Current Progress in Research into Environmentally Friendly Rigid Polyurethane Foams
Abstract
1. Introduction
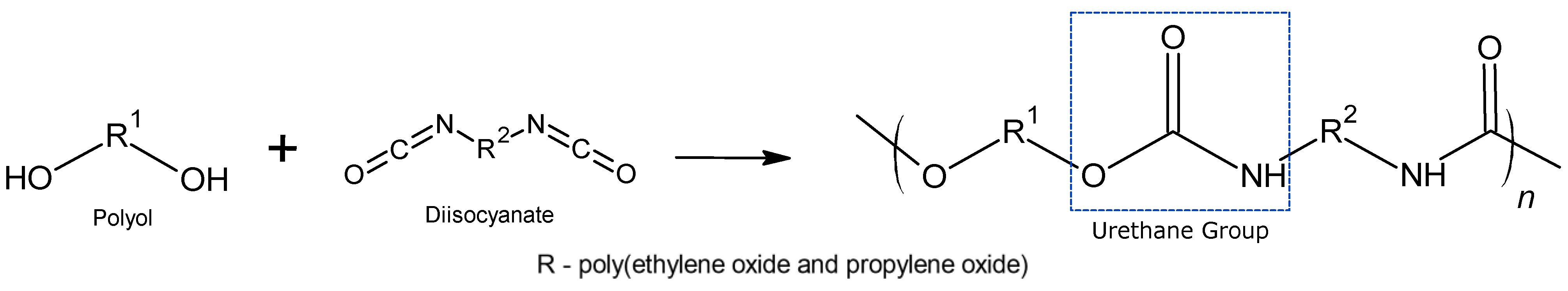
Definition of Polyurethanes
- Macrocells ,
- Microcells
- Ultracells
- Nanocells [10].
2. Materials
2.1. Description of Currently Used Compounds
2.1.1. Polyols
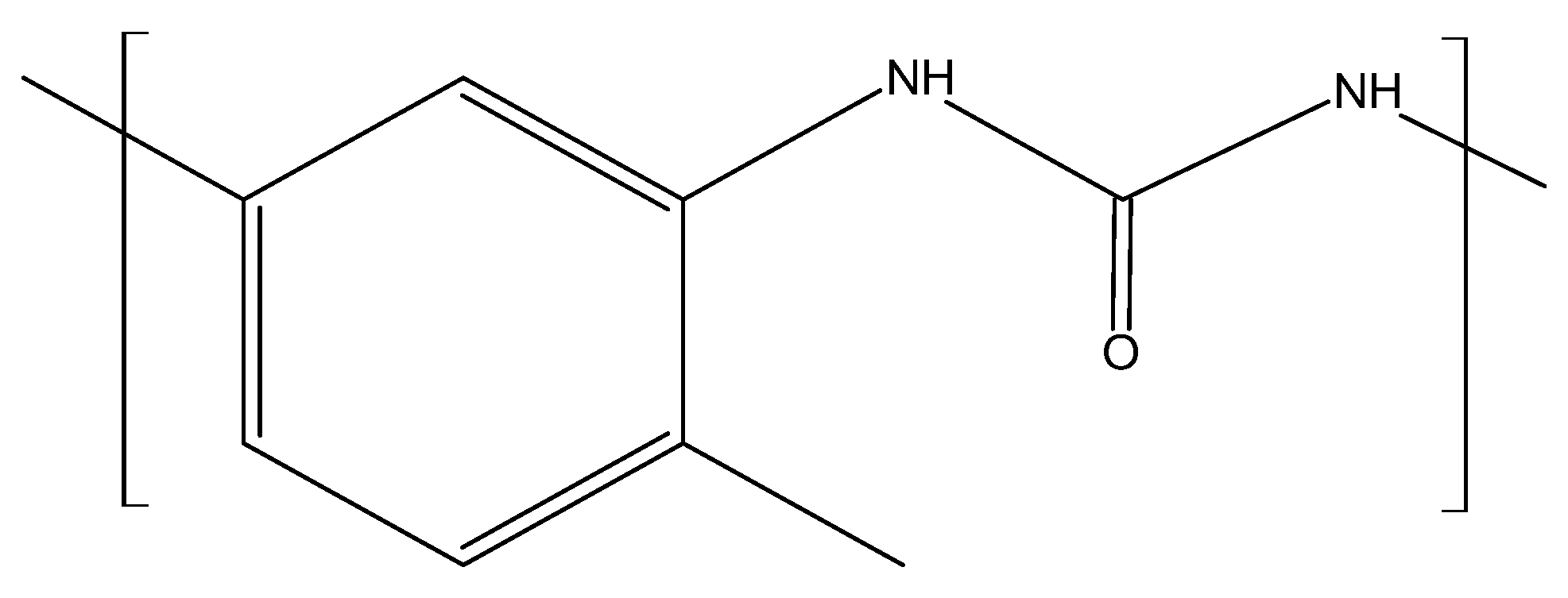
2.1.2. Isocyanates
- Reaction with −OH groups—catalyzed by tertiary amines and more strongly by organotin compounds, catalyst selection is of strong importance for foaming, organotin catalysts accelerate reactions with OH more strongly than amines, amines with H2O. Organotin catalysts, the most commonly used, are cinnamate and dibutyltin caprylates and laureates.
- The reaction with −NH2 groups proceeds faster and does not require catalysts. However, it requires the use of diols having NH2 groups or a mixture to control the synthesis reaction.
- By reacting diisocyanates and diepoxy compounds in the presence of suitable porophores and catalysts, it is possible to obtain foamed polyoxazolidones in the presence of suitable catalysts.
2.1.3. Blowing Agents
- Physical blowing agents, such as solvents with a low boiling point: pentane, acetone, or hexane. They form pore structures during evaporation.
- Chemical blowing agents, such as water, which expand the polymer by producing carbon dioxide [19].
2.1.4. Surfactants
2.1.5. Catalysts
2.1.6. Flame Retardants
2.2. Fillers for PU Foams
3. PU Foam Production
- The latent period, which lasts from the moment the components are mixed until the mixture starts to grow in volume.
- The growth period, which lasts from the moment the volume of the mixture begins to increase until it reaches its highest volume; here, an exothermic polymerization reaction takes place, causing the low-boiling liquids to evaporate, and the gas fluffs up the mixture while it is still in a plastic state, giving it a cellular structure.
- Stabilization (gelation) period, in which the foamed mixture is transformed into a property-stable plastic; the inherent reaction and side reactions of allophane and biuret bond formation still occur during this period.
- The maturation period of the foam, during which all ongoing chemical reactions take place to completion; the structure is finally established; and the properties, shape, and size of the foam are determined (this period generally lasts up to several hours).
4. Properties of Polyurethane Foams
4.1. Chemical Properties
4.2. Physical Properties
5. Recent Advances in the Production of PUR Foams
5.1. Rigid PUR Foams Derived from Bio-Based Polyols
5.1.1. Soybean Oil-Based Polyols
5.1.2. Rapeseed Oil-Based Polyols
5.1.3. Castor Oil-Based Polyols
5.1.4. Palm Oil-Based Polyols
5.1.5. Polyols Based on Various Compounds
5.2. Lignin-Based Polyol
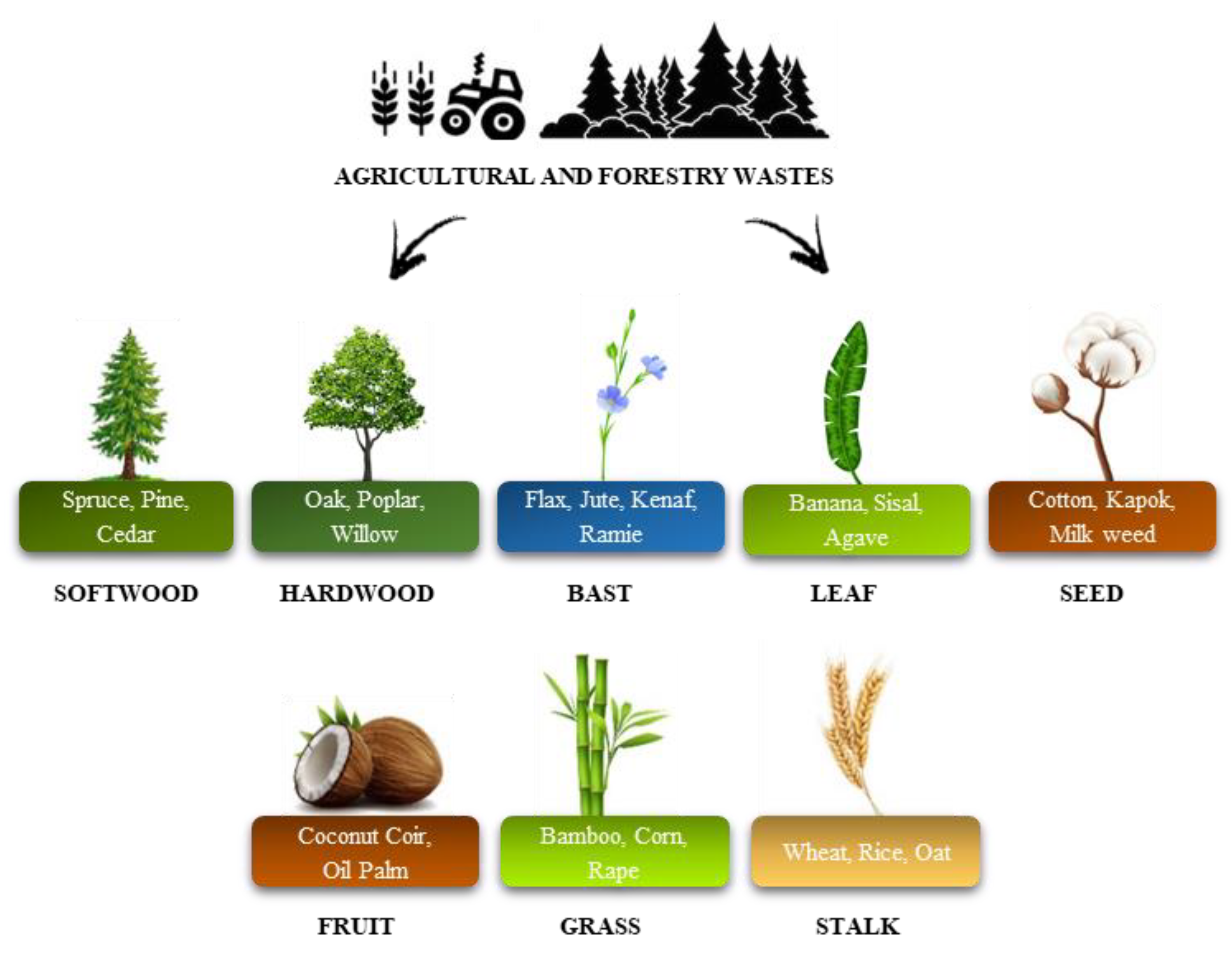
5.3. Rigid PUR Foams Reinforced with Natural Fillers
5.4. Use of Paper Waste in the Production of Polyurethane Foams
5.5. Improving the Sound Insulation Properties of Polyurethane Foams
6. Types and Applications of Polyurethane Materials
- Rigid and flexible foams,
- Thermoplastics,
- Thermosetting plastics,
- Coatings,
- Adhesives,
- Sealants,
- Elastomers.
7. Recycling of Polyurethane Foams
7.1. Chemical Recycling
7.2. Physical and Mechanical Recycling
7.3. Biological Recycling
8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Combating Climate Change. Available online: https://www.europarl.europa.eu/factsheets/en/sheet/72/walka-ze-zmiana-klimatu (accessed on 16 July 2024).
- EU Directive 2010/31/EU of the European Parliament and of the Council (on the Energy Performance of Buildings). 2010. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:153:0013:0035:EN:PDF (accessed on 16 July 2024).
- EU Directive 2012/27/EU of the European Parliament and of the Council. 2012. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:315:0001:0056:en:PDF (accessed on 16 July 2024).
- Paris Agreement on Climate Change. Available online: https://www.consilium.europa.eu/en/policies/climate-change/paris-agreement/ (accessed on 16 July 2024).
- Serrano, A.; Borreguero, A.M.; Garrido, I.; Rodríguez, J.F.; Carmona, M. Reducing Heat Loss through the Building Envelope by Using Polyurethane Foams Containing Thermoregulating Microcapsules. Appl. Therm. Eng. 2016, 103, 226–232. [Google Scholar] [CrossRef]
- Kim, C.; Youn, J.R. Environmentally Friendly Processing of Polyurethane Foam for Thermal Insulation. Polym. Plast. Technol. Eng. 2000, 39, 163–185. [Google Scholar] [CrossRef]
- Vest, N.A.; Kolibaba, T.J.; Afonso, A.O.; Kulatilaka, S.A.; Iverson, E.T.; Grunlan, J.C. Acid-Doped Biopolymer Nanocoatings for Flame-Retardant Polyurethane Foam. ACS Appl. Polym. Mater. 2022, 4, 1983–1990. [Google Scholar] [CrossRef]
- Palen, B.; Kolibaba, T.J.; Brehm, J.T.; Shen, R.; Quan, Y.; Wang, Q.; Grunlan, J.C. Clay-Filled Polyelectrolyte Complex Nanocoating for Flame-Retardant Polyurethane Foam. ACS Omega 2021, 6, 8016–8020. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yuen, A.C.Y.; Chen, T.B.Y.; Yu, B.; Yang, W.; Zhang, J.; Yao, Y.; Wu, S.; Wang, C.H.; Yeoh, G.H. Experimental and Numerical Perspective on the Fire Performance of MXene/Chitosan/Phytic Acid Coated Flexible Polyurethane Foam. Sci. Rep. 2021, 11, 4684. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Tapeh-Esmaeil, E.; Rodrigue, D. Morphological, Mechanical and Thermal Properties of Rubber Foams: A Review Based on Recent Investigations. Materials 2023, 16, 1934. [Google Scholar] [CrossRef] [PubMed]
- Brzeska, J.; Piotrowska-Kirschling, A. A Brief Introduction to the Polyurethanes According to the Principles of Green Chemis-try. Processes 2021, 9, 1929. [Google Scholar] [CrossRef]
- Oertel, G. Polyurethane Handbook: Chemistry, Raw Materials, Processing, Application, Properties; Hanser: Munchen, Germany, 1994. [Google Scholar]
- Szycher, M. Szycher’s Handbook of Polyurethanses; Szycher, M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429108907. [Google Scholar]
- Chattopadhyay, D.K.; Webster, D.C. Thermal Stability and Flame Retardancy of Polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Skleničková, K.; Abbrent, S.; Halecký, M.; Kočí, V.; Beneš, H. Biodegradability and Ecotoxicity of Polyurethane Foams: A Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 157–202. [Google Scholar] [CrossRef]
- Bayer, O. Das Di-Lsocganat-Poluadditionsverfahren (Polyurethane). Angew. Chem. 1947, 59, 257–272. [Google Scholar] [CrossRef]
- Crescentini, T.M.; May, J.C.; McLean, J.A.; Hercules, D.M. Mass Spectrometry of Polyurethanes. Polymer 2019, 181, 121624. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.S. Polyurethane Foam Chemistry. In Recycling of Polyurethane Foams; Elsevier: Amsterdam, The Netherlands, 2018; pp. 17–27. [Google Scholar]
- Gogoi, R.; Alam, M.S.; Niyogi, U.K. Effect of Soft Segment Chain Length on Tailoring the Properties of Isocyanate Terminated Oolyurethane Prepolymer, a Base Material for Polyurethane Bandage. Int. J. Res. Eng. Technol. 2013, 2, 395–398. [Google Scholar]
- Wang, Z.; Wang, C.; Gao, Y.; Li, Z.; Shang, Y.; Li, H. Porous Thermal Insulation Polyurethane Foam Materials. Polymers 2023, 15, 3818. [Google Scholar] [CrossRef] [PubMed]
- Janik, H.; Sienkiewicz, M.; Kucinska-Lipka, J. Polyurethanes. In Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2014; pp. 253–295. [Google Scholar]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane Types, Synthesis and Applications—A Review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Kaikade, D.S.; Sabnis, A.S. Polyurethane Foams from Vegetable Oil-Based Polyols: A Review. Polym. Bull. 2023, 80, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; Wiley: Hoboken, NJ, USA, 2014; ISBN 9781118737835. [Google Scholar]
- Randall, D.; Lee, S. The Polyurethanes Book; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Javni, I.; Zhang, W.; Petrović, Z.S. Effect of Different Isocyanates on the Properties of Soy-based Polyurethanes. J. Appl. Polym. Sci. 2003, 88, 2912–2916. [Google Scholar] [CrossRef]
- Allport, D.C.; Gilbert, D.S.; Outterside, S.M. MDI and TDI: Safety, Health and the Environment; Allport, D.C., Gilbert, D.S., Outterside, S.M., Eds.; Wiley: Hoboken, NJ, USA, 2003; ISBN 9780471958123. [Google Scholar]
- Liu, H.; Zhao, X. Thermal Conductivity Analysis of High Porosity Structures with Open and Closed Pores. Int. J. Heat Mass Transf. 2022, 183, 122089. [Google Scholar] [CrossRef]
- Zhao, X.; Brozena, A.H.; Hu, L. Critical Roles of Pores and Moisture in Sustainable Nanocellulose-Based Super-Thermal Insulators. Matter 2021, 4, 769–772. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, F.; Tekeei, A.; Suppes, G.J. Modeling Impact of Catalyst Loading on Polyurethane Foam Polymerization. Appl. Catal. A Gen. 2014, 469, 229–238. [Google Scholar] [CrossRef]
- Yasunaga, K.; Neff, R.A.; Zhang, X.D.; Macosko, C.W. Study of Cell Opening in Flexible Polyurethane Foam. J. Cell. Plast. 1996, 32, 427–448. [Google Scholar] [CrossRef]
- Dworakowska, S.; Bogdał, D.; Zaccheria, F.; Ravasio, N. The Role of Catalysis in the Synthesis of Polyurethane Foams Based on Renewable Raw Materials. Catal. Today 2014, 223, 148–156. [Google Scholar] [CrossRef]
- Yadav, A.; de Souza, F.M.; Dawsey, T.; Gupta, R.K. Recent Advancements in Flame-Retardant Polyurethane Foams: A Review. Ind. Eng. Chem. Res. 2022, 61, 15046–15065. [Google Scholar] [CrossRef]
- McKenna, S.T.; Hull, T.R. The Fire Toxicity of Polyurethane Foams. Fire Sci. Rev. 2016, 5, 3. [Google Scholar] [CrossRef]
- Cellulose. Available online: https://pl.wikipedia.org/wiki/Celuloza (accessed on 14 June 2024).
- Mort, R.; Peters, E.; Griffin, E.; Curtzwiler, G.; Vorst, K.; Jiang, S. Low-Isocyanate Polyurethane Foams with Improved Stability and Compression Modulus Prepared from Biosourced and Landfill-Diverted Materials. ACS Appl. Polym. Mater. 2023, 5, 7602–7613. [Google Scholar] [CrossRef]
- Stanzione, M.; Oliviero, M.; Cocca, M.; Errico, M.E.; Gentile, G.; Avella, M.; Lavorgna, M.; Buonocore, G.G.; Verdolotti, L. Tuning of Polyurethane Foam Mechanical and Thermal Properties Using Ball-Milled Cellulose. Carbohydr. Polym. 2020, 231, 115772. [Google Scholar] [CrossRef]
- Beaufils-Marquet, M.; Blanchet, P.; Hussain, A.; Landry, V. Investigation of Cellulose Filaments as Filler in Rigid Insulating Polyurethane Foam. BioResources 2023, 18, 6086–6117. [Google Scholar] [CrossRef]
- Sture, B.; Vevere, L.; Kirpluks, M.; Godina, D.; Fridrihsone, A.; Cabulis, U. Polyurethane Foam Composites Reinforced with Renewable Fillers for Cryogenic Insulation. Polymers 2021, 13, 4089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hori, N.; Takemura, A. Reinforcement of Agricultural Wastes Liquefied Polyols Based Polyurethane Foams by Agricultural Wastes Particles. J. Appl. Polym. Sci. 2021, 138, 50583. [Google Scholar] [CrossRef]
- Kairytė, A.; Kirpluks, M.; Ivdre, A.; Cabulis, U.; Vėjelis, S.; Balčiūnas, G. Paper Waste Sludge Enhanced Eco-efficient Polyurethane Foam Composites: Physical–Mechanical Properties and Microstructure. Polym. Compos. 2018, 39, 1852–1860. [Google Scholar] [CrossRef]
- Kairytė, A.; Vaitkus, S.; Vėjelis, S.; Girskas, G.; Balčiūnas, G. Rapeseed-Based Polyols and Paper Production Waste Sludge in Polyurethane Foam: Physical Properties and Their Prediction Models. Ind. Crops Prod. 2018, 112, 119–129. [Google Scholar] [CrossRef]
- Mills, N.J. The Wet Kelvin Model for Air Flow through Open-Cell Polyurethane Foams. J. Mater. Sci. 2005, 40, 5845–5851. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; iSmithers Rapra Publishing: Stropshire, UK, 2005; Volume 1, ISBN 9781910242131. [Google Scholar]
- Hejna, A. Clays as Inhibitors of Polyurethane Foams’ Flammability. Materials 2021, 14, 4826. [Google Scholar] [CrossRef]
- Ates, M.; Karadag, S.; Eker, A.A.; Eker, B. Polyurethane Foam Materials and Their Industrial Applications. Polym. Int. 2022, 71, 1157–1163. [Google Scholar] [CrossRef]
- Fagnani, D.E.; Tami, J.L.; Copley, G.; Clemons, M.N.; Getzler, Y.D.Y.L.; McNeil, A.J. 100th Anniversary of Macromolecular Science Viewpoint: Redefining Sustainable Polymers. ACS Macro Lett. 2021, 10, 41–53. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.X. Future Directions for Sustainable Polymers. Trends Chem. 2019, 1, 148–151. [Google Scholar] [CrossRef]
- Polyurethane Market|Global Research Report 2032. Available online: https://www.factmr.com/report/polyurethane-market (accessed on 17 September 2022).
- Peyrton, J.; Avérous, L. Structure-Properties Relationships of Cellular Materials from Biobased Polyurethane Foams. Mater. Sci. Eng. R Rep. 2021, 145, 100608. [Google Scholar] [CrossRef]
- Engels, H.W.; Pirkl, H.G.; Albers, R.; Albach, R.W.; Krause, J.; Hoffmann, A.; Casselmann, H.; Dormish, J. Polyurethanes: Versatile Materials and Sustainable Problem Solvers for Today’s Challenges. Angew. Chem. Int. Ed. 2013, 52, 9422–9441. [Google Scholar] [CrossRef] [PubMed]
- Furtwengler, P.; Avérous, L. Renewable Polyols for Advanced Polyurethane Foams from Diverse Biomass Resources. Polym. Chem. 2018, 9, 4258–4287. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From Vegetable Oils to Polyurethanes: Synthetic Routes to Polyols and Main Industrial Products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef]
- Fridrihsone, A.; Romagnoli, F.; Kirsanovs, V.; Cabulis, U. Life Cycle Assessment of Vegetable Oil Based Polyols for Polyurethane Production. J. Clean. Prod. 2020, 266, 121403. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Development of Completely Bio-based Epoxy Networks Derived from Epoxidized Linseed and Castor Oil Cured with Citric Acid. Polym. Adv. Technol. 2018, 29, 2080–2090. [Google Scholar] [CrossRef]
- Khandelwal, V.; Sahoo, S.K.; Kumar, A.; Sethi, S.K.; Manik, G. Bio-Sourced Electrically Conductive Epoxidized Linseed Oil Based Composites Filled with Polyaniline and Carbon Nanotubes. Compos. B Eng. 2019, 172, 76–82. [Google Scholar] [CrossRef]
- Singh, I.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Advancement in Plant Oil Derived Polyol-Based Polyurethane Foam for Future Perspective: A Review. Eur. J. Lipid Sci. Technol. 2020, 122, 1900225. [Google Scholar] [CrossRef]
- Tu, Y.; Kiatsimkul, P.; Suppes, G.; Hsieh, F. Physical Properties of Water-blown Rigid Polyurethane Foams from Vegetable Oil-based Polyols. J. Appl. Polym. Sci. 2007, 105, 453–459. [Google Scholar] [CrossRef]
- Guo, A.; Zhang, W.; Petrovic, Z.S. Structure–Property Relationships in Polyurethanes Derived from Soybean Oil. J. Mater. Sci. 2006, 41, 4914–4920. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zhang, W.; Javni, I. Structure and Properties of Polyurethanes Prepared from Triglyceride Polyols by Ozonolysis. Biomacromolecules 2005, 6, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Abraham, T.; Ference, D.; MacOsko, C.W. Rigid Polyurethane Foams from a Soybean Oil-Based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Tu, Y.C.; Fan, H.; Suppes, G.J.; Hsieh, F.H. Physical Properties of Water-Blown Rigid Polyurethane Foams Containing Epoxidized Soybean Oil in Different Isocyanate Indices. J. Appl. Polym. Sci. 2009, 114, 2577–2583. [Google Scholar] [CrossRef]
- Ji, D.; Fang, Z.; He, W.; Luo, Z.; Jiang, X.; Wang, T.; Guo, K. Polyurethane Rigid Foams Formed from Different Soy-Based Polyols by the Ring Opening of Epoxidised Soybean Oil with Methanol, Phenol, and Cyclohexanol. Ind. Crops Prod. 2015, 74, 76–82. [Google Scholar] [CrossRef]
- Narine, S.S.; Kong, X.; Bouzidi, L.; Sporns, P. Physical Properties of Polyurethanes Produced from Polyols from Seed Oils: II. Foams. J. Am. Oil Chem. Soc. 2006, 84, 65–72. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A. The Influence of Rapeseed Oil-Based Polyols on the Foaming Process of Rigid Polyurethane Foams. Ind. Crops Prod. 2016, 89, 182–187. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Kurańska, M.; Prociak, A.; Szczepkowski, L.; Krzyzowska, M.; Ryszkowska, J. Preparation and Characterisation of Rigid Polyurethane Foams Using a Rapeseed Oil-Based Polyol. Ind. Crops Prod. 2015, 74, 887–897. [Google Scholar] [CrossRef]
- Uram, K.; Prociak, A.; Kurańska, M. Influence of the Chemical Structure of Rapeseed Oil-Based Polyols on Selected Properties of Polyurethane Foams. Polimery 2020, 65, 698–707. [Google Scholar] [CrossRef]
- Kurańska, M.; Pinto, J.A.; Salach, K.; Barreiro, M.F.; Prociak, A. Synthesis of Thermal Insulating Polyurethane Foams from Lignin and Rapeseed Based Polyols: A Comparative Study. Ind. Crops Prod. 2020, 143, 111882. [Google Scholar] [CrossRef]
- Kairytė, A.; Vaitkus, S.; Vėjelis, S.; Pundienė, I. A Study of Rapeseed Oil-Based Polyol Substitution with Bio-Based Products to Obtain Dimensionally and Structurally Stable Rigid Polyurethane Foam. J. Polym. Environ. 2018, 26, 3834–3847. [Google Scholar] [CrossRef]
- Ogunniyi, D.S. Castor Oil: A Vital Industrial Raw Material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, H.; Zhang, L.; Hu, L.; Zhou, Y. Study of the Mechanical, Thermal Properties and Flame Retardancy of Rigid Polyurethane Foams Prepared from Modified Castor-Oil-Based Polyols. Ind. Crops Prod. 2014, 59, 135–143. [Google Scholar] [CrossRef]
- Ionescu, M.; Radojčić, D.; Wan, X.; Shrestha, M.L.; Petrović, Z.S.; Upshaw, T.A. Highly Functional Polyols from Castor Oil for Rigid Polyurethanes. Eur. Polym. J. 2016, 84, 736–749. [Google Scholar] [CrossRef]
- Carriço, C.S.; Fraga, T.; Carvalho, V.E.; Pasa, V.M.D. Polyurethane Foams for Thermal Insulation Uses Produced from Castor Oil and Crude Glycerol Biopolyols. Molecules 2017, 22, 1091. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The Influence of Crude Glycerol and Castor Oil-Based Polyol on the Structure and Performance of Rigid Polyurethane-Polyisocyanurate Foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Arniza, M.Z.; Hoong, S.S.; Idris, Z.; Yeong, S.K.; Hassan, H.A.; Din, A.K.; Choo, Y.M. Synthesis of Transesterified Palm Olein-Based Polyol and Rigid Polyurethanes from This Polyol. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 243–255. [Google Scholar] [CrossRef]
- Srihanum, A.; Tuan Noor, M.T.I.; Devi, K.P.P.; Hoong, S.S.; Ain, N.H.; Mohd, N.S.; Nek Mat Din, N.S.M.; Kian, Y.S. Low Density Rigid Polyurethane Foam Incorporated with Renewable Polyol as Sustainable Thermal Insulation Material. J. Cell. Plast. 2022, 58, 485–503. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Kurańska, M.; Prociak, A.; Malewska, E.; Kulpa, K. Open Cell Semi-Rigid Polyurethane Foams Synthesized Using Palm Oil-Based Bio-Polyol. Ind. Crops Prod. 2017, 102, 88–96. [Google Scholar] [CrossRef]
- Jaratrotkamjorn, R.; Tanrattanakul, V. Bio-based Flexible Polyurethane Foam Synthesized from Palm Oil and Natural Rubber. J. Appl. Polym. Sci. 2020, 137, 49310. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Isbrandt, M. Effect of Evening Primrose Oil-Based Polyol on the Properties of Rigid Polyurethane–Polyisocyanurate Foams for Thermal Insulation. Polymers 2018, 10, 1334. [Google Scholar] [CrossRef]
- Ionescu, M.; Petrović, Z.S. High Functionality Polyether Polyols Based on Polyglycerol. J. Cell. Plast. 2010, 46, 223–237. [Google Scholar] [CrossRef]
- Luo, X.; Hu, S.; Zhang, X.; Li, Y. Thermochemical Conversion of Crude Glycerol to Biopolyols for the Production of Polyurethane Foams. Bioresour. Technol. 2013, 139, 323–329. [Google Scholar] [CrossRef]
- Paruzel, A.; Michałowski, S.; Hodan, J.; Horák, P.; Prociak, A.; Beneš, H. Rigid Polyurethane Foam Fabrication Using Medium Chain Glycerides of Coconut Oil and Plastics from End-of-Life Vehicles. ACS Sustain. Chem. Eng. 2017, 5, 6237–6246. [Google Scholar] [CrossRef]
- Arbenz, A.; Perrin, R.; Avérous, L. Elaboration and Properties of Innovative Biobased PUIR Foams from Microalgae. J. Polym. Environ. 2018, 26, 254–262. [Google Scholar] [CrossRef]
- Pawar, M.S.; Kadam, A.S.; Dawane, B.S.; Yemul, O.S. Synthesis and Characterization of Rigid Polyurethane Foams from Algae Oil Using Biobased Chain Extenders. Polym. Bull. 2016, 73, 727–741. [Google Scholar] [CrossRef]
- Peyrton, J.; Chambaretaud, C.; Sarbu, A.; Avérous, L. Biobased Polyurethane Foams Based on New Polyol Architectures from Microalgae Oil. ACS Sustain. Chem. Eng. 2020, 8, 12187–12196. [Google Scholar] [CrossRef]
- Kosmela, P.; Kazimierski, P.; Formela, K.; Haponiuk, J.; Piszczyk, Ł. Liquefaction of Macroalgae Enteromorpha Biomass for the Preparation of Biopolyols by Using Crude Glycerol. J. Ind. Eng. Chem. 2017, 56, 399–406. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Wan, X.; Bilić, O.; Zlatanić, A.; Hong, J.; Javni, I.; Ionescu, M.; Milić, J.; Degruson, D. Polyols and Polyurethanes from Crude Algal Oil. JAOCS J. Am. Oil Chem. Soc. 2013, 90, 1073–1078. [Google Scholar] [CrossRef]
- Prociak, A.; Kurańska, M.; Cabulis, U.; Ryszkowska, J.; Leszczyńska, M.; Uram, K.; Kirpluks, M. Effect of Bio-Polyols with Different Chemical Structures on Foaming of Polyurethane Systems and Foam Properties. Ind. Crops Prod. 2018, 120, 262–270. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czupryński, B.; Tomaszewska, E.; Liszkowska, J. Oenothera Biennis Seed Oil as an Alternative Raw Material for Production of Bio-Polyol for Rigid Polyurethane-Polyisocyanurate Foams. Ind. Crops Prod. 2018, 126, 208–217. [Google Scholar] [CrossRef]
- Huang, X.; De Hoop, C.F.; Xie, J.; Wu, Q.; Boldor, D.; Qi, J. High Bio-Content Polyurethane (PU) Foam Made from Bio-Polyol and Cellulose Nanocrystals (CNCs) via Microwave Liquefaction. Mater. Des. 2018, 138, 11–20. [Google Scholar] [CrossRef]
- Ma, Y.; Xiao, Y.; Zhao, Y.; Bei, Y.; Hu, L.; Zhou, Y.; Jia, P. Biomass Based Polyols and Biomass Based Polyurethane Materials as a Route towards Sustainability. React. Funct. Polym. 2022, 175, 105285. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.K. Overview of the Recent Advances in Lignocellulose Liquefaction for Producing Biofuels, Bio-Based Materials and Chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Szpiłyk, M.; Lubczak, R.; Lubczak, J. The Biodegradable Cellulose-Derived Polyol and Polyurethane Foam. Polym. Test. 2021, 100, 107250. [Google Scholar] [CrossRef]
- Nadji, H.; Bruzzèse, C.; Belgacem, M.N.; Benaboura, A.; Gandini, A. Oxypropylation of Lignins and Preparation of Rigid Polyurethane Foams from the Ensuing Polyols. Macromol. Mater. Eng. 2005, 290, 1009–1016. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, Y.; Li, B.; Sun, C.; Guo, Z. Environmentally Friendly Alternative to Polyester Polyol by Corn Straw on Preparation of Rigid Polyurethane Composite. Compos. Commun. 2020, 17, 109–114. [Google Scholar] [CrossRef]
- D’Souza, J.; Camargo, R.; Yan, N. Polyurethane Foams Made from Liquefied Bark-Based Polyols. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Xie, J.; Zhai, X.; Hse, C.Y.; Shupe, T.F.; Pan, H. Polyols from Microwave Liquefied Bagasse and Its Application to Rigid Polyurethane Foam. Materials 2015, 8, 8496. [Google Scholar] [CrossRef]
- Yue, D.; Oribayo, O.; Rempel, G.L.; Pan, Q. Liquefaction of Waste Pine Wood and Its Application in the Synthesis of a Flame Retardant Polyurethane Foam. RSC Adv. 2017, 7, 30334–30344. [Google Scholar] [CrossRef]
- Fidan, M.S.; Ertaş, M. Biobased Rigid Polyurethane Foam Prepared from Apricot Stone Shell-Based Polyol for Thermal Insulation Application, Part 1: Synthesis, Chemical, and Physical Properties. Bioresources 2020, 15, 6061–6079. [Google Scholar] [CrossRef]
- Huang, G.; Wang, P. Effects of Preparation Conditions on Properties of Rigid Polyurethane Foam Composites Based on Liquefied Bagasse and Jute Fibre. Polym. Test. 2017, 60, 266–273. [Google Scholar] [CrossRef]
- Esteves, B.; Dulyanska, Y.; Costa, C.; Vicente, J.; Domingos, I.; Pereira, H.; de Lemos, L.T.; Cruz-Lopes, L. Cork Liquefaction for Polyurethane Foam Production. Bioresources 2017, 12, 2339–2353. [Google Scholar] [CrossRef]
- Wang, Q.; Tuohedi, N. Polyurethane Foams and Bio-Polyols from Liquefied Cotton Stalk Agricultural Waste. Sustainability 2020, 12, 4214. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Hse, C.Y.; Shupe, T.F. Preparation of Polyurethane Foams Using Fractionated Products in Liquefied Wood. J. Appl. Polym. Sci. 2014, 131, 40096. [Google Scholar] [CrossRef]
- Li, H.Q.; Shao, Q.; Luo, H.; Xu, J. Polyurethane Foams from Alkaline Lignin-Based Polyether Polyol. J. Appl. Polym. Sci. 2016, 133, 43261. [Google Scholar] [CrossRef]
- Arbenz, A.; Frache, A.; Cuttica, F.; Avérous, L. Advanced Biobased and Rigid Foams, Based on Urethane-Modified Isocyanurate from Oxypropylated Gambier Tannin Polyol. Polym. Degrad. Stab. 2016, 132, 62–68. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Formela, K.; Haponiuk, J.T.; Piszczyk, Ł. Biopolyols Obtained via Crude Glycerol-Based Liquefaction of Cellulose: Their Structural, Rheological and Thermal Characterization. Cellulose 2016, 23, 2929–2942. [Google Scholar] [CrossRef]
- Soares, B.; Gama, N.; Freire, C.S.R.; Barros-Timmons, A.; Brandão, I.; Silva, R.; Neto, C.P.; Ferreira, A. Spent Coffee Grounds as a Renewable Source for Ecopolyols Production. J. Chem. Technol. Biotechnol. 2015, 90, 1480–1488. [Google Scholar] [CrossRef]
- Domingos, I.J.; Fernandes, A.P.; Ferreira, J.; Cruz-Lopes, L.; Esteves, B.M. Polyurethane Foams from Liquefied Eucalyptus Globulus Branches. Bioresources 2019, 14, 31–43. [Google Scholar] [CrossRef]
- Kosmela, P.; Hejna, A.; Suchorzewski, J.; Piszczyk, Ł.; Haponiuk, J.T. Study on the Structure-Property Dependences of Rigid PUR-PIR Foams Obtained from Marine Biomass-Based Biopolyol. Materials 2020, 13, 1257. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, M.; Wan, X.; Bilić, N.; Petrović, Z.S. Polyols and Rigid Polyurethane Foams from Cashew Nut Shell Liquid. J. Polym. Environ. 2012, 20, 647–658. [Google Scholar] [CrossRef]
- Gandhi, T.S.; Patel, M.R.; Dholakiya, B.Z. Mechanical, Thermal and Fire Properties of Sustainable Rigid Polyurethane Foam Derived from Cashew Nut Shell Liquid. Int. J. Plast. Technol. 2015, 19, 30–46. [Google Scholar] [CrossRef]
- De Luca Bossa, F.; Santillo, C.; Verdolotti, L.; Campaner, P.; Minigher, A.; Boggioni, L.; Losio, S.; Coccia, F.; Iannace, S.; Lama, G.C. Greener Nanocomposite Polyurethane Foam Based on Sustainable Polyol and Natural Fillers: Investigation of Chemico-Physical and Mechanical Properties. Materials 2020, 13, 211. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of Agricultural Wastes Liquefaction Process and Preparing Bio-Based Polyurethane Foams by the Obtained Polyols. Ind. Crops Prod. 2019, 138, 111455. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, Y.; Elsayed, I.; Taylor, M.; Eberhardt, T.L.; Hassan, E.B.; Shmulsky, R. Rigid Polyurethane Foams Containing Lignin Oxyalkylated with Ethylene Carbonate and Polyethylene Glycol. Ind. Crops Prod. 2019, 141, 111797. [Google Scholar] [CrossRef]
- Duval, A.; Vidal, D.; Sarbu, A.; René, W.; Avérous, L. Scalable Single-Step Synthesis of Lignin-Based Liquid Polyols with Ethylene Carbonate for Polyurethane Foams. Mater. Today Chem. 2022, 24, 100793. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Kosugi, R.; Hatakeyama, T. Thermal Properties of Lignin-and Molasses-Based Polyurethane Foams. J. Therm. Anal. Calorim. 2008, 92, 419–424. [Google Scholar] [CrossRef]
- Gao, L.L.; Liu, Y.H.; Lei, H.; Peng, H.; Ruan, R. Preparation of Semirigid Polyurethane Foam with Liquefied Bamboo Residues. J. Appl. Polym. Sci. 2010, 116, 1694–1699. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y. Two-Step Sequential Liquefaction of Lignocellulosic Biomass by Crude Glycerol for the Production of Polyols and Polyurethane Foams. Bioresour. Technol. 2014, 161, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, M.; Huo, W.; Cai, D.; Qin, P.; Cao, H.; Tan, T. Fractionation and Oxypropylation of Corn-Stover Lignin for the Production of Biobased Rigid Polyurethane Foam. Ind. Crops Prod. 2020, 143, 111887. [Google Scholar] [CrossRef]
- Hwang, U.; Lee, B.; Oh, B.; Shin, H.S.; Lee, S.S.; Kang, S.G.; Kim, D.; Park, J.; Shin, S.; Suhr, J.; et al. Hydrophobic Lignin/Polyurethane Composite Foam: An Eco-Friendly and Easily Reusable Oil Sorbent. Eur. Polym. J. 2022, 165, 110971. [Google Scholar] [CrossRef]
- Kuranchie, C.; Yaya, A.; Bensah, Y.D. The Effect of Natural Fibre Reinforcement on Polyurethane Composite Foams—A Review. Sci. Afr. 2021, 11, e00722. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A Review of Recent Developments in Natural Fibre Composites and Their Mechanical Performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Zhou, X.; Sain, M.M.; Oksman, K. Semi-Rigid Biopolyurethane Foams Based on Palm-Oil Polyol and Reinforced with Cellulose Nanocrystals. Compos. Part A Appl. Sci. Manuf. 2016, 83, 56–62. [Google Scholar] [CrossRef]
- Zhou, X.; Sethi, J.; Geng, S.; Berglund, L.; Frisk, N.; Aitomäki, Y.; Sain, M.M.; Oksman, K. Dispersion and Reinforcing Effect of Carrot Nanofibers on Biopolyurethane Foams. Mater. Des. 2016, 110, 526–531. [Google Scholar] [CrossRef]
- Xue, B.L.; Wen, J.L.; Sun, R.C. Lignin-Based Rigid Polyurethane Foam Reinforced with Pulp Fiber: Synthesis and Characterization. ACS Sustain. Chem. Eng. 2014, 2, 1474–1480. [Google Scholar] [CrossRef]
- Ribeiro Da Silva, V.; Mosiewicki, M.A.; Yoshida, M.I.; Coelho Da Silva, M.; Stefani, P.M.; Marcovich, N.E. Polyurethane Foams Based on Modified Tung Oil and Reinforced with Rice Husk Ash II: Mechanical Characterization. Polym. Test. 2013, 32, 665–672. [Google Scholar] [CrossRef]
- Paberza, A.; Cabulis, U.; Arshanitsa, A. Wheat Straw Lignin as Filler for Rigid Polyurethane Foams on the Basis of Tall Oil Amide. Polimery 2014, 59, 477–481. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and Applicational Evaluation of the Rigid Polyurethane Foam Composites with Egg Shell Waste. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Silva, M.C.; Takahashi, J.A.; Chaussy, D.; Belgacem, M.N.; Silva, G.G. Composites of Rigid Polyurethane Foam and Cellulose Fiber Residue. J. Appl. Polym. Sci. 2010, 117, 3665–3672. [Google Scholar] [CrossRef]
- Prociak, A.; Kurañska, M.; Malewska, E.; Szczepkowski, L.; Zieleniewska, M.; Ryszkowska, J.; Ficon, J.; Rzasa, A. Biobased Polyurethane Foams Modified with Natural Fillers. Polimery 2015, 60, 592–599. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Dell’Arciprete, G.A.; Aranguren, M.I.; Marcovich, N.E. Polyurethane Foams Obtained from Castor Oil-Based Polyol and Filled with Wood Flour. J. Compos. Mater. 2009, 43, 3057–3072. [Google Scholar] [CrossRef]
- Joanna, P.S.; Bogusław, C.; Joanna, L. Application of Waste Products from Agricultural-Food Industry for Production of Rigid Polyurethane-Polyisocyanurate Foams. J. Porous Mater. 2011, 18, 631–638. [Google Scholar] [CrossRef]
- Qiu, Q.; Yang, X.; Zhang, P.; Wang, D.; Lu, M.; Wang, Z.; Guo, G.; Yu, J.; Tian, H.; Li, J. Effect of Fiber Surface Treatment on the Structure and Properties of Rigid Bagasse Fibers/Polyurethane Composite Foams. Polym. Compos. 2021, 42, 2766–2773. [Google Scholar] [CrossRef]
- de Avila Delucis, R.; Magalhães, W.L.E.; Petzhold, C.L.; Amico, S.C. Forest-Based Resources as Fillers in Biobased Polyurethane Foams. J. Appl. Polym. Sci. 2018, 135, 45684. [Google Scholar] [CrossRef]
- Jabber, L.J.Y.; Grumo, J.C.; Alguno, A.C.; Lubguban, A.A.; Capangpangan, R.Y. Influence of Cellulose Fibers Extracted from Pineapple (Ananas Comosus) Leaf to the Mechanical Properties of Rigid Polyurethane Foam. Mater. Today Proc. 2021, 46, 1735–1739. [Google Scholar] [CrossRef]
- Uram, K.; Kurańska, M.; Andrzejewski, J.; Prociak, A. Rigid Polyurethane Foams Modified with Biochar. Materials 2021, 14, 5616. [Google Scholar] [CrossRef] [PubMed]
- Uram, K.; Leszczyńska, M.; Prociak, A.; Czajka, A.; Gloc, M.; Leszczyński, M.K.; Michałowski, S.; Ryszkowska, J. Polyurethane Composite Foams Synthesized Using Bio-Polyols and Cellulose Filler. Materials 2021, 14, 3474. [Google Scholar] [CrossRef] [PubMed]
- Kuźnia, M.; Magiera, A.; Zygmunt-Kowalska, B.; Kaczorek-Chrobak, K.; Pielichowska, K.; Szatkowski, P.; Benko, A.; Ziabka, M.; Jerzak, W. Fly Ash as an Eco-Friendly Filler for Rigid Polyurethane Foams Modification. Materials 2021, 14, 6604. [Google Scholar] [CrossRef] [PubMed]
- Paciorek-Sadowska, J.; Czupryński, B.; Borowicz, M.; Liszkowska, J. Rigid Polyurethane–Polyisocyanurate Foams Modified with Grain Fraction of Fly Ashes. J. Cell. Plast. 2020, 56, 53–72. [Google Scholar] [CrossRef]
- Liszkowska, J. The Effect of Ground Coffee on the Mechanical and Application Properties of Rigid Polyurethane-Polyisocyanurate Foams. Polimery 2018, 63, 305–310. [Google Scholar] [CrossRef]
- Wrześniewska-Tosik, K.; Ryszkowska, J.; Mik, T.; Wesołowska, E.; Kowalewski, T.; Pałczyńska, M.; Sałasińska, K.; Walisiak, D.; Czajka, A. Composites of Semi-Rigid Polyurethane Foams with Keratin Fibers Derived from Poultry Feathers and Flame Retardant Additives. Polymers 2020, 12, 2943. [Google Scholar] [CrossRef] [PubMed]
- Kuranska, M.; Prociak, A.; Michalowski, S.; Cabulis, U.; Kirpluks, M. Microcellulose as a Natural Filler in Polyurethane Foams Based on the Biopolyol from Rapeseed Oil. Polimery 2016, 61, 625–632. [Google Scholar] [CrossRef]
- Luo, X.; Mohanty, A.; Misra, M. Water-Blown Rigid Biofoams from Soy-Based Biopolyurethane and Microcrystalline Cellulose. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 2057–2065. [Google Scholar] [CrossRef]
- Tian, H.; Wu, J.; Xiang, A. Polyether Polyol-Based Rigid Polyurethane Foams Reinforced with Soy Protein Fillers. J. Vinyl Addit. Technol. 2018, 24, E105–E111. [Google Scholar] [CrossRef]
- Khaleel, M.; Soykan, U.; Çetin, S. Influences of Turkey Feather Fiber Loading on Significant Characteristics of Rigid Polyurethane Foam: Thermal Degradation, Heat Insulation, Acoustic Performance, Air Permeability and Cellular Structure. Constr. Build. Mater. 2021, 308, 125014. [Google Scholar] [CrossRef]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; El Bouari, A. Mechanical and Thermal Conductivity Properties of Hemp Fiber Reinforced Polyurethane Composites. Case Stud. Constr. Mater. 2018, 8, 203–212. [Google Scholar] [CrossRef]
- de Avila Delucis, R.; Fischer Kerche, E.; Gatto, D.A.; Magalhães Esteves, W.L.; Petzhold, C.L.; Campos Amico, S. Surface Response and Photodegradation Performance of Bio-Based Polyurethane-Forest Derivatives Foam Composites. Polym. Test. 2019, 80, 106102. [Google Scholar] [CrossRef]
- Oushabi, A.; Sair, S.; Abboud, Y.; Tanane, O.; Bouari, A. El An Experimental Investigation on Morphological, Mechanical and Thermal Properties of Date Palm Particles Reinforced Polyurethane Composites as New Ecological Insulating Materials in Building. Case Stud. Constr. Mater. 2017, 7, 128–137. [Google Scholar] [CrossRef]
- Husainie, S.M.; Deng, X.; Ghalia, M.A.; Robinson, J.; Naguib, H.E. Natural Fillers as Reinforcement for Closed-Molded Polyurethane Foam Plaques: Mechanical, Morphological, and Thermal Properties. Mater. Today Commun. 2021, 27, 102187. [Google Scholar] [CrossRef]
- Kairytė, A.; Kizinievič, O.; Kizinievič, V.; Kremensas, A. Synthesis of Biomass-Derived Bottom Waste Ash Based Rigid Biopolyurethane Composite Foams: Rheological Behaviour, Structure and Performance Characteristics. Compos. Part A Appl. Sci. Manuf. 2019, 117, 193–201. [Google Scholar] [CrossRef]
- Husainie, S.M.; Khattak, S.U.; Robinson, J.; Naguib, H.E. A Comparative Study on the Mechanical Properties of Different Natural Fiber Reinforced Free-Rise Polyurethane Foam Composites. Ind. Eng. Chem. Res. 2020, 59, 21745–21755. [Google Scholar] [CrossRef]
- Kairytė, A.; Członka, S.; Boris, R.; Vėjelis, S. Vacuum-Based Impregnation of Liquid Glass into Sunflower Press Cake Particles and Their Use in Bio-Based Rigid Polyurethane Foam. Materials 2021, 14, 5351. [Google Scholar] [CrossRef]
- Głowacz-Czerwonka, D.; Zakrzewska, P.; Oleksy, M.; Pielichowska, K.; Kuźnia, M.; Telejko, T. The Influence of Biowaste-Based Fillers on the Mechanical and Fire Properties of Rigid Polyurethane Foams. Sustain. Mater. Technol. 2023, 36, e00610. [Google Scholar] [CrossRef]
- Anwar, M.F.; Yu, L.J.; Lim, Y.M.; Tarawneh, M.A.; Se Yong, E.N.; Lai, N.Y.G. Water Absorption Properties of Polyurethane Foam Reinforced with Paper Pulp. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Chanlert, P.; Ruamcharoen, P. Sound Absorption Properties of Rigid Polyurethane Foam Composites with Rubber-Wood Sawdust as a Natural Filler. J. Phys. Conf. Ser. 2021, 1719, 012062. [Google Scholar] [CrossRef]
- Olcay, H.; Kocak, E.D. The Mechanical, Thermal and Sound Absorption Properties of Flexible Polyurethane Foam Composites Reinforced with Artichoke Stem Waste Fibers. J. Ind. Text. 2022, 51, 8738S–8763S. [Google Scholar] [CrossRef]
- Olcay, H.; Kocak, E.D. Rice Plant Waste Reinforced Polyurethane Composites for Use as the Acoustic Absorption Material. Appl. Acoust. 2021, 173, 107733. [Google Scholar] [CrossRef]
- Ekici, B.; Kentli, A.; Küçük, H. Improving Sound Absorption Property of Polyurethane Foams by Adding Tea-Leaf Fibers. Arch. Acoust. 2012, 37, 515–520. [Google Scholar] [CrossRef]
- Polyurethane Market Size & Share Analysis—Growth Trends & Forecasts (2023–2028). Available online: https://www.mordorintelligence.com/industry-reports/polyurethane-market (accessed on 2 May 2024).
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the Thermo-Chemical Recycling of Waste Polyurethane Foam. J. Environ. Manag. 2021, 278, 111527. [Google Scholar] [CrossRef]
- Cregut, M.; Bedas, M.; Durand, M.-J.; Thouand, G. New Insights into Polyurethane Biodegradation and Realistic Prospects for the Development of a Sustainable Waste Recycling Process. Biotechnol. Adv. 2013, 31, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.T. Microbial Biodegradation of Polyurethane in: Polyurethane Biodegradation; Transworld Research Network: Kerala, India, 2011; pp. 215–238. [Google Scholar]
- Rossignolo, G.; Malucelli, G.; Lorenzetti, A. Recycling of Polyurethanes: Where We Are and Where We Are Going. Green Chem. 2024, 26, 1132–1152. [Google Scholar] [CrossRef]
- Liu, B.; Westman, Z.; Richardson, K.; Lim, D.; Stottlemyer, A.L.; Farmer, T.; Gillis, P.; Hooshyar, N.; Vlcek, V.; Christopher, P.; et al. Polyurethane Foam Chemical Recycling: Fast Acidolysis with Maleic Acid and Full Recovery of Polyol. ACS Sustain. Chem. Eng. 2024, 12, 4435–4443. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Ahmad Bhatti, I. Methods for Polyurethane and Polyurethane Composites, Recycling and Recovery: A Review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Sternberg, J.; Pilla, S. Chemical Recycling of a Lignin-Based Non-Isocyanate Polyurethane Foam. Nat. Sustain. 2023, 6, 316–324. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rojo, R.; Alameda, L.; Rodríguez, Á.; Calderón, V.; Gutiérrez-González, S. Characterization of Polyurethane Foam Waste for Reuse in Eco-Efficient Building Materials. Polymers 2019, 11, 359. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Magnin, A.; Entzmann, L.; Bazin, A.; Pollet, E.; Avérous, L. Green Recycling Process for Polyurethane Foams by a Chem-Biotech Approach. ChemSusChem 2021, 14, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Xu, B.; Xu, A.; Cao, S.; Wei, R.; Zhou, J.; Jiang, M.; Dong, W. Biodegradation of Polyether-Polyurethane Foam in Yellow Mealworms (Tenebrio Molitor) and Effects on the Gut Microbiome. Chemosphere 2022, 304, 135263. [Google Scholar] [CrossRef]
- Gunawan, N.R.; Tessman, M.; Zhen, D.; Johnson, L.; Evans, P.; Clements, S.M.; Pomeroy, R.S.; Burkart, M.D.; Simkovsky, R.; Mayfield, S.P. Biodegradation of Renewable Polyurethane Foams in Marine Environments Occurs through Depolymerization by Marine Microorganisms. Sci. Total Environ. 2022, 850, 158761. [Google Scholar] [CrossRef]





| Filler Type | Density, g/cm3 | Thermal Conductivity, W/m*K | Compressive Strength, Kpa |
|---|---|---|---|
| Tung oil-based polyol/rice-husk ash filler [127] | 0.09–0.05 | - | 3–5 |
| Tall oil-based polyol/wheat straw-lignin filler [128] | 0.045–0.06 | 0.0324 | 30–35 |
| Cellulose fibers filler [39] | 0.029–0.037 | 0.0220–0.0300 | 160–175 |
| Castor oil-based polyol/wood flour filler [132] | 0.021–0.037 | 0.0390 | 2100–3400 |
| Wheat-slop filler [133] | 0.033–0.025 | 0.0307 | 80–200 |
| Bagasse-fiber filler [134] | 0.041–0.060 | 0.025–0.030 | 180–240 |
| Pineapple filler [136] | 0.053–0.054 | - | 300–400 |
| Ground-coffee filler [141] | 0.046–0.054 | - | 150–240 |
| Turkey feather-fiber filler [146] | 0.038–0.040 | 0.0291 | - |
| Sunflower press-cake filler [154] | 0.066–0.086 | 0.0294–0.0321 | 160–320 |
| Liquid glass-impregnated sunflower press-cake filler [154] | 0.054–0.068 | 0.0319–0.0328 | 120–200 |
| Type of Recycling | Process | Products |
|---|---|---|
| Mechanical recycling | Extrusion, pressing, injection molding | Products with deteriorated properties compared to the original ones |
| Physical recycling | Pressing with glue | Products with deteriorated properties compared to the original ones |
| Chemical recycling | Hydrolysis, glycolysis, aminolysis, acidolysis, phosphorolysis | Monomers, oligomers |
| Thermochemical recycling | Pyrolysis, gasification, hydrogenation | Chemical compounds, fuels |
| Biological recycling | Biodegradation | CO2, H2O, CH4 |
| Energy recycling | Combustion | Energy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowska, S.; Szymborski, D.; Sienkiewicz, N.; Kairytė, A. Current Progress in Research into Environmentally Friendly Rigid Polyurethane Foams. Materials 2024, 17, 3971. https://doi.org/10.3390/ma17163971
Makowska S, Szymborski D, Sienkiewicz N, Kairytė A. Current Progress in Research into Environmentally Friendly Rigid Polyurethane Foams. Materials. 2024; 17(16):3971. https://doi.org/10.3390/ma17163971
Chicago/Turabian StyleMakowska, Sylwia, Dawid Szymborski, Natalia Sienkiewicz, and Agnė Kairytė. 2024. "Current Progress in Research into Environmentally Friendly Rigid Polyurethane Foams" Materials 17, no. 16: 3971. https://doi.org/10.3390/ma17163971
APA StyleMakowska, S., Szymborski, D., Sienkiewicz, N., & Kairytė, A. (2024). Current Progress in Research into Environmentally Friendly Rigid Polyurethane Foams. Materials, 17(16), 3971. https://doi.org/10.3390/ma17163971







