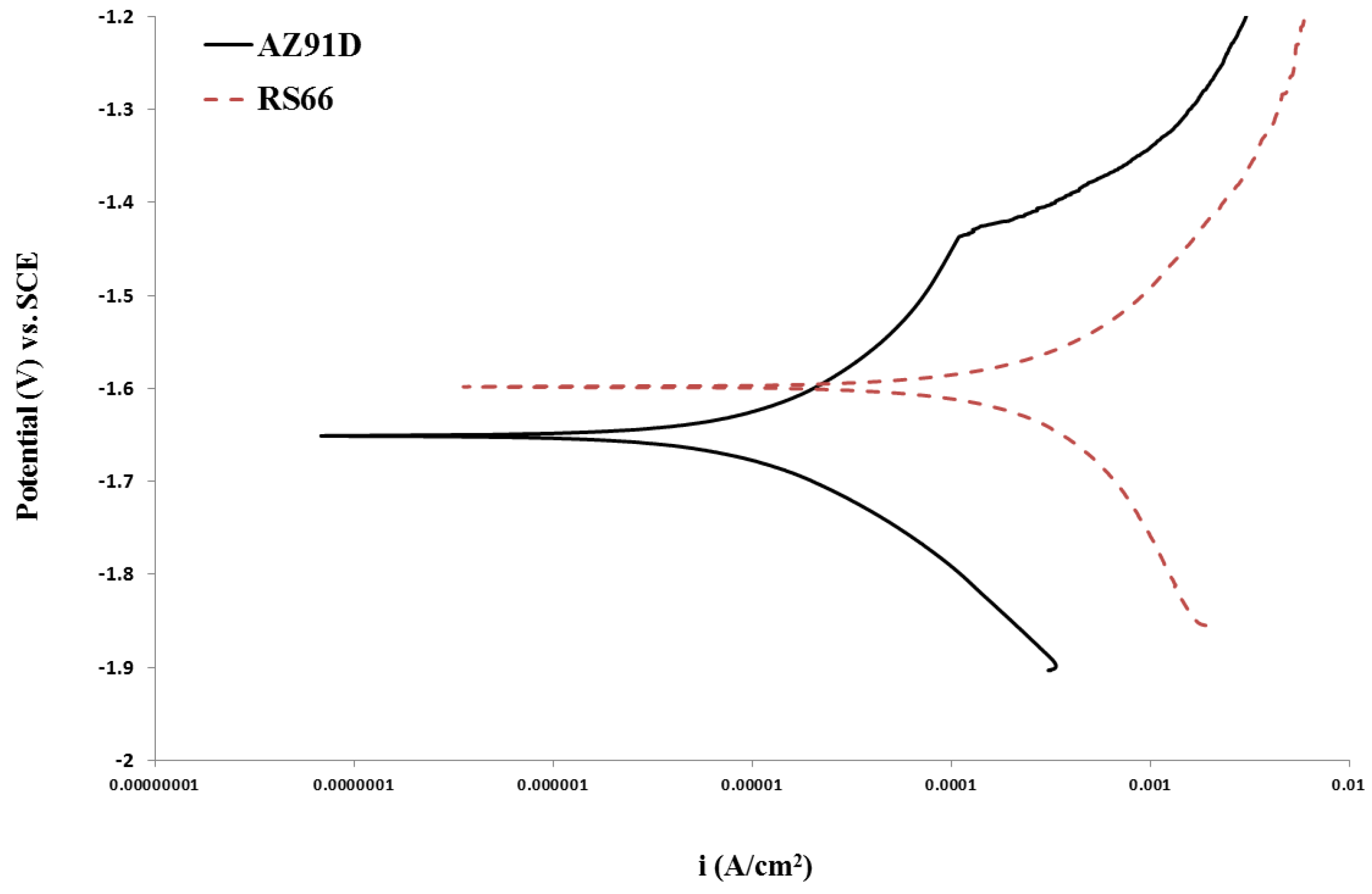
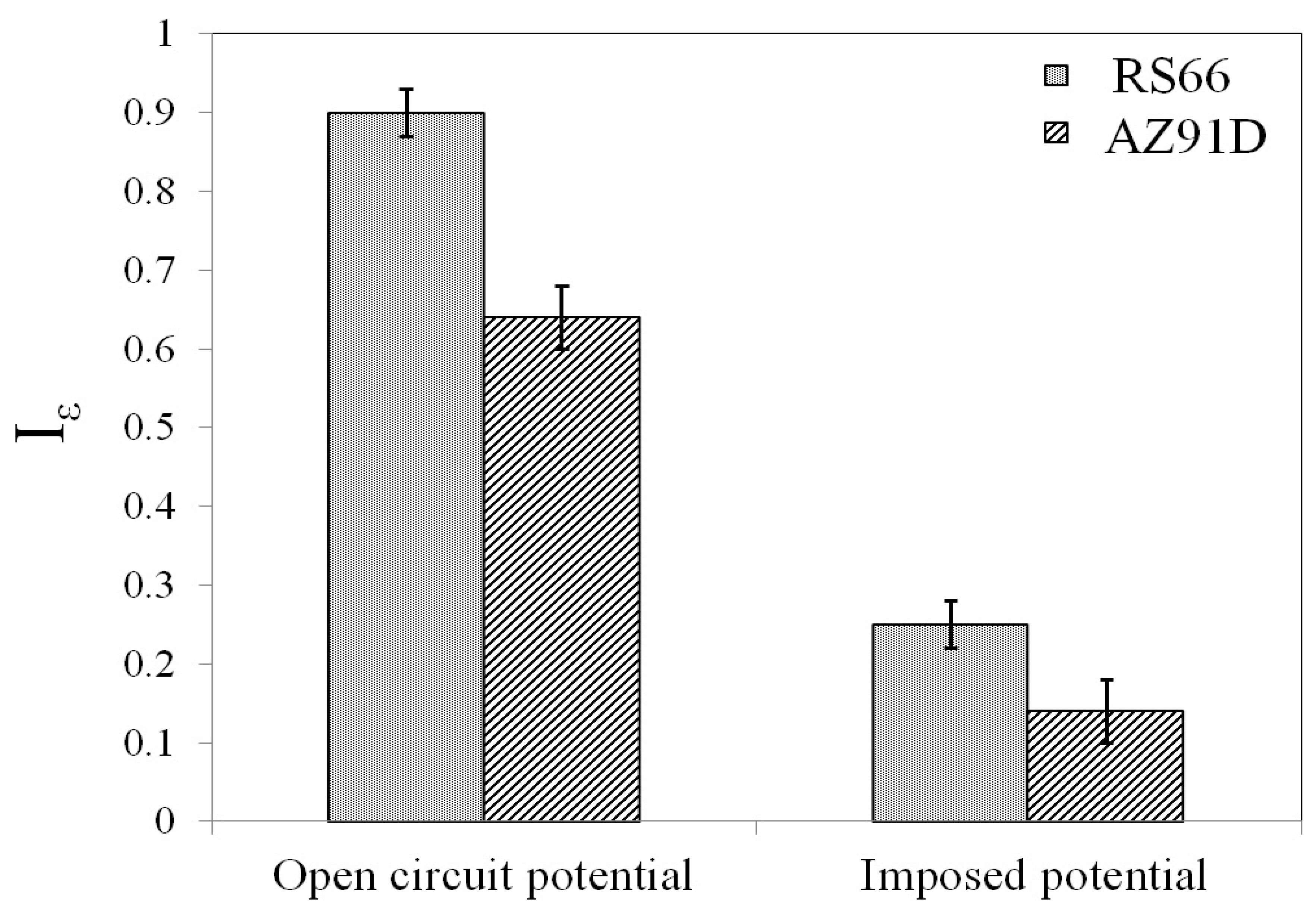
SCC Assessment by SSRT
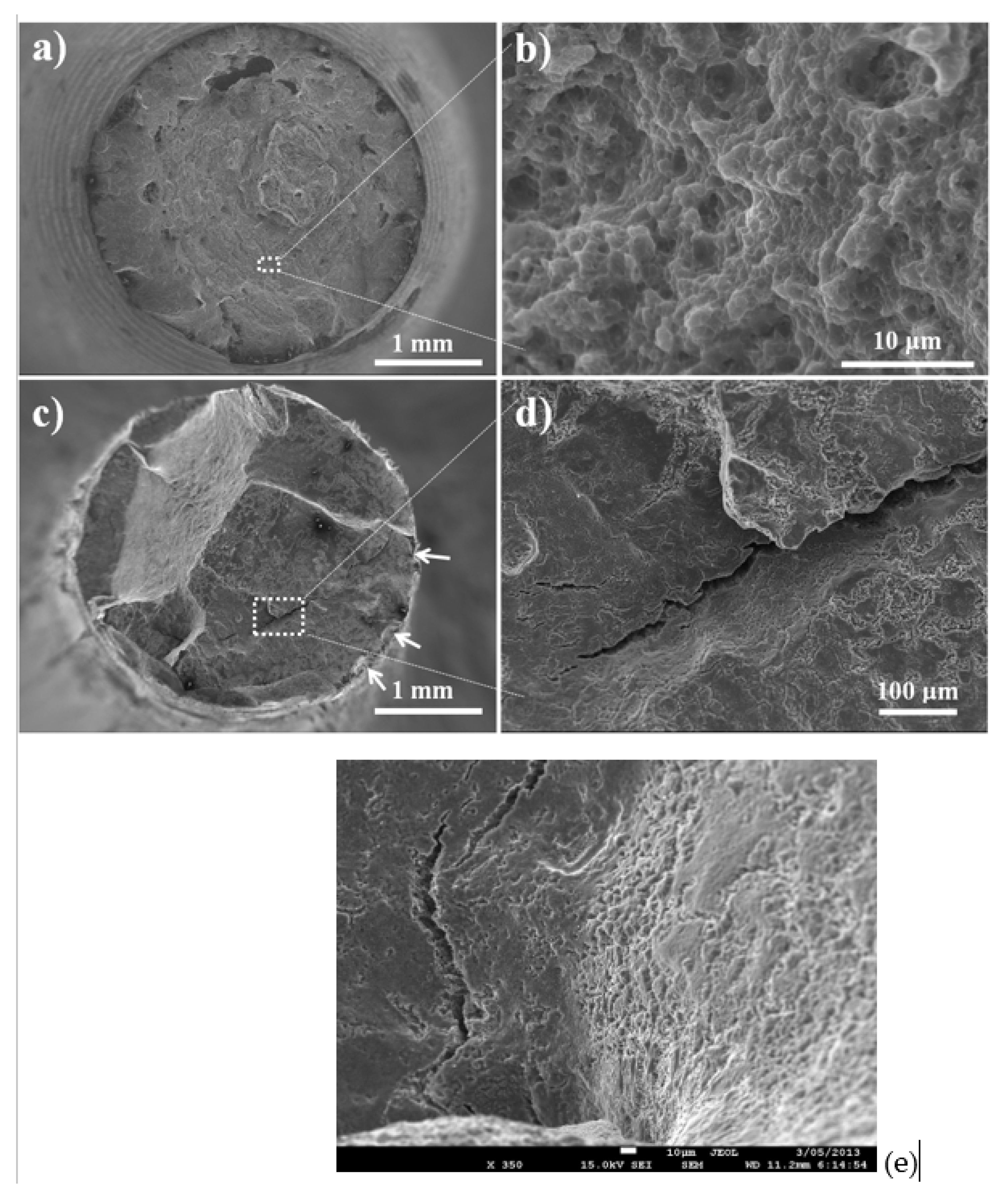
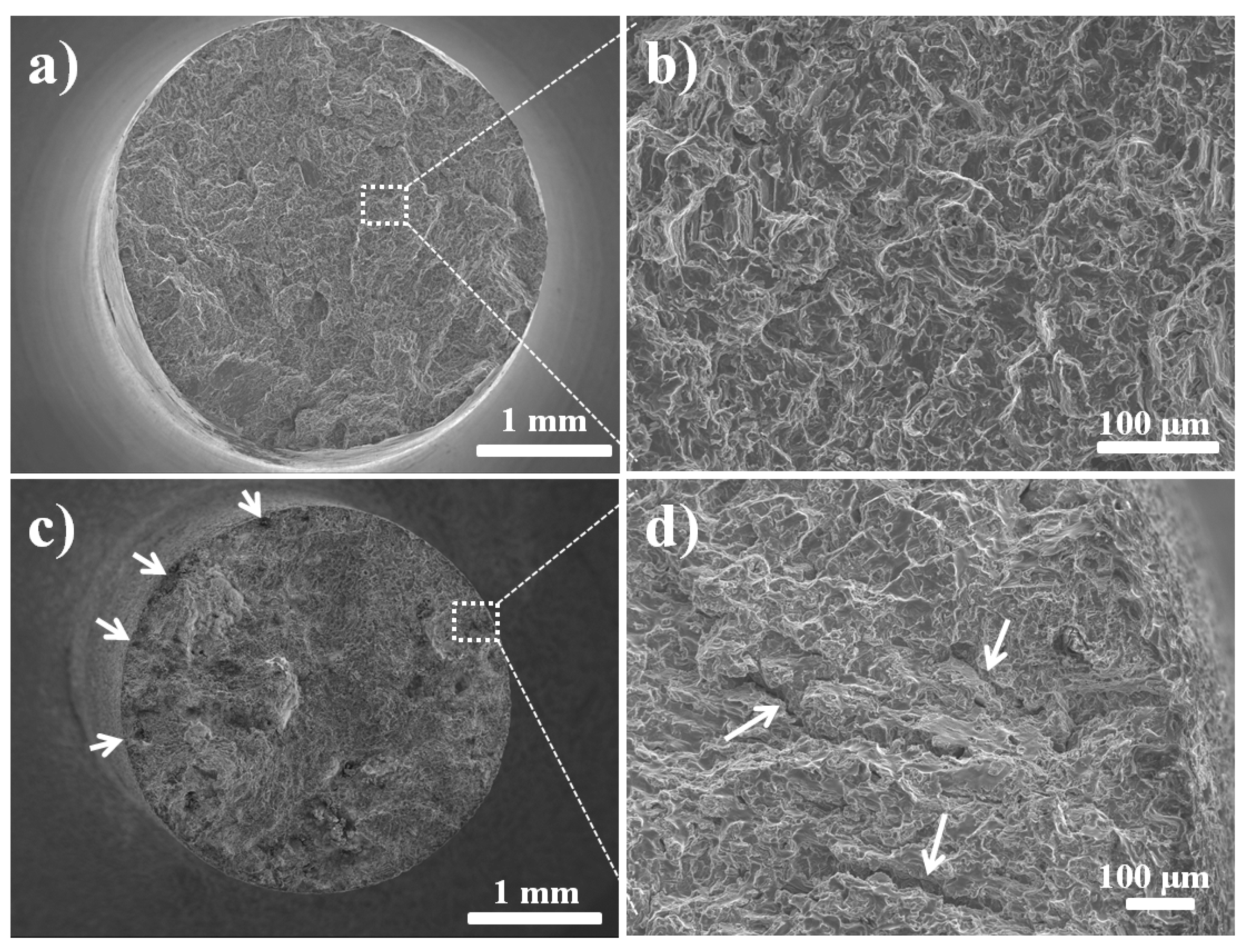
4. Fractography
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zberg, B.; Uggowitzer, P.J.; Löffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.; Xu, J. Biodegradable Alloys: The glass window of opportunities. Nat. Mater. 2009, 8, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Asp. Med. 2003, 24, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Hänzi, A.C.; Sologubenko, A.S.; Uggowitzer, P.J. Design strategy for new bioabsorbable Mg–Y–Zn alloys for medical applications. Int. J. Mater. Res. 2009, 100, 1127–1136. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hanzi, A.C.; Uggowitzer, P.J.; Loffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Sivakumar, M.; Rajeswari, S. Investigation of failures in stainless steel orthopaedic implant devices: Pit-induced stress corrosion cracking. J. Mater. Sci. Lett. 1992, 11, 1039–1042. [Google Scholar] [CrossRef]
- Amel-Farzad, H.; Peivandi, M.T.; Yusof-Sani, S.M.R. In-body corrosion fatigue failure of a stainless steel orthopaedic implant with a rare collection of different damage mechanisms. Eng. Fail. Anal. 2007, 14, 1205. [Google Scholar] [CrossRef]
- Antunes, R.A.; de Oliveira, M.C. Corrosion fatigue of biomedical metallic alloys: Mechanisms and mitigation. Acta Biomater. 2012, 8, 937–962. [Google Scholar] [CrossRef] [PubMed]
- Ambat, R.; Aung, N.N.; Zhou, W. Evaluation of microstructural effects on corrosion behaviour of AZ91D magnesium alloy. Corros. Sci. 2000, 42, 1433–1455. [Google Scholar] [CrossRef]
- Raman, R.K.S. The role of microstructure in localized corrosion of magnesium alloys. Metall. Mater. Trans. A 2004, 35, 2525–2531. [Google Scholar] [CrossRef]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.U.; Hort, N.; Blawert, C. A Critical Review of the Stress Corrosion Cracking (SCC) of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, L.; Raman, R.K.S. Magnesium alloys as body implants: Fracture mechanism under dynamic and static loadings in a physiological environment. Acta Biomater. 2012, 8, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.B.; Raman, R.K.S. Evaluation of SCC behaviour of AZ91 alloy in modified-simulated body fluid for orthopaedic implant application. Scr. Mater. 2008, 59, 175–178. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Choudhary, L. Cracking of magnesium-based biodegradable implant alloys under the combined action of stress and corrosive body fluid: A review. Emerg. Mater. Res. 2013, 2, 219–228. [Google Scholar]
- Jafari, S.; Raman, R.S.; Davies, C.H. Corrosion Fatigue Cracking of a Magnesium Alloy in Modified Simulated Body Fluid. Eng. Fract. Mech. (Spl Issue Corros. Fatigue) 2015, 137, 2–11. [Google Scholar] [CrossRef]
- Jafari, S.; Raman, R.S.; Davies, C.H.; Hofstetter, J.; Uggowitzer, P.J.; Löffler, J.F. Corrosion Fatigue and Stress Corrosion Cracking Characterization of MgZn1Ca0.3 (ZX10) in a Simulated Physiological Environment. J. Mech. Behav. Biomed. Mater. 2017, 65, 634–643. [Google Scholar] [CrossRef]
- Harandi, S.E.; Raman, R.S. Corrosion Fatigue of a Magnesium Alloy Under Appropriate Human Physiological Conditions for Bio-Implant Applications. Eng. Fract. Mech. 2017, 186, 134–142. [Google Scholar] [CrossRef]
- Harandi, S.E.; Banerjee, P.C.; Easton, C.D.; Raman, R.S. Influence of Bovine Serum Albumin in Hanks’ Solution on the Corrosion and Stress Corrosion Cracking of a Magnesium Alloy. Mater. Sci. Eng. C 2017, 80, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.S.; Jafari, S.; Harandi, S.E. Corrosion Fatigue Fracture of Magnesium Alloys in Bioimplant Applications: A Review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.; Chen, L. Corrosion fatigue behaviors of two biomedical Mg alloys AZ91D and WE43 in simulated body fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef]
- Hanzi, A.C.; Gerber, I.; Schinhammer, M.; Loffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg–Y–Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651–656. [Google Scholar] [CrossRef]
- Matias, T.B.; Asato, G.H.; Ramasco, B.T.; Botta, W.J.; Kiminami, C.S.; Bolfarini, C. Processing and characterization of amorphous magnesium based alloy for application in biomedical implants. J. Mater. Res. Technol. 2014, 3, 203–209. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Kirkland, N.T. Magnesium biomaterials: Past, present and future. Corros. Engng Sci. Technol. 2012, 47, 322–328. [Google Scholar] [CrossRef]
- El-Rahman, S.S.A. Neuropathology of aluminum toxicity in rats. Pharmacol. Res. 2003, 47, 189. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary MgCa alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Song, Y.; Zhao, C.; Zhang, X.; Xie, C.; Zhang, Y.; Tao, H.; He, Y.; Jiang, Y.; et al. In vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg-Zn alloy. Mater. Sci. Eng. C 2009, 29, 1907. [Google Scholar] [CrossRef]
- Bobby Kannan, M.; Dietzel, W.; Blawert, C.; Atrens, A.; Lyon, P. Stress corrosion cracking of rare-earth containing magnesium alloys ZE41, QE22 and Elektron 21 (EV31A) compared with AZ80. Mater. Sci. Eng. A 2008, 480, 529–539. [Google Scholar] [CrossRef]
- Cihova, M.; Martinelli, E.; Schmutz, P.; Myrissa, A.; Schäublin, R.; Weinberg, A.M.; Uggowitzer, P.J.; Löffler, J.F. The role of zinc in the biocorrosion behavior of resorbable Mg–Zn–Ca alloys. Acta Biomater. 2019, 100, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Schäublin, R.E.; Becker, M.; Cihova, M.; Gerstl, S.S.A.; Deiana, D.; Hébert, C.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. Precipitation in lean Mg–Zn–Ca alloys. Acta Mater. 2022, 239, 118223. [Google Scholar] [CrossRef]
- Xue, D.; Yun, Y.; Tan, Z.; Dong, Z.; Schulz, M.J. In vivo and in vitro degradation behavior of magnesium alloys as biomaterials. J. Mater. Sci. Technol. 2012, 28, 261–267. [Google Scholar] [CrossRef]
- Hofstetter, J.; Becker, M.; Martinelli, E.; Weinberg, A.M.; Mingler, B.; Kilian, H.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. High-strength low-alloy (HSLA) Mg–Zn–Ca alloys with excellent, biodegradation performance. JOM 2014, 66, 566–572. [Google Scholar] [CrossRef]
- Guo, X.; Remennik, S.; Xu, C.; Shechtman, D. Dvelopment of Mg-6.0%Zn-1.0%Y-0.6%Ce-0.6%Zr magnesium alloy and its microstructure evolution during processing. Mater. Sci. Eng. A 2008, 473, 255–273. [Google Scholar] [CrossRef]
- Willbold, E.; Kalla, K.; Bartsch, I.; Bobe, K.; Brauneis, M.; Remennik, S.; Shechtman, D.; Nellesen, J.; Tillmann, W.; Vogt, C.; et al. Biocompatibility of rapidly solidified magnesium alloy RS66 as a temporary biodefradabel metal. Acta Biomater. 2013, 9, 8509–8517. [Google Scholar] [CrossRef]
- Koike, J.; Kobayashi, T.; Mukai, T.; Watanabe, H.; Suzuki, M.; Maruyama, K.; Higashi, K. The activity of non-basal slip systems and dynamic recovery at room temperature in fine-grained AZ31B magnesium alloys. Acta Mater. 2003, 51, 2055–2065. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Stjohn, D.; Nairn, J.; Li, Y. The electrochemical corrosion of pure magnesium in 1 N NaCl. Corros. Sci. 1997, 39, 855–875. [Google Scholar] [CrossRef]
- Padekar, B.S.; Singh Raman, R.K.; Raja, V.S.; Paul, L. Stress corrosion cracking of a recent rare-earth containing magnesium alloy, EV31A, and a common Al-containing alloy, AZ91E. Corros. Sci. 2013, 71, 1–9. [Google Scholar] [CrossRef]
- Stampella, R.; Procter, R.; Ashworth, V. Environmentally-induced cracking of magnesium. Corros. Sci. 1984, 24, 325–341. [Google Scholar] [CrossRef]
- Raja, V.; Padekar, B.S. Role of chlorides on pitting and hydrogen embrittlement of Mg–Mn wrought alloy. Corros. Sci. 2013, 75, 176–183. [Google Scholar] [CrossRef]
- Yang, L.; Hort, N.; Willumeit, R.; Feyerabend, F. Effects of corrosion environment and proteins on magnesium corrosion, Corrosion Engineering. Corros. Eng. Sci. Technol. 2012, 47, 335–339. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hiromoto, S. Effect of inorganic salts, amino acids and proteins on the degradation of pure magnesium in vitro. Mater. Sci. Eng. C 2009, 29, 1559–1568. [Google Scholar] [CrossRef]
| Reagents | Amount |
|---|---|
| NaCl | 5.403 g |
| NaHCO3 | 0.504 g |
| Na2CO3 | 0.426 g |
| KCl | 0.225 g |
| K2HPO4.3H2O | 0.23 g |
| MgCl2.6H2O | 0.311 g |
| 0.2 mol L−1 NaOH | 100 mL |
| HEPES | 17.892 g |
| CaCl2 | 0.293 g |
| Na2SO4 | 0.072 g |
| 1 mol L−1 NaOH | 15 mL |
| Alloy | In Air | In m-SBF | ||
|---|---|---|---|---|
| UTS (MPa) | Elongation to Failure (%) | UTS (MPa) | Elongation to Failure (%) | |
| RS66 | 305.8 ± 4.6 | 27.2 ± 1.8 | 248.4 ± 17.2 | 2.7 ± 0.2 |
| AZ91D | 165.5 ± 4.5 | 5.4 ± 0.4 | 83.2 ± 8.7 | 1.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh Raman, R.K.; Choudhary, L.; Shechtman, D. Simulated Body Fluid-Assisted Stress Corrosion Cracking of a Rapidly Solidified Magnesium Alloy RS66. Materials 2024, 17, 3967. https://doi.org/10.3390/ma17163967
Singh Raman RK, Choudhary L, Shechtman D. Simulated Body Fluid-Assisted Stress Corrosion Cracking of a Rapidly Solidified Magnesium Alloy RS66. Materials. 2024; 17(16):3967. https://doi.org/10.3390/ma17163967
Chicago/Turabian StyleSingh Raman, R. K., Lokesh Choudhary, and Dan Shechtman. 2024. "Simulated Body Fluid-Assisted Stress Corrosion Cracking of a Rapidly Solidified Magnesium Alloy RS66" Materials 17, no. 16: 3967. https://doi.org/10.3390/ma17163967
APA StyleSingh Raman, R. K., Choudhary, L., & Shechtman, D. (2024). Simulated Body Fluid-Assisted Stress Corrosion Cracking of a Rapidly Solidified Magnesium Alloy RS66. Materials, 17(16), 3967. https://doi.org/10.3390/ma17163967







