A Multiscale Inelastic Internal State Variable Corrosion Model
Abstract
:1. Introduction
2. Process–Structure–Property Experiments Used to Calibrate the Corrosion Model
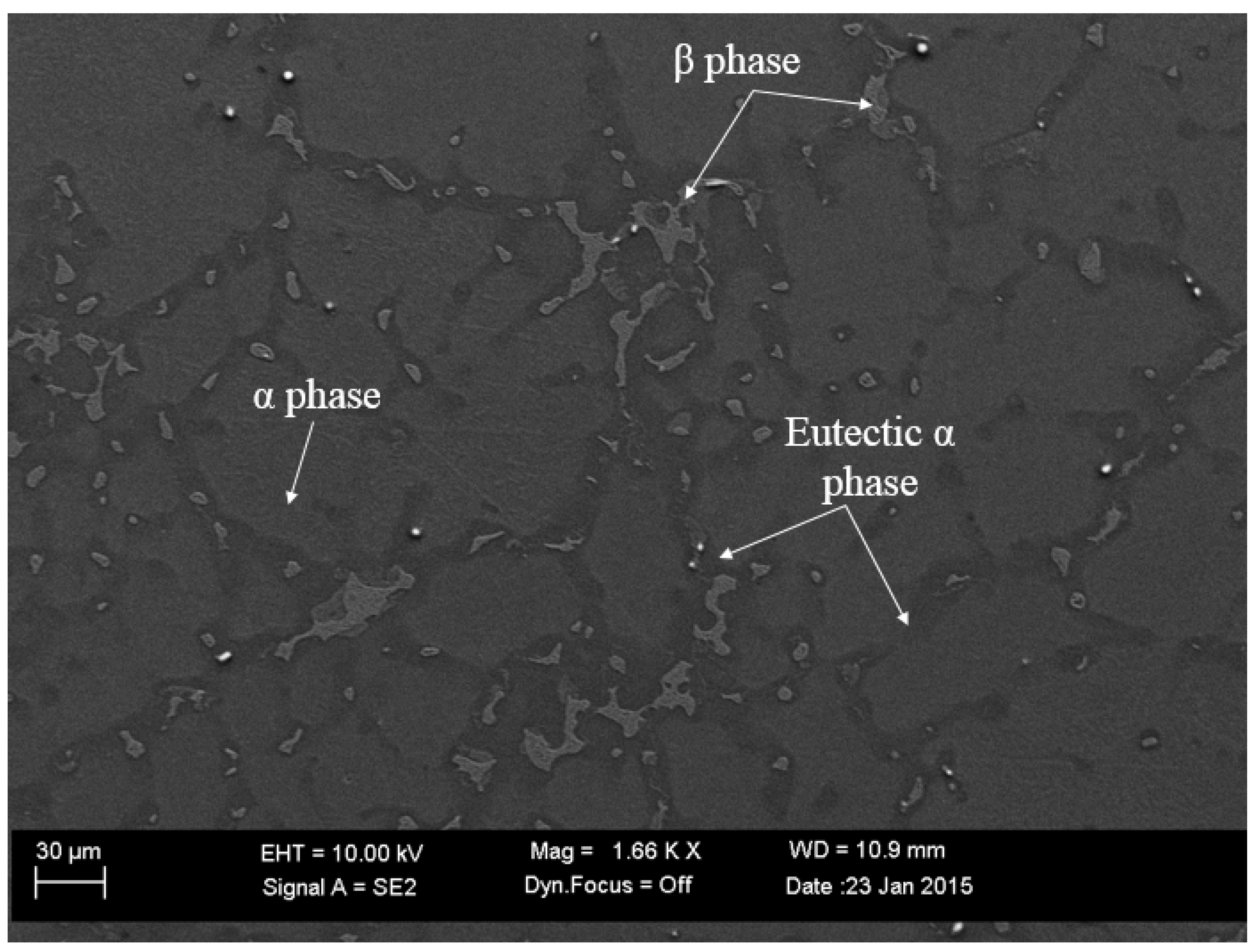
2.1. Materials Processing of Magnesium Castings
2.2. Microstructural Analysis
2.3. Corrosion Properties
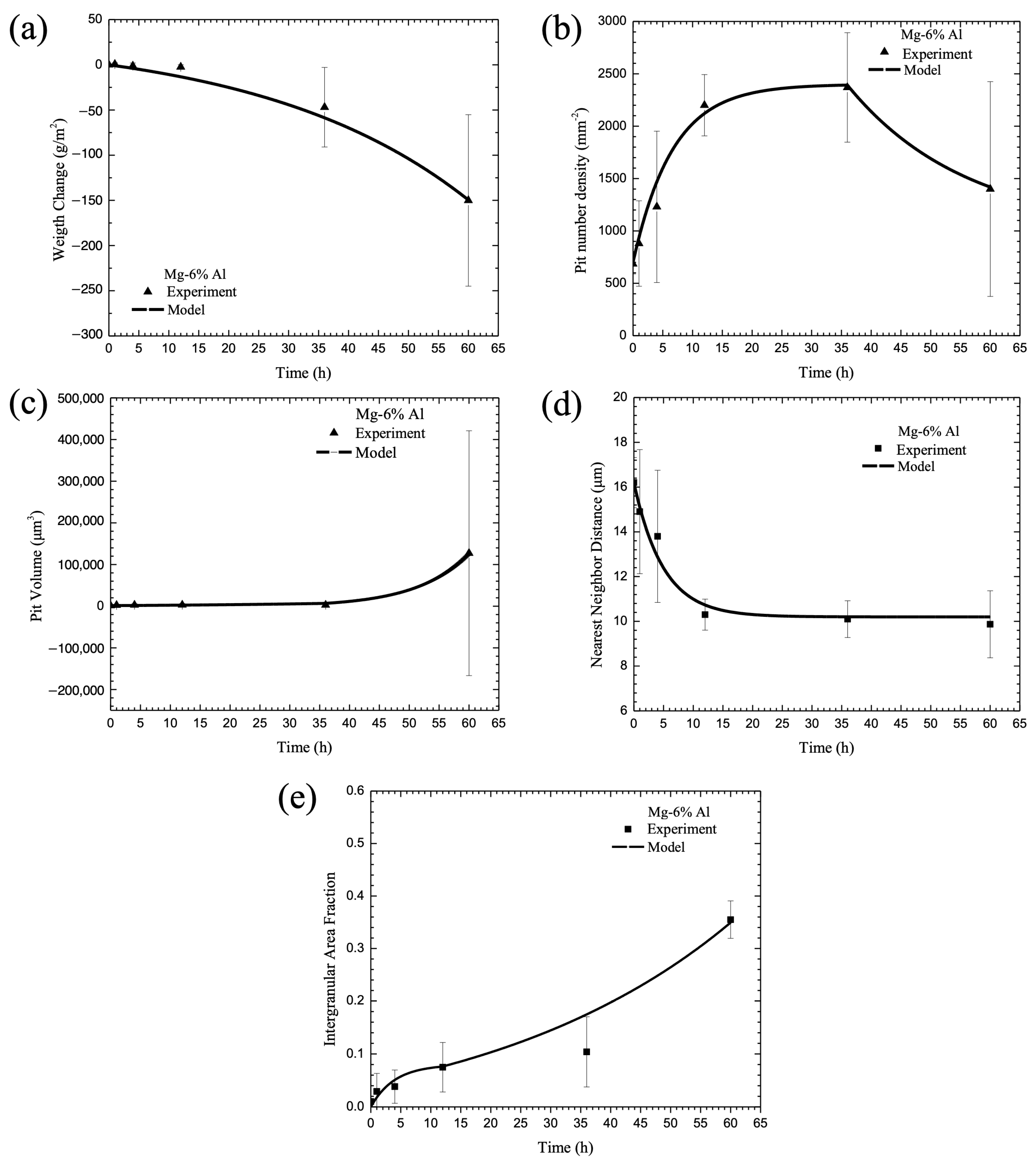
3. Macroscale Corrosion Damage
3.1. Macroscale General Corrosion Rate
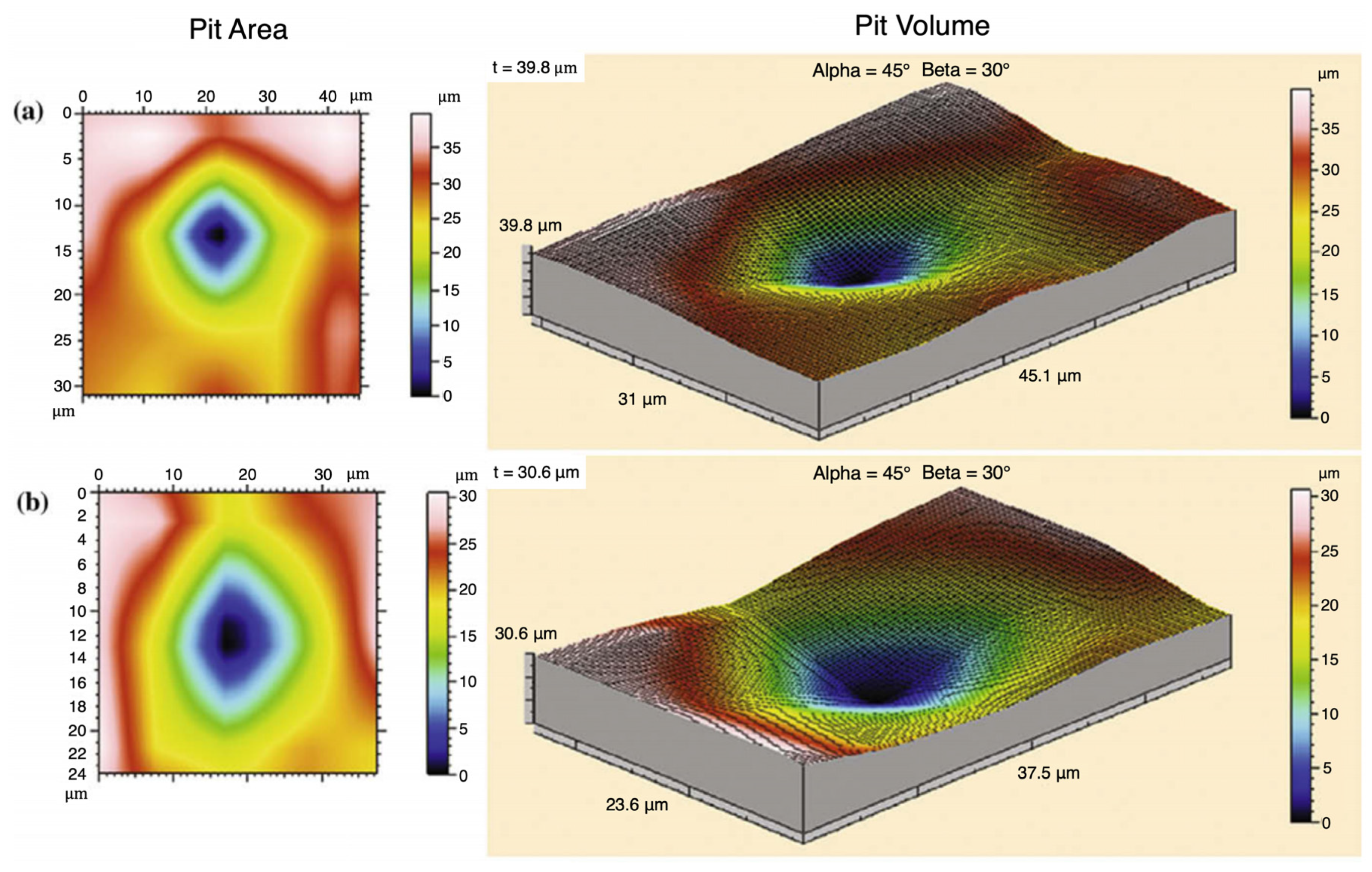
3.2. Macroscale Pitting Corrosion
3.3. Macroscale Intergranular Corrosion
3.4. Mesoscale Electrochemical Kinetics
3.5. Bridging Different Length Scales
3.5.1. General Corrosion Rate at the Mesoscale
3.5.2. Pit Nucleation Rate
3.5.3. Internal State Variable (ISV) Pit Growth Rate with Butler–Volmer Equation
3.5.4. Internal State Variable (ISV) Pit Coalescence Rate with Butler–Volmer Equation
3.5.5. Internal State Variable (ISV) Intergranular Corrosion Rate with Butler–Volmer Equation
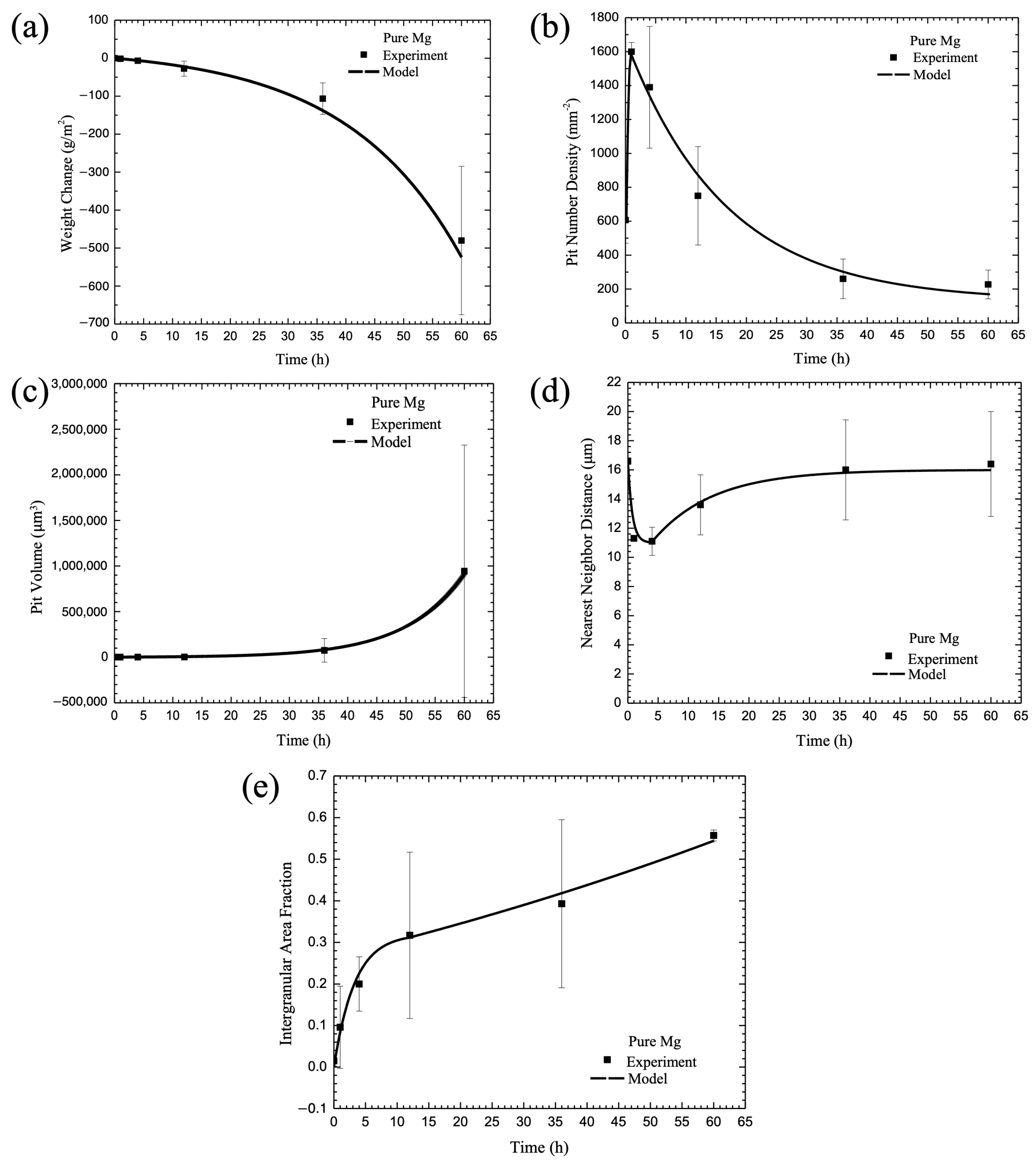
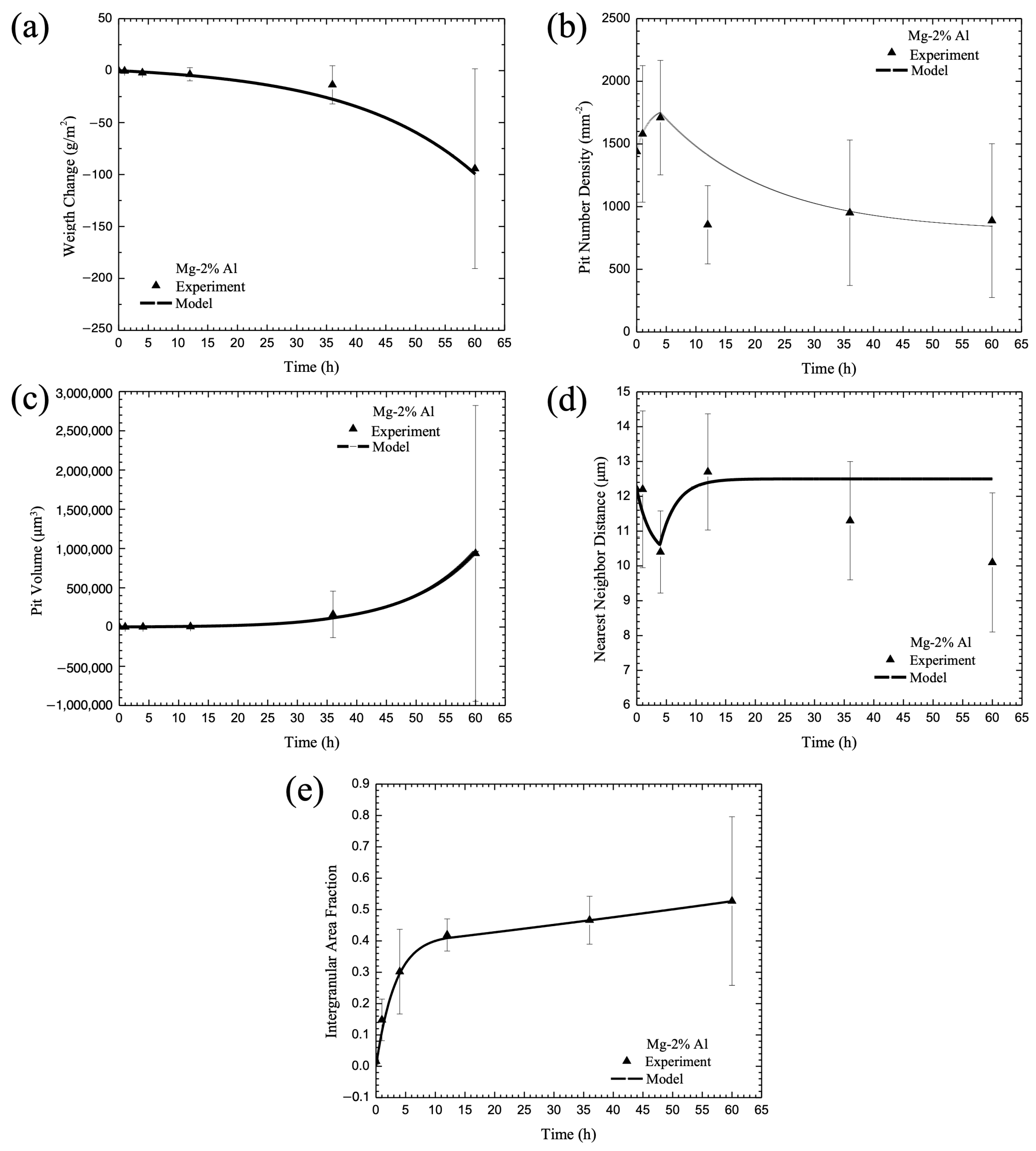
3.6. Calibration of Internal State Variable (ISV) Corrosion Equations with Experimental Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, B.M. Corrosion and Corrosion Control in Light Water Reactors. JOM 2013, 65, 1043–1056. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Perez, T.E. Corrosion in the Oil and Gas Industry: An Increasing Challenge for Materials. JOM 2013, 65, 1033–1042. [Google Scholar] [CrossRef]
- Aghion, E.; Bronfin, B. Magnesium Alloys Development towards the 21st Century. Mater. Sci. Forum 2000, 350–351, 19–30. [Google Scholar] [CrossRef]
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium” in the automotive industry. J. Mater. Process. Technol. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Froats, A.; Aune, T.K.; Hawke, D.; Unsworth, W.; Hillis, J. Corrosion of magnesium and magnesium alloys. In ASM Handbook; ASM International: Almere, The Netherlands, 1987; Volume 13, pp. 740–754. [Google Scholar]
- Cho, H.E.; Hammi, Y.; Francis, D.K.; Stone, T.; Mao, Y.; Sullivan, K.; Wilbanks, J.; Zelinka, R.; Horstemeyer, M.F. Mi-crostructure-sensitive, history-dependent internal state variable plasticity-damage model for a sequential tubing process. In Integrated Computational Materials Engineering (ICME) for Metals: Concepts and Case Studies; Wiley: Hoboken, NJ, USA, 2018; pp. 199–234. [Google Scholar] [CrossRef]
- Cho, H.; Hammi, Y.; Bowman, A.; Karato, S.-I.; Baumgardner, J.; Horstemeyer, M. A unified static and dynamic recrystallization Internal State Variable (ISV) constitutive model coupled with grain size evolution for metals and mineral aggregates. Int. J. Plast. 2019, 112, 123–157. [Google Scholar] [CrossRef]
- Horstemeyer, M.F.; Bammann, D.J. Historical review of internal state variable theory for inelasticity. Int. J. Plast. 2010, 26, 1310–1334. [Google Scholar] [CrossRef]
- Coleman, B.D.; Gurtin, M.E. Thermodynamics with Internal State Variables. J. Chem. Phys. 1967, 47, 597–613. [Google Scholar] [CrossRef]
- Gurson, A.L. Continuum Theory of Ductile Rupture by Void Nucleation and Growth: Part I—Yield Criteria and Flow Rules for Porous Ductile Media. J. Eng. Mater. Technol. 1977, 99, 2–15. [Google Scholar] [CrossRef]
- Bammann, D.J.; Chiesa, M.L.; Horstemeyer, M.F.; Weingarten, L.I. Failure in ductile materials using finite element methods. Struct. Crashworthiness Fail. 1993, 1–54. [Google Scholar]
- Cocks, A.C.F.; Ashby, M.F. Intergranular fracture during power-law creep under multiaxial stresses. Met. Sci. 1980, 14, 395–402. [Google Scholar] [CrossRef]
- Horstemeyer, M.F.; Gokhale, A.M. A void–crack nucleation model for ductile metals. Int. J. Solids Struct. 1999, 36, 5029–5055. [Google Scholar] [CrossRef]
- Horstemeyer, M.F.; Lathrop, J.; Gokhale, A.; Dighe, M. Modeling stress state dependent damage evolution in a cast Al–Si–Mg aluminum alloy. Theor. Appl. Fract. Mech. 2000, 33, 31–47. [Google Scholar] [CrossRef]
- Chandler, M.Q.; Bammann, D.J.; Horstemeyer, M.F. A Continuum Model for Hydrogen-Assisted Void Nucleation in Ductile Materials. Model. Simul. Mater. Sci. Eng. 2013, 21, 055028. [Google Scholar] [CrossRef]
- Peterson, L.A.; Horstemeyer, M.F.; Lacy, T.E.; Moser, R.D. Experimental Characterization and Constitutive Modeling of an Aluminum 7085-T711 Alloy Under Large Deformations at Varying Strain Rates, Stress States, and Temperatures. Mech. Mater. 2020, 151, 103602. [Google Scholar] [CrossRef]
- Cho, H.; Zbib, H.M.; Horstemeyer, M.F. An Internal State Variable Elastoviscoplasticity-Damage Model for Irradiated Metals. J. Eng. Mater. Technol. 2022, 144, 011017. [Google Scholar] [CrossRef]
- Dimitrov, N.; Liu, Y.; Horstemeyer, M.F. An electroplastic internal state variable (ISV) model for nonferromagnetic ductile metals. Mech. Adv. Mater. Struct. 2020, 29, 761–772. [Google Scholar] [CrossRef]
- Malki, M.; Horstemeyer, M.F.; Cho, H.E.; Peterson, L.A.; Dickel, D.; Capolungo, L.; Baskes, M.I. A Multiphysics Thermoelastoviscoplastic Damage Internal State Variable Constitutive Model including Magnetism. Materials 2024, 17, 2412. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.A.; Horstemeyer, M.; Martin, H.J.; Francis, D. Formulation of a macroscale corrosion damage internal state variable model. Int. J. Solids Struct. 2014, 51, 1235–1245. [Google Scholar] [CrossRef]
- Amiri, M.; Arcari, A.; Airoldi, L.; Naderi, M.; Iyyer, N. A continuum damage mechanics model for pit-to-crack transition in AA2024-T3. Corros. Sci. 2015, 98, 678–687. [Google Scholar] [CrossRef]
- Chen, Z.; Bobaru, F. Peridynamic modeling of pitting corrosion damage. J. Mech. Phys. Solids 2015, 78, 352–381. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Chen, Z.; Li, S.; Bobaru, F. A peridynamic mechano-chemical damage model for stress-assisted corrosion. Electrochimica Acta 2019, 323, 134795. [Google Scholar] [CrossRef]
- Xia, D.; Deng, C.; Chen, Z.; Li, T.; Hu, W. Modeling localized corrosion propagation of metallic materials by peri-dynamics: Progresses and challenges. Acta Metall. Sin. 2022, 58, 1093–1107. [Google Scholar]
- Cui, C.; Ma, R.; Martínez-Pañeda, E. A phase field formulation for dissolution-driven stress corrosion cracking. J. Mech. Phys. Solids 2020, 147, 104254. [Google Scholar] [CrossRef]
- Mai, W.; Soghrati, S.; Buchheit, R.G. A phase field model for simulating the pitting corrosion. Corros. Sci. 2016, 110, 157–166. [Google Scholar] [CrossRef]
- Mai, W.; Soghrati, S. A phase field model for simulating the stress corrosion cracking initiated from pits. Corros. Sci. 2017, 125, 87–98. [Google Scholar] [CrossRef]
- Hu, P.; Meng, Q.; Hu, W.; Shen, F.; Zhan, Z.; Sun, L. A continuum damage mechanics approach coupled with an improved pit evolution model for the corrosion fatigue of aluminum alloy. Corros. Sci. 2016, 113, 78–90. [Google Scholar] [CrossRef]
- Lemaitre, J.; Chaboche, J.-L. Mechanics of Solid Materials; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar] [CrossRef]
- Li, A.; Hu, W.; Zhan, Z.; Meng, Q. A novel continuum damage mechanics-based approach for thermal corrosion fatigue (TCF) life prediction of aluminum alloys. Int. J. Fatigue 2022, 163, 107065. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Qi, W.; Wang, J.; Xie, J.; Wang, H.; Chang, L.; Liu, B.; Zeng, G.; Gao, Q.; et al. Modeling of Pitting Corrosion Damage Based on Electrochemical and Statistical Methods. J. Electrochem. Soc. 2019, 166, C539–C549. [Google Scholar] [CrossRef]
- Gutman, E.M. Mechanochemistry of Materials; Cambridge Int Science Publishing: Cambridge, UK, 1998. [Google Scholar]
- Valor, A.; Caleyo, F.; Alfonso, L.; Rivas, D.; Hallen, J. Stochastic modeling of pitting corrosion: A new model for initiation and growth of multiple corrosion pits. Corros. Sci. 2007, 49, 559–579. [Google Scholar] [CrossRef]
- Wei, R.P. Environmental considerations for fatigue cracking. Fatigue Fract. Eng. Mater. Struct. 2002, 25, 845–854. [Google Scholar] [CrossRef]
- Liu, C.; Kelly, R.G. A review of the application of finite element method (FEM) to localized corrosion modeling. Corrosion 2019, 75, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Bailly-Salins, L.; Borrel, L.; Jiang, W.; Spencer, B.W.; Shirvan, K.; Couet, A. Modeling of high-temperature corrosion of zirconium alloys using the extended finite element method (X-FEM). Corros. Sci. 2021, 189, 109603. [Google Scholar] [CrossRef]
- Jin, H.; Yu, S. Study on corrosion-induced cracks for the concrete with transverse cracks using an improved CDM-XFEM. Constr. Build. Mater. 2022, 318, 126173. [Google Scholar] [CrossRef]
- Zhang, X.; Okodi, A.; Tan, L.; Leung, J.; Adeeb, S. Failure Pressure Prediction of Cracks in Corrosion Defects Using XFEM. In International Pipeline Conference; American Society of Mechanical Engineers: New York, NY, USA, 2020; Volume 84447, p. V001T03A006. [Google Scholar]
- Guo, S.; Wang, H.; Han, E.H. Cellular Automata Simulation of Pitting Corrosion of Metals: A Review. Corrosion Modelling with Cellular Automata; Elsevier: Amsterdam, The Netherlands, 2024; pp. 155–181. [Google Scholar]
- Reinoso-Burrows, J.C.; Toro, N.; Cortés-Carmona, M.; Pineda, F.; Henriquez, M.; Madrid, F.M.G. Cellular Automata Modeling as a Tool in Corrosion Management. Materials 2023, 16, 6051. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Jiang, Y.; Ke, D.; Hu, X.; Liu, X. Review on Cellular Automata for Microstructure Simulation of Metallic Materials. Materials 2024, 17, 1370. [Google Scholar] [CrossRef] [PubMed]
- Horstemeyer, M.F. Integrated Computational Materials Engineering (ICME) for Metals: Reinvigorating Engineering Design with Science; Wiley Press: New York, NY, USA, 2012. [Google Scholar]
- Horstemeyer, M.F. Integrated Computational Materials Engineering (ICME) for Metals: Concepts and Case Studies; Wiley Press: New York, NY, USA, 2018. [Google Scholar]
- Butler, J.A.V. Studies in heterogeneous equilibria. Part II.—The kinetic interpretation of the nernst theory of electromotive force. Trans. Faraday Soc. 1924, 19, 729–733. [Google Scholar] [CrossRef]
- Butler, J.A.V. The thermodynamics of the surfaces of solutions. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1932, 135, 348–375. [Google Scholar]
- Butler, J.A.V. Hydrogen overvoltage and the reversible hydrogen electrode. Proc. R. Soc. Lond. Ser. A. Math. Phys. Sci. 1936, 157, 423–433. [Google Scholar] [CrossRef]
- Erdey-Gruz, T.; Volmer, M. The theory of hydrogen overvoltage. Z. Phys. Chem 1930, 150, 203–213. [Google Scholar]
- Gurtin, M.E.; Drugan, W.J. An Introduction to Continuum Mechanics. J. Appl. Mech. 1981, 51, 949–950. [Google Scholar] [CrossRef]
- Martin, H.J.; Horstemeyer, M.F.; Wang, P.T. Structure–property quantification of corrosion pitting under immersion and salt-spray environments on an extruded AZ61 magnesium alloy. Corros. Sci. 2011, 53, 1348–1361. [Google Scholar] [CrossRef]
- Martin, H.J.; Horstemeyer, M.F.; Wang, P.T. Effects of Variations in Salt-Spray Conditions on the Corrosion Mechanisms of an AE44 Magnesium Alloy. Int. J. Corros. 2010, 1, 602342. [Google Scholar] [CrossRef]
- Walton, C.A.; Martin, H.J.; Horstemeyer, M.F.; Wang, P.T. Quantification of corrosion mechanisms under immersion and salt-spray environments on an extruded AZ31 magnesium alloy. Corros. Sci. 2012, 56, 194–208. [Google Scholar] [CrossRef]
- Liu, M.; Uggowitzer, P.J.; Nagasekhar, A.; Schmutz, P.; Easton, M.; Song, G.-L.; Atrens, A. Calculated phase diagrams and the corrosion of die-cast Mg–Al alloys. Corros. Sci. 2009, 51, 602–619. [Google Scholar] [CrossRef]
- ASTM B117–07a; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM: West Conshohocken, PA, USA, 2007.
- Faraday, M. Experimental Researches in Electricity, Vols. I. and II. Richard and John Edward Taylor; Richard Taylor and William Francis: London, UK, 1855; Volume III. [Google Scholar]
- Faraday, M. Experimental Researches in Chemistry and Physics; Taylor and Francis: London, UK, 1859. [Google Scholar]
- ASM Handbook; ASM International: Almere, The Netherlands, 1987; Volume 13, ISBN 0-87170-007-7.
- Zhu, L. Study of Corrosion Pits in Chloride Solution. Ph.D. Thesis, University of Illinois, Champaign, IL, USA, 2002. [Google Scholar]
- Ernst, P.; Newman, R.C. Pit growth studies in stainless steel foils. I. Introduction and pit growth kinetics. Corros. Sci. 2002, 44, 927–941. [Google Scholar] [CrossRef]
- Coulomb, C.A. Premier mémoire sur l‘électricité et le magnétisme. In Histoire de l’Académie Royale des Sciences; Imprimerie Royale: Paris, France, 1785; pp. 569–775. [Google Scholar]
- Coulomb, C.A. Second mémoire sur l‘électricité et le magnétisme. In Histoire de l’Académie Royale des Sciences; Imprimerie Royale: Paris, France, 1785; pp. 578–611. [Google Scholar]
- Coulomb, C.A. Troisième mémoire sur l‘électricité et le magnétisme. In Histoire de l’Académie Royale des Sciences; Imprimerie Royale: Paris, France, 1785; pp. 612–638. [Google Scholar]
- Maxwell, J.C. On Stresses in Rarified Gases Arising from Inequalities of Temperature. R. Soc. Proc. 1878, 27, 304–308. [Google Scholar]
- Tedmon, C.S.; Vermilyea, D.A.; Rosolowski, J.H. Intergranular Corrosion of Austenitic Stainless Steel. J. Electrochem. Soc. 1971, 118, 192–202. [Google Scholar] [CrossRef]
- Birbilis, N.; Ralston, K.D.; Virtanen, S.; Fraser, H.L.; Davies, C.H.J. Grain character influences on corrosion of ECAPed pure magnesium. Corros. Eng. Sci. Technol. 2010, 45, 224–230. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Tafel, J.; Hahl, H. Vollständige Reduktion des Benzylacetessigesters. Berichte Der Dtsch. Chem. Gesell-Schaft 1907, 40, 3312–3318. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Deshpande, K.B. Numerical modeling of micro-galvanic corrosion. Electrochimica Acta 2011, 56, 1737–1745. [Google Scholar] [CrossRef]

| Parameters | Pure Mg | Mg-2% Al | Mg-6% Al | Units |
|---|---|---|---|---|
| GS | 1000 | 500 | 161 | mm |
| 0 | 9.79 | 22 | mm | |
| 0 | 120 | 410 | mm−2 | |
| 0 | 36 | 31.43 | mm | |
| 0 | 0.3363 | 0.20 | - | |
| 0 | 0.03 | 0.10 | - | |
| 0 | 0.015 | 0.03 | - | |
| 0 | 2.49 | 5.2 | mm | |
| 0 | 769 | 1434 | mm−2 | |
| 0 | 0 | 13.172 | mm | |
| 0 | 0.001 | 0.049 | - |
| Parameters | Values | Units |
|---|---|---|
| −1.67 | Volts | |
| −1.63 | Volts | |
| −1.61 | Volts | |
| −1.59 | Volts | |
| −1.57 | Volts | |
| −1.52 | Volts | |
| −1.43 | Volts | |
| Rp | 137 | Ohm/cm2 |
| Parameter | Values |
|---|---|
| F (C/mol) | 96,485 |
| M (g/mol) | 24.305 |
| z | 2 |
| ke (Nμm2/C2) | 8.987 × 1021 |
| q1 | 1 × 10−19 |
| q2 | 1 × 10−19 |
| (C2/Nμm2) | 8.85419 × 10−24 |
| MO/MO0 | 1 |
| Zic | 1 |
| Parameter | Mg-0%Al | Mg-2%Al | Mg-6%Al |
|---|---|---|---|
| C1 | −250 | −157 | −190 |
| C2 | 85 | 40 | 45 |
| C3 | 2.5 | 0.5 | 0.15 |
| C4 | 1650 | 180 | 2400 |
| C5 | 0.06 | 0.05 | 0.05 |
| C6 | 130 | 800 | 1000 |
| C7 | 0.04473 | 0.075 | 0.3 |
| C8 | 1585 | 950 | 400 |
| C9 | 0 | 0 | 0.08 |
| C10 | 0 | 0 | 40 |
| C11 | 1.3 | 0.4 | 0.2 |
| C12 | 11 | 10.2 | 10.2 |
| C13 | 0.1 | 0.35 | 0 |
| C14 | 16 | 12.5 | 0 |
| C15 | 0.3 | 0.3 | 0.25 |
| C16 | 0.32 | 0.42 | 0.08 |
| C17 | 0.007 | 0.003 | 0.024 |
| C18 | 0.27 | 0.35 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horstemeyer, M.F.; Song, W.; Cho, H.E.; Wipf, D.; Martin, H.J.; Francis, D.K.; Chaudhuri, S. A Multiscale Inelastic Internal State Variable Corrosion Model. Materials 2024, 17, 3995. https://doi.org/10.3390/ma17163995
Horstemeyer MF, Song W, Cho HE, Wipf D, Martin HJ, Francis DK, Chaudhuri S. A Multiscale Inelastic Internal State Variable Corrosion Model. Materials. 2024; 17(16):3995. https://doi.org/10.3390/ma17163995
Chicago/Turabian StyleHorstemeyer, M. F., W. Song, H. E. Cho, D. Wipf, H. J. Martin, D. K. Francis, and S. Chaudhuri. 2024. "A Multiscale Inelastic Internal State Variable Corrosion Model" Materials 17, no. 16: 3995. https://doi.org/10.3390/ma17163995






