Preparation of Supercapacitor Carbon Electrode Materials by Low-Temperature Carbonization of High-Nitrogen-Doped Raw Materials from Food Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Experimental Equipment
2.2. FW Hydrothermal Doping Experiment
2.3. FW Pyrolysis Doping Experiment
2.4. Preparation of Supercapacitor Electrode Materials with Doped Carbon
2.5. Characterization of the Activated Doped Carbon
2.5.1. Physicochemical Properties
2.5.2. Electrochemistry and Product Yield Calculations
3. Results and Discussion
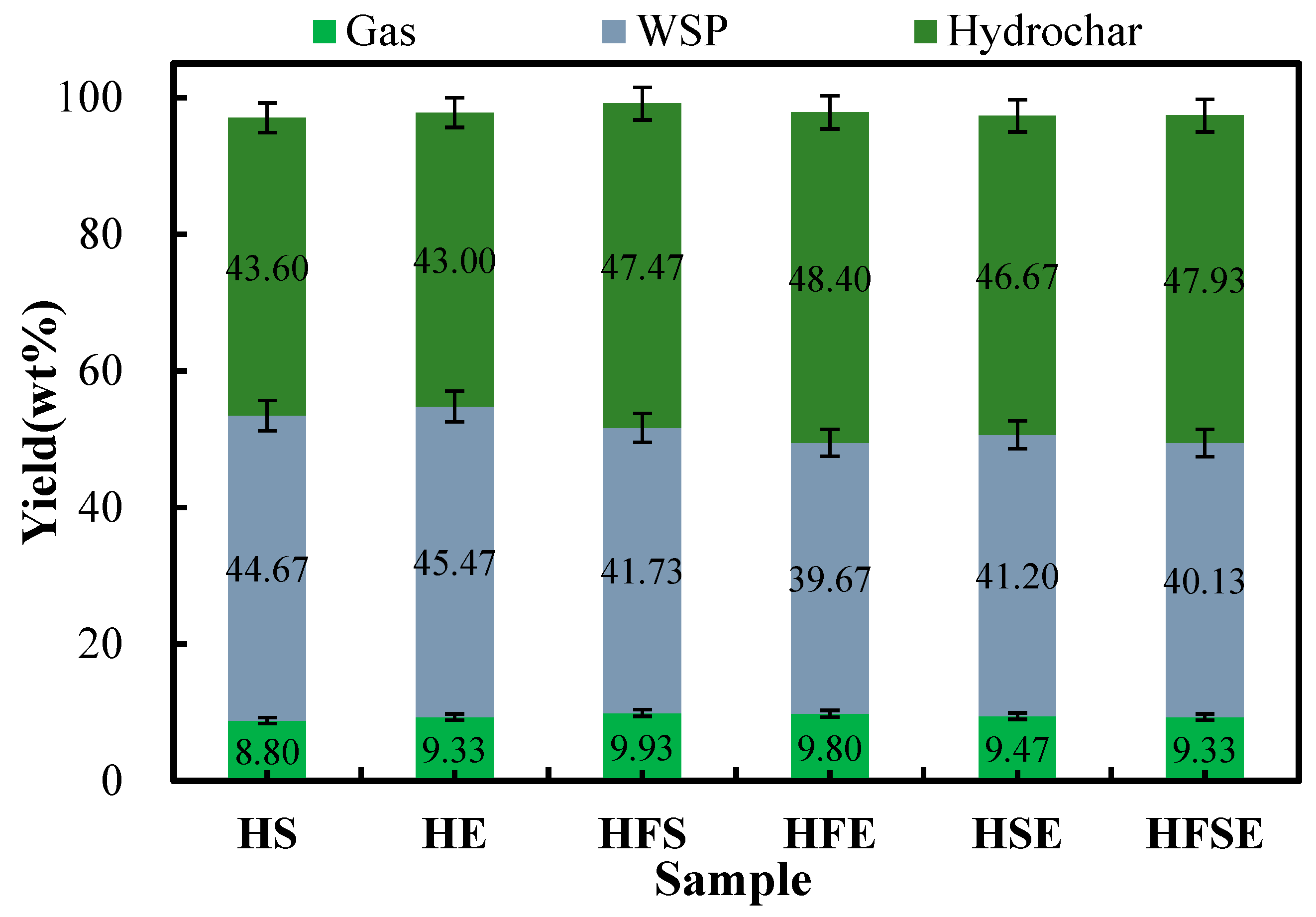
3.1. Distribution of Hydrothermal Product Yields of FW, SM, and EW
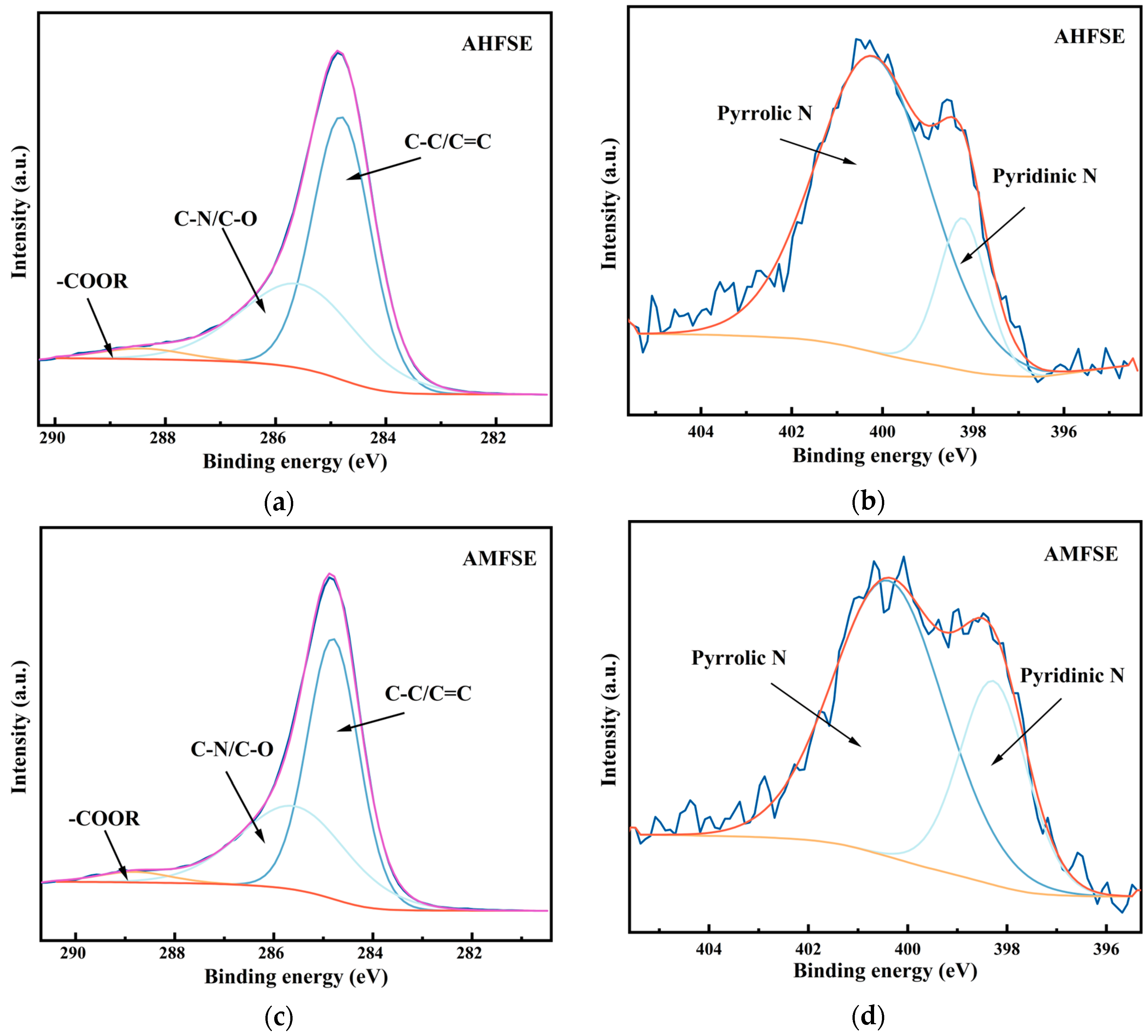
3.2. Carbon Doping and Activated Carbon Doping Element Analysis
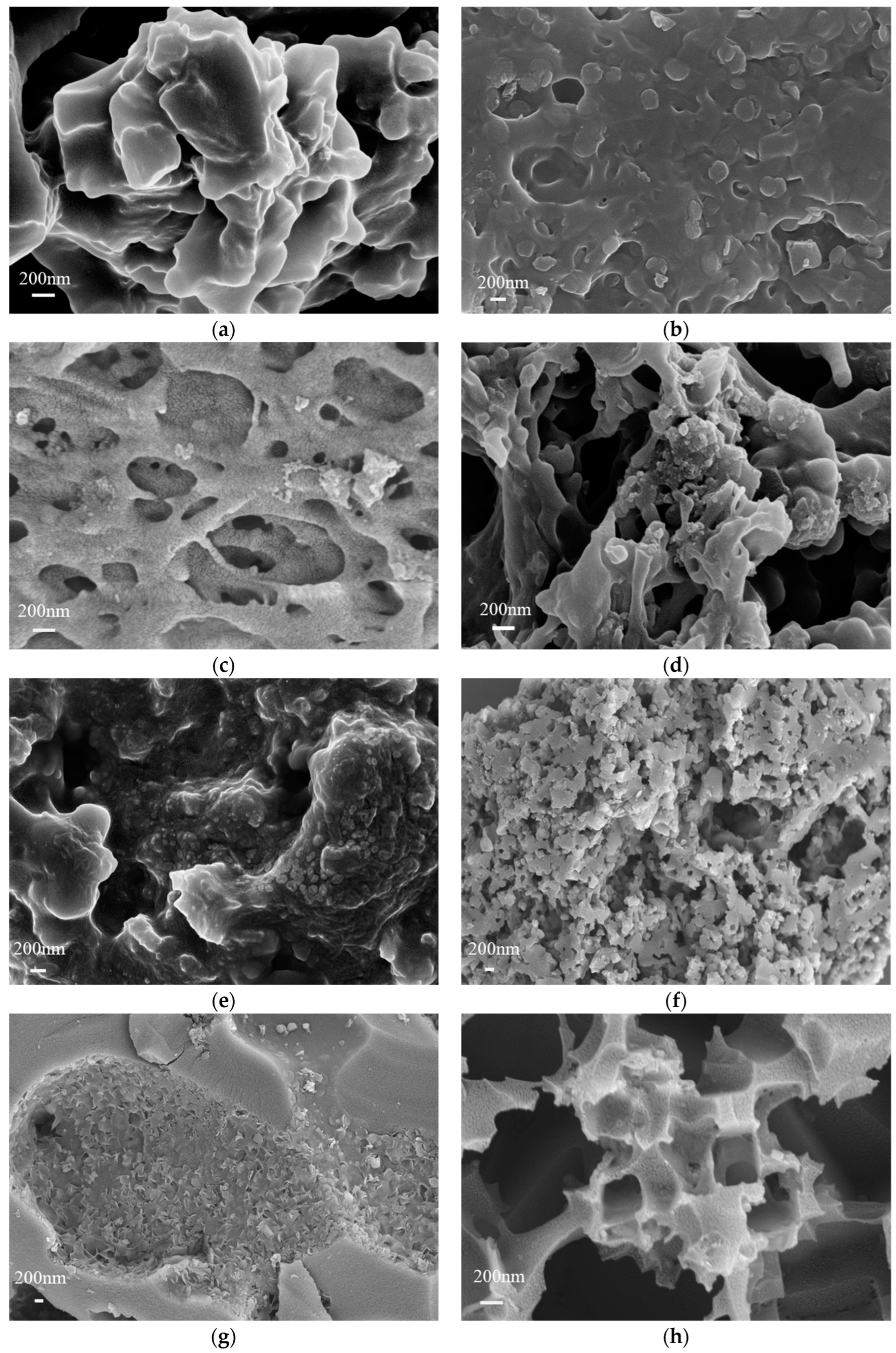
3.3. Carbon Doping and Activated Carbon Doping Morphology and Structural Characterization
3.4. Electrochemical Performance of Doped Carbon
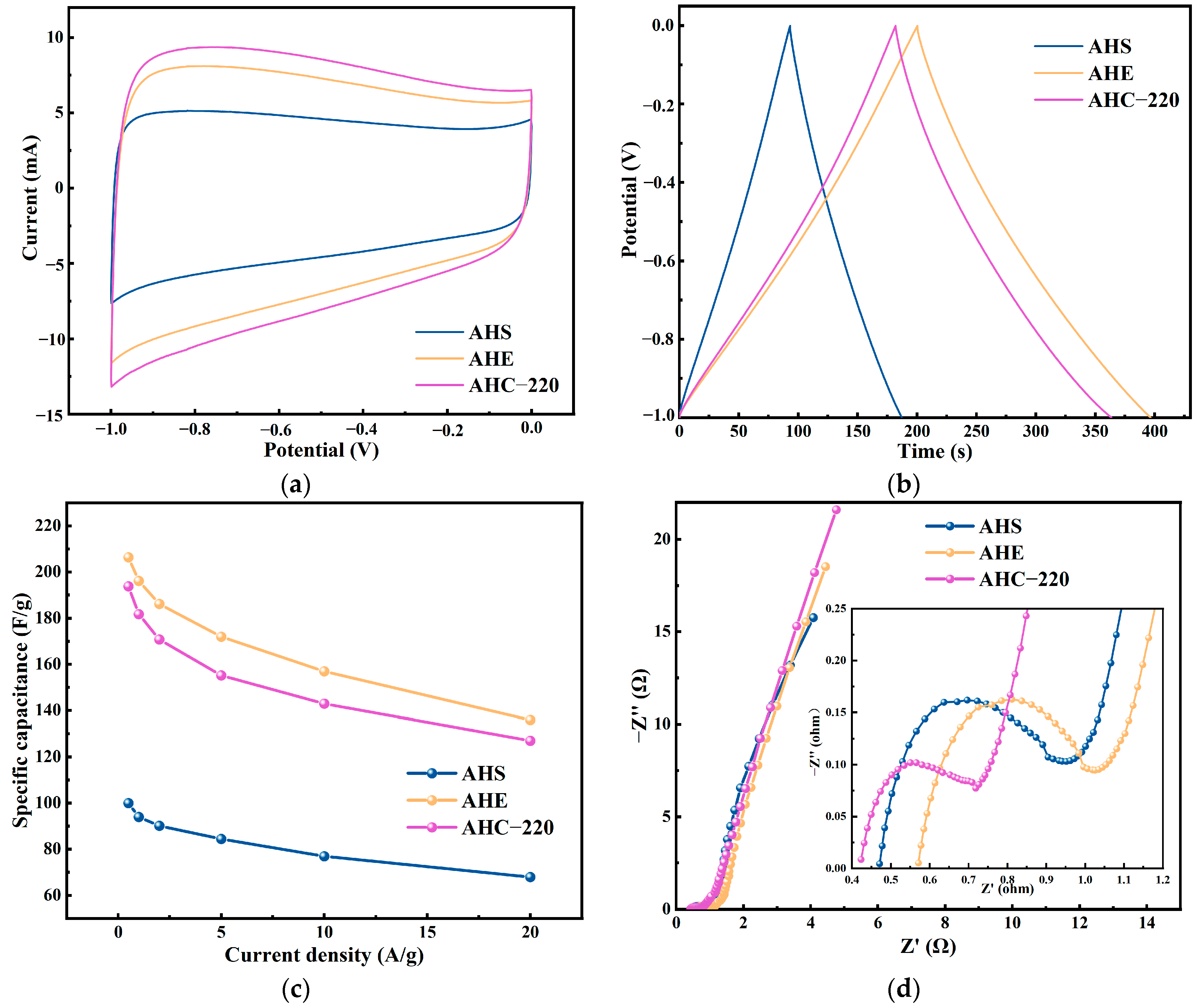
3.4.1. Electrochemical Performance of the AHS and AHE
3.4.2. Electrochemical Performance of AHFS, AHFE, AHSE, and AHFSE
3.4.3. Electrochemical Performance of AMFSE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2020, 56, 173–200. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Tong, Y.; Liu, X.; Zheng, J.; Han, D.; Li, H.; Niu, L. Recent advances in potassium-ion hybrid capacitors: Electrode materials, storage mechanisms and performance evaluation. Energy Storage Mater. 2021, 41, 108–132. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L. Recent advances of cathode materials for zinc-ion hybrid capacitors. Nano Energy 2023, 109, 108290. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of postmodified biochar in supercapacitors. J. Clean Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, L.; Hu, G.; Mai, L.; Cui, Y. Nanowires for Electrochemical Energy Storage. Chem. Rev. 2019, 119, 11042–11109. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Lyu, S.; Xu, H.; Ren, X.; Levendis, Y.A. Activated coke preparation by physical activation of coal and biomass cocarbonized chars. J. Anal. Appl. Pyrolysis 2021, 156, 105137. [Google Scholar] [CrossRef]
- Jin, H.; Li, J.; Yuan, Y.; Wang, J.; Lu, J.; Wang, S. Recent Progress in Biomass-Derived Electrode Materials for High Volumetric Performance Supercapacitors. Adv. Energy Mater. 2018, 8, 1801007. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.T.; Tran, T.T.V.; Van, C.N.; Vo, D.-V.N.; Kongparakul, S.; Zhang, H.; Guan, G.; Samart, C. Carbon sequestration through hydrothermal carbonization of expired fresh milk and its application in supercapacitor. Biomass Bioenergy 2020, 143, 105836. [Google Scholar] [CrossRef]
- K A, S.R.; Rout, C.S. Recent developments, challenges and future prospects of magnetic field effects in supercapacitors. J. Mater. Chem. A 2023, 11, 5495–5519. [Google Scholar] [CrossRef]
- Djandja, O.S.; Yin, L.; Wang, Z.; Duan, P.-G.; Xu, D.; Kapusta, K. Synthesis of N-doped carbon material via hydrothermal carbonization: Effects of reaction solvent and nitrogen source. J. Energy Storage 2023, 60, 106588. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Badhulika, S. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications. J. Power Sources 2020, 480, 228830. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Han, M.; Pang, J.; Xiang, Y.; Cao, G.; Yang, Y. Synthesis of nitrogen-doped polymeric resin-derived porous carbon for high performance supercapacitors. Microporous Mesoporous Mater. 2018, 270, 204–210. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Park, J.-S. Biomass-derived N-doped porous carbon nanosheets for energy technologies. Chem. Eng. J. 2021, 425, 129017. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Deng, X.-Q.; Zhang, S.; Shen, Y.-F.; Shan, Y.-Q.; Zhang, Z.-M.; Luque, R.; Duan, P.-G.; Hu, X. Benign-by-design N-doped carbonaceous materials obtained from the hydrothermal carbonization of sewage sludge for supercapacitor applications. Green Chem. 2020, 22, 3885–3895. [Google Scholar] [CrossRef]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. Biomass derived nitrogen-doped hierarchical porous carbon sheets for supercapacitors with high performance. J. Colloid Interface Sci. 2018, 523, 133–143. [Google Scholar] [CrossRef]
- Shan, Y.-Q.; Xu, Z.-X.; Duan, P.-G.; Fan, H.-L.; Hu, X.; Luque, R. Nitrogen- and Sulfur-Doped Carbon Obtained from Direct Hydrothermal Carbonization of Cellulose and Ammonium Sulfate for Supercapacitor Applications. ACS Sustain. Chem. Eng. 2020, 8, 15809–15814. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Yin, L.-X.; Zhang, X.-X.; Shan, Y.-Q.; Duan, P.-G. Preparation of supercapacitor carbon materials from food waste via low-temperature pyrolysis. J. Anal. Appl. Pyrolysis 2023, 170, 105880. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M. From Soybean residue to advanced supercapacitors. Sci Rep. 2015, 5, 16618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, J.; Gao, Z.; Li, X. Converting eggs to flexible, all-solid supercapacitors. Nano Energy 2019, 65, 104045. [Google Scholar] [CrossRef]
- Wang, J.C.; Kaskel, S. KOH activation of carbon-basedmaterials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.-X.; Sheng, J.-X.; Wang, K.; Yin, L.-X.; Shan, Y.-Q.; Cao, C.-Q.; Duan, P.-G.; Duo, J.; Kapusta, K. Cohydrothermal carbonization of defatted microalgae and glucose for the production of supercapacitor carbon material. J. Energy Storage 2024, 80, 110258. [Google Scholar] [CrossRef]
- Sim, C.-K.; Majid, S.R.; Mahmood, N.Z. Electrochemical Performance of Activated Carbon Derived from Treated Food-Waste. Int. J. Electrochem. Sci. 2015, 10, 10157–10172. [Google Scholar] [CrossRef]
- Grycová, B.; Koutník, I.; Pryszcz, A. Pyrolysis process for the treatment of food waste. Bioresour. Technol. 2016, 218, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, X.; Li, L.; Hu, Z.; Guo, P.; Jiang, Y. The catalytic pyrolysis of food waste by microwave heating. Bioresour. Technol. 2014, 166, 45–50. [Google Scholar] [CrossRef]
- Tarimo, D.J.; Oyedotun, K.O.; Sylla, N.F.; Mirghni, A.A.; Ndiaye, N.M.; Manyala, N. Waste chicken bone-derived porous carbon materials as high performance electrode for supercapacitor applications. J. Energy Storage 2022, 51, 104378. [Google Scholar] [CrossRef]
- Zhan, C.; Yu, X.; Liang, Q.; Liu, W.; Wang, Y.; Lv, R.; Huang, Z.-H.; Kang, F. Flour food waste derived activated carbon for high-performance supercapacitors. RSC Adv. 2016, 6, 89391–89396. [Google Scholar] [CrossRef]
- Li, P.; Xie, H.; Liu, Y.; Wang, J.; Xie, Y.; Hu, W.; Xie, T.; Wang, Y.; Zhang, Y. Molten salt and air induced nitrogen-containing graphitic hierarchical porous biocarbon nanosheets derived from kitchen waste hydrolysis residue for energy storage. J. Power Sources 2019, 439, 227096. [Google Scholar] [CrossRef]
- Khan, Z.; Park, S.O.; Yang, J.; Park, S.; Shanker, R.; Song, H.-K.; Kim, Y.; Kwak, S.K.; Ko, H. Binary N,S-doped carbon nanospheres from bioinspired artificial melanosomes: A route to efficient air electrodes for seawater batteries. J. Mater. Chem. A 2018, 6, 24459–24467. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.; Wang, M.; Ji, D.; Tan, W. XPS and two-dimensional FTIR correlation analysis on the binding characteristics of humic acid onto kaolinite surface. Sci. Total Environ. 2020, 724, 138145. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Chen, X.; Hou, X.; Lin, H.; Jia, L. One step synthesis of N, P codoped hierarchical porous carbon nanosheets derived from pomelo peel for high performance supercapacitors. J. Colloid Interface Sci. 2022, 605, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, N.; Liu, X.-H.; Shang, H. High-performance N-doped activated carbon derived from walnut green peel for supercapacitors. Biomass Convers. Biorefinery 2022, 14, 14641–14651. [Google Scholar] [CrossRef]
- Yang, X.; Lv, T.; Qiu, J. High Mass-Loading Biomass-Based Porous Carbon Electrodes for Supercapacitors: Review and Perspectives. Small 2023, 19, 2300336. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Du, J.; Chen, A.; Gao, X.; Otun, K.O.; Liu, X.; Jewell, L.L. N-doped crumpled carbon nanotubes as advanced electrode material for supercapacitor. J. Alloys Compd. 2022, 928, 167222. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Szkoda, M.; Zarach, Z.; Lukaszewicz, J.P. N-doped carbon materials as electrodes for highly stable supercapacitors. Mater. Res. Lett. 2022, 11, 213–221. [Google Scholar] [CrossRef]
- Li, H.; Bai, Q.; Li, C.; Wei, X.; Shu, Y.; Chen, B.; Shen, Y. N-Doped Two-Dimensional Carbon Nanosheets with Micropore-Dominant Porosity for High-Performance Supercapacitors. Energy Fuels 2022, 36, 13246–13255. [Google Scholar] [CrossRef]
- Li, H.; Du, T.; Wang, Q.; Guo, J.; Zhang, S.; Lu, Y. A new synthesis of O/N-doped porous carbon material for supercapacitors. J. Energy Storage 2023, 66, 107397. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Shen, F.; Tian, D.; Huang, M.; He, J.; Zou, J.; Zhao, L.; Zeng, Y. Trichoderma bridges waste biomass and ultrahigh specific surface area carbon to achieve a high-performance supercapacitor. J. Power Sources 2021, 497, 229880. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, H.; Lan, Y.; Qiu, C.; Du, G.; Su, X. Preparation of high-performance, three-dimensional, hierarchical porous carbon Supercapacitor materials and high-value-added potassium Humate from cotton stalks. Diam. Relat. Mat. 2021, 116, 108375. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Song, C.-L.; Ren, K.; Long, S.-W.; Zhu, Y.-Q.; Teng, Z.-C.; Wang, M.-M.; Zhang, J.-G.; Han, K.-h. Preparation of activated carbon from unburned carbon in biomass fly ash and its supercapacitor performance. J. Fuel Chem. Technol. 2021, 49, 1936–1942. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, K.; Wu, C. Recent progress in supercapacitors based on the advanced carbon electrodes. Nanotechnol. Rev. 2019, 8, 299–314. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Wang, X.; Wang, X.; Ju, Y.; Ge, S.; Lu, X.; Ding, J.; Yuan, N.; Gogotsi, Y. Low-Temperature pseudocapacitive energy storage in Ti3C2T MXene. Energy Storage Mater. 2020, 33, 382–389. [Google Scholar] [CrossRef]
- Shinde, P.A.; Abbas, Q.; Chodankar, N.R.; Ariga, K.; Abdelkareem, M.A.; Olabi, A.G. Strengths, weaknesses, opportunities, and threats (SWOT) analysis of supercapacitors: A review. J. Energy Chem. 2023, 79, 611–638. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, G.; Li, A. Edge defects-enriched porous carbon derived from food waste for high-performance supercapacitors. Mater. Lett. 2019, 253, 74–77. [Google Scholar] [CrossRef]




| Samples Name | C (%) | H (%) | N (%) | S (%) | O (%) |
|---|---|---|---|---|---|
| FW | 48.17 | 7.04 | 2.72 | 0.19 | 41.02 |
| SM | 45.51 | 6.44 | 7.69 | 0.35 | 38.90 |
| EW | 44.61 | 6.97 | 3.98 | 0.43 | 43.38 |
| HFS | 64.10 | 7.17 | 6.74 | 0.37 | 21.30 |
| HFE | 66.38 | 6.42 | 5.42 | 0.43 | 21.21 |
| HSE | 64.54 | 6.71 | 7.54 | 0.45 | 20.53 |
| HFSE | 64.40 | 6.56 | 6.79 | 0.15 | 21.49 |
| AHFS | 77.30 | 1.58 | 6.59 | 0.23 | 14.06 |
| AHFE | 78.27 | 1.46 | 5.44 | 0.31 | 14.08 |
| AHSE | 79.13 | 2.11 | 5.29 | 0.22 | 12.68 |
| AHFSE | 77.58 | 1.66 | 5.67 | 0.19 | 14.41 |
| AMFSE | 74.69 | 1.43 | 5.05 | 0.20 | 17.98 |
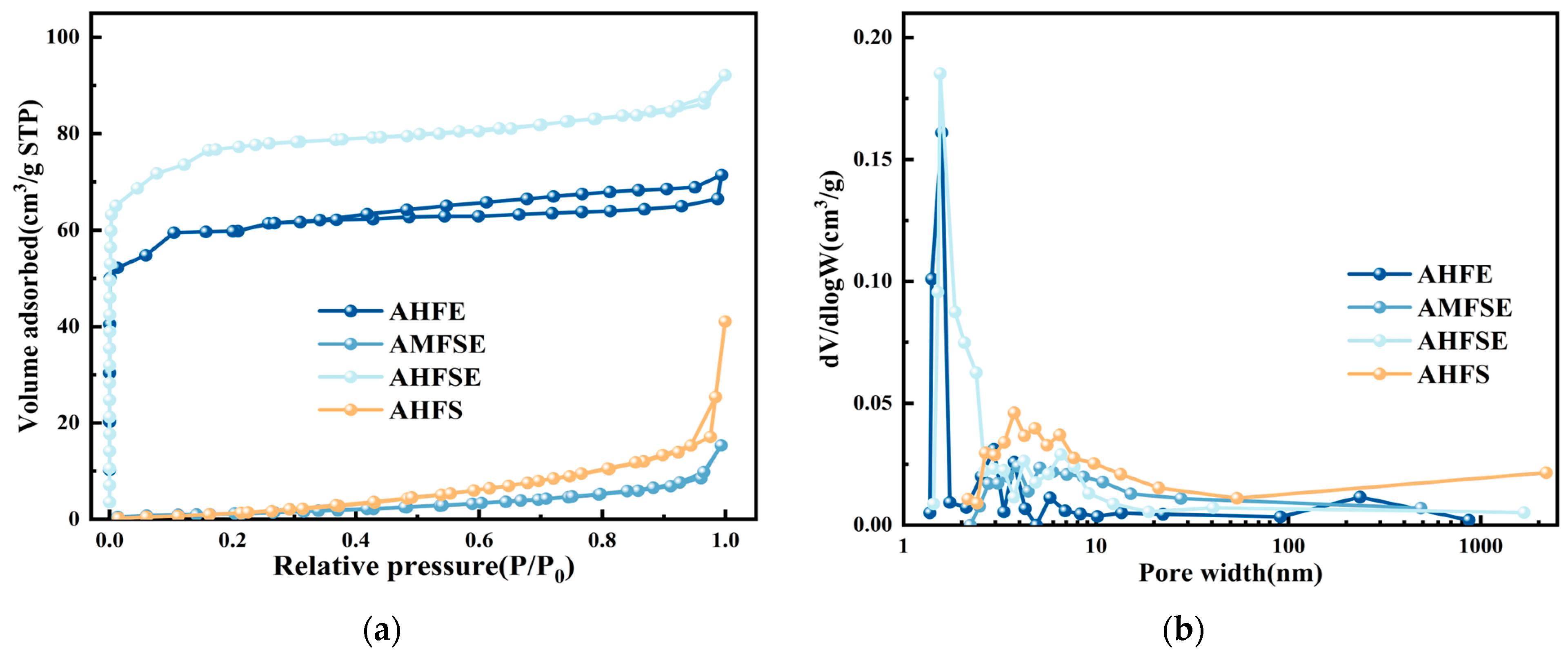
| Samples Name | SBET (m2/g) | Smicro (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Dpores (nm) |
|---|---|---|---|---|---|
| AHFE | 207.8 | 179.8 | 0.11 | 0.09 | 2.20 |
| AMFSE | 11.8 | 2.95 | 0.03 | 0.0012 | 26.7 |
| AHFSE | 268.6 | 248.2 | 0.14 | 0.12 | 2.38 |
| AHFS | 22.4 | 4.42 | 0.07 | 0.0015 | 21.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Q.; Liu, C.; Guo, Y.; Wang, K.; Gao, Z.; Du, Y.; Cao, C.; Duan, P.; Kapusta, K. Preparation of Supercapacitor Carbon Electrode Materials by Low-Temperature Carbonization of High-Nitrogen-Doped Raw Materials from Food Waste. Materials 2024, 17, 3984. https://doi.org/10.3390/ma17163984
Mu Q, Liu C, Guo Y, Wang K, Gao Z, Du Y, Cao C, Duan P, Kapusta K. Preparation of Supercapacitor Carbon Electrode Materials by Low-Temperature Carbonization of High-Nitrogen-Doped Raw Materials from Food Waste. Materials. 2024; 17(16):3984. https://doi.org/10.3390/ma17163984
Chicago/Turabian StyleMu, Qingnan, Chang Liu, Yao Guo, Kun Wang, Zhijie Gao, Yuhan Du, Changqing Cao, Peigao Duan, and Krzysztof Kapusta. 2024. "Preparation of Supercapacitor Carbon Electrode Materials by Low-Temperature Carbonization of High-Nitrogen-Doped Raw Materials from Food Waste" Materials 17, no. 16: 3984. https://doi.org/10.3390/ma17163984
APA StyleMu, Q., Liu, C., Guo, Y., Wang, K., Gao, Z., Du, Y., Cao, C., Duan, P., & Kapusta, K. (2024). Preparation of Supercapacitor Carbon Electrode Materials by Low-Temperature Carbonization of High-Nitrogen-Doped Raw Materials from Food Waste. Materials, 17(16), 3984. https://doi.org/10.3390/ma17163984








