Long-Term Evaluation of a Ternary Mixture of Molten Salts in Solar Thermal Storage Systems: Impact on Thermophysical Properties and Corrosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Corrosion Process
3. Results and Discussion
3.1. Thermophysical Properties
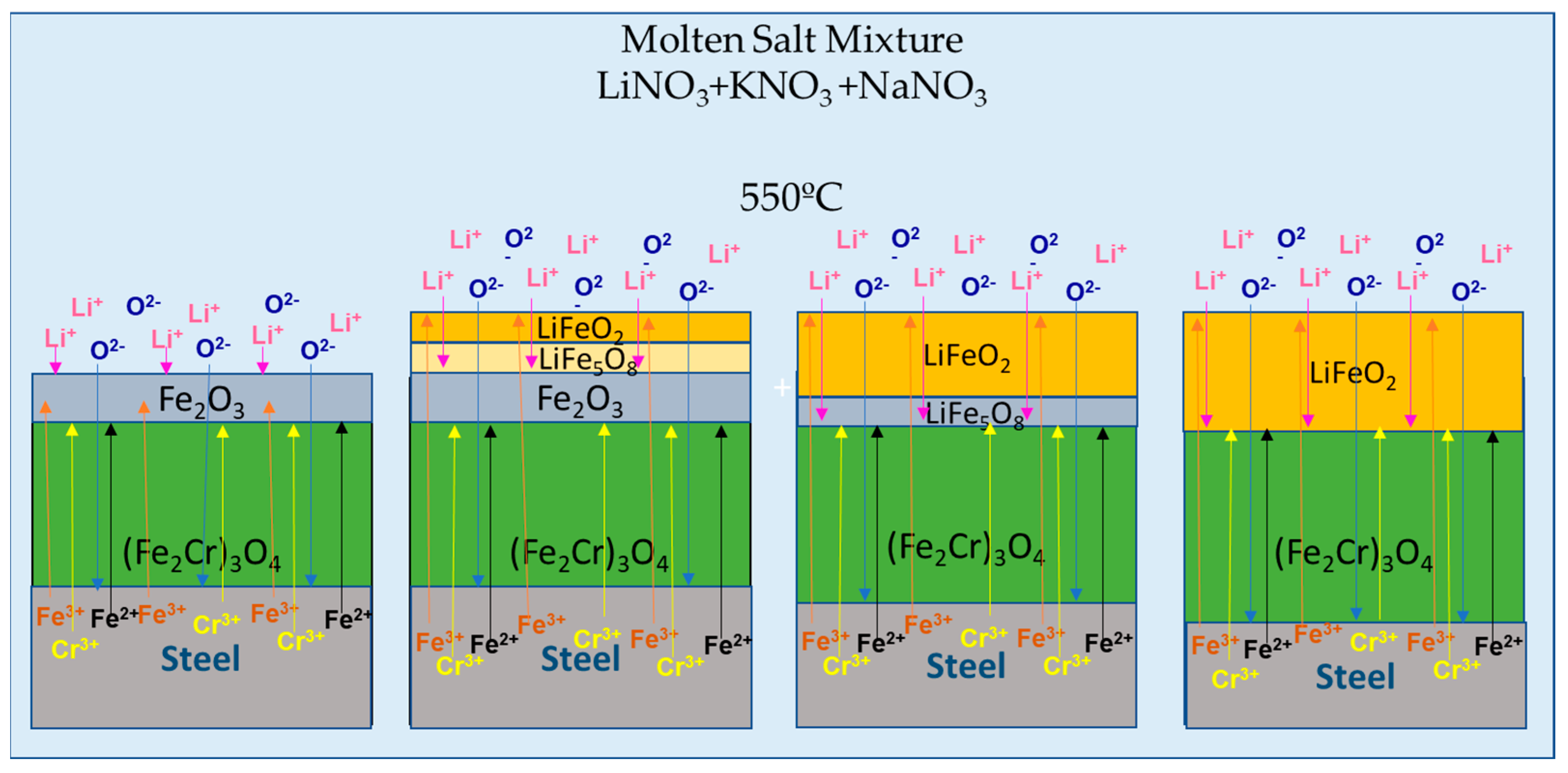
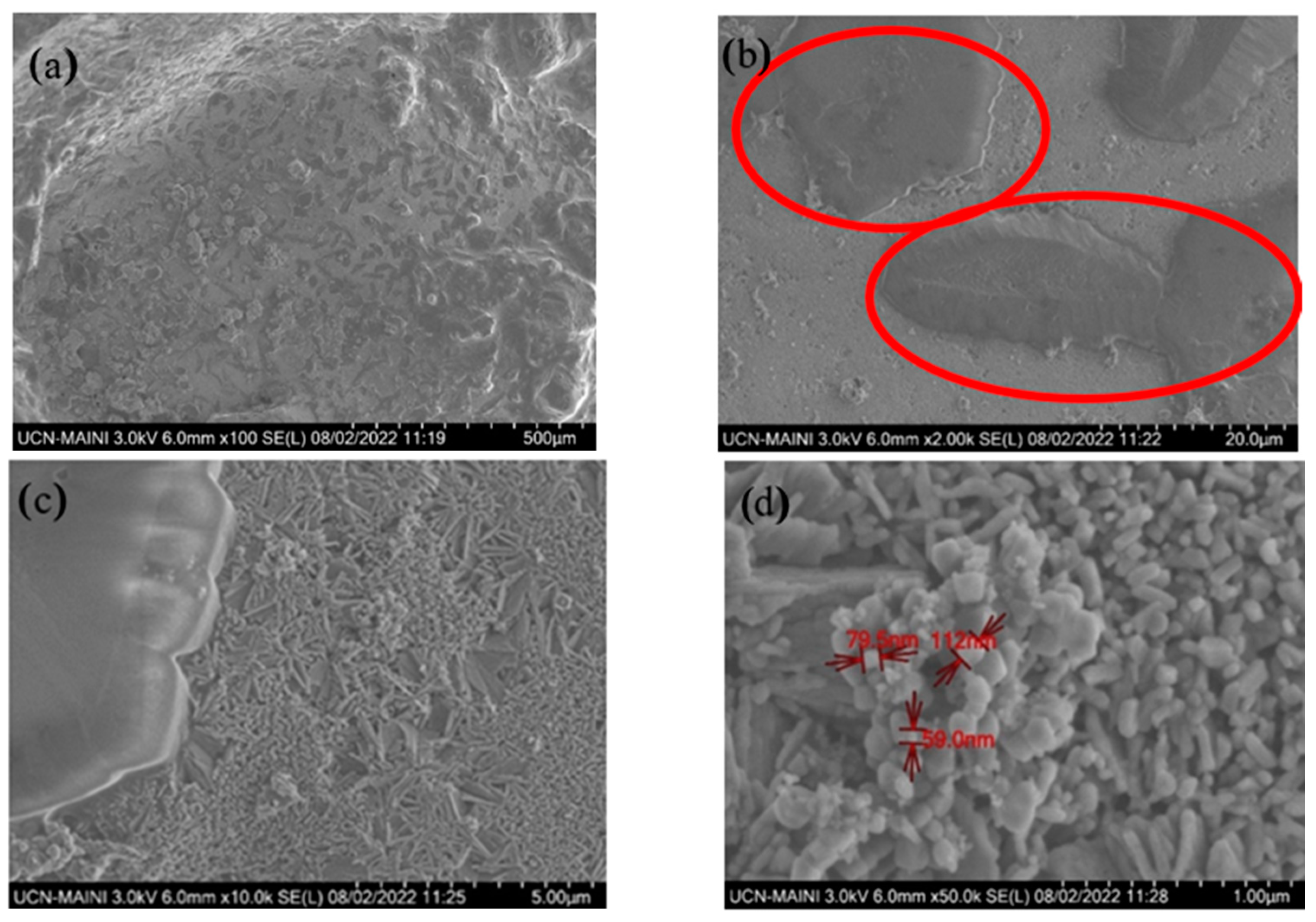
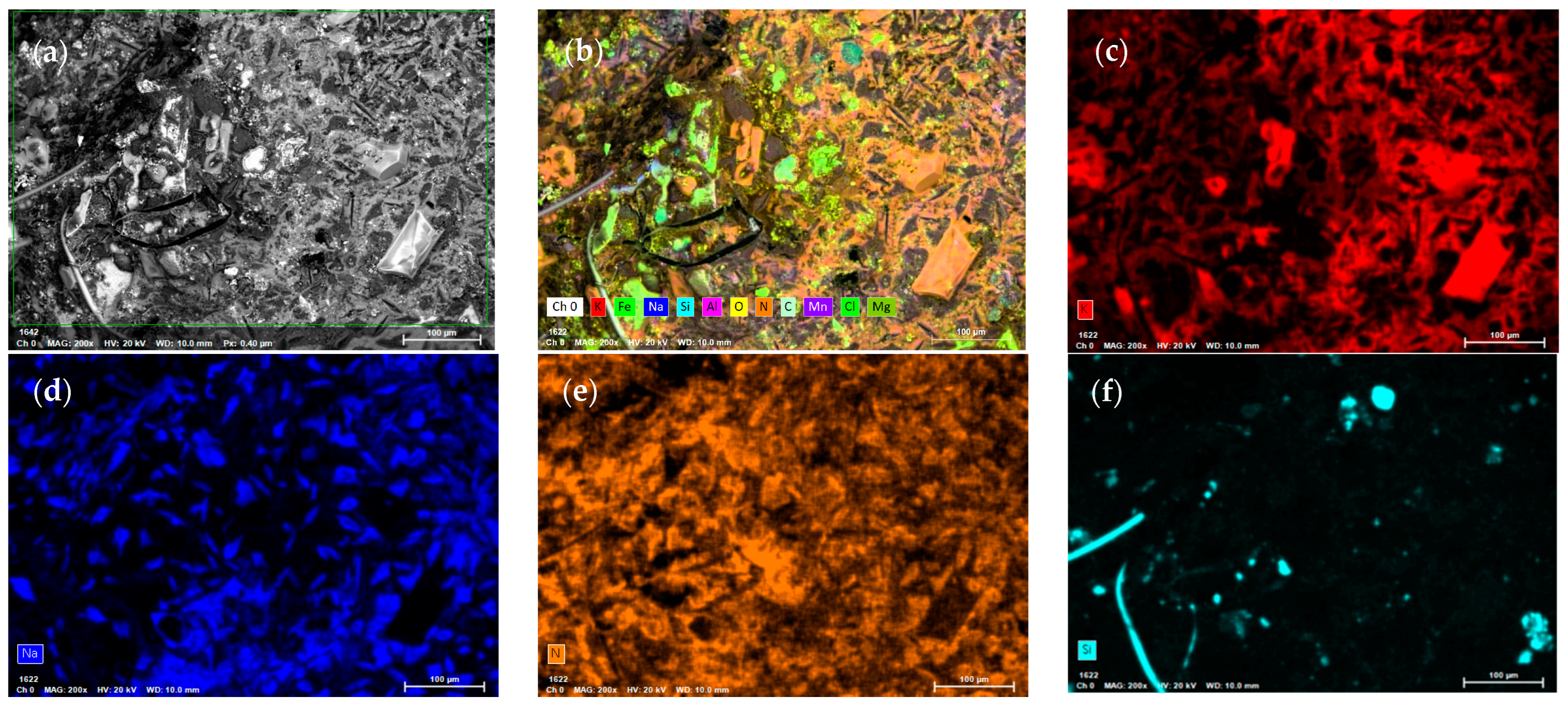
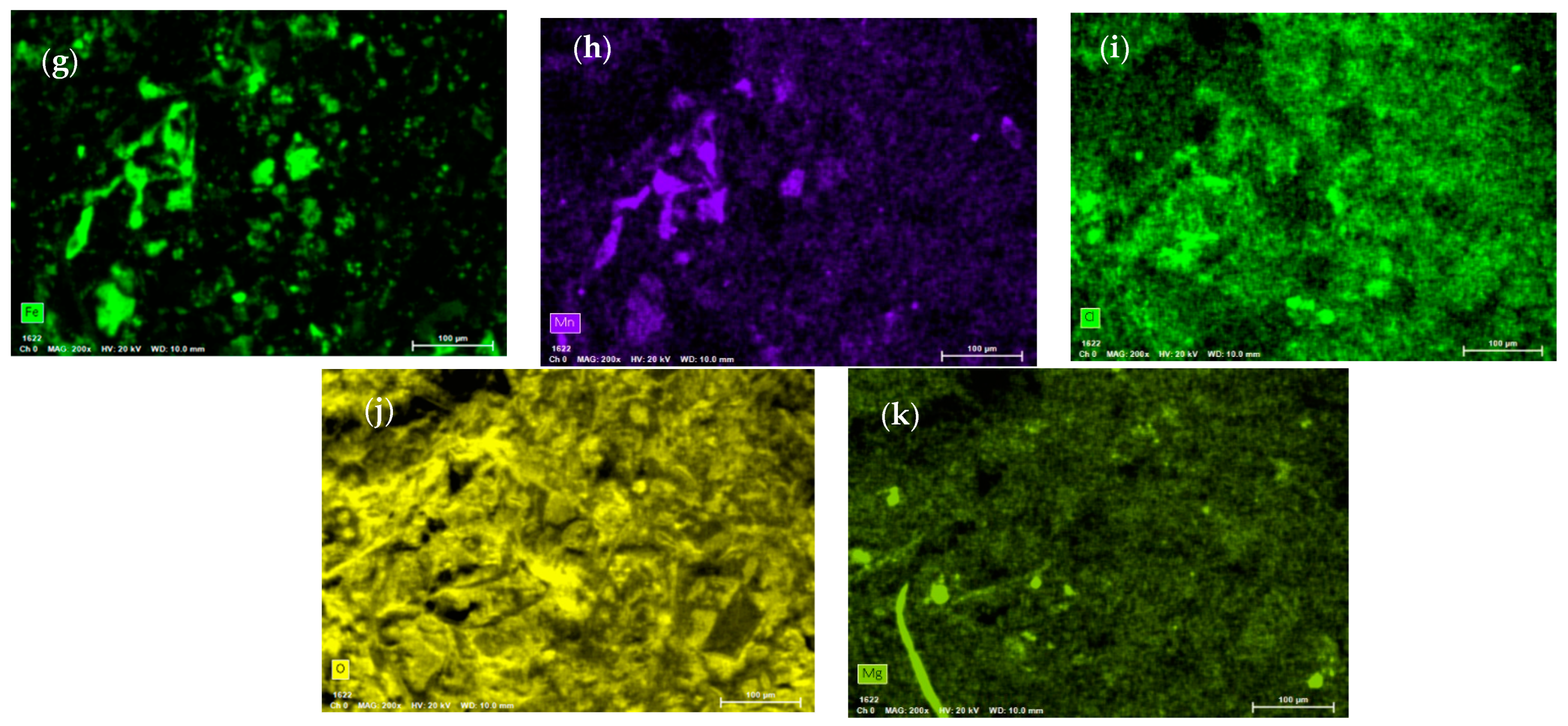
3.2. Corrosion Process and Decomposition of the Ternary Mixture
4. Conclusions
- (1)
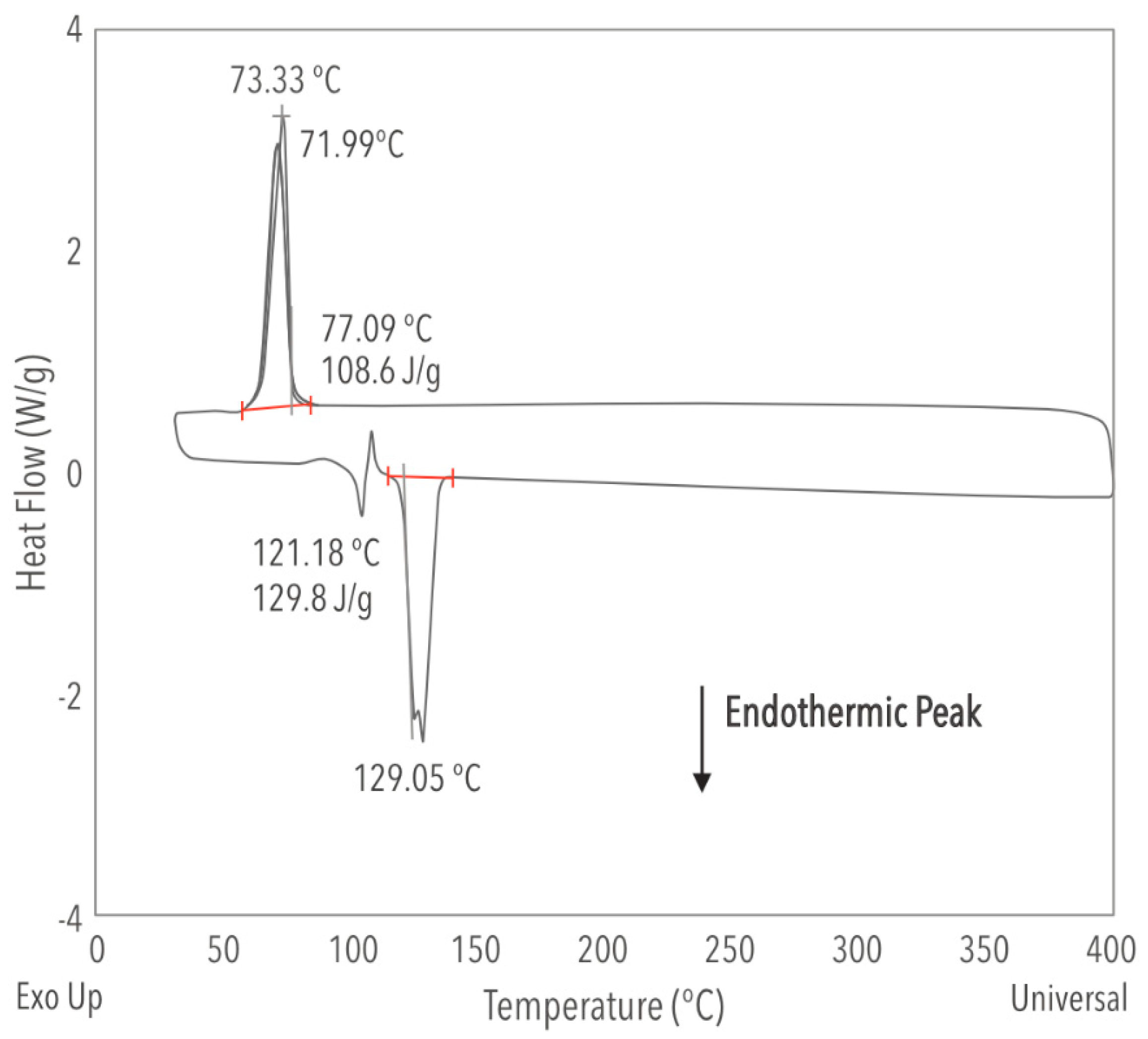
- After 15,000 h, the heat capacity decreased from 1.794 to 1.409 J g−1 °C−1. We found this to be the property most sensitive to long-term usage, which might be especially relevant if our mixture is utilized in thermal energy storage.
- (2)
- The results for the immersed salt inside the salt tank at time t = 0 and t = 15,000 h showed that the ternary mixture offers a low melting point of 124 and 129 °C, respectively, which is lower than the conventional solar salt. The latter could potentially reduce the CSP plant’s operating gap and would allow for maintaining a lower operating temperature with evident cost savings.
- (3)
- For both times, its decomposition temperature is higher than 590 °C, considering that the weight loss remained within 3%.
- (4)
- The viscosity decreases as the temperature increases, and above 200 °C, the viscosity values are below 12 cP, which approximates the viscosity range of the solar salt (4 to 7 cP).
- (5)
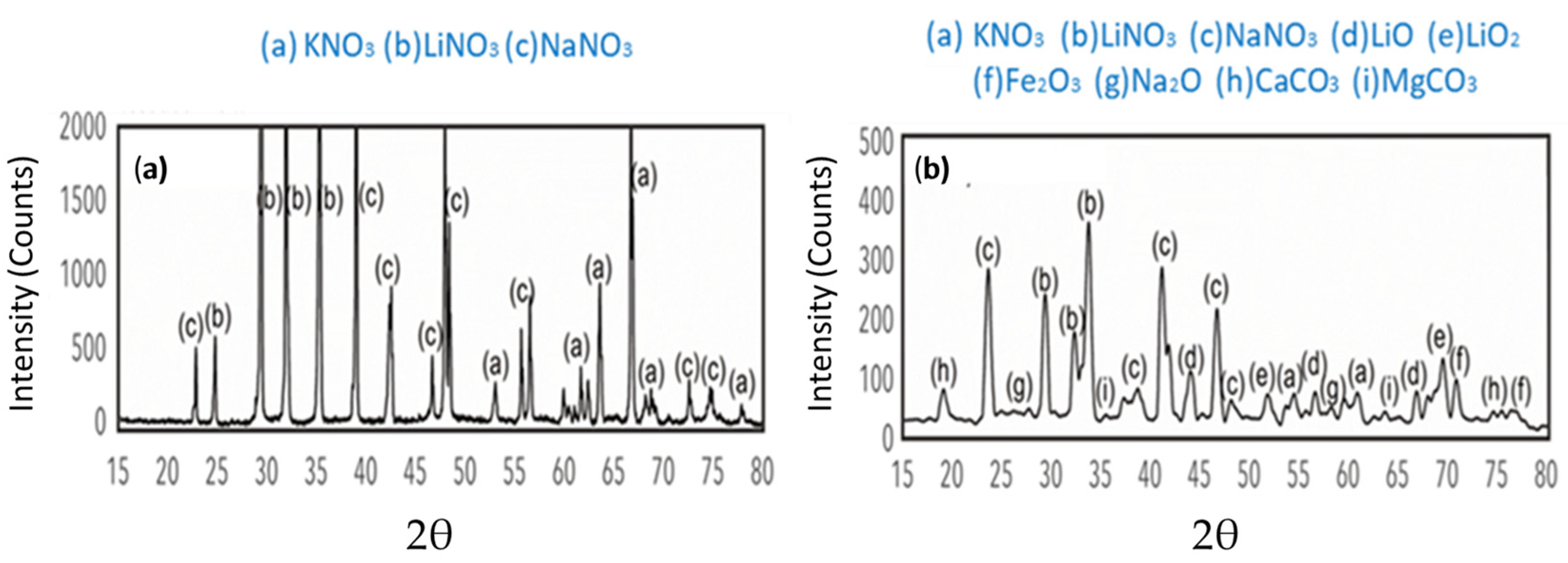
- After 30,000 h, we detected saline components, such as CaCO3 and MgCO3, produced by the decomposition of the ternary mixture. These can generate clogging problems in the pipes due to the formation of insoluble solids. We also identified lithium oxides (LiO and LiO2) produced by the decomposition of the unstable species of the ternary mixture as LiNO3.
- (6)
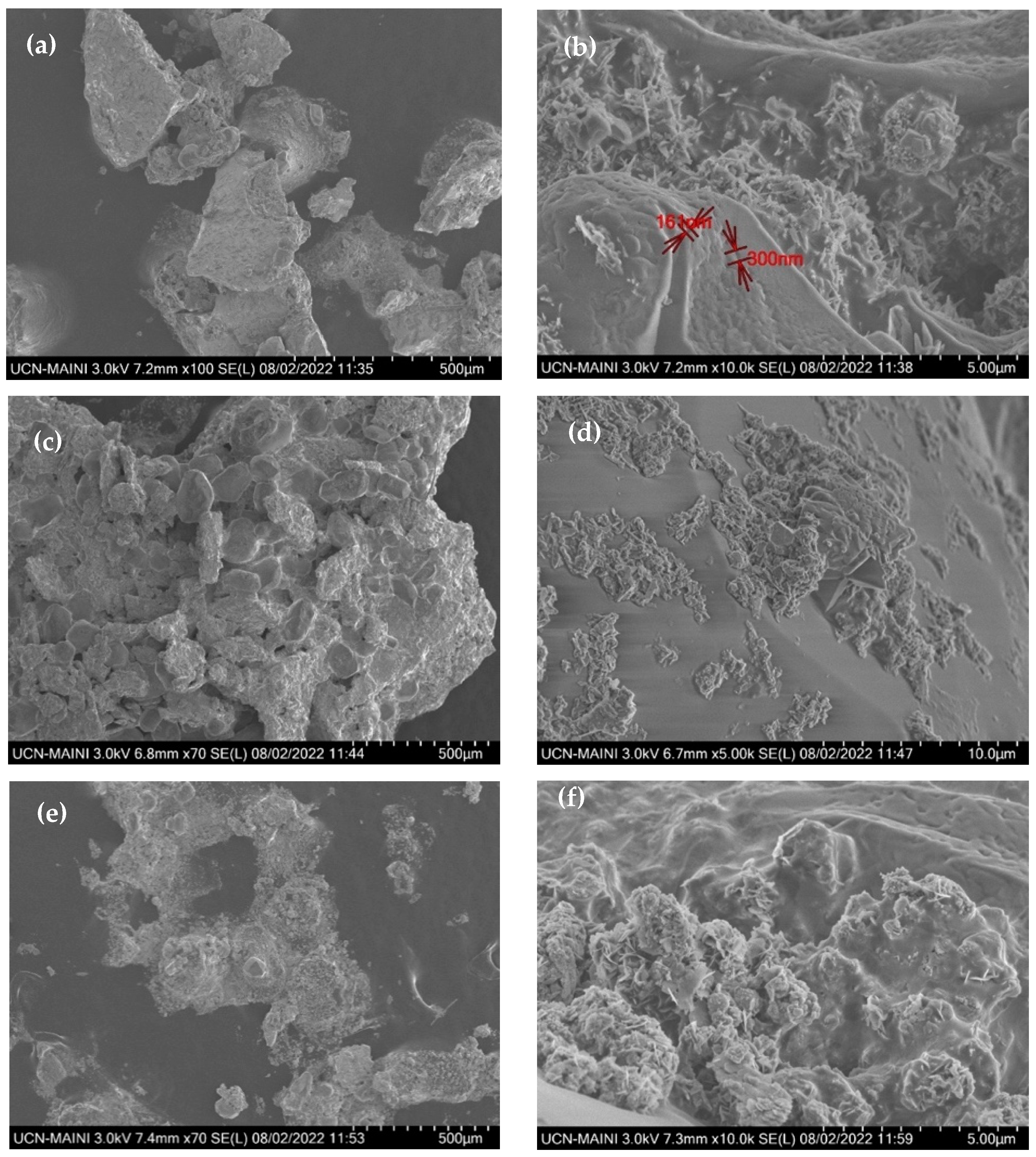
- After 30,000 h of contact between the ternary mixture of nitrates and various components, such as the internal surface of the steel tank and the suction of the molten salt pump, Fe2O3 was identified as a corrosion product. Additionally, Cl was detected, probably from the local marine atmosphere, where salts can be transported in the air from nearby seas. These findings highlighted the relevance of the interaction of these components with the liquid salt mixture.
- (7)
- Due to the low melting point, viscosity, and heat capacity, the ternary mixture could be an excellent substitute for solar salt for thermal storage applications. However, lithium currently has its market in electrochemical batteries, which is undergoing vigorous development, and as prices are very volatile, it could not compete with solar salt.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Energía. “Plan de Retiro y/o Reconversiones Centrales Carbon”, Report. 2020, pp. 1–38. Available online: https://energia.gob.cl/sites/default/files/plan_de_retiro_y_o_reconversion_centrales_carbon.pdf (accessed on 30 March 2024).
- Coordinador Eléctrico Nacional. Estudio de Operación y Desarrollo del SEN sin Centrales a Carbon. 2018. Available online: www.coordinadorelectrico.cl (accessed on 30 March 2024).
- Beaudin, M.; Zareipour, H.; Schellenberglabe, A.; Rosehart, W. Energy storage for mitigating the variability of renewable electricity sources: An updated review. Energy Sustain. Dev. 2010, 14, 302–314. [Google Scholar] [CrossRef]
- Muñoz, F.D.; Suazo-Martínez, C.; Pereira, E.; Moreno, R. Electricity market design for low-carbon and flexible systems: Room for improvement in Chile. Energy Policy 2020, 148, 111997. [Google Scholar] [CrossRef]
- Medrano, M.; Gil, A.; Martorell, I.; Potau, X.; Cabeza, L.F. State of the art on high-temperature thermal energy storage for power generation. Part 2—Case studies. Renew. Sustain. Energy Rev. 2010, 14, 56–72. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Fernández, A.G.; Gomez-Vidal, J.; Oró, E.; Kruizenga, A.; Solé, A.; Cabeza, L.F. Mainstreaming commercial CSP systems: A technology review. Renew. Energy 2019, 140, 152–176. [Google Scholar] [CrossRef]
- González-Roubaud, E.; Pérez-Osorio, D.; Prieto, C. Review of commercial thermal energy storage in concentrated solar power plants: Steam vs. molten salts. Renew. Sustain. Energy 2017, 80, 133–148. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Novel low melting point quaternary eutectic system for solar thermal energy storage. Appl. Energy 2013, 102, 1422–1429. [Google Scholar] [CrossRef]
- Fernández, A.G.; Galleguillos, H.; Pérez, F.J. Corrosion Ability of a Novel Heat Transfer Fluid for Energy Storage in CSP Plants. Oxid. Met. 2014, 82, 331–345. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Li, Y.; Lu, Y.-W.; Wang, H.-F.; Ma, C.-F. Novel low melting point binary nitrates for thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2016, 164, 114–121. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Ren, N.; Wang, T.; Ma, C.-F. Experimental study on optimized composition of mixed carbonate salt for sensible heat storage in solar thermal power plant. Sol. Energy 2011, 85, 1957–1966. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Siegel, N.P. Molten Nitrate Salt Development for Thermal Energy Storage in Parabolic Trough Solar Power Systems. In Proceedings of the ASME 2008 2nd International Conference on Energy Sustainability, Jacksonville, FL, USA, 10–14 August 2008; pp. 631–637. [Google Scholar]
- Reddy, R.G.; Wang, T.; Mantha, D. Thermodynamic properties of potassium nitrate–magnesium nitrate compound [2KNO3·Mg(NO3)2]. Thermochim. Acta 2012, 531, 6–11. [Google Scholar] [CrossRef]
- Trujillo, U. Novel Molten Salts Thermal Energy Storage for Concentrating Solar Power Generation. 2013. Available online: https://www.osti.gov/biblio/1111584 (accessed on 30 March 2024).
- Bradshaw, R.; Meeker, D. High-temperature stability of ternary nitrate molten salts for solar thermal energy systems. Sol. Energy Mater. 1990, 21, 51–60. [Google Scholar] [CrossRef]
- Bin Mohammad, M.; Brooks, G.A.; Rhamdhani, M.A. Thermal analysis of molten ternary lithium-sodium-potassium nitrates. Renew. Energy 2017, 104, 76–87. [Google Scholar] [CrossRef]
- Olivares, R.I. The thermal stability of molten nitrite/nitrates salt for solar thermal energy storage in different atmospheres. Sol. Energy 2012, 86, 2576–2583. [Google Scholar] [CrossRef]
- Henríquez, M.; Guerreiro, L.; Fernández, G.; Fuentealba, E. Lithium nitrate purity influence assessment in ternary molten salts as thermal energy storage material for CSP plants. Renew. Energy 2020, 149, 940–950. [Google Scholar] [CrossRef]
- Nissen, D.A. Thermophysical Properties of the Equimolar Mixture NaN0,-KNO, from 300 to 600 °C. J. Chem. Eng. Data 1982, 27, 269–273. [Google Scholar] [CrossRef]
- Bradshaw, R.W. Low Melting Point Heat Transfer Fluid. Patent N° US 7,828,990 B1, 17 December 2008. [Google Scholar]
- Bradshaw, R.W. Viscosity of Multi-component Molten Nitrate Salts—Liquidus to 200 °C. Sandia Rep. 2010, 21. [Google Scholar]
- Jin, Y.; Cheng, J.; An, X.; Su, T.; Zhang, P.; Li, Z. Accurate viscosity measurement of nitrates/nitrites salts for concentrated solar power. Sol. Energy 2016, 137, 385–392. [Google Scholar] [CrossRef]
- Coscia, K.; Nelle, S.; Elliott, T.; Mohapatra, S.; Oztekin, A.; Neti, S. Thermophysical Properties of LiNO3–NaNO3–KNO3 Mixtures for Use in Concentrated Solar Thermophysical Properties of for Use in Concentrated Solar Power. J. Sol. Energy Eng. 2013, 135, 034506. [Google Scholar] [CrossRef]
- Morin, G. Optimization of Concentrating Solar Power Plant Designs through Integrated Techno-Economic Modelling; Elsevier Ltd.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 86, 22–44. [Google Scholar] [CrossRef]
- Prieto, C.; Blindu, A.; Cabeza, L.F.; Valverde, J.; García, G. Molten Salts Tanks Thermal Energy Storage: Aspects to Consider during Design. Energies 2024, 17, 22. [Google Scholar] [CrossRef]
- Mallco, A.; Portillo, C.; Kogan, M.J.; Galleguillos, F.; Fernández, A.G. A Materials Screening Test of Corrosion Monitoring in LiNO3 Containing Molten Salts as a Thermal Energy Storage Material for CSP Plants. Appl. Sci. 2020, 10, 3160. [Google Scholar] [CrossRef]
- Cheng, W.-J.; Chen, D.-J.; Wang, C.-J. High-temperature corrosion of Cr–Mo steel in molten LiNO3–NaNO3–KNO3 eutectic salt for thermal energy storage. Sol. Energy Mater. Sol. Cells 2015, 132, 563–569. [Google Scholar] [CrossRef]
- Mallco, A.; Fernández, A.G. Corrosion Monitoring Assessment on Lithium Nitrate Molten Salts as Thermal Energy Storage Material Applied to CSP Plants. Oxid. Met. 2020, 94, 383–396. [Google Scholar] [CrossRef]
- Perkin-Elmer Ltd. Differential scanning calorimeter DSC-1. J. Sci. Instrum. 1963, 40, 470. [Google Scholar] [CrossRef]
- ASTM E 1269-11; Standard Test Method for Determining Specific Heat Capacity by Differential Scanning. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011.
- Bradshaw, R.W.; Brosseau, D.A. Low-Melting Point Inorganic Nitrate Salt Heat Transfer Fluid. Patent US 7,588,694 B1, 15 September 2009. [Google Scholar]
- Wang, T.; Mantha, D.; Reddy, R.G. Thermal stability of the eutectic composition in LiNO3–NaNO3–KNO3 ternary system used for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 100, 162–168. [Google Scholar] [CrossRef]
- Hoshino, Y.; Utsunomiya, T.; Abe, O. The Thermal Descomposition of Sodium Nitrate and the effects of Several Oxides on the Descomposition. Bull. Chem. Soc. Jpn. 1981, 54, 1385–1391. [Google Scholar] [CrossRef]
- Fernández, A.; Pérez, F. Improvement of the corrosion properties in ternary molten nitrate salts for direct energy storage in CSP plants. Sol. Energy 2016, 134, 468–478. [Google Scholar] [CrossRef]
- Ag, F.; Benav, C.-J.; De, P.; Aguaquim, S.A. Division Rheinhütte Pumpen Documentación para bomba de sales. 2014. [Google Scholar]
- Specification, S.; Stainless, M. Material—ASTM A217 Grade WC6. p. 217. Available online: https://gravitycastindia.com/images/resources/ferrouspdf/ASTM%20A217%20Grade%20WC6.pdf (accessed on 30 May 2024).
- Fernández, A.G.; Cabeza, L.F. Molten salt corrosion mechanisms of nitrate based thermal energy storage materials for concentrated solar power plants: A review. Sol. Energy Mater. Sol. Cells 2019, 194, 160–165. [Google Scholar] [CrossRef]
- Candelaria, T.-R.M.; Ebelia, D.-M.; Germán, B.-M.R.; Elena, H.-M.N. Corrosividad atmosférica del cobre y del acero en dos localidades de Villahermosa, Tabasco. Ing. Investig. Tecnol. 2015, 16, 197–206. [Google Scholar] [CrossRef]
- Rivero, S.; Chico, B.; De La Fuente, D.; Morcillo, M. Corrosión atmosférica del acero bajo en carbono en un ambiente marino polar. Estudio del efecto del régimen de vientos. Rev. Metal. 2007, 43, 370–383. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Wu, Y.; Lu, Y. Comparative review of different influence factors on molten salt corrosion characteristics for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 235, 111485. [Google Scholar] [CrossRef]
- Prieto, C.; Ruiz-Cabañas, J.; Madina, V.; Fernández, A.I.; Cabeza, L.F. Lessons learned from corrosion of materials with molten salts during molten salt tank preheating. Sol. Energy Mater. Sol. Cells 2022, 247, 111943. [Google Scholar] [CrossRef]
- Sarvghad, M.; Will, G.; Steinberg, T.A. Corrosion of Inconel 601 in molten salts for thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 172, 220–229. [Google Scholar] [CrossRef]
- Sarvghad, M.; Will, G.; Steinberg, T.A. Corrosion of steel alloys in molten NaCl + Na2SO4 at 700 °C for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 179, 207–216. [Google Scholar] [CrossRef]




| Mixture | Melting Point (°C) | Decomposition Temperature (°C) | Solidification Point (°C) | Heat Capacity (J g−1 °C−1) | (% wt) Heat Capacity/Heat Capacity Solar Salt |
|---|---|---|---|---|---|
| 30 wt% LiNO3 + 13 wt% NaNO3 + 57 wt% KNO3 Todini (99% Li time 0 h) | 125 | 596.3 | 76.05 | 1.794 | +21.6 |
| 30 wt% LiNO3 + 13 wt% NaNO3 + 57 wt% KNO3 Todini (99% Li time 15,000 h) | 129 | 596.57 | 73.33 | 1.409 | −6.0 |
| Solar salt | 223 | 565 | - | 1.50 | 0 |
| Zone 1 (Supramolecular Water Losses) | Zone 2 (Intramolecular Water Losses) | Zone 3 (Stability) | Zone 4 (Decomposition) | Total Weight Loss (wt%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time | T °C | Weight Loss (wt%) | T °C | Weight Loss (wt%) | T °C | ∆T | T °C | Weight Loss (1–3 wt%) | |
| 0 h | 40–100 | 1.39% | 100–264 | 0.664% | 264–368 | 104 | 368–596 | 2.84% | 4.3 |
| 15,000 h | 34–89 | 1.32% | 89–303 | 2.62% | 303–475 | 172 | 475–596 | 1.22% | 5.2 |
| T (°C) | Viscosity (cP) LiNO3 99% (t = 0 h) | Viscosity (cP) LiNO3 99% (t = 15,000 h) | Viscosity (cP) Solar Salt |
|---|---|---|---|
| 150 | 20.4 | 20.53 | - |
| 170 | 13.81 | 14.25 | - |
| 200 | 9.78 | 12.20 | 6.7 |
| 230 | 8.74 | 9.22 | 5.51 |
| 260 | 8.55 | 8.76 | 4.83 |
| 300 | 7.13 | 7.2 | 4.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henríquez, M.; Reinoso-Burrows, J.C.; Pastén, R.; Soto, C.; Duran, C.; Olivares, D.; Guerreiro, L.; Cardemil, J.M.; Galleguillos Madrid, F.M.; Fuentealba, E. Long-Term Evaluation of a Ternary Mixture of Molten Salts in Solar Thermal Storage Systems: Impact on Thermophysical Properties and Corrosion. Materials 2024, 17, 4053. https://doi.org/10.3390/ma17164053
Henríquez M, Reinoso-Burrows JC, Pastén R, Soto C, Duran C, Olivares D, Guerreiro L, Cardemil JM, Galleguillos Madrid FM, Fuentealba E. Long-Term Evaluation of a Ternary Mixture of Molten Salts in Solar Thermal Storage Systems: Impact on Thermophysical Properties and Corrosion. Materials. 2024; 17(16):4053. https://doi.org/10.3390/ma17164053
Chicago/Turabian StyleHenríquez, Mauro, Juan Carlos Reinoso-Burrows, Raúl Pastén, Carlos Soto, Carlos Duran, Douglas Olivares, Luis Guerreiro, José Miguel Cardemil, Felipe M. Galleguillos Madrid, and Edward Fuentealba. 2024. "Long-Term Evaluation of a Ternary Mixture of Molten Salts in Solar Thermal Storage Systems: Impact on Thermophysical Properties and Corrosion" Materials 17, no. 16: 4053. https://doi.org/10.3390/ma17164053





