Microstructure and Mechanical Properties of As-Cast Al-10Ce-3Mg-xZn Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
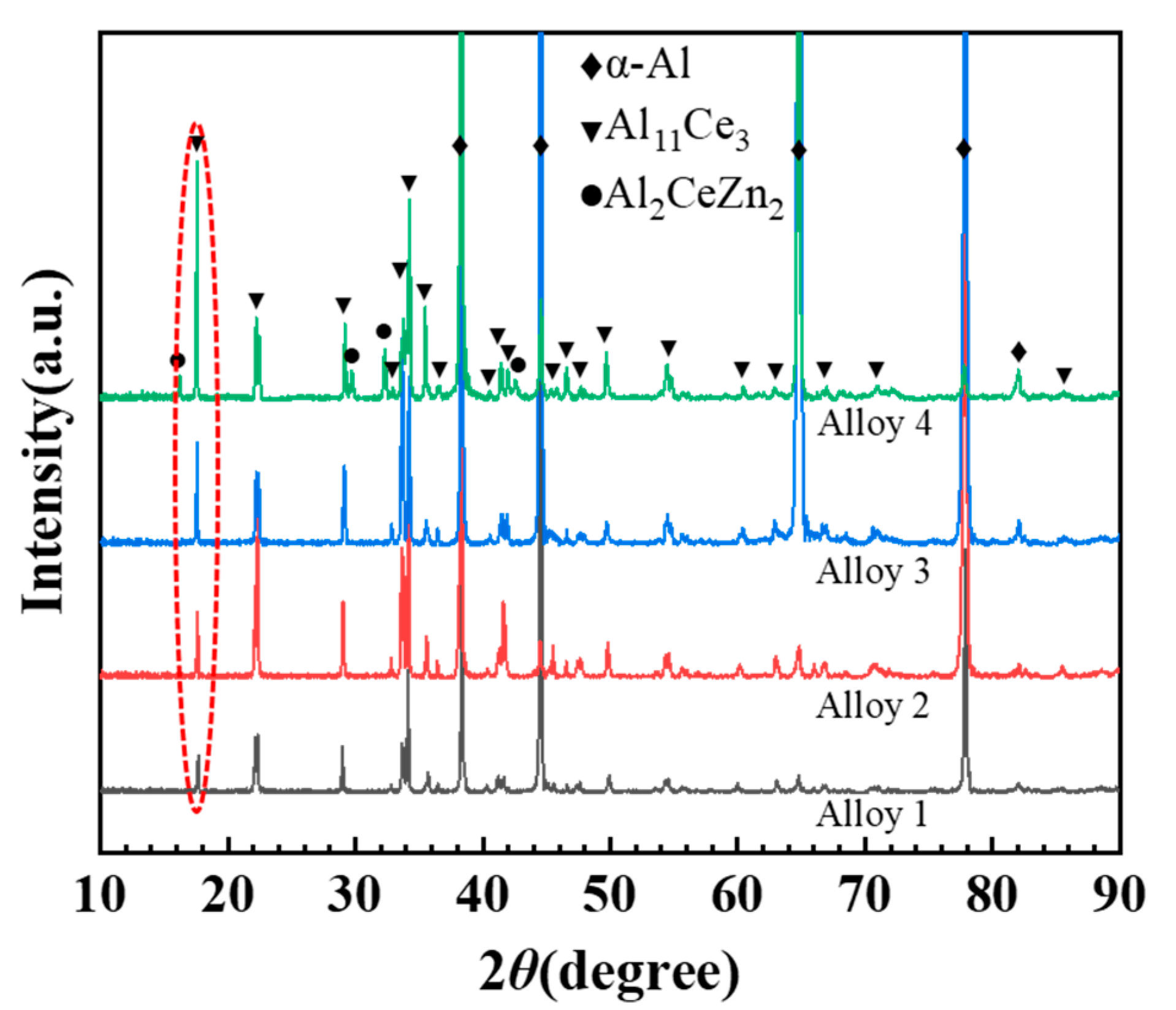
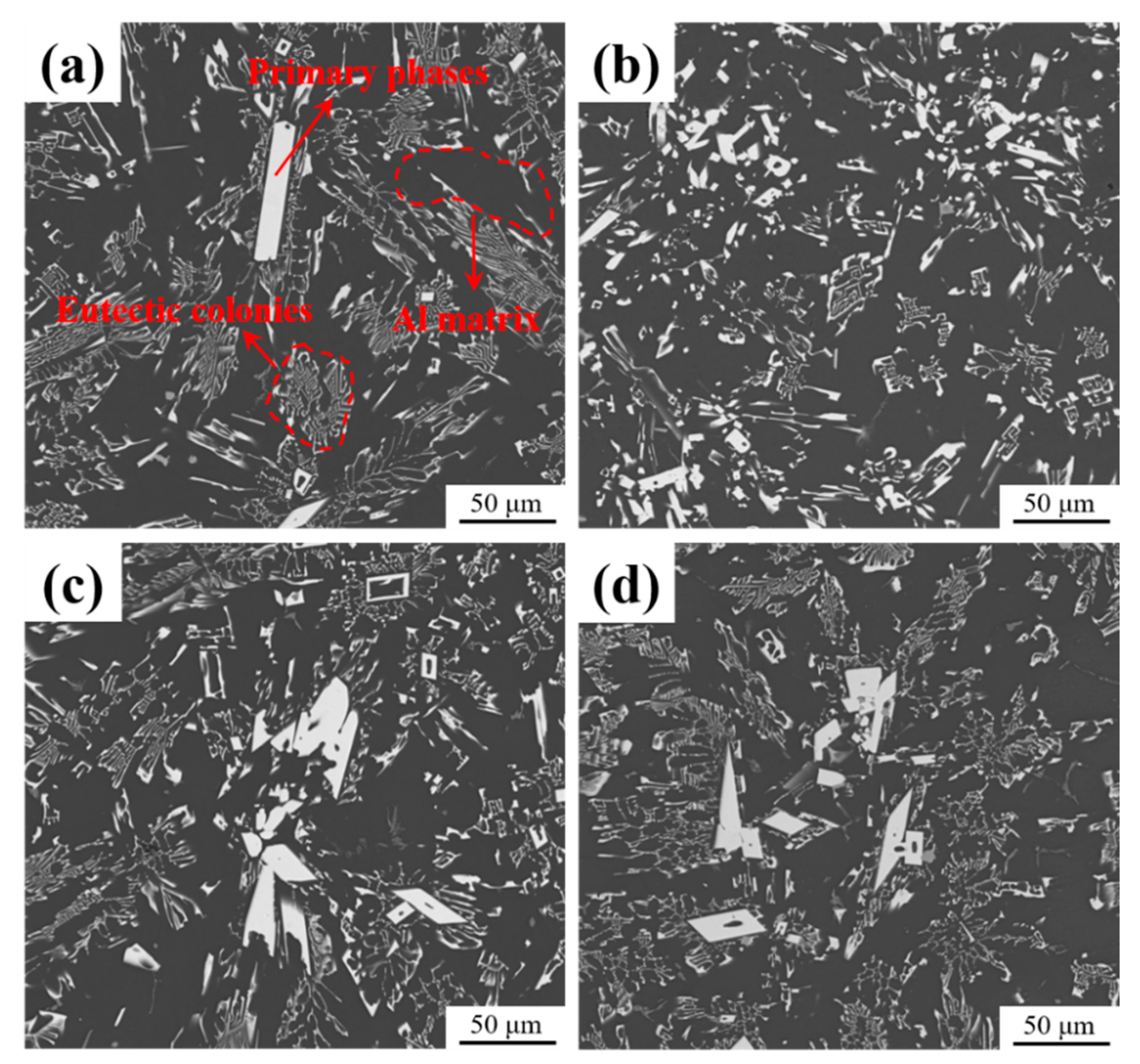
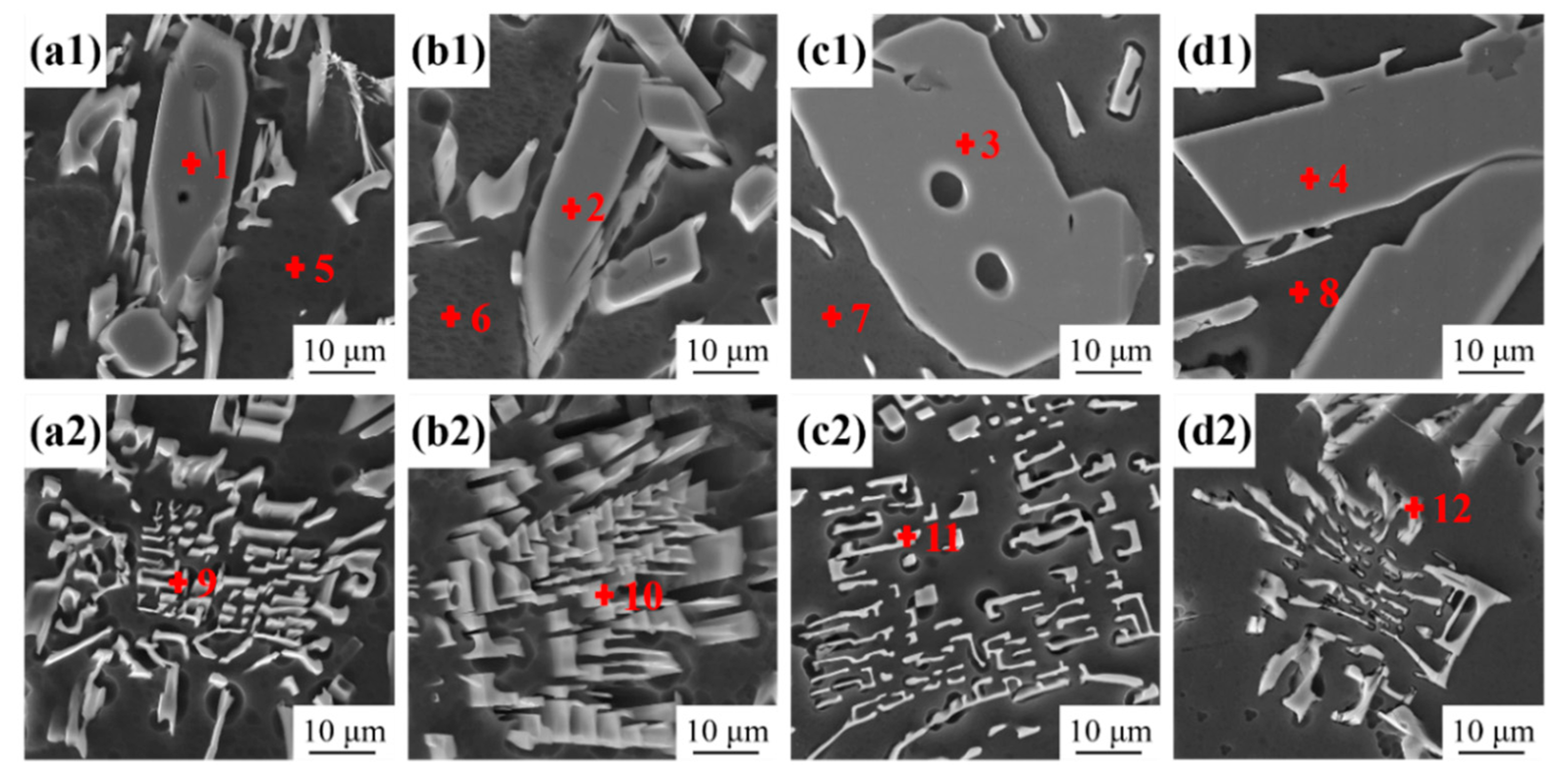
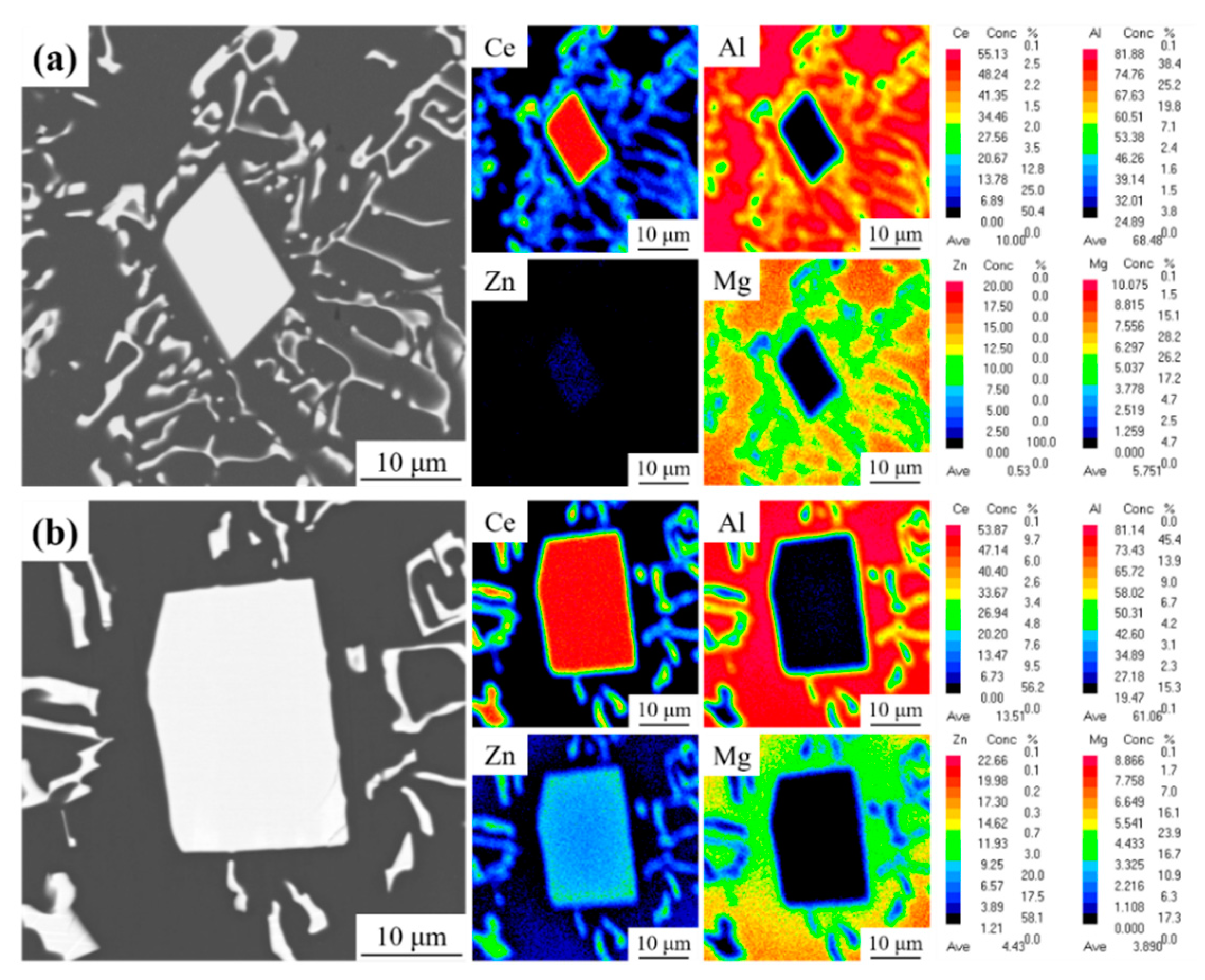
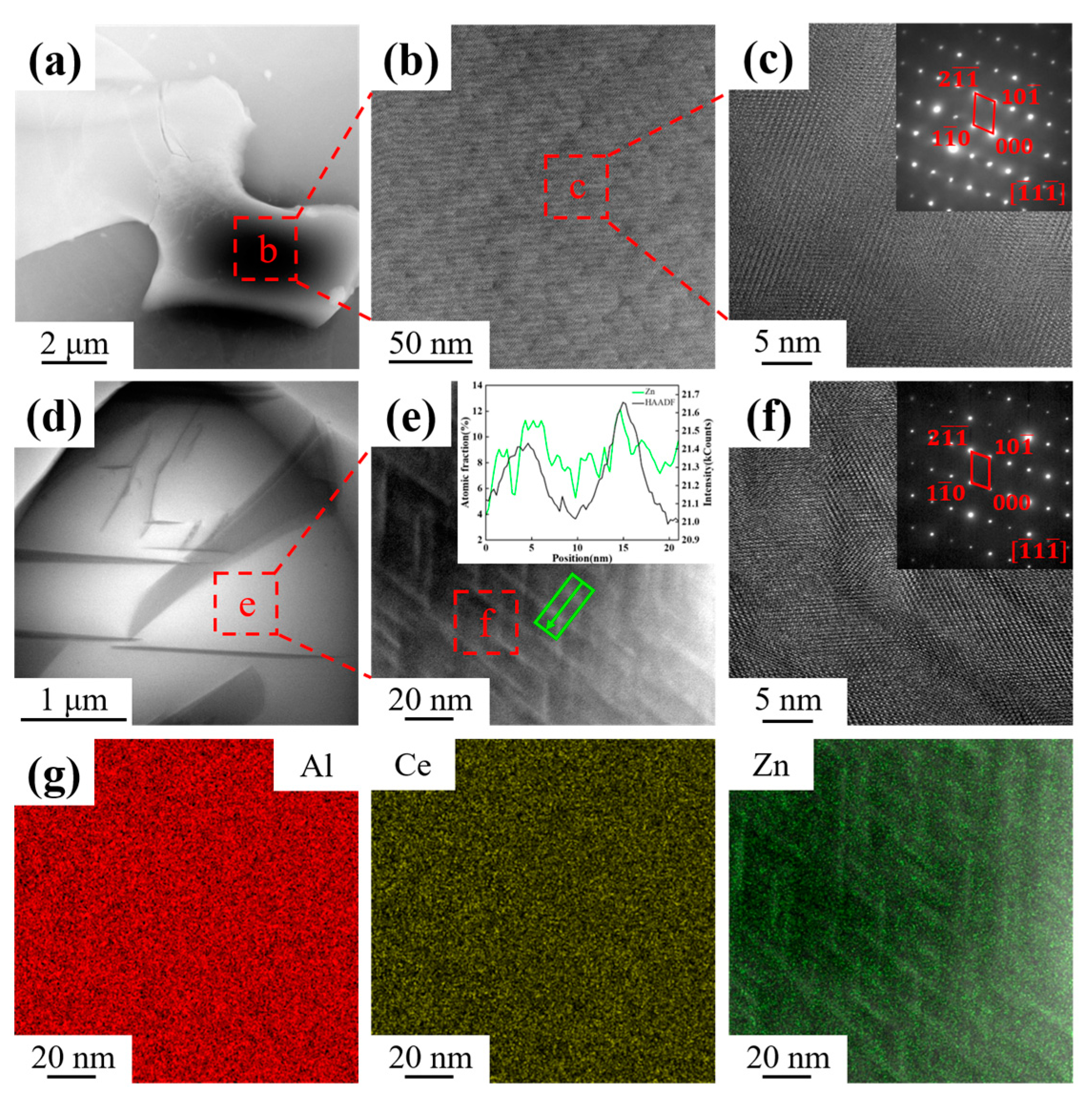
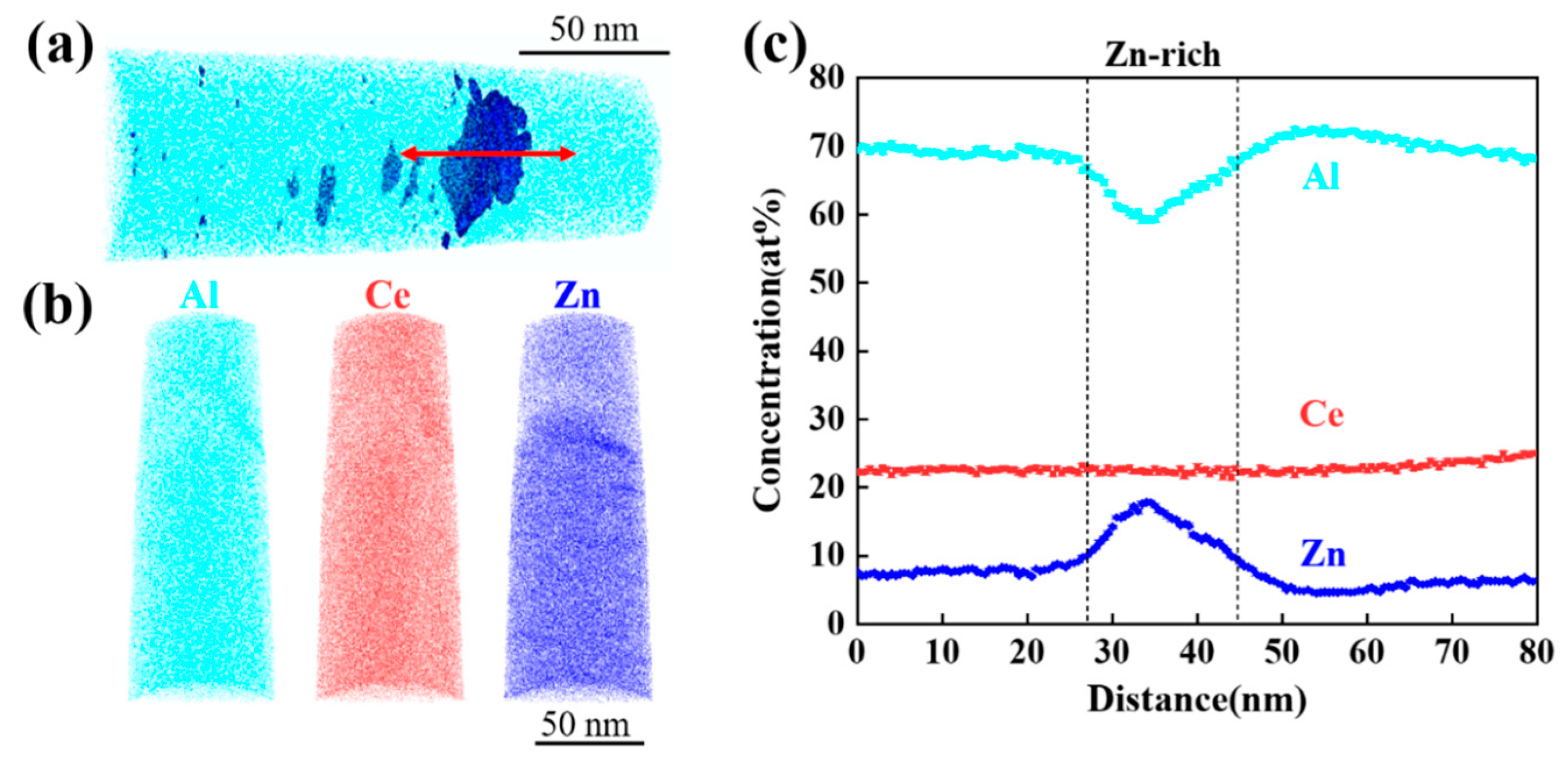
3.1. As-Cast Microstructure Characterization
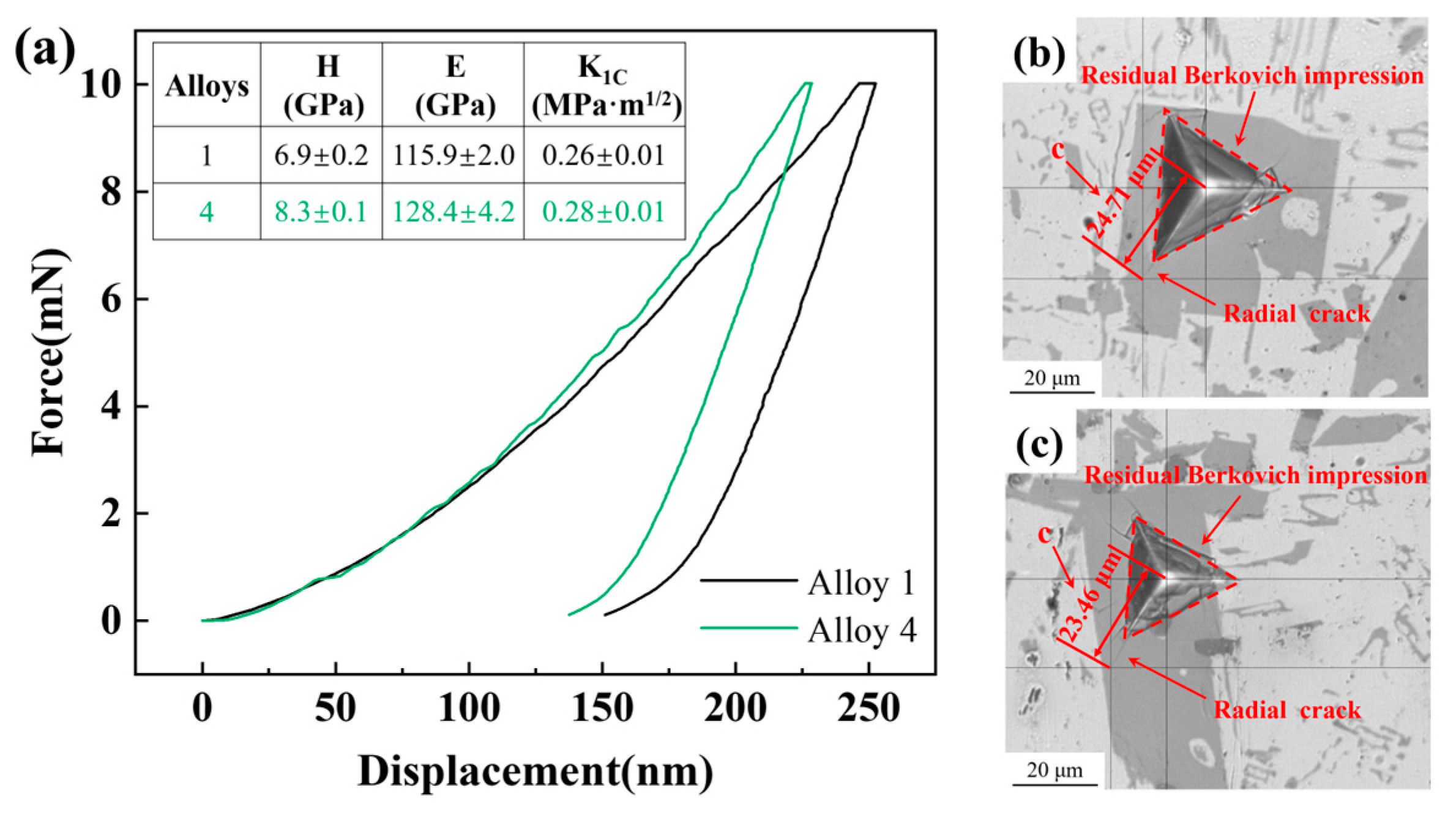
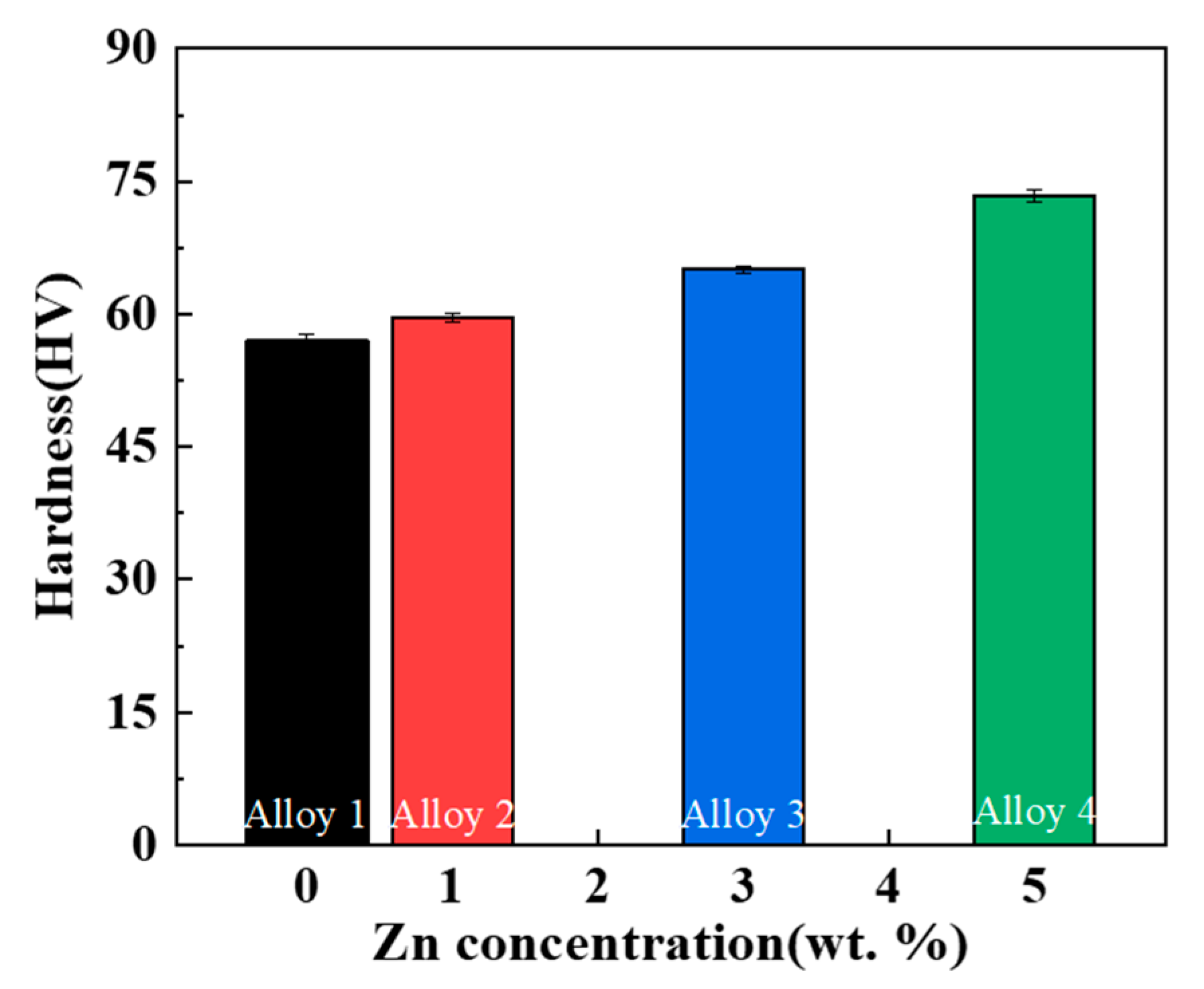
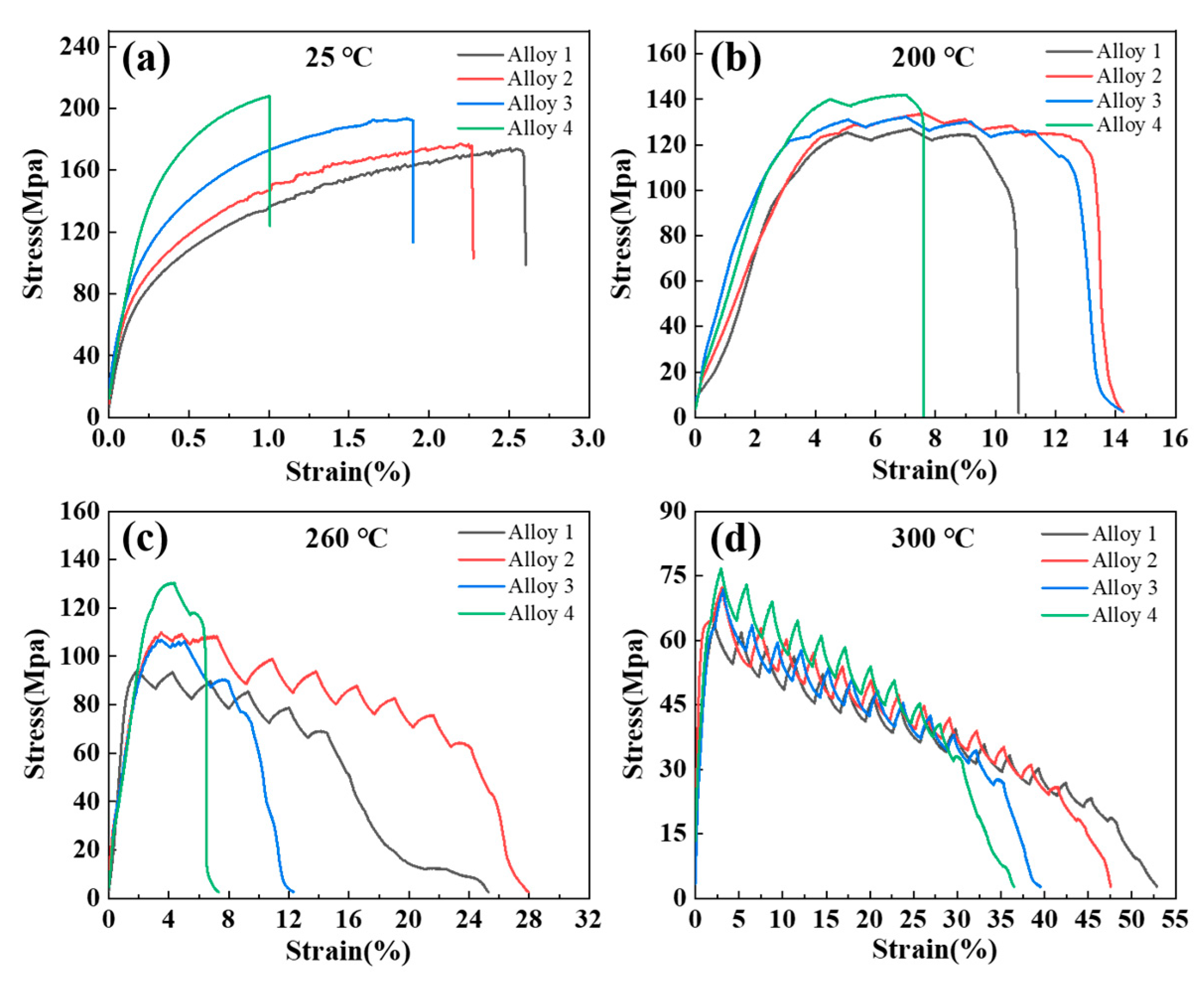
3.2. Mechanical Properties
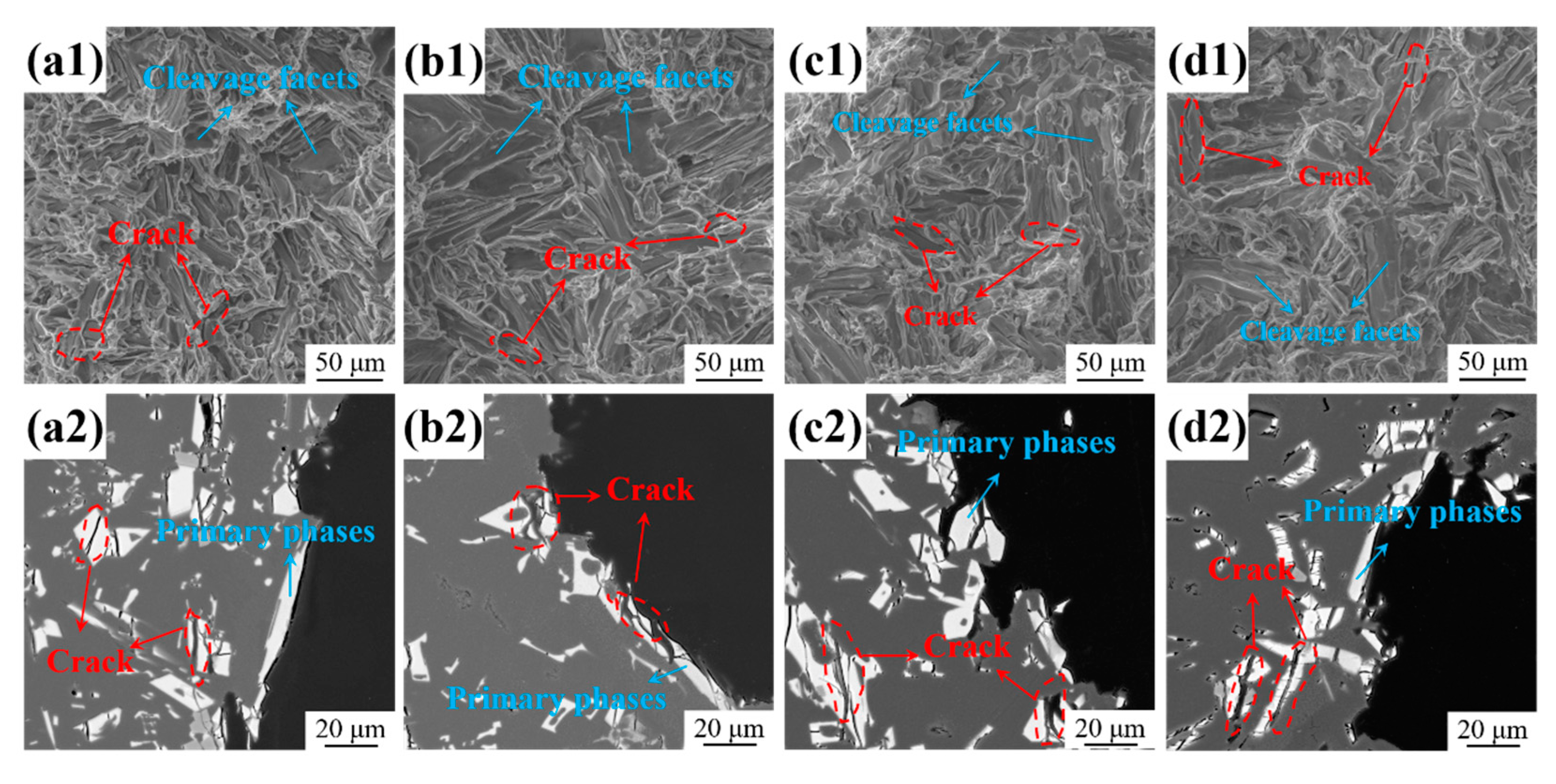
4. Discussion
4.1. Effect of Zn Addition on Microstructure
4.2. Effect of Zn Addition on Strength
5. Conclusions
- (1)
- The as-cast Al-10Ce-3Mg-xZn alloys consist of α-Al, a Chinese-script eutectic phase, and a bulk primary phase. The volume fraction of the Al11Ce3 phase in the alloy increases as the Zn content increases, and the majority of the Zn element is concentrated in the Al11Ce3 phase; when the Zn addition is increased to 5 wt.%, the regularly distributed acicular Al2CeZn2 phase is formed within the Al11Ce3 phase.
- (2)
- The alloy with 5 wt.% Zn exhibited a significant increase in microhardness, elastic modulus, and fracture toughness of the Al11Ce3 phase compared to the alloy without Zn addition; this indicates that the increase in the Zn content significantly enhances the second-phase strengthening effect of the alloy.
- (3)
- The addition of Zn can significantly enhance the hardness and mechanical properties of Al-10Ce-3Mg alloys through the combined effect of solid solution strengthening and second-phase strengthening. The UTS and YS of the Al-10Ce-3Mg alloy increase with increasing Zn content at room temperature, while the EL gradually decreases. The decrease in EL is due to the increase in the volume fraction of the Al11Ce3 phase as a source of fracture; the alloy with 5 wt.% Zn exhibits the highest UTS at all test temperatures, indicating that the alloy has superior heat resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakata, I.F.; Langenbeck, S.L. Elevated Temperature Aluminum Alloys for Aerospace Applications; SAE International: Warrendale, PA, USA, 1983. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, K.; Chen, S.; Ding, Y.; Fan, S. Correlation between stress corrosion cracking resistance and grain-boundary precipitates of a new generation high Zn-containing 7056 aluminum alloy by non-isothermal aging and re-aging heat treatment. J. Alloys Compd. 2021, 850, 156717. [Google Scholar] [CrossRef]
- Xiao, D.H.; Wang, J.N.; Ding, D.Y. Effect of minor cerium additions on microstructure and mechanical properties of cast Al-Cu-Mg-Ag alloy. Mater. Sci. Technol. 2013, 20, 1237–1240. [Google Scholar] [CrossRef]
- Mondol, S.; Alam, T.; Banerjee, R.; Kumar, S.; Chattopadhyay, K. Development of a high temperature high strength Al alloy by addition of small amounts of Sc and Mg to 2219 alloy. Mater. Sci. Eng. A 2017, 687, 221–231. [Google Scholar] [CrossRef]
- Du, J.; Ding, D.; Zhang, W.; Xu, Z.; Gao, Y.; Chen, G.; Chen, W.; You, X.; Chen, R.; Huang, Y.; et al. CeLa enhanced corrosion resistance of Al-Cu-Mn-Mg-Fe alloy for lithium battery shell. Appl. Surf. Sci. 2017, 422, 221–227. [Google Scholar] [CrossRef]
- Wang, Y.; Yue, C.; Su, M.; Yuan, X.; Zheng, B. Effect of Ce on hot tearing sensitivity of as-cast Al-Cu-Mg-Y alloy. J. Mater. Eng. Perform. 2022, 31, 6349–6359. [Google Scholar] [CrossRef]
- Weiss, D. Improved high-temperature aluminum alloys containing cerium. J. Mater. Eng. Perform. 2019, 28, 1903–1908. [Google Scholar] [CrossRef]
- Weiss, D. Castability and characteristics of high cerium aluminum alloys. In Advanced Casting Technologies; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Sun, Y.; Hung, C.; Hebert, R.J.; Fennessy, C.; Tulyani, S.; Aindow, M. Eutectic microstructures in dilute Al-Ce and Al-Co alloys. Mater. Charact. 2019, 154, 269–276. [Google Scholar] [CrossRef]
- De Giovanni, M.; Kaduk, J.A.; Srirangam, P. Modification of Al-Si Alloys by Ce or Ce with Sr. JOM 2018, 71, 426–434. [Google Scholar] [CrossRef]
- Song, M.; Xiao, D.; Zhang, F. Effect of Ce on the thermal stability of the Ω phase in an Al-Cu-Mg-Ag alloy. Rare Met. 2009, 28, 156–159. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, M.; Li, Z.; Xiao, D.; Huang, Y.; Huang, L.; Liu, W. Effect of combined addition of CeLa and GdY on microstructure and mechanical properties of as-cast Al-Cu-Mn alloys. Materials 2023, 16, 7332. [Google Scholar] [CrossRef] [PubMed]
- Sims, Z.C.; Rios, O.R.; Weiss, D.; Turchi, P.E.A.; Perron, A.; Lee, J.R.I.; Li, T.T.; Hammons, J.A.; Bagge-Hansen, M.; Willey, T.M.; et al. High performance aluminum–cerium alloys for high-temperature applications. Mater. Horiz. 2017, 4, 1070–1078. [Google Scholar] [CrossRef]
- Czerwinski, F. Thermal stability of aluminum–cerium binary alloys containing the Al–Al11Ce3 eutectic. Mater. Sci. Eng. A 2021, 809, 140973. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, P.; Lv, H.; Gao, H.; Wang, Y.; Wang, J.; Sun, B. Orientation relationships and interface structure between Al11Ce3 and Al in Al–Ce eutectic. J. Mater. Res. Technol. 2022, 18, 693–704. [Google Scholar] [CrossRef]
- Kozakevich, J.; Stroh, J.; Mallouhi, V.; Sediako, D.; Weiss, D. Interplay Between Cooling Rate, Microstructure, and mechanical properties of an Al–Ce–Ni–Mn alloy. In Light Metals 2022; Springer International Publishing: Cham, Switzerland, 2022; pp. 83–91. [Google Scholar] [CrossRef]
- Lei, Z.; Wen, S.; Huang, H.; Wei, W.; Nie, Z. Grain refinement of aluminum and aluminum alloys by Sc and Zr. Metals 2023, 13, 751. [Google Scholar] [CrossRef]
- Ye, J.; Dai, K.; Wang, Z.; Chen, J.; Gao, M.; Guan, R. Beneficial effects of Sc/Zr addition on hypereutectic Al–Ce alloys: Modification of primary phases and precipitation hardening. Mater. Sci. Eng. A 2022, 835, 142611. [Google Scholar] [CrossRef]
- El-Hadad, S.; Moussa, M.E.; Waly, M.A. Effects of solidification under ultrasonic vibrations on Al11Ce3 phase fragmentation and mechanical properties of Al-10 wt.% Ce alloy. J. Mater. Eng. Perform. 2022, 31, 5393–5408. [Google Scholar] [CrossRef]
- Wang, L.; Qi, R.; Ye, B.; Bai, Y.; Huang, R.; Jiang, H.; Ding, W. Improved tensile strength of Al-5Ce alloy by permanent magnet stirring. Metall. Mater. Trans. A 2020, 51, 1972–1977. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Lv, H.; Gao, H.; Wang, J.; Sun, B. Enhanced load transfer and ductility in Al–9Ce alloy through heterogeneous lamellar microstructure design by cold rolling and annealing. Mater. Sci. Eng. A 2021, 821, 141591. [Google Scholar] [CrossRef]
- Murashkin, M.Y.; Sabirov, I.; Medvedev, A.E.; Enikeev, N.A.; Lefebvre, W.; Valiev, R.Z.; Sauvage, X. Mechanical and electrical properties of an ultrafine grained Al–8.5 wt. % RE (RE = 5.4 wt.% Ce, 3.1 wt.% La) alloy processed by severe plastic deformation. Mater. Des. 2016, 90, 433–442. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, F.; Fu, Z.; Mao, H.; Xu, H. Microstructure and mechanical properties of novel heat-resistant Al-12Ce-xCu-yMg alloy under laser melting. J. Alloys Compd. 2022, 924, 166359. [Google Scholar] [CrossRef]
- Wang, L.; Song, H.; Ye, B.; Zhao, B.; Bai, Y.; Ding, W. Microstructure and mechanical properties of high performance die cast Al-8Ce-3Y aluminum alloy containing Al4(Ce,Y) phase. Mater. Lett. 2021, 305, 130742. [Google Scholar] [CrossRef]
- Zhou, W.B.; Teng, G.B.; Liu, C.Y.; Qi, H.Q.; Huang, H.F.; Chen, Y.; Jiang, H.J. Microstructures and mechanical properties of binary Al-Zn alloys fabricated by casting and heat treatment. J. Mater. Eng. Perform. 2017, 26, 3977–3982. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, L.; Xu, M.; Yi, J. The Effect of Zn incorporation on the structure and mechanical properties of the Al–Zn alloy system. Trans. Indian Inst. Met. 2021, 75, 79–90. [Google Scholar] [CrossRef]
- Hu, G.; Zhu, C.; Xu, D.; Dong, P.; Chen, K. Effect of cerium on microstructure, mechanical properties and corrosion properties of Al-Zn-Mg alloy. J. Rare Earths 2021, 39, 208–216. [Google Scholar] [CrossRef]
- Khan, M.A.; Wang, Y.; Afifi, M.A.; Tabish, M.; Hamza, M.; Yasin, G.; Ahmad, T.; Liao, W.-B. The formation of new (Al, Zn)3Zr precipitates in an Al–Zn–Mg–Cu aluminum alloy after aging treatment and their response to dynamic compression. Arch. Civ. Mech. Eng. 2022, 23, 33. [Google Scholar] [CrossRef]
- Czerwinski, F. Cerium in aluminum alloys. J. Mater. Sci. 2020, 55, 24–72. [Google Scholar] [CrossRef]
- Sims, Z.C.; Kesler, M.S.; Henderson, H.B.; Castillo, E.; Fishman, T.; Weiss, D.; Singleton, P.; Eggert, R.; McCall, S.K.; Rios, O. How cerium and lanthanum as coproducts promote stable rare earth production and new alloys. J. Sustain. Metall. 2022, 8, 1225–1234. [Google Scholar] [CrossRef]
- Nguyen, R.T.; Imholte, D.D.; Rios, O.R.; Weiss, D.; Sims, Z.; Stromme, E.; McCall, S.K. Anticipating impacts of introducing aluminum-cerium alloys into the United States automotive market. Resour. Conserv. Recycl. 2019, 144, 340–349. [Google Scholar] [CrossRef]
- Iyer, A.V.; Lim, H.; Rios, O.; Sims, Z.; Weiss, D. An economic model and experiments to understand aluminum-cerium alloy recycling. JOM 2018, 70, 547–552. [Google Scholar] [CrossRef]
- Gschneidner, K.A.; Calderwood, F.W. The Al−Ce (Aluminum-Cerium) system. Bull. Alloy Phase Diagr. 1988, 9, 669–672. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Ce (Aluminum-Cerium). J. Phase Equilibria Diffus. 2011, 32, 392–393. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, J.; Han, Q.; Sun, B.; Wang, J.; Liu, C. Microstructure diversity dominated by the interplay between primary intermetallics and eutectics for Al-Ce heat-resistant alloys. J. Alloys Compd. 2022, 899, 162914. [Google Scholar] [CrossRef]
- Anstis, G.R.; Chantikul, P.; Lawn, B.R.; Marshall, D.B. A critical evaluation of indentation techniques for measuring fracture toughness: I, direct crack measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Yang, T.; Wu, C.; Su, X.; Tu, H.; Liu, Y.; Peng, H.; Wang, J. The 450 and 600 °C isothermal sections of the Al-Ce-Zn (0-33.3 at.%Ce) ternary system. J. Phase Equilibria Diffus. 2015, 36, 198–208. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, R.; Zhao, Y.; Liu, D.; Yang, L.; Hou, H. Serrated flow behaviors in a Ni-based superalloy. Mater. Res. Express 2021, 8, 026515. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.P.; Chen, S.Y.; Xie, X.; Liaw, P.K.; Dahmen, K.A.; Qiao, J.W.; Wang, Y.L. Serration and noise behaviors in materials. Prog. Mater. Sci. 2017, 90, 358–460. [Google Scholar] [CrossRef]
- Weiss, D. Composites and Alloys Based on the Al-Ce System. In Aluminium Alloys and Composites; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Sabirov, I.; Murashkin, M.Y.; Valiev, R.Z. Nanostructured aluminium alloys produced by severe plastic deformation: New horizons in development. Mater. Sci. Eng. A 2013, 560, 1–24. [Google Scholar] [CrossRef]
- Embury, J.D. Strengthening Mechanisms in AL Alloys—An Overview of Natural Limits and Engineering Possibilities. Mater. Sci. Forum 1996, 217–222, 57–70. [Google Scholar] [CrossRef]
- Cáceres, C.H.; Poole, W.J.; Bowles, A.L.; Davidson, C.J. Section thickness, macrohardness and yield strength in high-pressure diecast magnesium alloy AZ91. Mater. Sci. Eng. A 2005, 402, 269–277. [Google Scholar] [CrossRef]
- Brown, L.M.; Clarke, D.R. Work hardening due to internal stresses in composite materials. Acta Metall. 1975, 23, 821–830. [Google Scholar] [CrossRef]
- Cáceres, C.H.; Davidson, C.J.; Griffiths, J.R.; Newton, C.L. Effects of solidification rate and ageing on the microstructure and mechanical properties of AZ91 alloy. Mater. Sci. Eng. A 2002, 325, 344–355. [Google Scholar] [CrossRef]
- Hu, B.; Quan, B.; Li, D.; Wang, X.; Li, Z.; Zeng, X. Solid solution strengthening mechanism in high pressure die casting Al–Ce–Mg alloys. Mater. Sci. Eng. A 2021, 812, 141109. [Google Scholar] [CrossRef]
- Sun, P.; Huang, Y.; Sun, L.; Li, Y.; Zheng, X.; Li, B.; Li, X.; Yan, H.; Liu, Y.; Du, Y. Effect of heat treatment on microstructure evolution, strengthening mechanisms and mechanical properties of Zn modified Al–Mg alloys with Sc and Zr additions. Mater. Sci. Eng. A 2024, 896, 146206. [Google Scholar] [CrossRef]
| Alloys | Ce | Mg | Zr | Y | Zn | Al |
|---|---|---|---|---|---|---|
| Alloy 1 | 10.0 | 2.8 | 0.1 | 0.1 | 0.0 | Balance |
| Alloy 2 | 10.5 | 2.9 | 0.1 | 0.1 | 1.0 | Balance |
| Alloy 3 | 10.1 | 3.0 | 0.1 | 0.1 | 3.0 | Balance |
| Alloy 4 | 10.3 | 2.8 | 0.1 | 0.1 | 4.9 | Balance |
| Point | Al | Ce | Mg | Zn | Phase |
|---|---|---|---|---|---|
| 1 | 66.4 | 33.6 | - | - | Al11Ce3 |
| 2 | 61.4 | 34.6 | - | 4.0 | Al11−xCe3Znx |
| 3 | 58.6 | 32.8 | - | 8.6 | Al11−xCe3Znx |
| 4 | 54.2 | 33.3 | - | 12.5 | Al2CeZn2 |
| 5 | 96.4 | - | 3.6 | - | α-Al |
| 6 | 96.4 | - | 3.3 | 0.3 | α-Al |
| 7 | 96.2 | - | 3.1 | 0.7 | α-Al |
| 8 | 94.8 | - | 3.6 | 1.6 | α-Al |
| 9 | 80.3 | 17.6 | 2 | - | AlCeMg |
| 10 | 79.3 | 14.7 | 2.9 | 3.1 | AlCeMgZn |
| 11 | 78.1 | 15.1 | 2.2 | 4.6 | AlCeMgZn |
| 12 | 77.5 | 13.4 | 2.2 | 6.9 | AlCeMgZn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wu, M.; Li, Z.; Xiao, D.; Huang, Y.; Huang, L.; Liu, W. Microstructure and Mechanical Properties of As-Cast Al-10Ce-3Mg-xZn Alloys. Materials 2024, 17, 3999. https://doi.org/10.3390/ma17163999
Zhang H, Wu M, Li Z, Xiao D, Huang Y, Huang L, Liu W. Microstructure and Mechanical Properties of As-Cast Al-10Ce-3Mg-xZn Alloys. Materials. 2024; 17(16):3999. https://doi.org/10.3390/ma17163999
Chicago/Turabian StyleZhang, Haiyang, Mingdong Wu, Zeyu Li, Daihong Xiao, Yang Huang, Lanping Huang, and Wensheng Liu. 2024. "Microstructure and Mechanical Properties of As-Cast Al-10Ce-3Mg-xZn Alloys" Materials 17, no. 16: 3999. https://doi.org/10.3390/ma17163999
APA StyleZhang, H., Wu, M., Li, Z., Xiao, D., Huang, Y., Huang, L., & Liu, W. (2024). Microstructure and Mechanical Properties of As-Cast Al-10Ce-3Mg-xZn Alloys. Materials, 17(16), 3999. https://doi.org/10.3390/ma17163999







