Effect of Crevice Size on Crevice Corrosion of N80 Carbon Steel in CO2-Saturated NaCl-HAc Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Solution
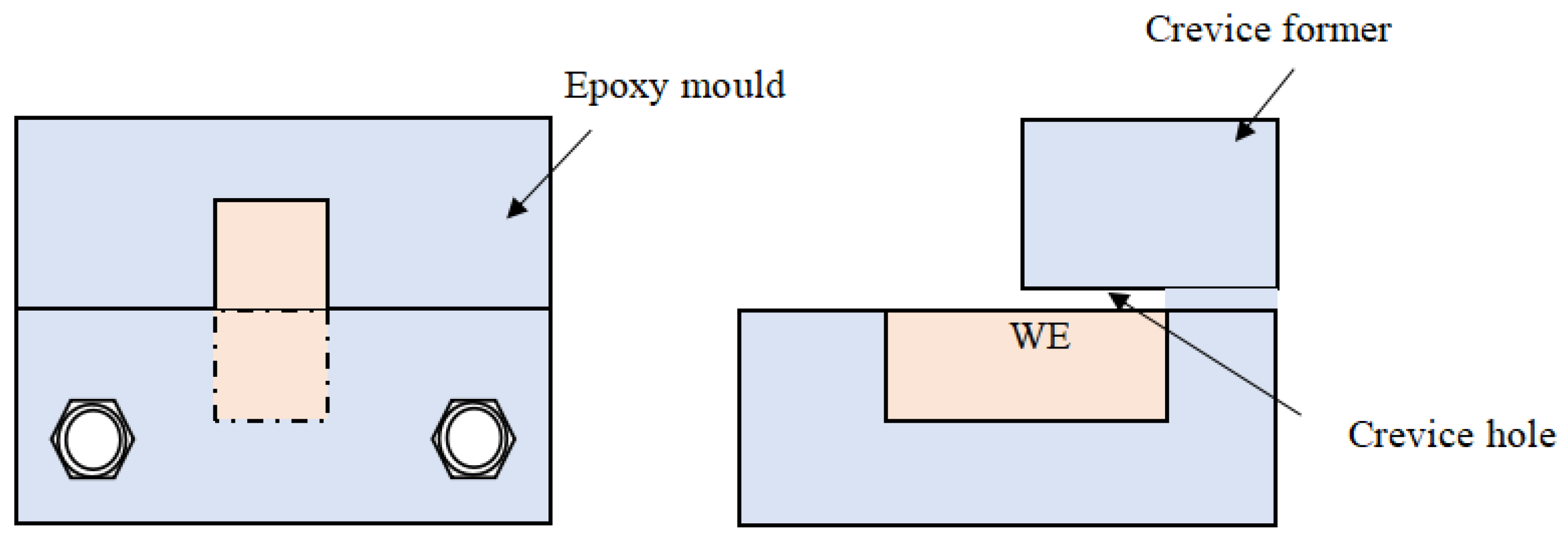
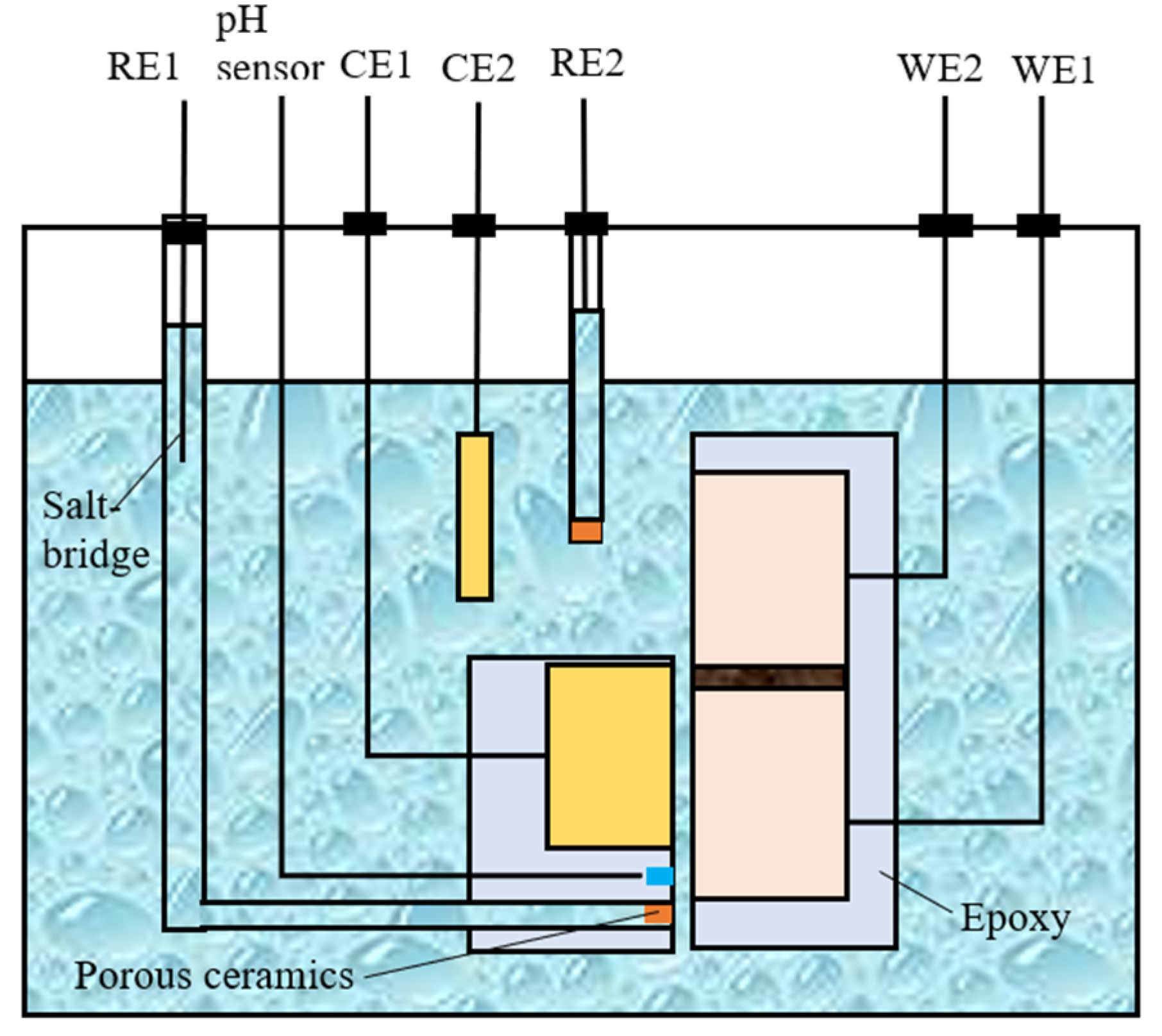
2.2. Configuration of the Crevice and Electrochemical Measurements
2.3. Morphology Analysis
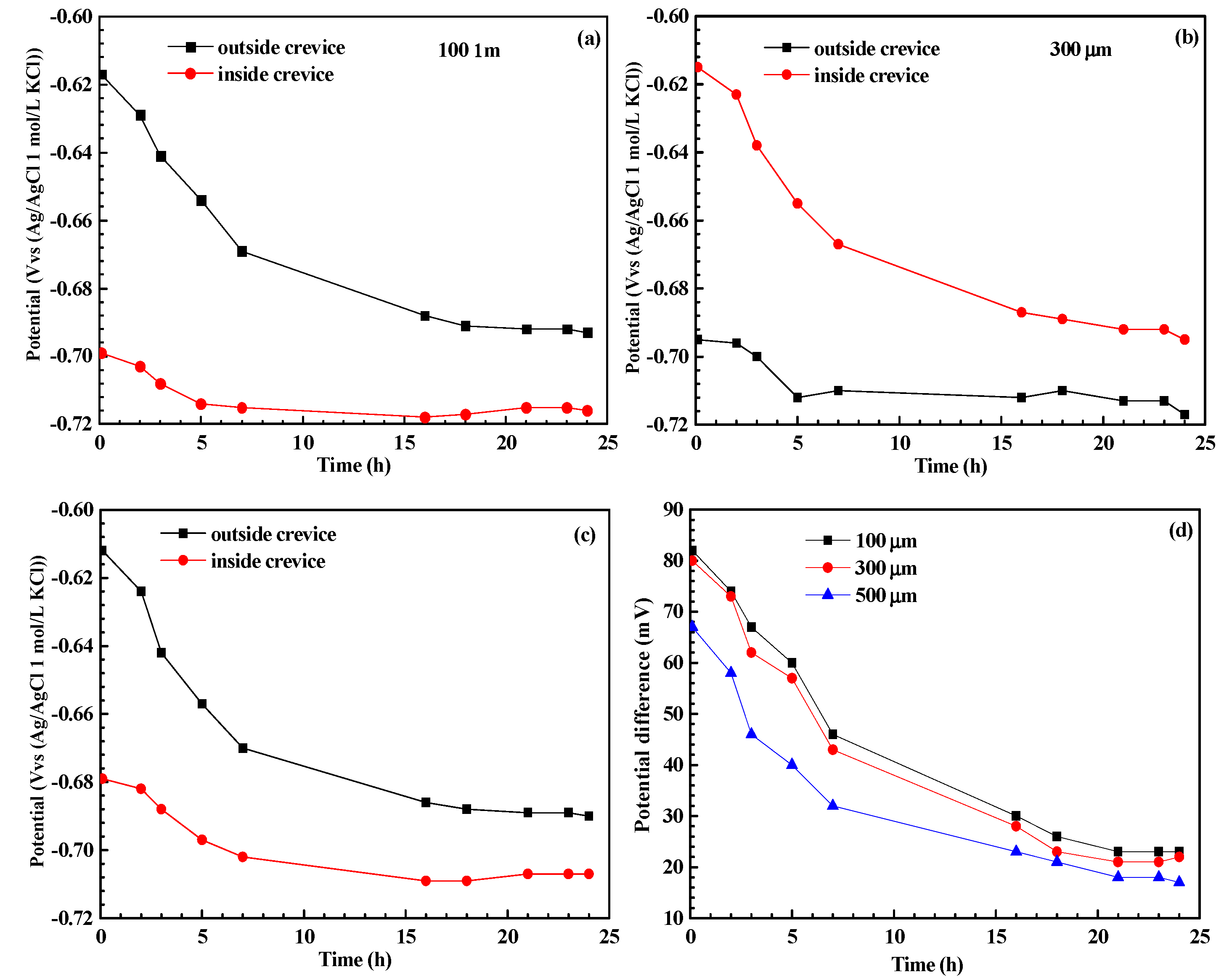
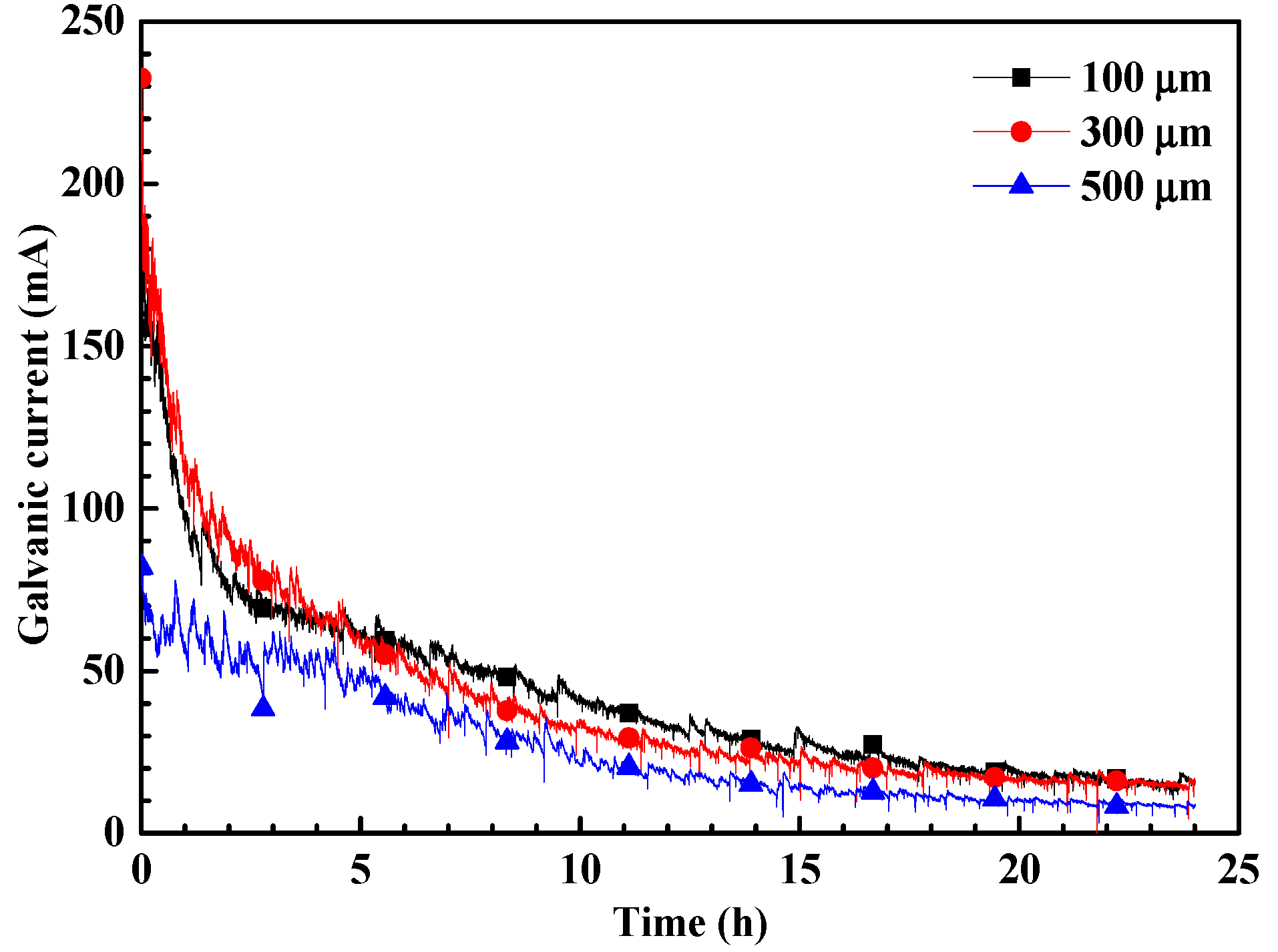
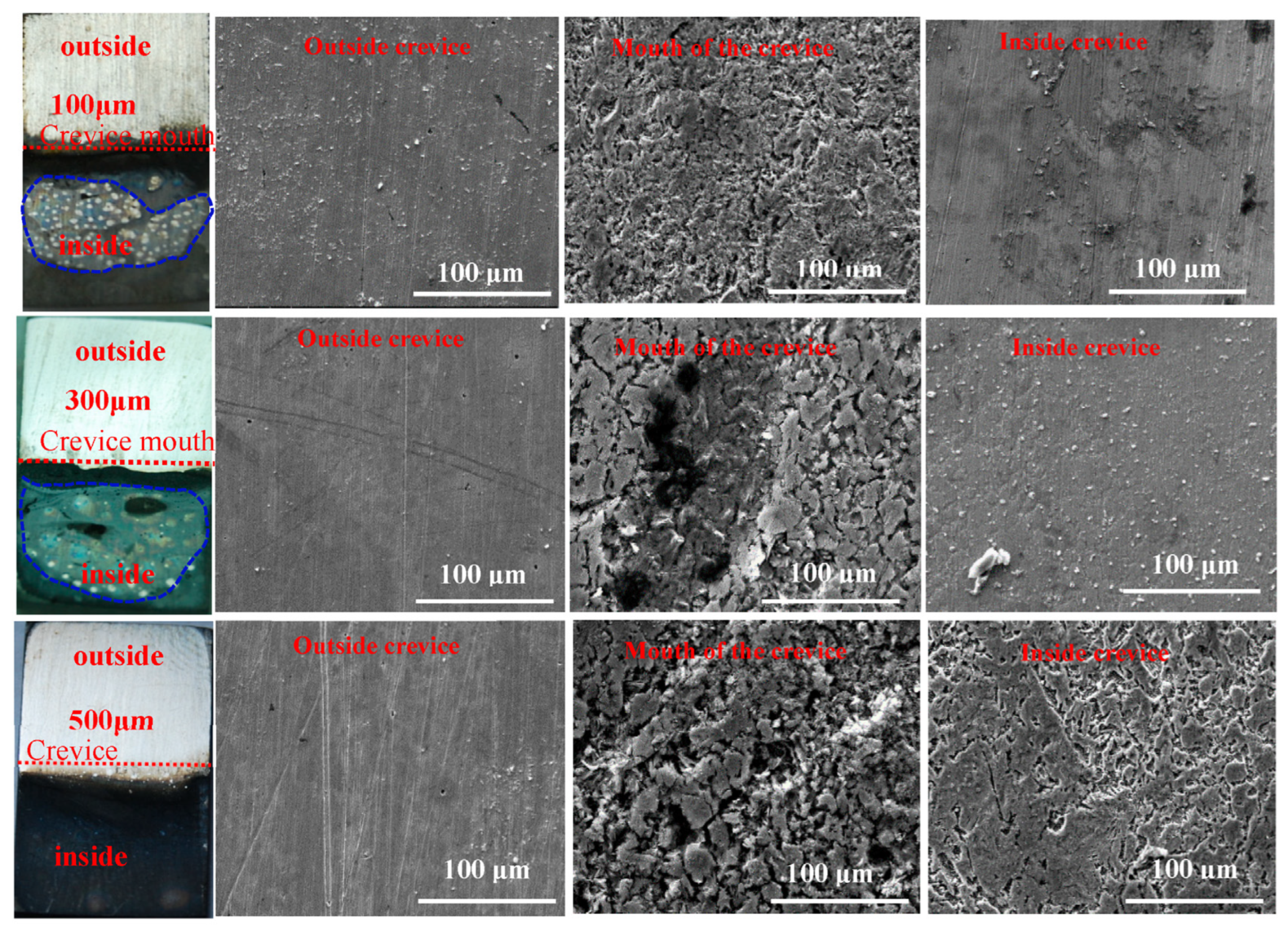
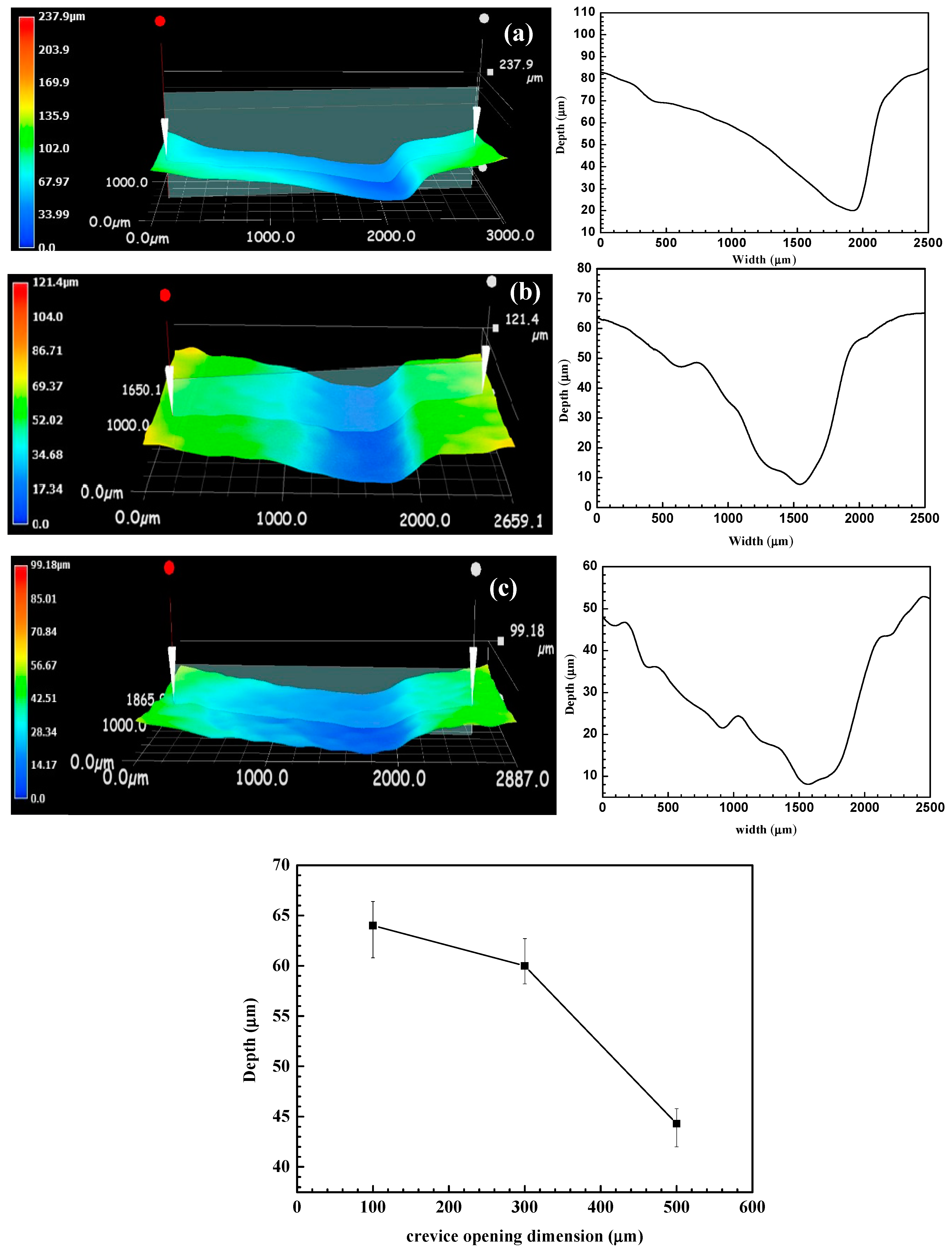
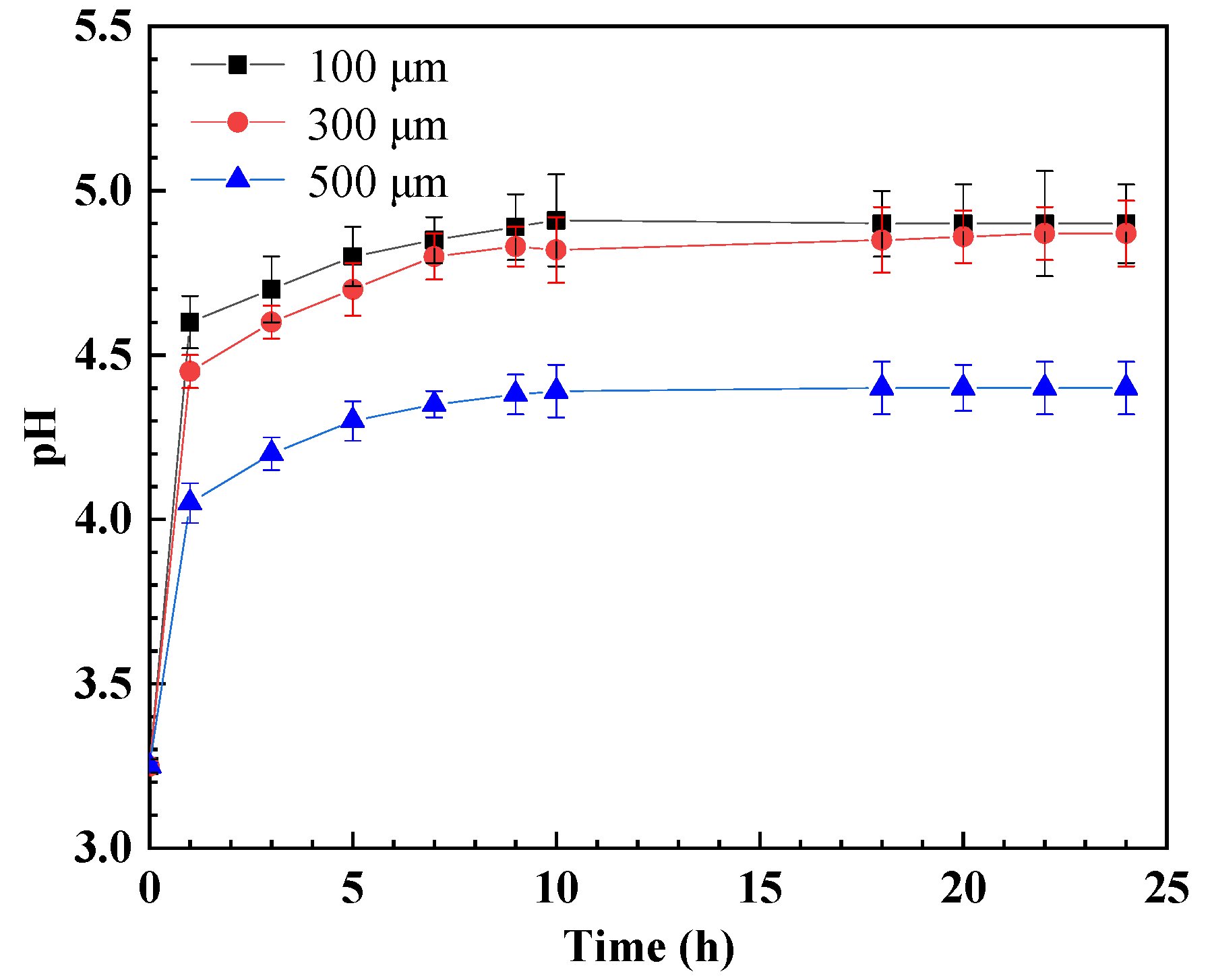
3. Results
4. Discussion
Effect of Crevice Size on Crevice Corrosion of N80 Carbon Steel
5. Conclusions
- 1
- The crevice corrosion of N80 carbon steel has a high crevice corrosion susceptibility in a CO2-saturated NaCl-HAc solution which could be immediately initiated without an induction period for specimens with 100 μm, 300 μm, and 500 μm crevice sizes.
- 2
- The pH inside the crevice increases rather than decreases after crevice corrosion. This contributes to the negative shift in the corrosion potential of steel inside the crevice, which then triggers the galvanic corrosion effect between the inner and outer metals. Deep corrosion grooves could be formed near the crevice mouth after crevice corrosion.
- 3
- The crevice corrosion phenomenon of N80 carbon steel between specimens with 100 μm and 300 μm crevice sizes has no significant difference, but it is more severe than in the specimen with a 500 μm crevice size.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, G.Y.; Li, Y.Y.; Hou, B.S.; Zhang, Q.H.; Zhang, G.A. Corrosion behavior of 13Cr stainless steel under stress and crevice in high pressure CO2/O2 environment. J. Mater. Sci. Technol. 2021, 88, 79–89. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, D.; Xiao, G.; Shang, J.; Liu, Y.; Long, D.; He, Q.; Singh, A. Effect of Cl− accumulation on corrosion behavior of steels in H2S/CO2 methyldiethanolamine (MDEA) gas sweetening aqueous solution. J. Nat. Gas. SCI. Eng. 2016, 30, 444–454. [Google Scholar] [CrossRef]
- Wu, G.; Gu, X.; Zhao, W.; Fan, R.; Mao, T. Corrosion behavior of carbon steel in H2S-CO2-H2O-methyldiethanolamine (MDEA) solution under the presence of chloride ions. Anti-Corros. Msthod. Mater. 2021, 68, 284–292. [Google Scholar] [CrossRef]
- Da Silva, C.A.; Filho, D.; Pimentel, T.; Panossian, Z. Analysis of crude oil effect for CO2 corrosion of carbon steel—A rotating cylinder electrode approach, Geoenergy. Sci. Eng. 2023, 229, 212085. [Google Scholar]
- Sun, C.; Yan, X.; Sun, J.; Pang, J.; Zhao, W.; Lin, X. Unraveling the effect of O2, NO2 and SO2 impurities on the stress corrosion behavior of X65 steel in water-saturated supercritical CO2 streams. Corros. Sci. 2022, 209, 110729. [Google Scholar] [CrossRef]
- Lin, X.; Liu, W.; Wu, F.; Xu, C.; Dou, J.; Lu, M. Effect of O2 on corrosion of 3Cr steel in high temperature and high pressure CO2–O2 environment. Appl. Surf. Sci. 2015, 329, 104–115. [Google Scholar] [CrossRef]
- Li, Y.; Mu, J.; Cui, Z.; Wang, X. The crevice corrosion behavior of N80 carbon steel in acidic NaCl solution: The effect of O2. Mater. Corrosion 2022, 73, 281–290. [Google Scholar] [CrossRef]
- He, L.; Zhang, Q.; Chen, W.; Wang, Y.; Wang, M.; Huang, Y.; Xu, Y. Unraveling short-term O2 contamination on under deposit corrosion of X65 pipeline steel in CO2 saturated solution. Corros. Sci. 2024, 233, 112113. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, L.; Li, Y.; Du, Z.; Zhu, Q.; Han, Z. The synergistic effect of temperature, H2S/CO2 partial pressure and stress toward corrosion of X80 pipeline steel. Eng. Fail. Anal. 2023, 146, 107079. [Google Scholar] [CrossRef]
- Van der Merwe, J.W.; Du Toit, M.; Klenam, D.E.P.; Bodunrin, M.O. Prediction of stress-corrosion cracking using electrochemical noise measurements: A case study of carbon steels exposed to H2O-CO-CO2 environment. Eng. Fail. Anal. 2023, 144, 106948. [Google Scholar] [CrossRef]
- He, X.; Wang, L.; Kong, D.; Zhang, W.; Dai, K.; Ni, X.; Zhang, L.; Zhou, Y.; Dong, C. The kinetics of pitting corrosion in a defect-contained Hastelloy X alloy fabricated by laser powder bed fusion. Corros. Sci. 2024, 237, 112321. [Google Scholar] [CrossRef]
- Li, H.-B.; Jiang, Z.-H.; Yang, Y.; Cao, Y.; Zhang, Z.-R. Pitting corrosion and crevice corrosion behaviors of high nitrogen austenitic stainless steels. Int. J. Miner. Met. Mater. 2009, 16, 517–524. [Google Scholar] [CrossRef]
- Wang, R. Influence of ultrasound on pitting corrosion and crevice corrosion of SUS304 stainless steel in chloride sodium aqueous solution. Corros. Sci. 2008, 50, 325–328. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Cui, Z.Y.; Cui, H.Z.; Wang, X.; Li, Y.Z. The effect of applied stress on the crevice corrosion of 304 stainless steel in 3.5 wt% NaCl solution. Corros. Sci. 2022, 196, 110039. [Google Scholar] [CrossRef]
- Oldfield, J.W.; Sutton, W.H. Crevice Corrosion of Stainless Steels: I. A Mathematical Model. Br. Corros. J. 1978, 13, 13–22. [Google Scholar] [CrossRef]
- Oldfield, J.W.; Sutton, W.H. Crevice Corrosion of Stainless Steels: II. Experimental studies. Br. Corros. J. 1978, 13, 104–111. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Bahn, C.B.; Baek, S.H.; Choi, W.; Song, G.D. Crevice chemistry and corrosion in high temperature water: A review. Nucl. Eng. Technol. 2024, 56, 3112–3122. [Google Scholar] [CrossRef]
- Luo, B.; Hu, Q.; Liu, J.; Huang, F. Effect of crevice gap on crevice corrosion initiation and development of 2205 duplex stainless steel in NaCl solution. J. Mater. Res. Technol. 2022, 21, 2584–2597. [Google Scholar] [CrossRef]
- He, P.; Suo, D.; Wu, W.; Yin, L.; Dai, W.; Shang, B.; Liu, Y.; Sun, Y.; Jiang, Y.; Li, J. Effects of hydrogen on the crevice corrosion behaviors of duplex stainless steel 2205. J. Mater. Res. Technol. 2022, 19, 101–120. [Google Scholar] [CrossRef]
- Calabokis, O.P.; de la Rosa, Y.N.; Lepienski, C.M.; Cardoso, R.P.; Borges, P.C. Crevice and pitting corrosion of low temperature plasma nitrided UNS S32750 super duplex stainless steel. Surf. Coat. Technol. 2021, 413, 127095. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, G.; Qiu, Y.; Guo, X. The crevice corrosion behaviour of stainless steel in sodium chloride solution. Corros. Sci. 2011, 53, 4065–4072. [Google Scholar] [CrossRef]
- Li, Y.Z.; Guo, X.P.; Zhang, G.A. Synergistic effect of stress and crevice on the corrosion of N80 carbon steel in the CO2 -saturated NaCl solution containing acetic acid. Corros. Sci. 2017, 123, 228–242. [Google Scholar] [CrossRef]
- Li, Y.Z.; Xu, N.; Guo, X.P.; Zhang, G.A. The role of acetic acid or H+ in initiating crevice corrosion of N80 carbon steel in CO2-saturated NaCl solution. Corros. Sci. 2017, 128, 9–22. [Google Scholar] [CrossRef]
- Mu, J.; Li, Y.Z.; Wang, X. Crevice corrosion behavior of X70 steel in NaCl solution with different pH. Corros. Sci. 2021, 182, 109310. [Google Scholar] [CrossRef]
- Li, Y.Z.; Xu, N.; Liu, G.R.; Guo, X.P.; Zhang, G.A. Crevice corrosion of N80 carbon steel in CO2 -saturated environment containing acetic acid. Corros. Sci. 2016, 112, 426–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, P.; Cai, G.; Li, Y. Effect of Crevice Size on Crevice Corrosion of N80 Carbon Steel in CO2-Saturated NaCl-HAc Solution. Materials 2024, 17, 4078. https://doi.org/10.3390/ma17164078
Hu P, Cai G, Li Y. Effect of Crevice Size on Crevice Corrosion of N80 Carbon Steel in CO2-Saturated NaCl-HAc Solution. Materials. 2024; 17(16):4078. https://doi.org/10.3390/ma17164078
Chicago/Turabian StyleHu, Pengfei, Guangyi Cai, and Yizhou Li. 2024. "Effect of Crevice Size on Crevice Corrosion of N80 Carbon Steel in CO2-Saturated NaCl-HAc Solution" Materials 17, no. 16: 4078. https://doi.org/10.3390/ma17164078
APA StyleHu, P., Cai, G., & Li, Y. (2024). Effect of Crevice Size on Crevice Corrosion of N80 Carbon Steel in CO2-Saturated NaCl-HAc Solution. Materials, 17(16), 4078. https://doi.org/10.3390/ma17164078




