Phase Transformation and Mechanical Optimization of Eggshell-Derived Hydroxyapatite across a Wide Sintering Temperature Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of HA by Precipitation Method
2.2. Sintering of HA
2.3. X-ray Diffraction Analysis, Phase Content, and Crystallinity
2.4. Microstructural Observations and Grain Size Calculation
2.5. Compressive Strength Measurements
2.6. Apparent Density Measurements
2.7. Microhardness Testing
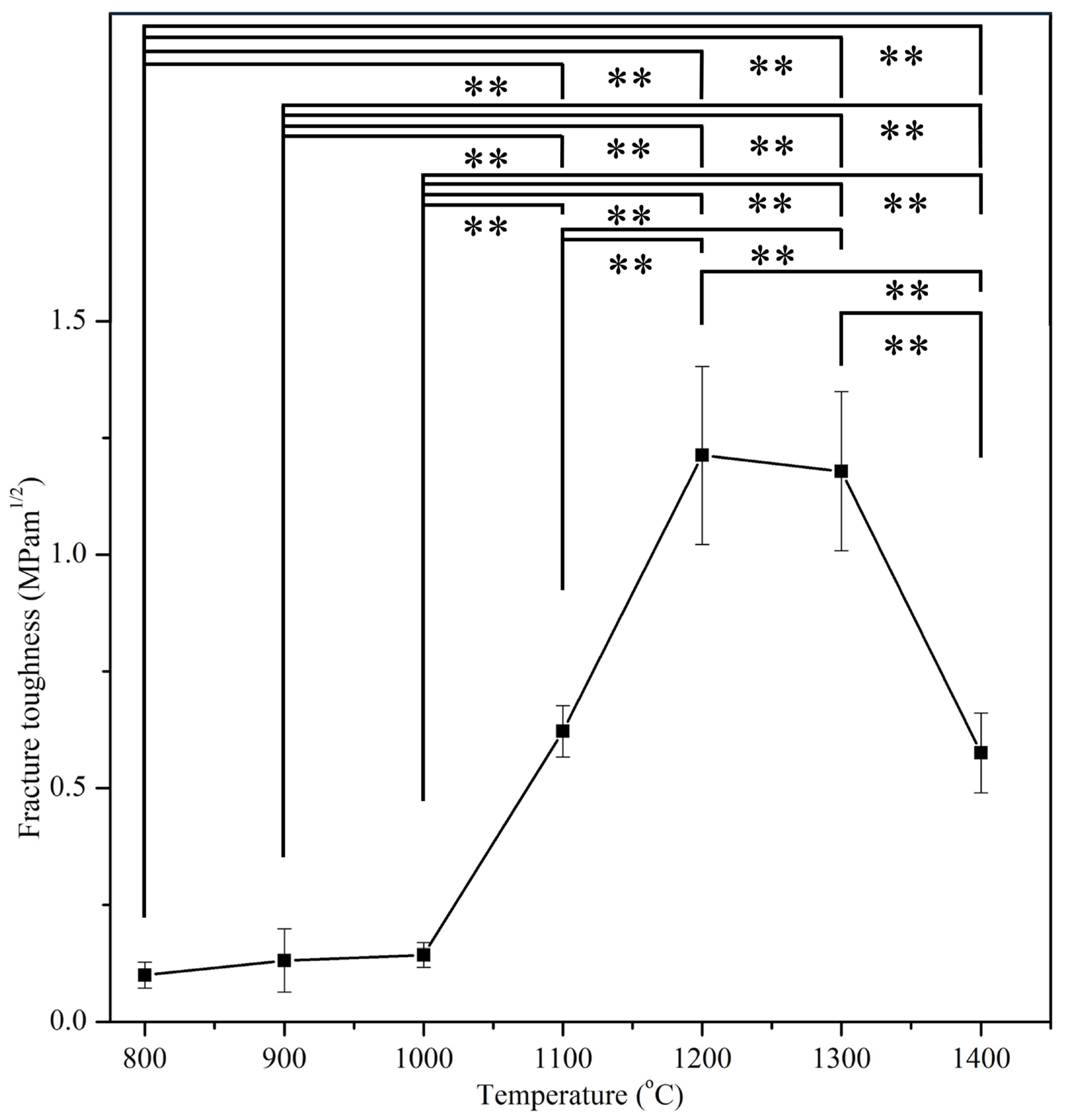
2.8. Fracture Toughness Evaluation
2.9. Antimicrobial Activity Assessment
2.10. Experimental Design and Statistical Analysis
3. Results
3.1. XRD Analysis of HA Post-Sintering
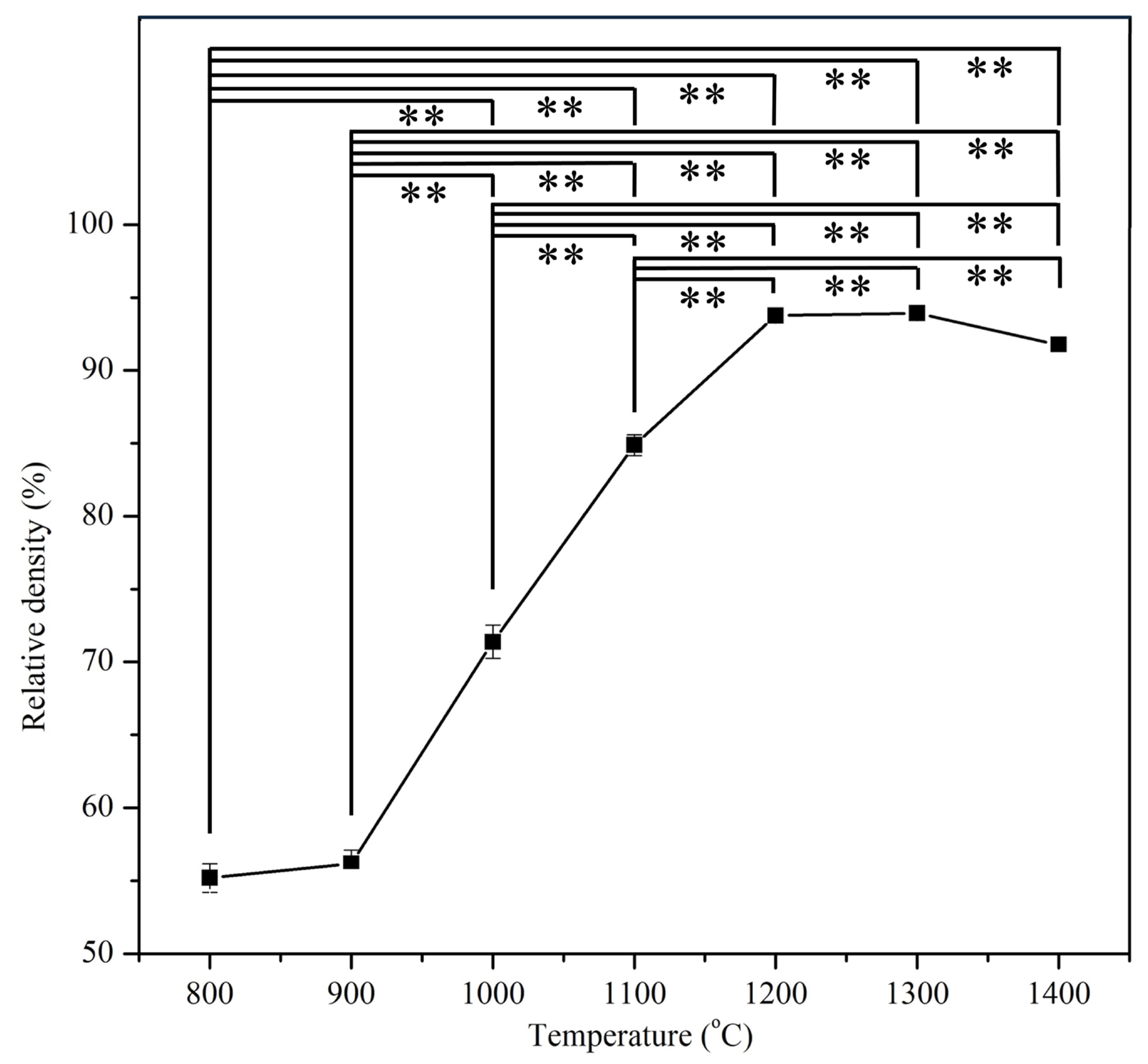
3.2. Relative Density
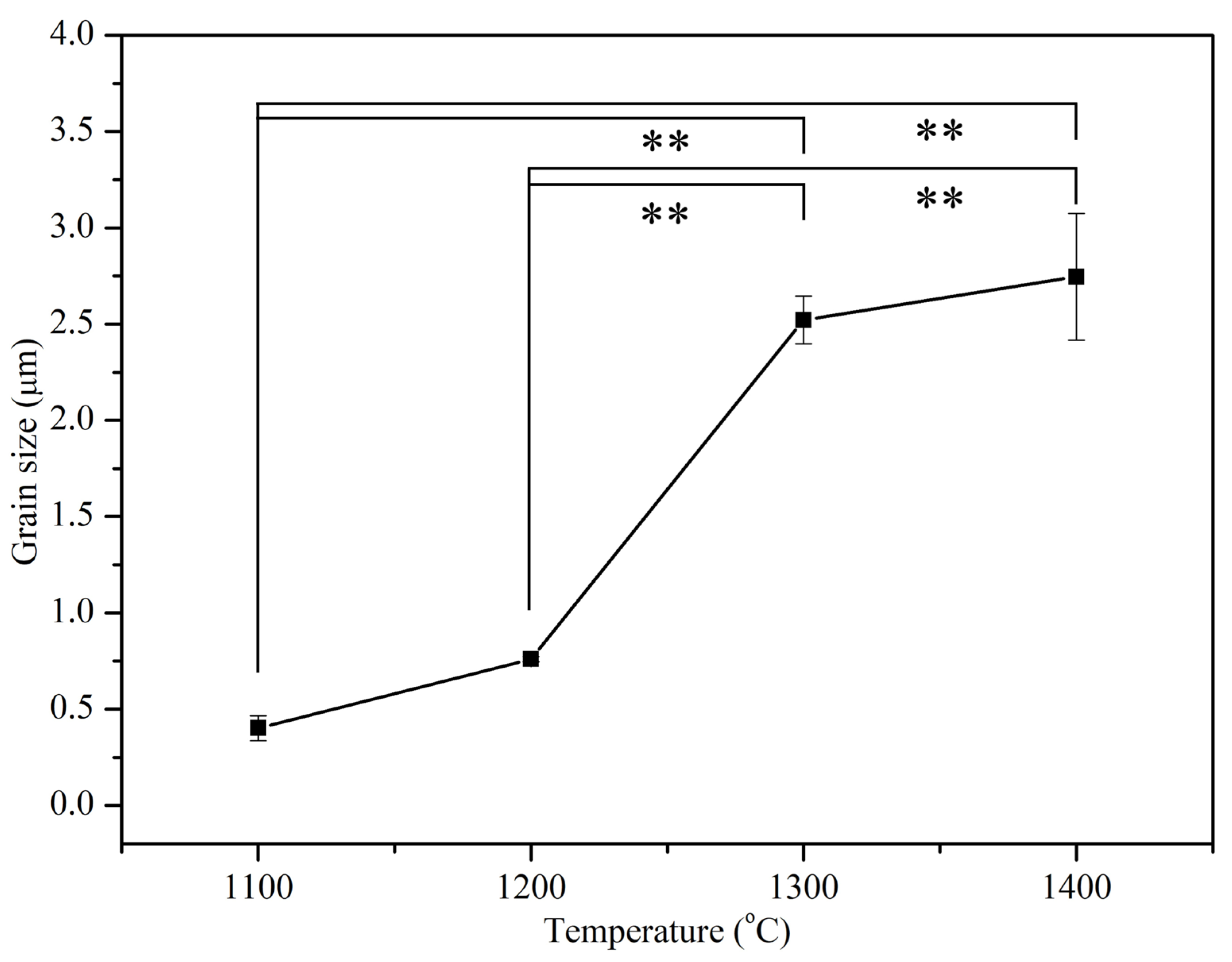
3.3. Microstructural Observation
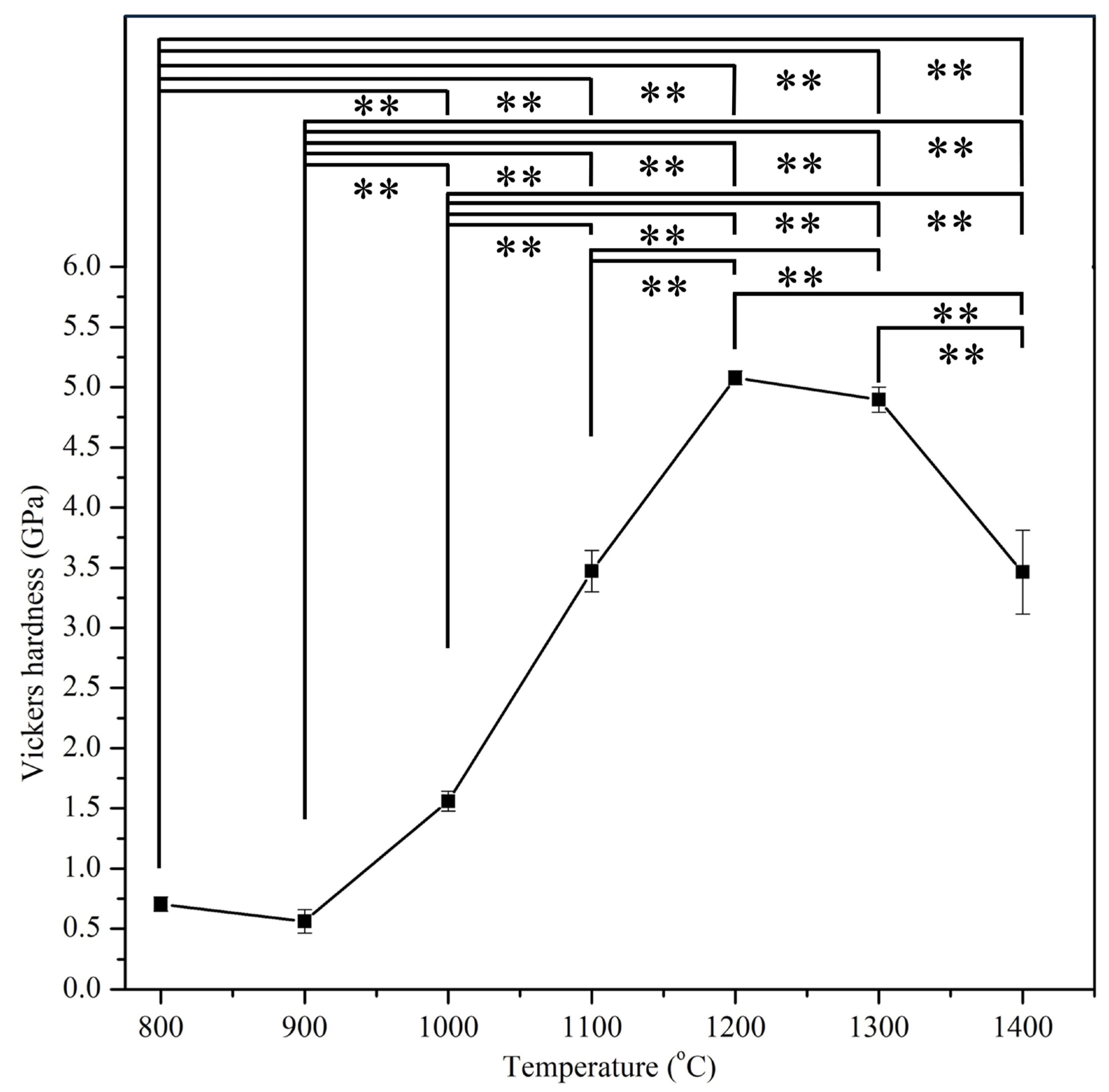
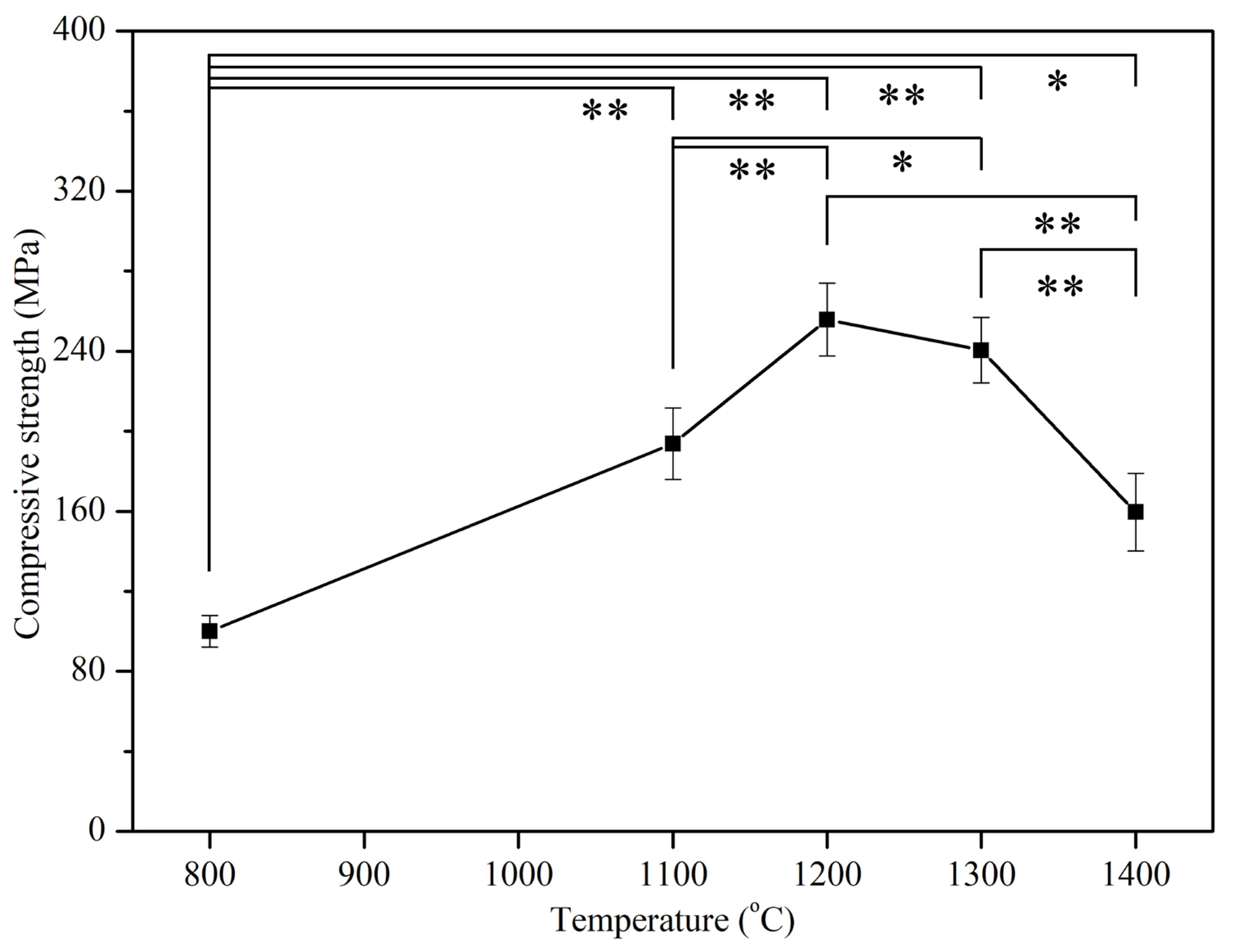
3.4. Mechanical Properties
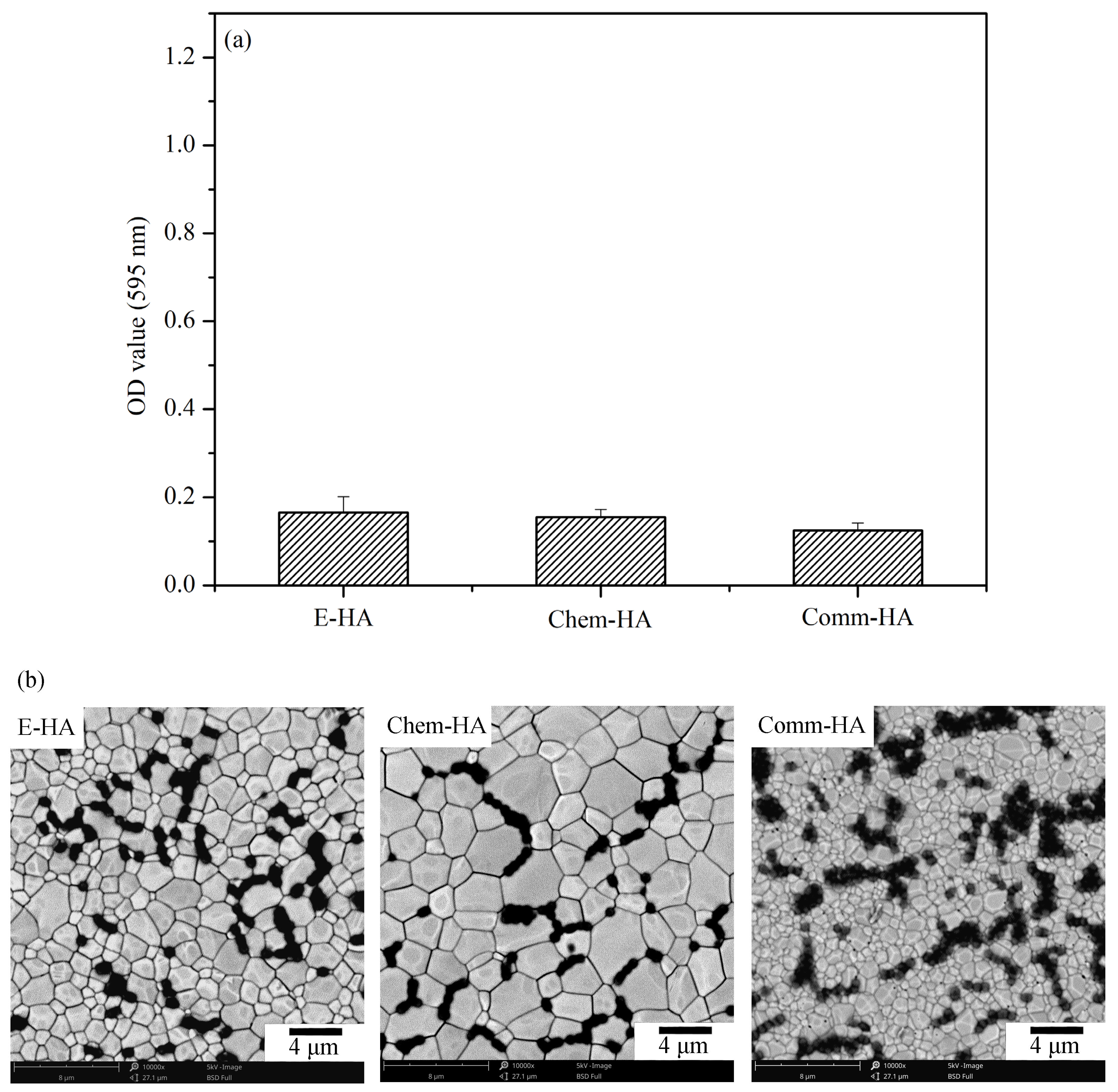
3.5. Antibacterial Activity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Cell Selective apoptosis induced by polymorphic alteration of Self-assembled Silica Nano-webs. ACS Appl. Mater. Interfaces 2017, 9, 6292–6305. [Google Scholar] [CrossRef] [PubMed]
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef]
- Fendi, F.; Abdullah, B.; Suryani, S.; Usman, A.N.; Tahir, D. Development and application of hydroxyapatite-based scaffolds for bone tissue regeneration: A systematic literature review. Bone 2024, 183, 117075. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Pikos, M.A.; Nakamura, T.; Imafuji, T.; Zhang, Y.; Shinohara, Y.; Sculean, A.; Shirakata, Y. The development of non-resorbable bone allografts: Biological background and clinical perspectives. Periodontology 2000 2024, 94, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Bang, L.T.; Long, B.D.; Lan, P.X.; Shi, X.; Thanh, N.T.; Ramesh, S. Effect of strontium substitution on the properties of mesoporous carbonate apatite for biomedical applications. J. Aust. Ceram. Soc. 2024, 60, 1–15. [Google Scholar] [CrossRef]
- Ramesh, S.; Aw, K.L.; Tolouei, R.; Amiriyan, M.; Tan, C.Y.; Hamdi, M.; Purbolaksono, J.; Hassan, M.A.; Teng, W.D. Sintering properties of hydroxyapatite powders prepared using different methods. Ceram. Int. 2013, 39, 111–119. [Google Scholar] [CrossRef]
- Kamalanathan, P.; Ramesh, S.; Bang, L.T.; Niakan, A.; Tan, C.Y.; Purbolaksono, J.; Chandran, H.; Teng, W.D. Synthesis and sintering of hydroxyapatite derived from eggshells as a calcium precursor. Ceram. Int. 2014, 40, 16349–16359. [Google Scholar] [CrossRef]
- Zusho, Y.; Kobayashi, S. Sintering behavior and mechanical properties of calcium phosphate–alumina composite porous scaffolds. Adv. Compos. Mater. 2023, 32, 591–601. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Q.; Zhang, J.; Sheng, J. Effect of MgF2 addition on sinterability and mechanical properties of fluorapatite ceramic composites fabricated by wollastonite and phosphate glass. Ceram. Int. 2022, 48, 20400–20408. [Google Scholar] [CrossRef]
- Solonenko, A.P.; Blesman, A.I.; Polonyankin, D.A. Poorly crystallized hydroxyapatite and calcium silicate hydrate composites: Synthesis, characterization and soaking in simulated body fluid. Mater. Charact. 2020, 161, 110158. [Google Scholar] [CrossRef]
- Quan, V.M.; Dang-Ngoc, T.N.; Nguyen, T.H. Rapid fabrication of mechanical-improved hydroxyapatite scaffolds from the microwave sintering with homogenous incorporated nanosilver particles. Mater. Lett. 2024, 367, 136555. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 1291. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Tarafder, S.; Bandyopadhyay, A.; Bose, S. Effect of grain size on mechanical, surface and biological properties of microwave sintered hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Kalita, S.J.; Bhardwaj, A.; Bhatt, H.A. Nanocrystalline calcium phosphate ceramics in biomedical engineering. Mater. Sci. Eng. C 2007, 27, 441–449. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wang, H.-F.; Liou, S.-P.; Ho, W.-F. Synthesis and Characterization of Nano-Hydroxyapatite Obtained from Eggshell via the Hydrothermal Process and the Precipitation Method. Molecules 2023, 28, 4926. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Kao, Y.L.; Lu, Y.C.; Hsu, H.-C.; Ho, W.-F. Preparation and characterization of microrod hydroxyapatite bundles obtained from oyster shells through microwave irradiation. J. Aust. Ceram. Soc. 2021, 57, 1541–1551. [Google Scholar] [CrossRef]
- Wu, S.C.; Hsu, H.C.; Liu, M.Y.; Ho, W.F. Characterization of nanosized hydroxyapatite prepared by an aqueous precipitation method using eggshells and mulberry leaf extract. J. Korean Ceram. Soc. 2021, 58, 116–122. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Yu, H.C.; Shen, C.E.; Ho, W.-F. Preparation and evaluation of osteoinductive porous biphasic calcium phosphate granules obtained from eggshell for bone tissue engineering. Adv. Powder Technol. 2023, 34, 103909. [Google Scholar] [CrossRef]
- Hsieh, K.-H.; Hsu, H.-C.; Kao, Y.-L.; Wu, S.-C.; Yang, T.-Y.; Ho, W.-F. Nanohydroxyapatite/Peptide Composite Coatings on Pure Titanium Surfaces with Nanonetwork Structures Using Oyster Shells. Nanomaterials 2024, 14, 577. [Google Scholar] [CrossRef]
- Wu, S.-C.; Tsou, H.K.; Liu, H.C.; Hsu, S.K.; Liou, S.P.; Ho, W.F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Shafaghi, R.; Rodriguez, O.; Phull, S.; Schemitsch, E.H.; Zalzal, P.; Waldman, S.D.; Papini, M.; Towler, M.R. Effect of TiO2 doping on degradation rate, microstructure and strength of borate bioactive glass scaffolds. Mater. Sci. Eng. C 2020, 107, 110351. [Google Scholar] [CrossRef] [PubMed]
- Kolanthai, E.; Sindu, P.A.; Arul, K.T.; Chandra, V.S.; Manikandan, E.; NKalkura, S.N. Agarose encapsulated mesoporous carbonated hydroxyapatite nanocomposites powder for drug delivery. J. Photochem. Photobiol. B Biol. 2017, 166, 220–231. [Google Scholar] [CrossRef]
- Jamil, M.; Elouahli, A.; Abida, F.; Khallok, H.; Gourri, E.; Kheribech, A.; Hatim, Z. Development of triphasic hydroxyapatite/(α and β)-tricalcium phosphate based composites by sintering powder of calcium-apatite in the presence of montmorillonite. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2489–2498. [Google Scholar] [CrossRef]
- Elmourabit, M.; Zarki, Y.; Arfoy, B.; Allaoui, I.; Aghzzaf, A.A.; Raissouni, I.; Bouchta, D.; Chaouket, F.; Draoui, K. Phosphate sludge valorization as new alternative precursor for carbonated hydroxyapatite nanostructures: Synthesis and characterization. J. Mater. Cycles Waste Manag. 2024, 26, 602–619. [Google Scholar] [CrossRef]
- ASTM E112-96; Standard Test Methods for Determining Average Grain Size. ASTM International: Philadelphia, PA, USA, 1996.
- ASTM C1424-10; Standard Test Method for Monotonic Compressive Strength of Advanced Ceramics at Ambient Temperature. ASTM International: West Conshohocken, PA, USA, 2009.
- Horikawa, S.; Suzuki, K.; Motojima, K.; Nakano, K.; Nagaya, M.; Nagashima, H.; Kaneko, H.; Aizawa, M. Material Design of Porous Hydroxyapatite Ceramics via Inverse Analysis of an Estimation Model for Bone-Forming Ability Based on Machine Learning and Experimental Validation of Biological Hard Tissue Responses. Materials 2024, 17, 571. [Google Scholar] [CrossRef]
- Shah, J.; Verma, K.C.; Agarwal, A.; Kotnala, R.K. Novel application of multiferroic compound for green electricity generation fabricated as hydroelectric cell. Mater. Chem. Phys. 2020, 239, 122068. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Baciu, D.; Koltsakidis, S.; Tzetzis, D.; Garmpi, E.; Roussi, E.; Kitsou, I.; Tsetsekou, A.; Andreouli, C.-D. Additive Manufacturing of Zirconia-Based Pastes for Dental Prosthesis Via Robocasting Method. J. Mater. Eng. Perform. 2024, 33, 1–15. [Google Scholar] [CrossRef]
- Sachiko, H.S.; Jun, S.; Makoto, S.; Hideaki, E. Determination of fracture toughness of human permanent and primary enamel using an indentation microfracture method. J. Mater. Sci. Mater. Med. 2012, 23, 2047–2054. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, C.; Li, Y.; Gao, J.; Yang, Y.; Zhao, S.; Che, H.; Yang, Y.; Yao, S.; Li, W.; et al. Mimicking the mechanical properties of cortical bone with an additively manufactured biodegradable Zn-3Mg alloy. Acta Biomater. 2024, 182, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Beighton, D.; Al-Haboubi, M.; Mantzourani, M.; Gilbert, S.C.; Clark, D.; Zoitopoulos, L.; Gallagher, J.E. Oral Bifidobacteria: Caries-associated Bacteria in Older Adults. J. Dent. Res. 2010, 89, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, M.; Wu, Y.; He, Y.; Fu, Z. Specific chemiluminescent protocol for dual-site recognition of Streptococcus mutans utilizing strong affinity between teicoplanin and Grampositive bacteria. Talanta 2018, 179, 350–355. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.Y.; Tolouei, R.; Amiriyan, M.; Purbolaksono, J.; Sopyan, I.; Teng, W.D. Sintering behavior of hydroxyapatite prepared from different routes. Mater. Des. 2012, 34, 148–154. [Google Scholar] [CrossRef]
- Thuault, A.; Savary, E.; Hornez, J.C.; Moreau, C.; Descamps, M.; Marinel, S.; Leriche, A. Improvement of the hydroxyapatite mechanical properties by direct microwave sintering in single mode cavity. J. Eur. Ceram. Soc. 2014, 34, 1865–1871. [Google Scholar] [CrossRef]
- Aminzare, M.; Eskandari, A.; Baroonian, M.H.; Berenov, A.; Razavi Hesabi, Z.; Taheri, M.; Sadrnezhaad, S.K. Hydroxyapatite nanocomposites: Synthesis, sintering and mechanical properties. Ceram. Int. 2013, 39, 2197–2206. [Google Scholar] [CrossRef]
- Khodaei, M.; Valanezhad, A.; Watanabe, I.; Yousefi, R. Surface and mechanical properties of modified porous titanium scaffold. Surf. Coat. Technol. 2017, 315, 61–66. [Google Scholar] [CrossRef]
- Ou, S.L.; Chiou, S.Y.; Ou, K.L. Phase transformation on hydroxyapatite decomposition. Ceram. Int. 2013, 39, 3809–3816. [Google Scholar] [CrossRef]
- Ge, J.; Cui, F.Z.; Wang, X.M.; Feng, H.L. Property variations in the prism and the organic sheath within enamel by nanoindentation. Biomaterials 2005, 26, 3333–3339. [Google Scholar] [CrossRef]
- Ayatollahi, M.R.; Karimzadeh, A. Nano-indentation measurement of fracture toughness of dental enamel. Int. J. Fract. 2013, 183, 113–118. [Google Scholar] [CrossRef]
- El-Rab, S.M.F.G.; Fadl-allah, S.A.; Montser, A.A. Improvement in antibacterial properties of Ti by electrodeposition of biomimetic Ca–P apatite coat on anodized titania. Appl. Surf. Sci. 2012, 261, 1–7. [Google Scholar] [CrossRef]



| Group | Sintering Temperature (°C) | Sample Number | Test Performed |
|---|---|---|---|
| E-HA | 800 °C, 900 °C, 1000 °C, 1100 °C, 1200 °C, 1300 °C, and 1400 °C | Five specimens for each sintering temperature | Relative density |
| E-HA | 1100 °C, 1200 °C, 1300 °C, and 1400 °C | Five specimens for each sintering temperature | Grain size |
| E-HA | 800 °C, 900 °C, 1000 °C, 1100 °C, 1200 °C, 1300 °C, and 1400 °C | Five specimens for each sintering temperature | Vickers hardness |
| E-HA | 800 °C, 1100 °C, 1200 °C, 1300 °C, and 1400 °C | Five specimens for each sintering temperature | Compressive strength |
| E-HA | 800 °C, 900 °C, 1000 °C, 1100 °C, 1200 °C, 1300 °C, and 1400 °C | Five specimens for each sintering temperature | Fracture toughness |
| E-HA | 1200 °C | Three specimens | Antimicrobial activity |
| Chem-HA | 1200 °C | Three specimens | Antimicrobial activity |
| Comm-HA | 1200 °C | Three specimens | Antimicrobial activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-C.; Hsu, H.-C.; Liu, M.-Y.; Ho, W.-F. Phase Transformation and Mechanical Optimization of Eggshell-Derived Hydroxyapatite across a Wide Sintering Temperature Range. Materials 2024, 17, 4062. https://doi.org/10.3390/ma17164062
Wu S-C, Hsu H-C, Liu M-Y, Ho W-F. Phase Transformation and Mechanical Optimization of Eggshell-Derived Hydroxyapatite across a Wide Sintering Temperature Range. Materials. 2024; 17(16):4062. https://doi.org/10.3390/ma17164062
Chicago/Turabian StyleWu, Shih-Ching, Hsueh-Chuan Hsu, Mei-Yi Liu, and Wen-Fu Ho. 2024. "Phase Transformation and Mechanical Optimization of Eggshell-Derived Hydroxyapatite across a Wide Sintering Temperature Range" Materials 17, no. 16: 4062. https://doi.org/10.3390/ma17164062
APA StyleWu, S.-C., Hsu, H.-C., Liu, M.-Y., & Ho, W.-F. (2024). Phase Transformation and Mechanical Optimization of Eggshell-Derived Hydroxyapatite across a Wide Sintering Temperature Range. Materials, 17(16), 4062. https://doi.org/10.3390/ma17164062








