In Situ Synthesis of (Mo,Cr)Si2 Composites by Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Sample Fabrication
2.2. Sample Characterization
2.3. Oxidation Tests
3. Results and Discussion
3.1. Thermodynamic Analysis
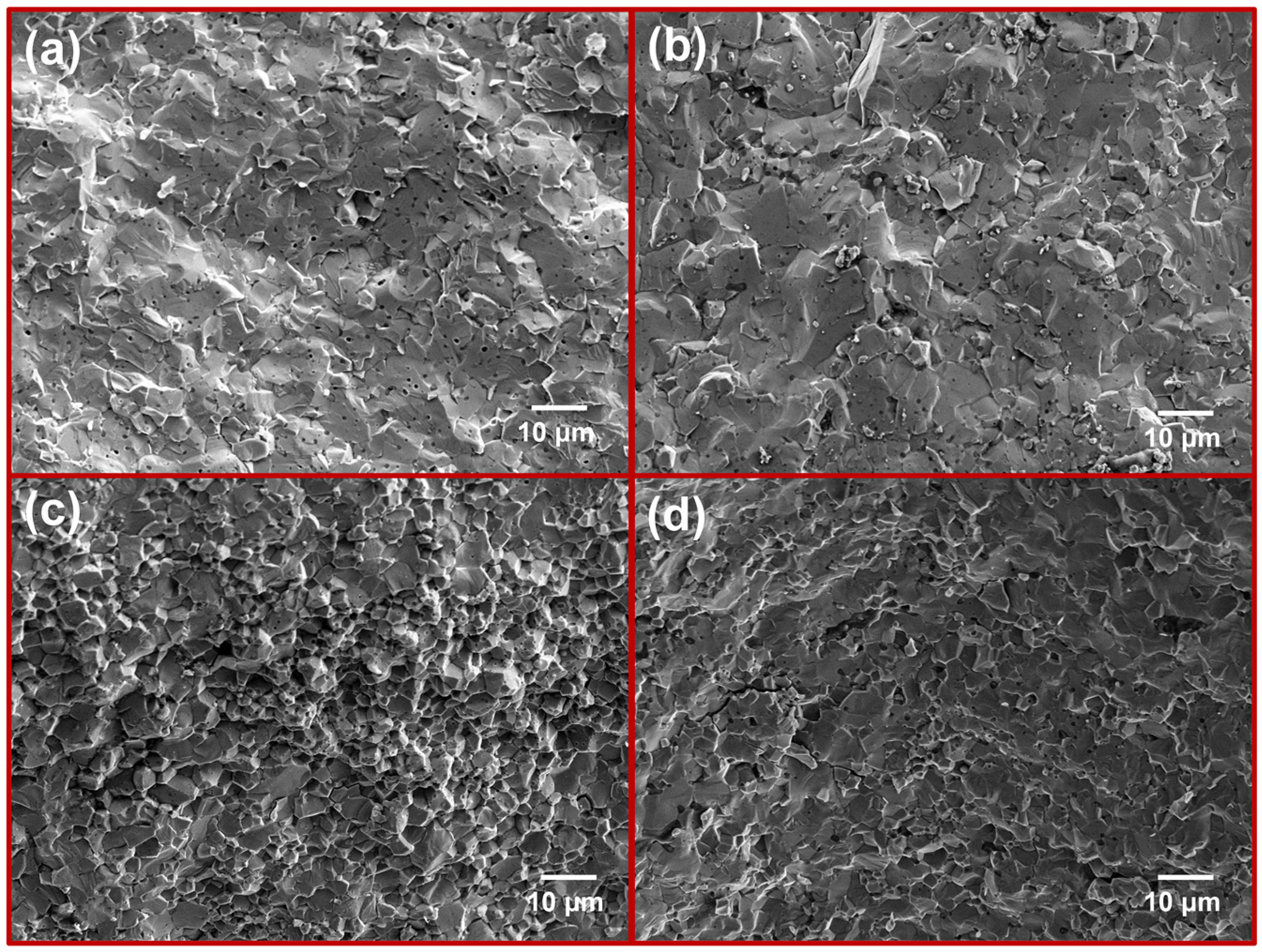
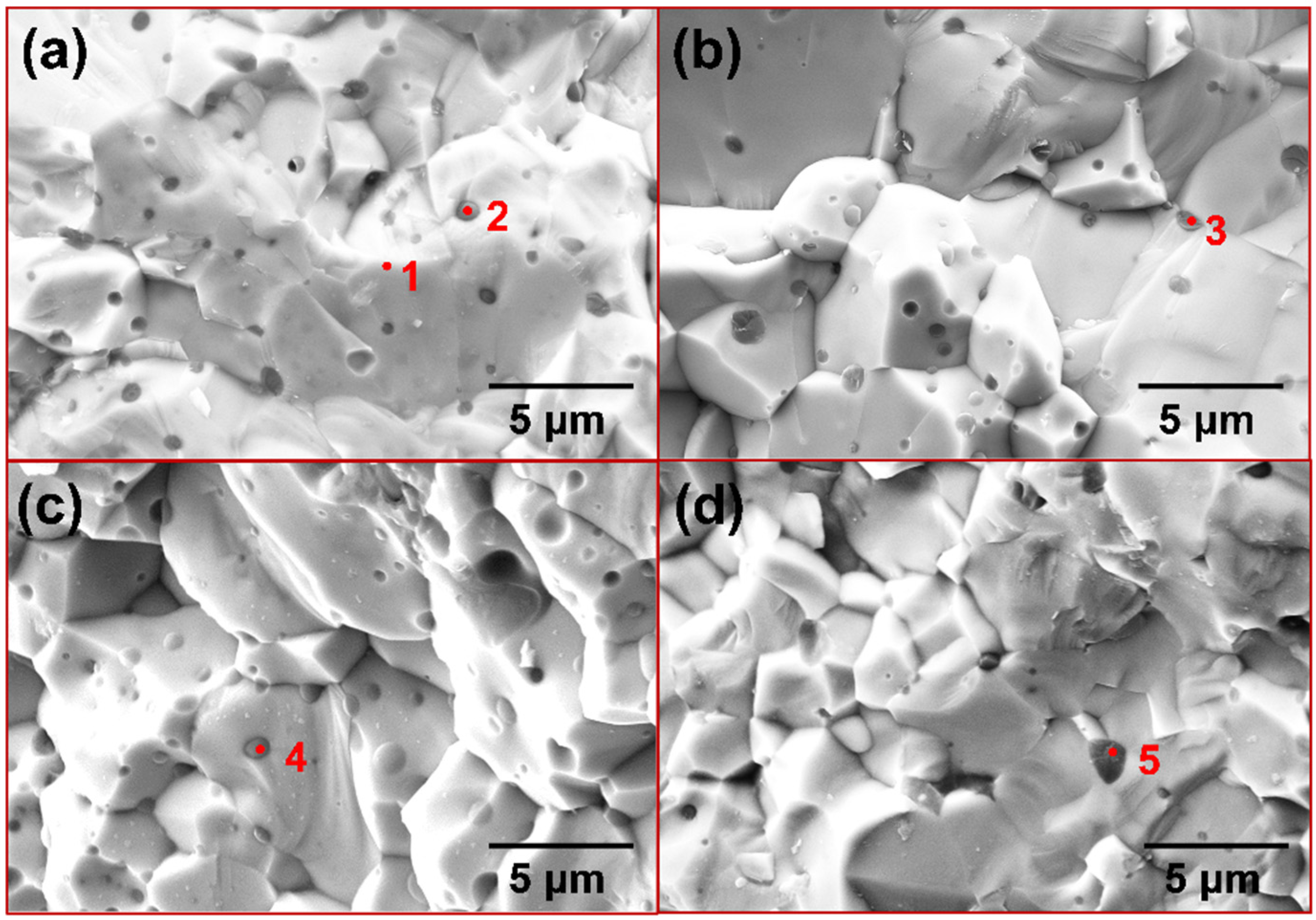
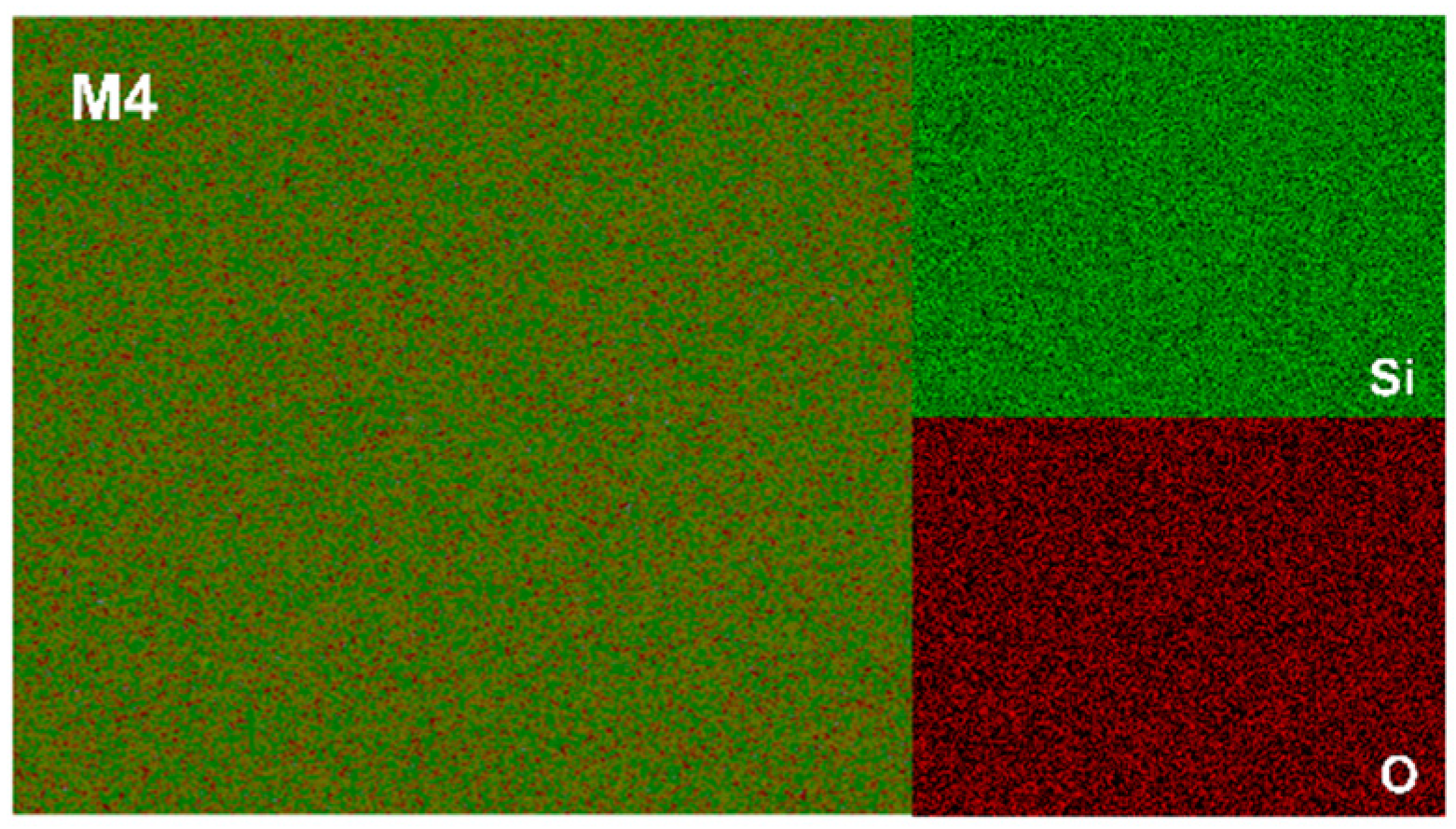
3.2. The XRD Patterns and Microstructures of the Sintered Composites
3.3. Sintering Densification
3.4. Properties of the Sintered Composites
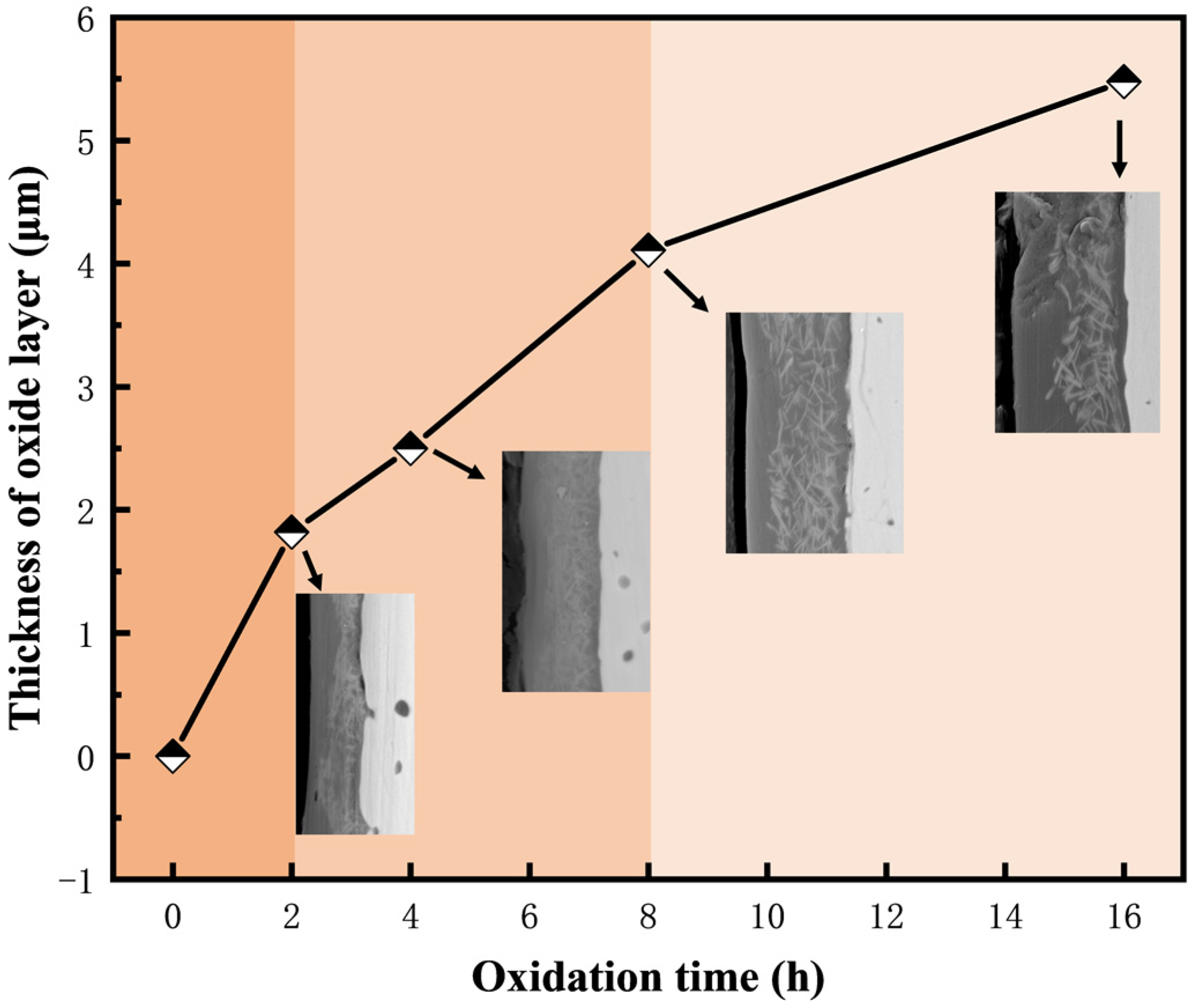
3.5. Oxidation Behavior at 1500 °C
4. Conclusions
- (1)
- In this study, (Mo,Cr)Si2 composites were prepared at 1400 °C using SPS technology. A moderate amount of Cr can be solidly dissolved into the MoSi2 matrix. Simultaneously, a small amount of oxide is distributed as a second phase in the matrix.
- (2)
- The addition of the Cr element has the potential to enhance the mechanical performance of the composites. The (Mo95,Cr5)Si2 samples exhibit the most favorable comprehensive mechanical properties, with a Vickers hardness of 11.6 GPa, a fracture toughness of 4.6 MPa·m1/2, and a flexural strength of 397 MPa.
- (3)
- During the oxidation at 1500 °C, the oxidation products of Cr-added samples produce Cr2O3 alongside SiO2. Nevertheless, the mismatch in thermal expansion coefficients between Cr2O3 and the matrix can cause Cr2O3 to delaminate and separate from the oxide layer, resulting in layer failure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ovalı, D.; Tarraste, M.; Kaba, M.; Ağaoğulları, D.; Kollo, L.; Prashanth, K.; Öveçoğlu, M.L. Spark plasma sintering of molybdenum silicides synthesized from oxide precursors. Ceram. Int. 2021, 47, 13827–13836. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, P.; Yan, Y. Influence of spark plasma sintering temperature on sintering behavior and microstructures of dense bulk MoSi2. J. Alloys Compd. 2008, 459, 163–168. [Google Scholar] [CrossRef]
- Zhu, L.; Ren, X.; Wang, X.; Kang, X.; Zheng, R.; Feng, P. Microstructure and high-temperature oxidation resistance of MoSi2-ZrO2 composite coatings for Niobium substrate. J. Eur. Ceram. Soc. 2021, 41, 1197–1210. [Google Scholar] [CrossRef]
- Knittel, S.; Mathieu, S.; Vilasi, M. Oxidation behaviour of arc-melted and uniaxial hot pressed MoSi2 at 500 °C. Intermetallics 2010, 18, 2267–2274. [Google Scholar] [CrossRef]
- Nie, X.; Lu, Q. Fracture toughness of ZrO2–SiC/MoSi2 composite ceramics prepared by powder metallurgy. Ceram. Int. 2021, 47, 19700–19708. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Li, B. Synthesis of MoSi2/WSi2 nanocrystalline powder by mechanical-assistant combustion synthesis method. Int. J. Refract. Met. Hard Mater. 2010, 28, 217–220. [Google Scholar] [CrossRef]
- Petrovic, J.; Vasudevan, A. Key developments in high temperature structural silicides. Mater. Sci. Eng. A 1999, 261, 1–5. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Zhuang, S.; Chen, Y.; Gu, S. Effect of WSi2 and Si3N4 contents on the thermal expansion behaviors of (Mo,W)Si2-Si3N4 composites. Ceram. Int. 2017, 43, 2847–2852. [Google Scholar] [CrossRef]
- Taleghani, P.; Bakhshi, S.; Erfanmanesh, M.; Borhani, G.; Vafaei, R. Improvement of MoSi2 oxidation resistance via boron addition: Fabrication of MoB/MoSi2 composite by mechanical alloying and subsequent reactive sintering. Powder Technol. 2014, 254, 241–247. [Google Scholar] [CrossRef]
- Patel, M.; Subramanyuam, J.; Prasad, V.B. Synthesis and mechanical properties of nanocrystalline MoSi2–SiC composite. Scr. Mater. 2008, 58, 211–214. [Google Scholar] [CrossRef]
- Chen, F.; Xu, J.; Liu, Y.; Cai, L. In situ reactive spark plasma sintering of WSi2/MoSi2 composites. Ceram. Int. 2016, 42, 11165–11169. [Google Scholar] [CrossRef]
- Wang, J.; Feng, P.; Niu, J.; Guo, R.; Liu, Z.; Akhtar, F. Synthesis, microstructure and properties of MoSi2–5 vol% Al2O3 composites. Ceram. Int. 2014, 40, 16381–16387. [Google Scholar] [CrossRef]
- Yanagihara, K.; Przybylski, K.; Maruyama, T. The role of microstructure on pesting during oxidation of MoSi2 and Mo(Si,Al)2 at 773 K. Oxid. Met. 1997, 47, 277–293. [Google Scholar] [CrossRef]
- Frankwicz, P.; Perepezko, J.; Anton, D. Phase Stability of MoSi2 with Cr Additions. MRS Online Proc. Libr. (OPL) 1992, 288, 159. [Google Scholar] [CrossRef]
- Feng, P.; Liu, W.; Farid, A.; Wu, J.; Niu, J.; Wang, X.; Qiang, Y. Combustion synthesis of (Mo1−xCrx)Si2 (x = 0.00–0.30) alloys in SHS mode. Adv. Powder Technol. 2012, 23, 133–138. [Google Scholar] [CrossRef]
- Yazdani-rad, R.; Mirvakili, S.; Zakeri, M. Synthesis of (Mo1−x–Crx)Si2 nanostructured powders via mechanical alloying and following heat treatment. J. Alloys Compd. 2010, 489, 379–383. [Google Scholar] [CrossRef]
- Ström, E.; Cao, Y.; Yao, Y. Low temperature oxidation of Cr-alloyed MoSi2. Trans. Nonferrous Met. Soc. China 2007, 17, 1282–1286. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, S. Insight into the oxidation mechanism of MoSi2: Ab-initio calculations. Ceram. Int. 2018, 44, 19583–19589. [Google Scholar] [CrossRef]
- Chu, Y.; Fu, Q.; Li, H.; Li, K. Thermal fatigue behavior of C/C composites modified by SiC–MoSi2–CrSi2 coating. J. Alloys Compd. 2011, 509, 8111–8115. [Google Scholar] [CrossRef]
- Peng, K.; Yi, M.; Ran, L.; Ge, Y. Reactive hot pressing of SiC/MoSi2 nanocomposites. J. Am. Ceram. Soc. 2007, 90, 3708–3711. [Google Scholar] [CrossRef]
- Zakeri, M.; Yazdani-Rad, R.; Enayati, M.; Rahimipour, M. Synthesis of nanocrystalline MoSi2 by mechanical alloying. J. Alloys Compd. 2005, 403, 258–261. [Google Scholar] [CrossRef]
- Jo, S.-W.; Lee, G.-W.; Moon, J.-T.; Kim, Y.-S. On the formation of MoSi2 by self-propagating high-temperature synthesis. Acta Mater. 1996, 44, 4317–4326. [Google Scholar] [CrossRef]
- Kermani, M.; Razavi, M.; Rahimipour, M.R.; Zakeri, M. The effect of temperature on the in situ synthesis–sintering and mechanical properties of MoSi2 prepared by spark plasma sintering. J. Alloys Compd. 2014, 585, 229–233. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Cabouro, G.; Chevalier, S.; Gaffet, E.; Grin, Y.; Bernard, F. Reactive sintering of molybdenum disilicide by spark plasma sintering from mechanically activated powder mixtures: Processing parameters and properties. J. Alloys Compd. 2008, 465, 344–355. [Google Scholar] [CrossRef]
- Ding, Z.; Brouwer, J.C.; Zhu, J.-N.; Popovich, V.; Hermans, M.J.; Sloof, W.G. Effects of boron addition on the high temperature oxidation of MoSi2 alloys. Scr. Mater. 2023, 234, 115580. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Zhang, H. Effect of Si3N4 content on microstructures and antioxidant properties of MoSi2/Si3N4 composite coatings on Mo substrate. Ceram. Int. 2015, 41, 13903–13907. [Google Scholar] [CrossRef]
- Dasgupta, T.; Etourneau, J.; Chevalier, B.; Matar, S.F.; Umarji, A. Structural, thermal, and electrical properties of CrSi2. J. Appl. Phys. 2008, 103, 11. [Google Scholar] [CrossRef]
- Khalil, M.; Beaudhuin, M.; Villeroy, B.; Ravot, D.; Viennois, R. A modeling approach for new CrSi2 based alloys: Application to metastable Cr1-xZrxSi2 as a potential thermoelectric material. J. Alloys Compd. 2016, 662, 150–156. [Google Scholar] [CrossRef]
- Pan, Y.; Pu, D.; Yu, E. Structural, electronic, mechanical and thermodynamic properties of Cr–Si binary silicides from first-principles investigations. Vacuum 2021, 185, 110024. [Google Scholar] [CrossRef]
- Mitra, R.; Khanna, R.; Rao, V.R. Microstructure, mechanical properties and oxidation behavior of a multiphase (Mo,Cr)(Si,Al)2 intermetallic alloy–SiC composite processed by reaction hot pressing. Mater. Sci. Eng. A 2004, 382, 150–161. [Google Scholar] [CrossRef]
- Pan, Y. Structural prediction and overall performances of CrSi2 disilicides: DFT investigations. ACS Sustain. Chem. Eng. 2020, 8, 11024–11030. [Google Scholar] [CrossRef]
- Sun, G.-D.; Zhang, G.-H.; Ji, X.-P.; Liu, J.-K.; Zhang, H.; Chou, K.-C. Size-controlled synthesis of nano Mo powders via reduction of commercial MoO3 with carbon black and hydrogen. Int. J. Refract. Met. Hard Mater. 2019, 80, 11–22. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, G.; Li, W.; Zhuang, H.; Zhang, B.; Chen, L. In situ synthesis of SiCW/MoSi2 composite through SPS process. J. Alloys Compd. 2008, 462, 170–174. [Google Scholar] [CrossRef]
- Ren, B.; Lu, D.-H.; Zhou, R.; Ji, D.-P.; Hu, M.-Y.; Feng, J. First principles study of stability, mechanical, and electronic properties of chromium silicides. Chin. Phys. B 2018, 27, 107102. [Google Scholar] [CrossRef]
- Anstis, G.; Chantikul, P.; Lawn, B.R.; Marshall, D. A critical evaluation of indentation techniques for measuring fracture toughness: I, direct crack measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Han, X.-x.; Wang, Y.-l.; Xiong, X.; Heng, L.; Chen, Z.-k.; Wei, S. Microstructure, sintering behavior and mechanical properties of SiC/MoSi2 composites by spark plasma sintering. Trans. Nonferrous Met. Soc. China 2018, 28, 957–965. [Google Scholar] [CrossRef]
- Shimizu, H.; Yoshinaka, M.; Hirota, K.; Yamaguchi, O. Fabrication and mechanical properties of monolithic MoSi2 by spark plasma sintering. Mater. Res. Bull. 2002, 37, 1557–1563. [Google Scholar] [CrossRef]
- Kermani, M.; Razavi, M.; Rahimipour, M.R.; Zakeri, M. The effect of mechanical alloying on microstructure and mechanical properties of MoSi2 prepared by spark plasma sintering. J. Alloys Compd. 2014, 593, 242–249. [Google Scholar] [CrossRef]
- Huang, J.-B.; Zhang, G.-H.; Yang, X.-H. Effect of WSi2 content on oxidation behavior of MoSi2-20 vol% Al2O3 composites. Powder Technol. 2023, 430, 119012. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Wang, H.; Kumar, V. Coarsening, densification, and grain growth during sintering of nano-sized powders—A perspective. Int. J. Refract. Met. Hard Mater. 2017, 62, 110–117. [Google Scholar] [CrossRef]
- Harada, Y.; Murata, Y.; Morinaga, M. Solid solution softening and hardening in alloyed MoSi2. Intermetallics 1998, 6, 529–535. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Luo, J.; Wang, S.; Liang, B.; Chen, W. Microstructure, properties and toughening mechanisms of MoSi2@ZrO2 core shell composites prepared by spark plasma sintering. Mater. Charact. 2023, 195, 112510. [Google Scholar] [CrossRef]
- He, R.; Tong, Z.; Zhang, K.; Fang, D. Mechanical and electrical properties of MoSi2-based ceramics with various ZrB2-20 vol% SiC as additives for ultra high temperature heating element. Ceram. Int. 2018, 44, 1041–1045. [Google Scholar] [CrossRef]
- Nishida, I.; Sakata, T. Semiconducting properties of pure and Mn-doped chromium disilicides. J. Phys. Chem. Solids 1978, 39, 499–505. [Google Scholar] [CrossRef]
- Lu, Z.; Peng, C.; Cai, Z.-m.; Feng, P.-z.; Kang, X.-q.; Akhtar, F.; Wang, X.-h. Fabrication of MoSi2 coatings on molybdenum and its high-temperature anti-oxidation properties. Trans. Nonferrous Met. Soc. China 2022, 32, 935–946. [Google Scholar]
- Liu, L.; Zhang, L.; Liu, D. Complete elimination of pest oxidation by high entropy refractory metallic silicide (Mo0.2W0.2Cr0.2Ta0.2Nb0.2)Si2. Scr. Mater. 2020, 189, 25–29. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Liu, L.; Wang, Y. Oxidation and interdiffusion behavior of Niobium substrate coated MoSi2 coating prepared by spark plasma sintering. Appl. Surf. Sci. 2014, 320, 791–797. [Google Scholar] [CrossRef]
- Yan-Hui, C.; Qian-Gang, F.; Cui-Wei, C.; He-Jun, L.; Ke-Zhi, L.; Qin, L. SiC nanowire-toughened SiC–MoSi2–CrSi2 oxidation protective coating for carbon/carbon composites. Surf. Coat. Technol. 2010, 205, 413–418. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Ling, Y.; Wang, R.; Zhou, Q.; Wang, J.; Li, Y.; Zhang, Z. Preparation of Cr2O3/Al2O3 bipolar oxides as hydrogen permeation barriers by selective oxide removal on SS and atomic layer deposition. Int. J. Hydrog. Energy 2019, 44, 12277–12287. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, S.; Ye, F.; Ren, X.; Feng, P. Recycling of MoSi2-based industrial solid wastes for the fabrication and high-temperature oxidation behavior of MoSi2–ZrSi2–SiC composite coating. Compos. Part B Eng. 2024, 274, 111281. [Google Scholar] [CrossRef]
- Li, H.-J.; Feng, T.; Fu, Q.-G.; Wu, H.; Shen, X.-T. Oxidation and erosion resistance of MoSi2–CrSi2–Si/SiC coated C/C composites in static and aerodynamic oxidation environment. Carbon 2010, 48, 1636–1642. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, H.-J.; Fu, Q.-G.; Yao, X.-Y.; Li, K.-Z.; Jiao, G.-S. An oxidation protective Si-Mo-Cr coating for C/SiC coated carbon/carbon composites. Carbon 2008, 46, 179–182. [Google Scholar] [CrossRef]
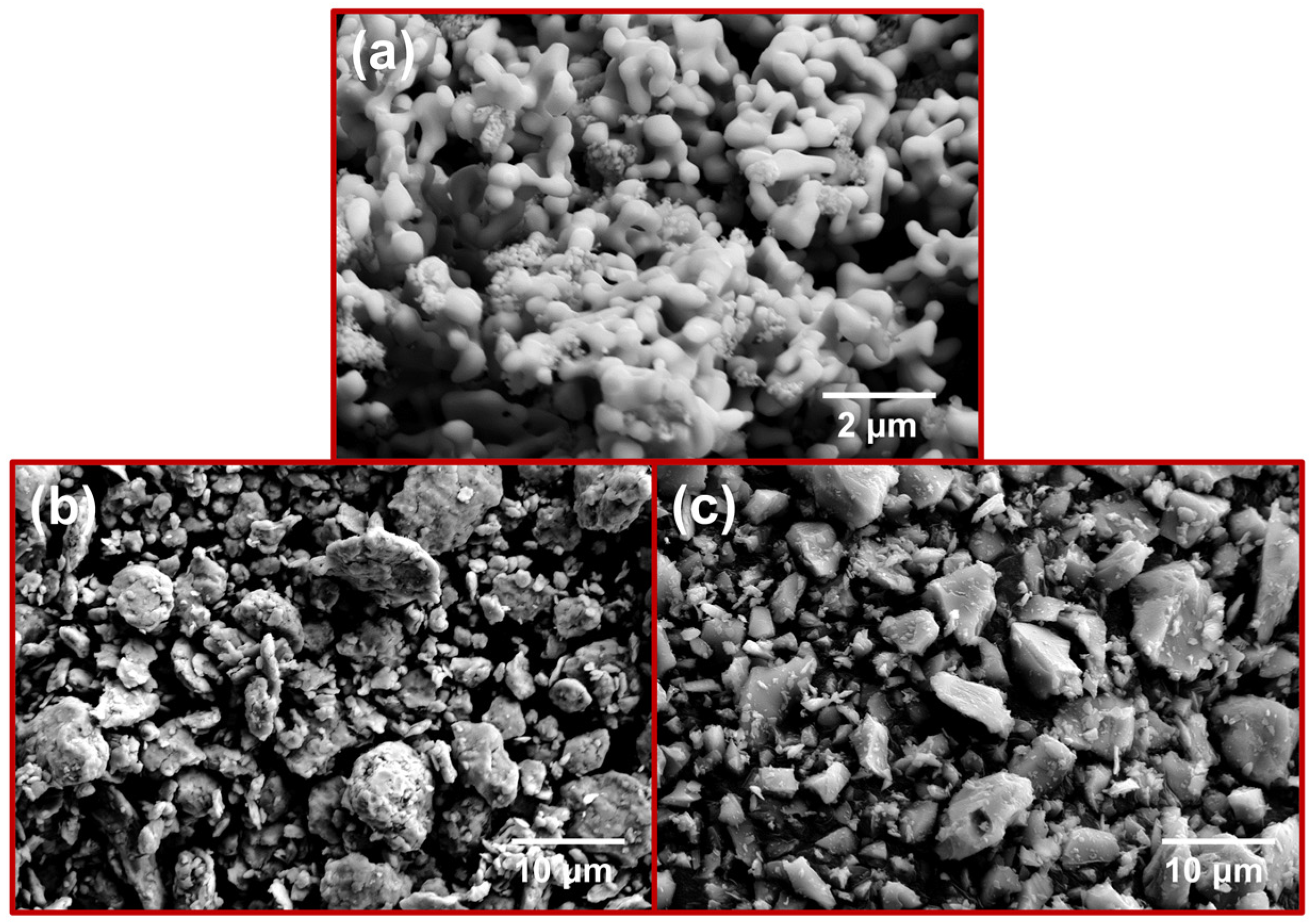
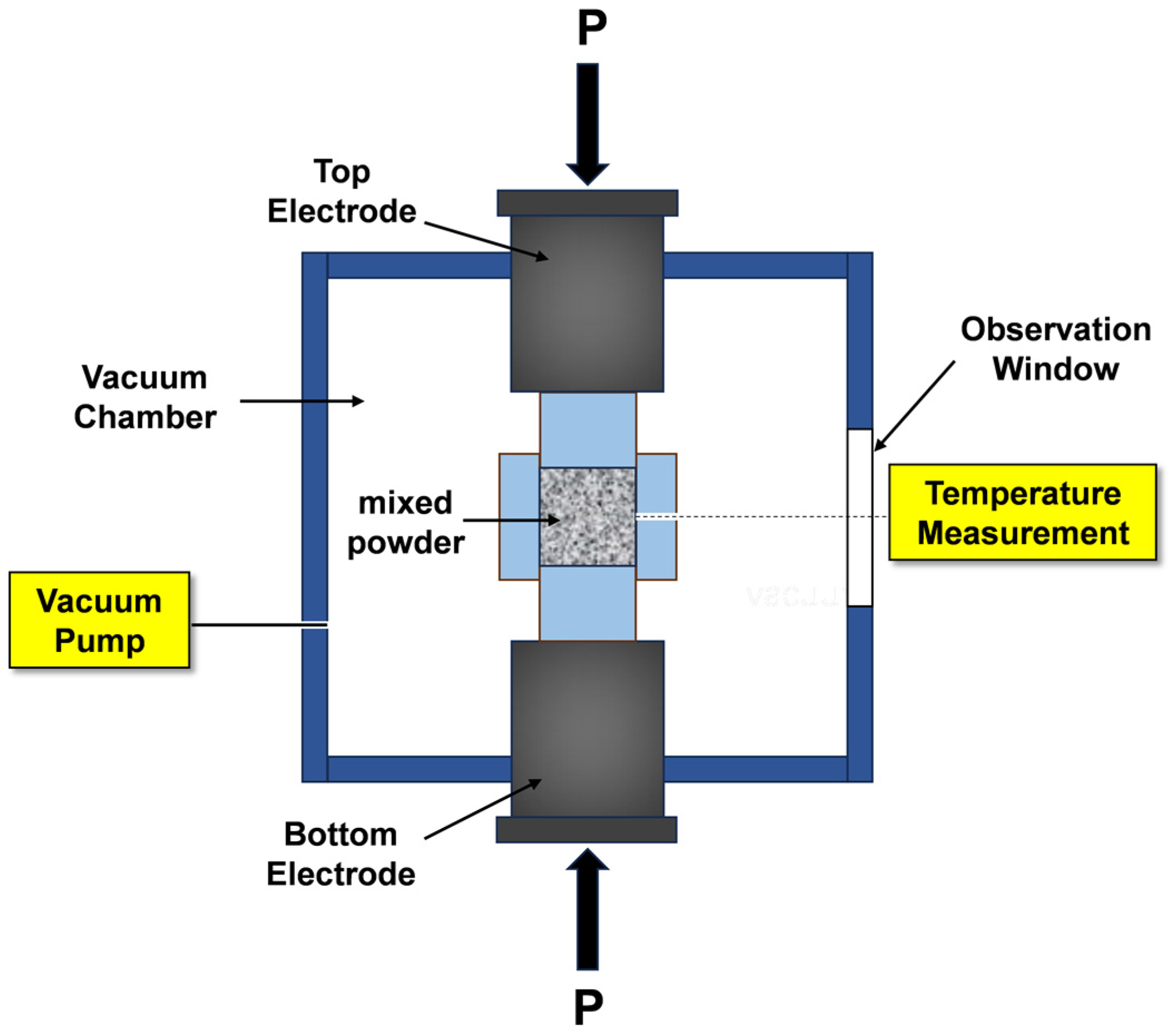
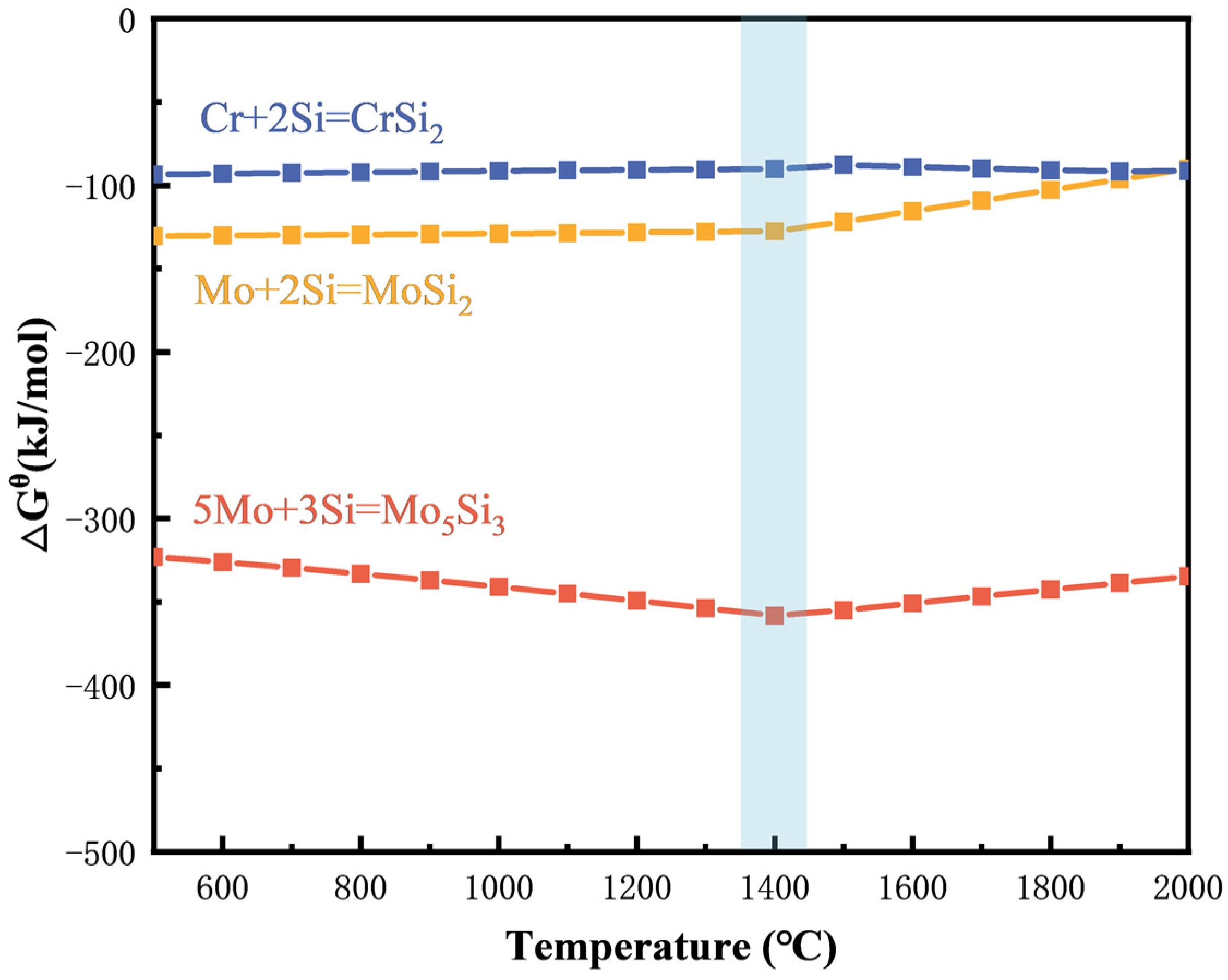
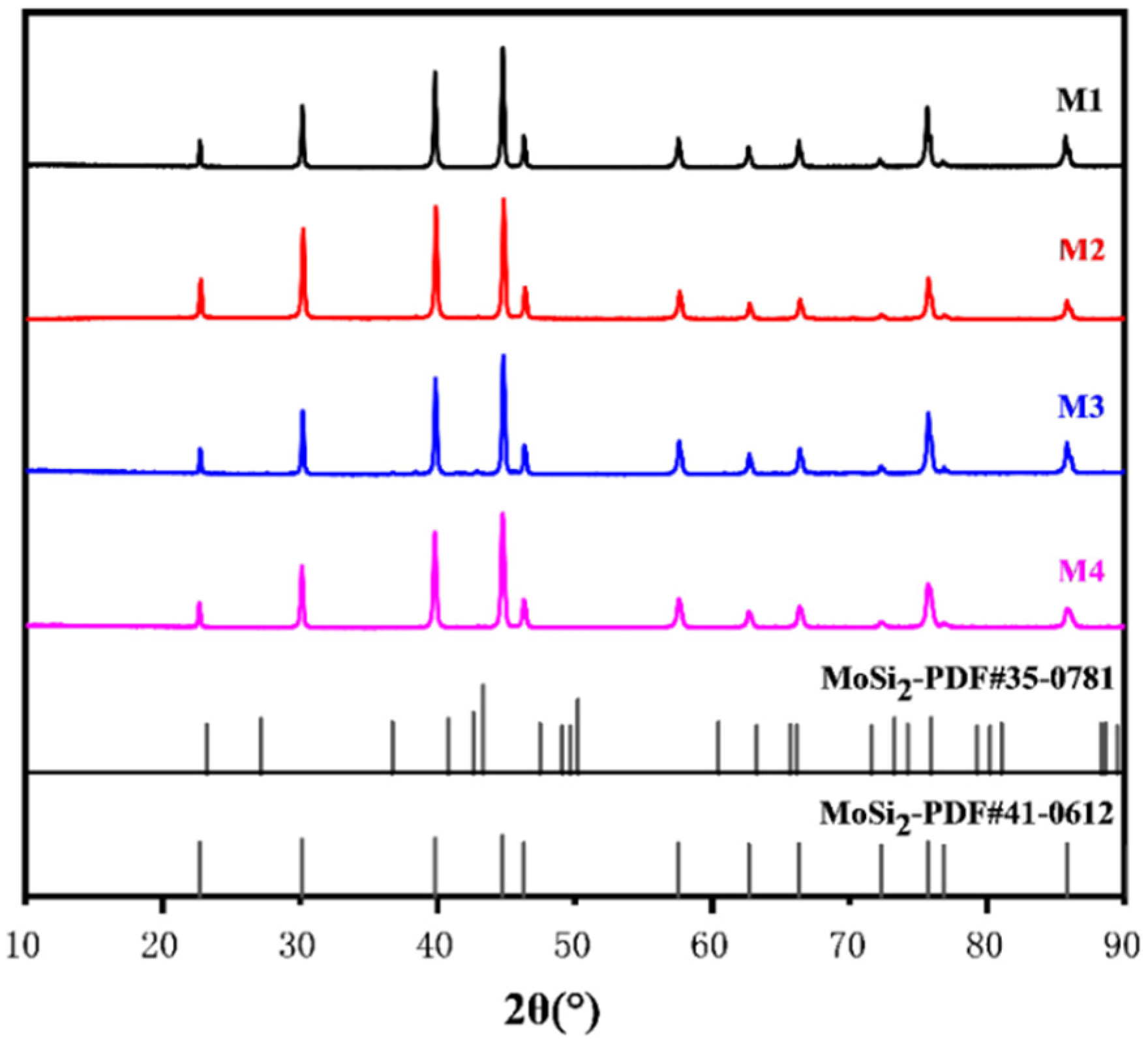

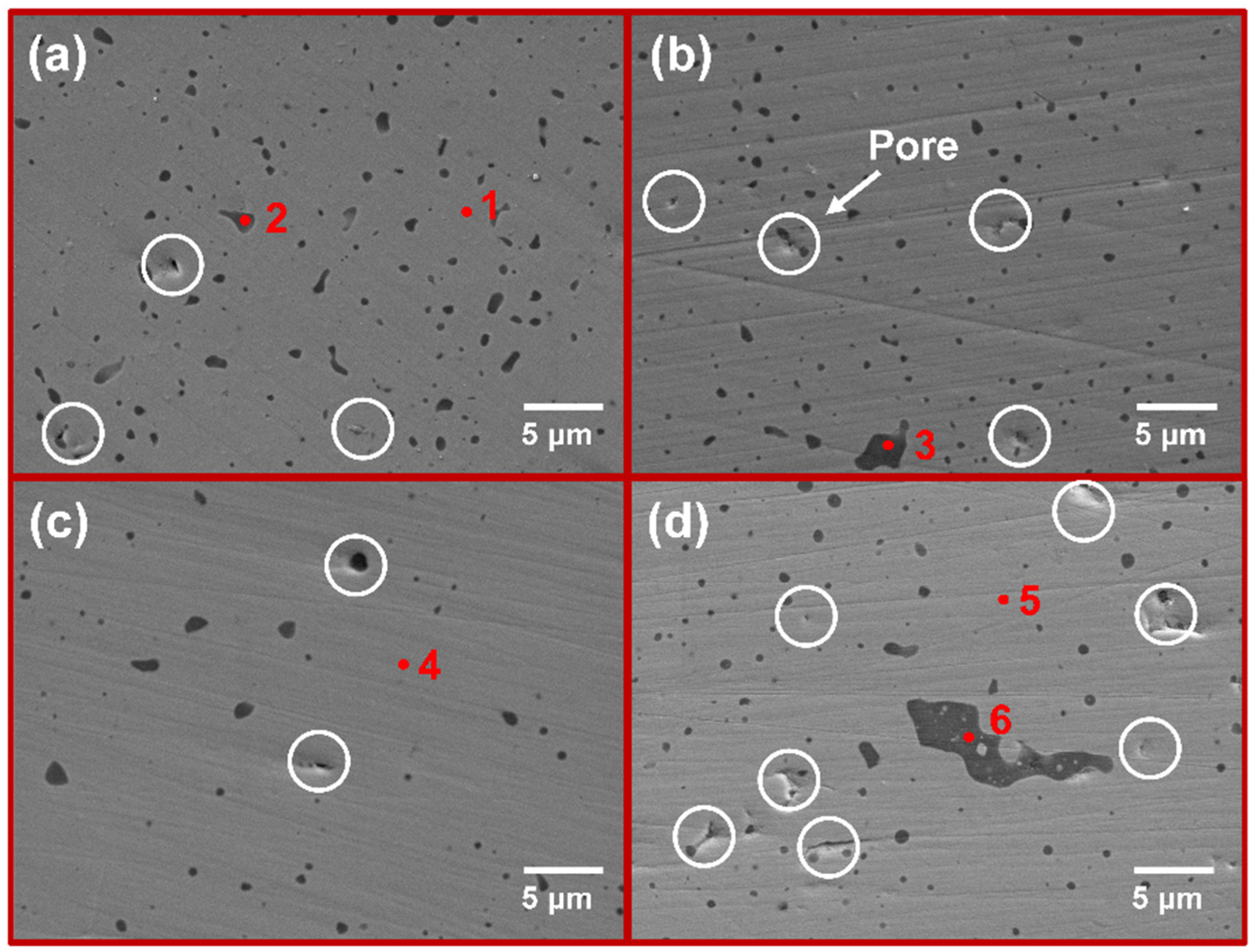
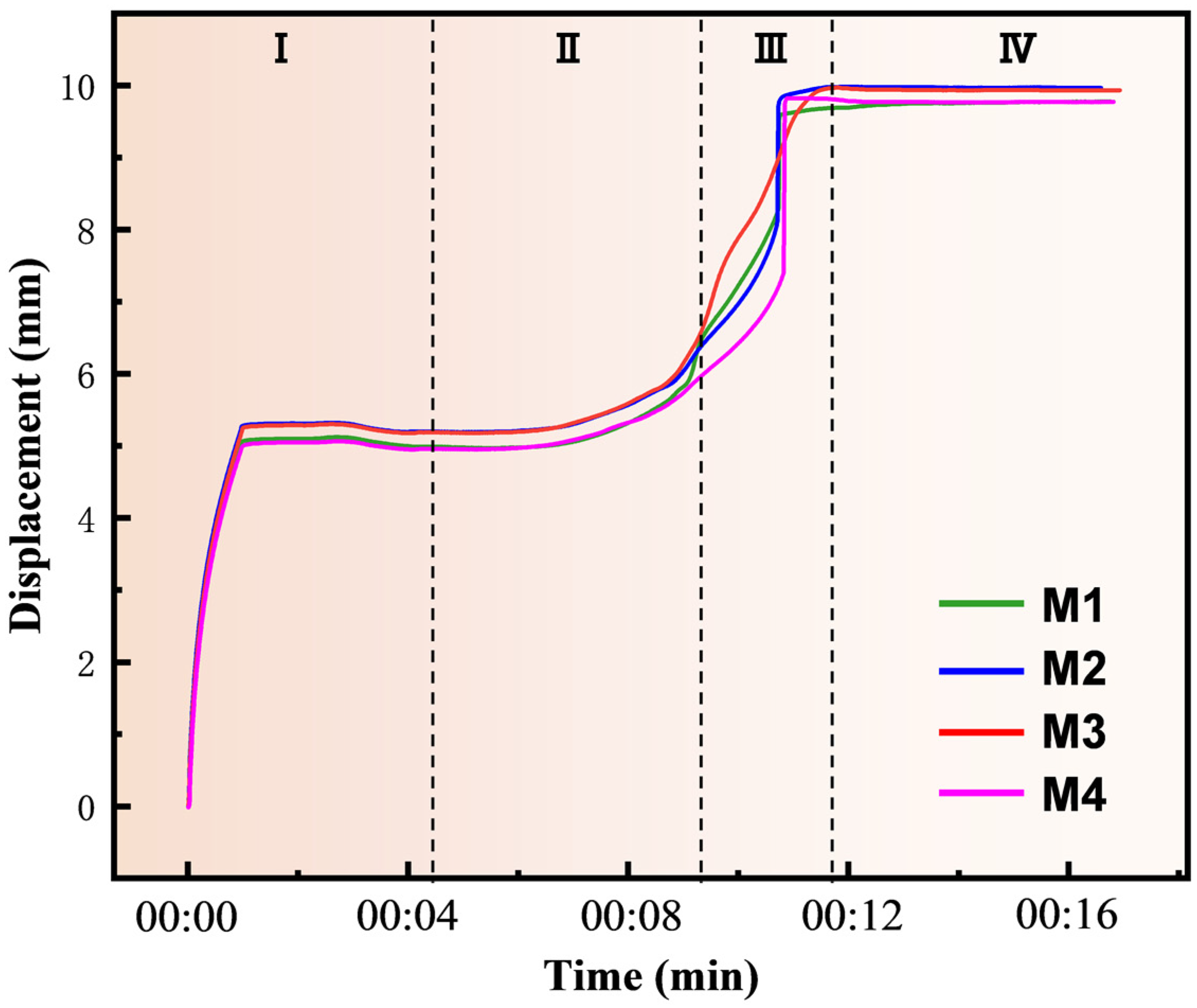
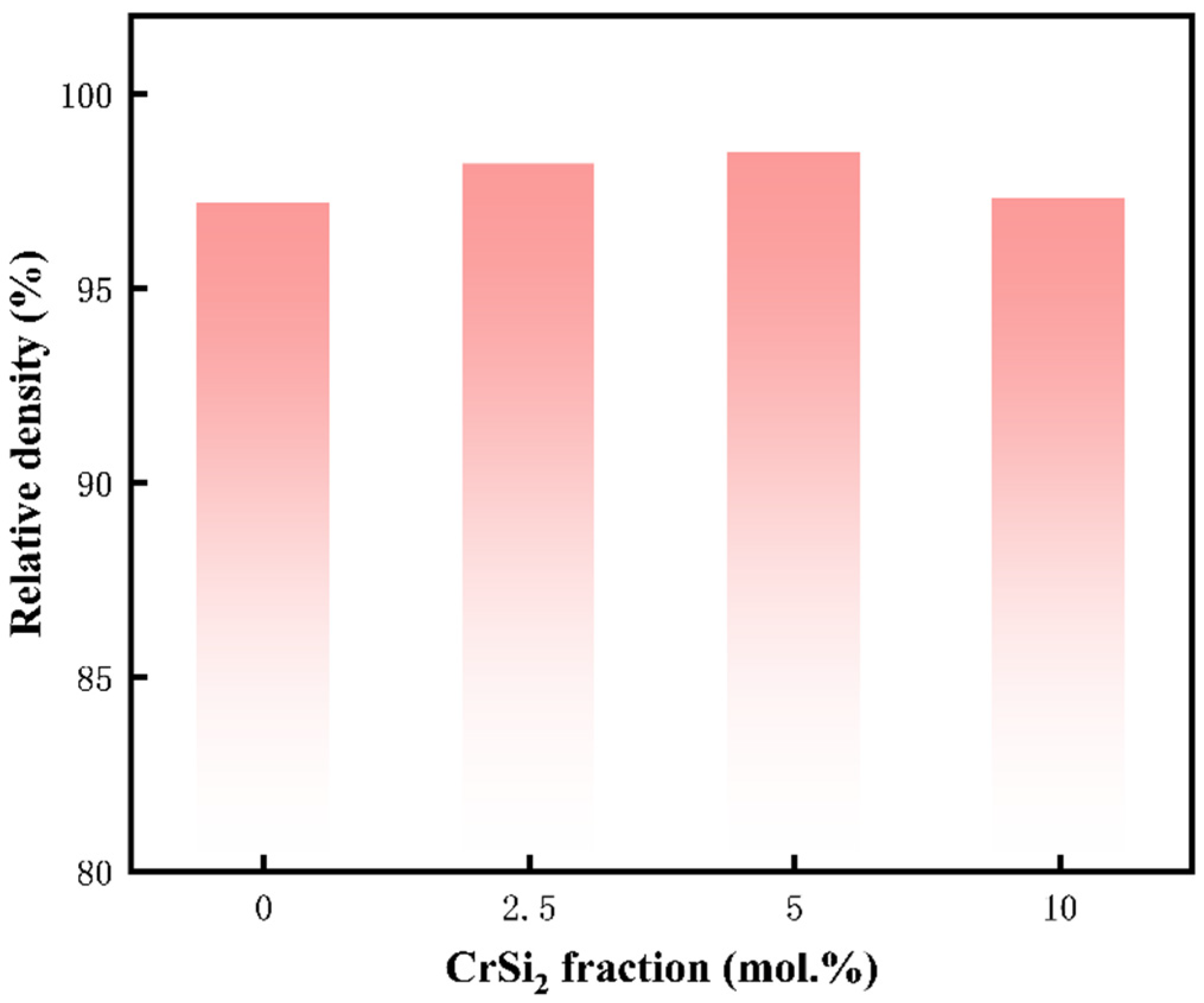
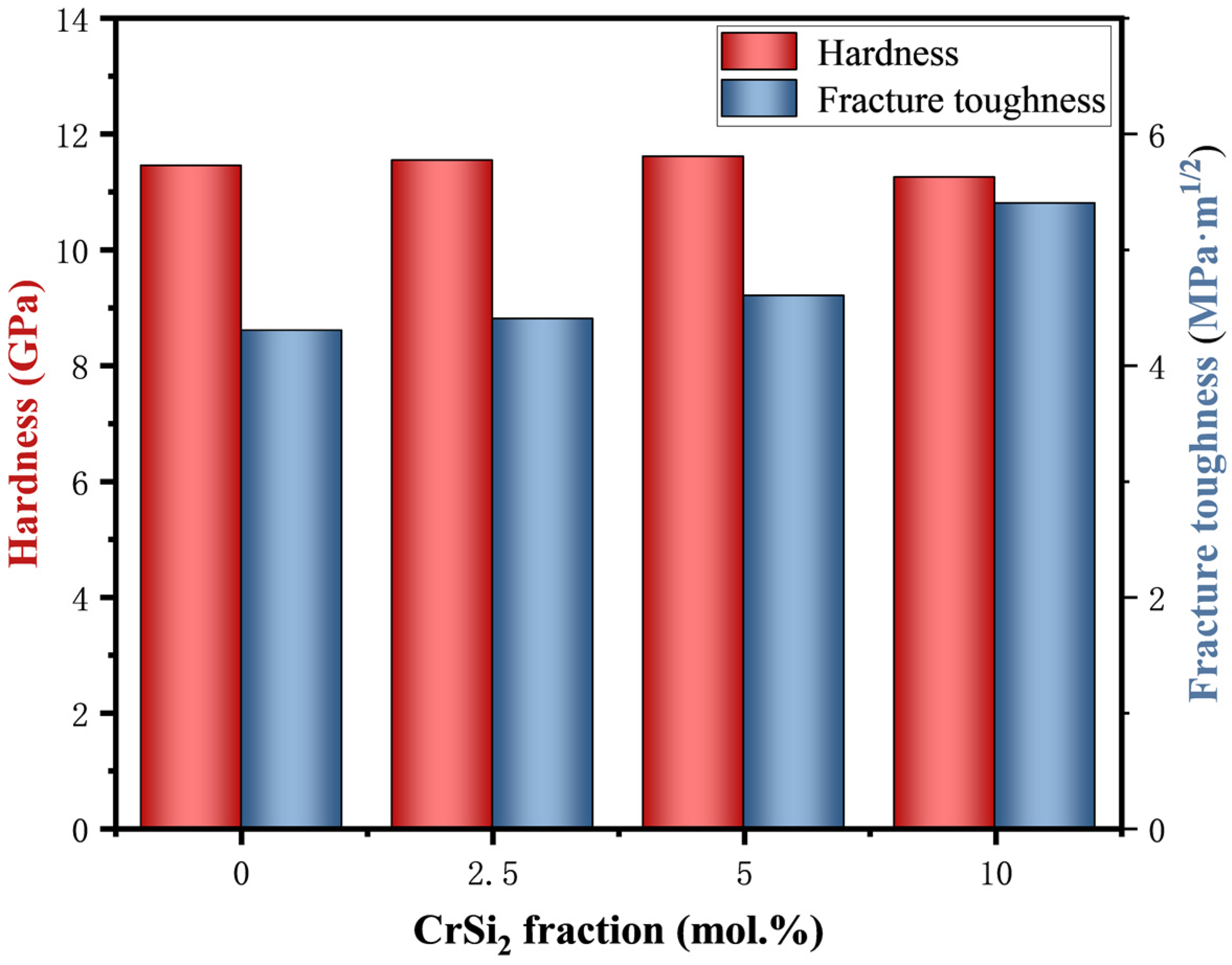





| Sample Number | MoSi2/mol. % | CrSi2/mol. % |
|---|---|---|
| M1 | 100 | 0 |
| M2 | 97.5 | 2.5 |
| M3 | 95 | 5 |
| M4 | 90 | 10 |
| Point | Element (at. %) | |||
|---|---|---|---|---|
| Mo | Si | O | Cr | |
| 1 | 32.4 | 67.6 | ||
| 2 | 7.0 | 39.9 | 53.2 | |
| 3 | 39.0 | 61.0 | ||
| 4 | 32.1 | 67.1 | 0.9 | |
| 5 | 29.9 | 67.7 | 2.3 | |
| 6 | 41.0 | 58.3 | 0.6 | |
| Point | Element (wt. %) | |||
|---|---|---|---|---|
| Mo | Si | O | Cr | |
| 1 | 60.5 | 39.5 | ||
| 2 | 33.0 | 40.1 | 26.9 | |
| 3 | 39.4 | 40.5 | 19.5 | 0.6 |
| 4 | 23.2 | 57.3 | 17.1 | 2.4 |
| 5 | 28.0 | 47.0 | 24.1 | 0.9 |
| Sample Number | Electrical Resistivity (µΩ·cm) |
|---|---|
| M1 | 16.9 |
| M2 | 18.7 |
| M3 | 21.5 |
| M4 | 24.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Y.; Zhang, G.-H. In Situ Synthesis of (Mo,Cr)Si2 Composites by Spark Plasma Sintering. Materials 2024, 17, 4105. https://doi.org/10.3390/ma17164105
Wang Y-Y, Zhang G-H. In Situ Synthesis of (Mo,Cr)Si2 Composites by Spark Plasma Sintering. Materials. 2024; 17(16):4105. https://doi.org/10.3390/ma17164105
Chicago/Turabian StyleWang, Yue-Yao, and Guo-Hua Zhang. 2024. "In Situ Synthesis of (Mo,Cr)Si2 Composites by Spark Plasma Sintering" Materials 17, no. 16: 4105. https://doi.org/10.3390/ma17164105





