Effect of Scaffold Geometrical Structure on Macrophage Polarization during Bone Regeneration Using Honeycomb Tricalcium Phosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Honeycomb TCP
2.2. Animals and Implantation Procedure
2.3. Histological Procedure
2.4. Immunohistochemical (IHC) Staining
3. Results
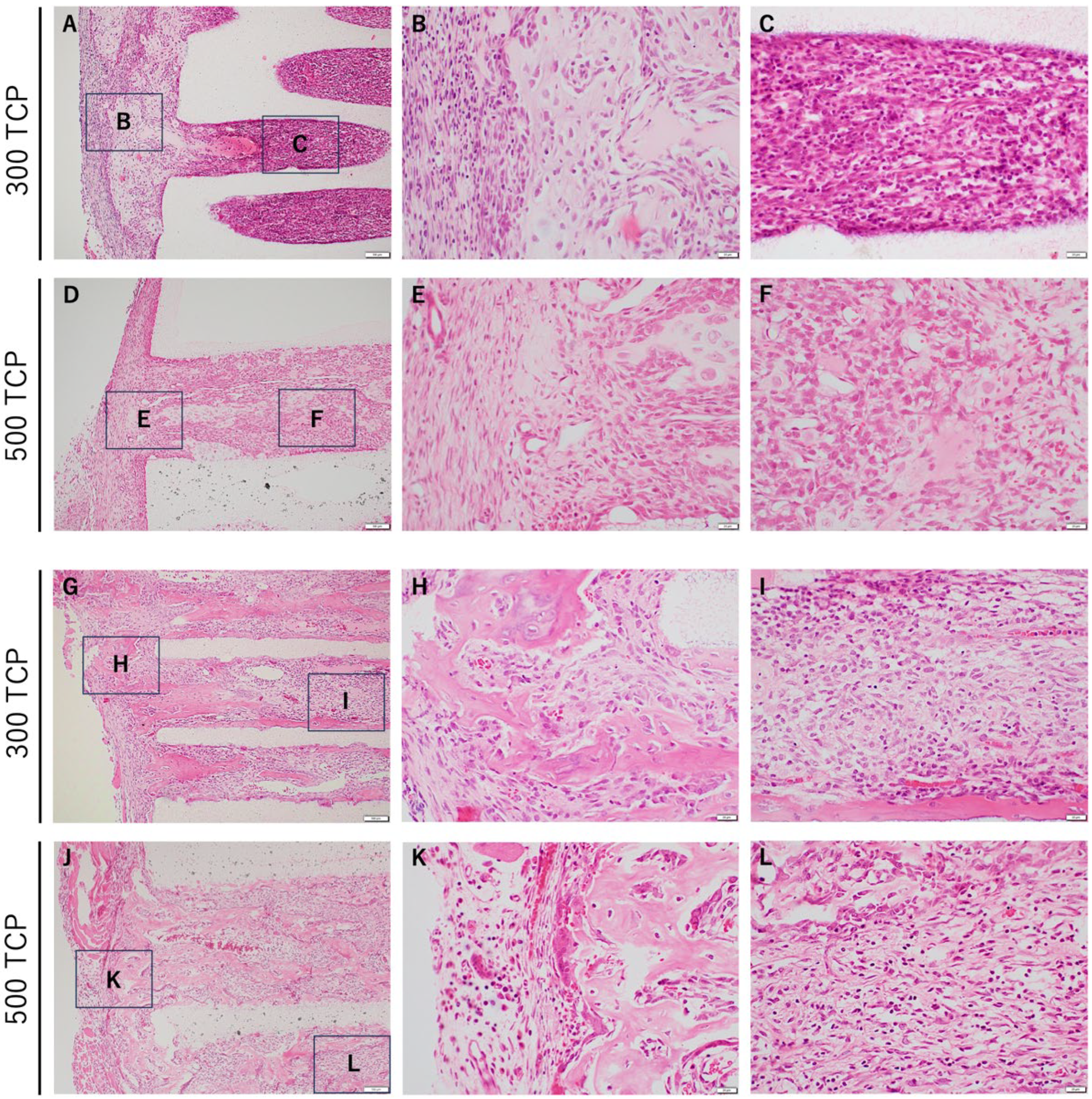
3.1. Comparison of HE Staining between 300 TCP and 500 TCP
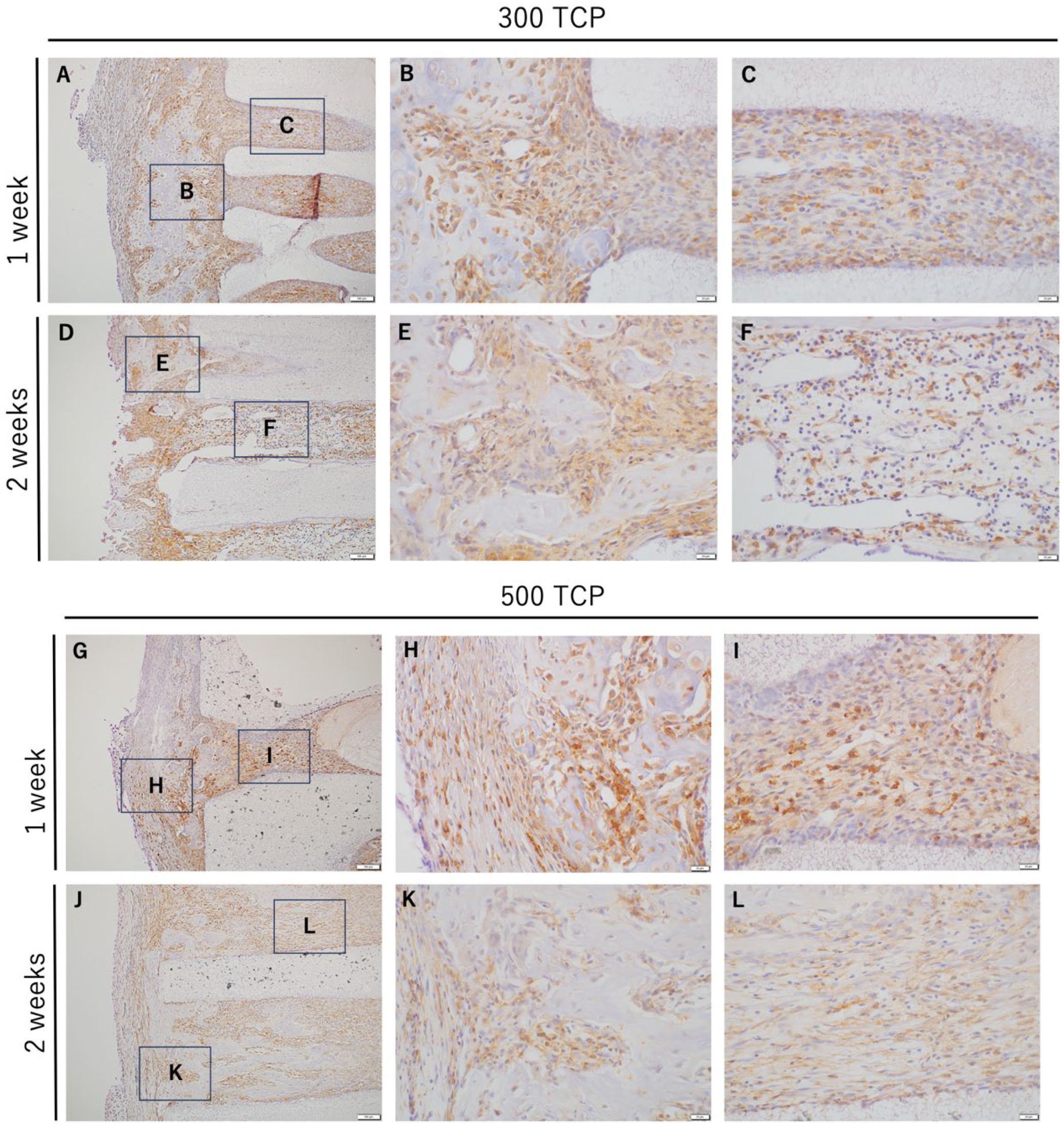
3.2. Immunohistochemistry Analysis of M0 Macrophage Induced by 300 TCP and 500 TCP
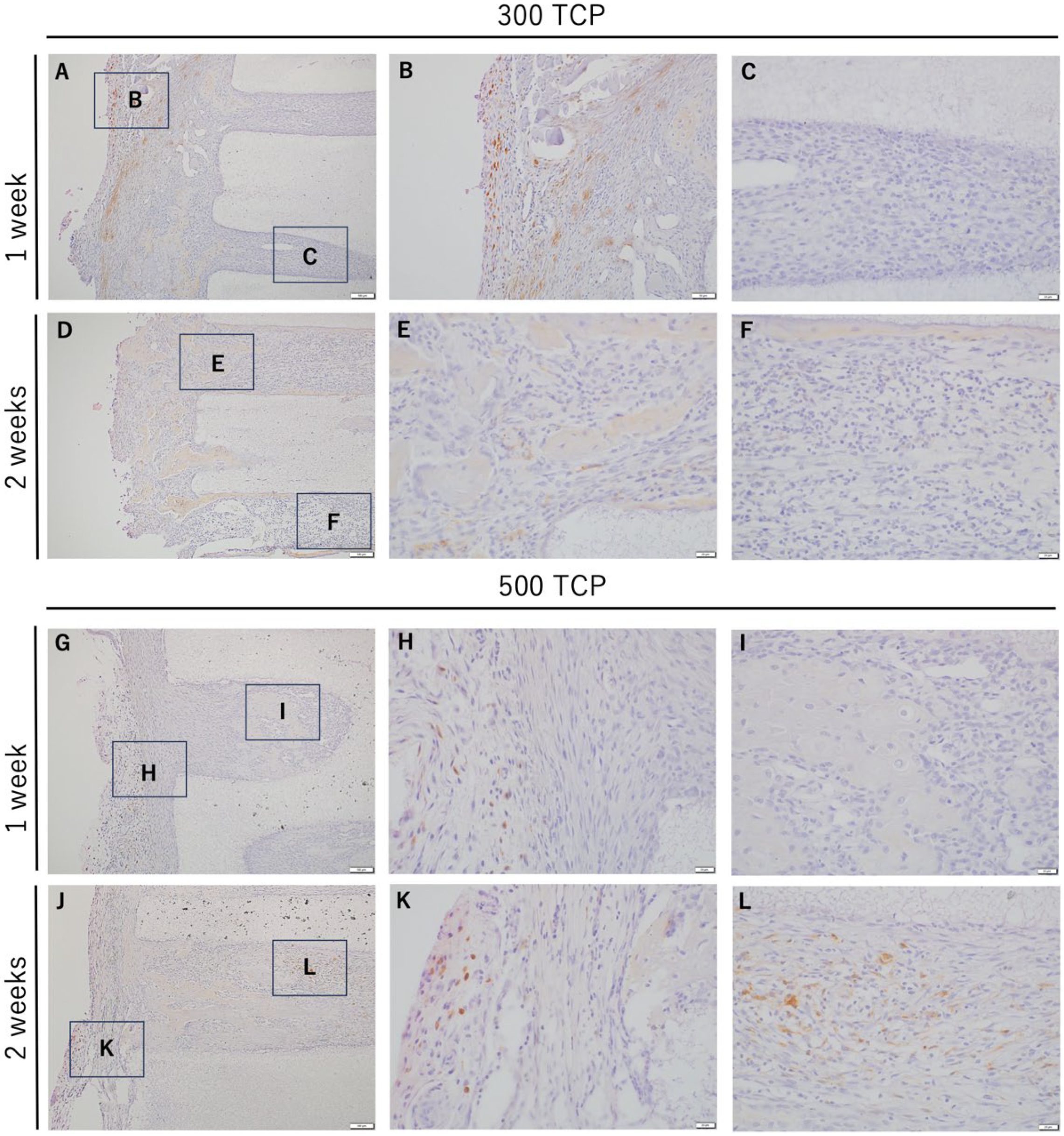
3.3. Immunohistochemistry Analysis of M1 Macrophages Induced by 300 TCP and 500 TCP
3.4. Immunohistochemistry Analysis of M2 Macrophages Induced by 300 TCP and 500 TCP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tommaso, G.; Luigi, S.; Giovanni, G. Narrow implants (2.75 and 3.25 mm diameter) supporting a fixed splinted prostheses in posterior regions of mandible: One-year results from a prospective cohort study. Int. J. Implant Dent. 2017, 3, 43. [Google Scholar]
- Zeeshan, S.; Nader, H.; Yuichi, I.; Marc, G.; Bernhard, G.; Michael, G. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar]
- Toshiyuki, K.; Eiji, N.; Tomohiro, F.; Toshiaki, H.; Kohei, S.; Haruyoshi, K.; Ayana, K.; Toshifumi, O. Clinical application of unidirectional porous hydroxyapatite to bone tumor surgery and other orthopedic surgery. Biomimetics 2024, 9, 294. [Google Scholar] [CrossRef]
- Lauren, A.B.; Stefanie, M.S.; David, C.F.; Sun, H.P.; Jonathan, G.S.; Craig, D.; Joseph, C.W.; Scott, A.G. Effect of nanocrystalline hydroxyapatite concentration and skeletal site on bone and cartilage formation in rats. Acta Biomater. 2021, 130, 485–496. [Google Scholar]
- Jian-Hua, Z.; Shi-Wei, L.; Long, X.; Peng, Q.; Ling-Hua, D.; Shi-Lang, X.; Jing-Tang, L.; Xin-Gen, L.; Zhi-Ming, T. Scaffolds for the repair of bone defects in clinical studies: A systematic review. J. Orthop. Surg. Res. 2018, 13, 33. [Google Scholar]
- Qiao, Z.; Dai, J.; Yufeng, Z.; Richard, J.M. Histomorphometric Study of New Bone Formation Comparing Defect Healing with Three Bone Grafting Materials: The Effect of Osteoporosis on Graft Consolidation. Int. J. Oral Maxilofac. Implant. 2018, 33, 645–652. [Google Scholar]
- Lara, S.; Tim, F.; Maximilian, F.G.; Anja, S.K.; Alexandra, C.M.; Julian, L.; Henrik, H.; Daniel, R. Influence of different carrier materials on biphasic calcium phosphate induced bone regeneration. Clin. Oral Investig. 2021, 25, 3729–3737. [Google Scholar]
- Cai, Y.; Tan, X.; Zhao, L.; Zhang, R.; Zhu, T.; Du, Y.; Wang, X. Withdrawal: Synthesis of a Novel bFGF/nHAP/COL Bone Tissue Engineering Scaffold for Mandibular Defect Regeneration in a Rabbit Model. J. Hard Tissue Biol. 2022, 31, 61. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Jt. Surg. Am. 2001, 83A, S105–S115. [Google Scholar] [CrossRef]
- Jin, Q.M.; Takita, H.; Kohgo, T.; Itoh, H.; Kuboki, Y. Effect of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 51, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hongpu, W.; Jinjie, C.; Kaili, L.; Jing, X.; Xudong, W. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022, 10, 17. [Google Scholar]
- Hoe-Jin, K.; Preeti, M.; Andrew, R.P.; Gun-Hee, L.; Soo-Bin, I.; Byong-Taek, L. Comparative study on biodegradation and biocompatibility of multichannel calcium phosphate-based bone substitutes. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110694. [Google Scholar]
- Ya-Meng, Y.; Yu-Pu, L.; Ting, Z.; Yu-Feng, Z.; Yun-Song, L.; Dan-Dan, X. Biomaterials science and surface engineering strategies for dental peri-implantitis management. Mil. Med. Res. 2024, 11, 29. [Google Scholar]
- Kiyofumi, T.; Eiki, Y.; Hidetsugu, T.; Yasushi, T.; Mariko, K.; Shin, T.; Hitoshi, N. Effect of geometry of microstructure of honeycomb TCP scaffolds on bone regeneration. J. Biomed. Mater. Res. A 2013, 102, 2952–2960. [Google Scholar]
- Hiroyuki, M.; Kiyofumi, T.; Hidetsugu, T.; Satoko, W.; Satoshi, I.; Hotaka, K.; Mei, H.; Saori, Y.; Keisuke, N.; Hitoshi, N. Effects of the Geometrical Structure of a Honeycomb TCP on Relationship between Bone/Cartilage Formation and Angiogenesis. Int. J. Med. Sci. 2018, 15, 1582–1590. [Google Scholar]
- Satoko, W.; Kiyofumi, T.; Hidetsugu, T.; Toshiyuki, W.; Eijiro, T.; Satoshi, I.; Hitoshi, N.; Yoshihiro, K. Efficacy of Honeycomb TCP-induced Microenvironment on Bone Tissue Regeneration in Craniofacial Area. Int. J. Med. Sci. 2016, 13, 466–476. [Google Scholar]
- Ryoko, N.; Satoko, W.; Kiyofumi, T.; Hidetsugu, T.; Toshiyuki, W.; Hiroshi, M.; Yoshihiko, K. Long-Term Effect of Honeycomb β-Tricalcium Phosphate on Zygomatic Bone Regeneration in Rats. Materials 2020, 13, 5374. [Google Scholar] [CrossRef] [PubMed]
- Vi, L.; Baht, G.S.; Soderblom, E.J. Macrophage cells secrete factors including LRP1 that orchestrate the rejuvenation of bone repair in mice. Nat. Commun. 2018, 9, 5191. [Google Scholar] [CrossRef]
- Siamon, G. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar]
- Osamu, T.; Shizuo, A. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar]
- Claudia, S.; Thaqif, E.l.K.; Alessandro, S.; Anke, D.; Sebastian, W.; Hanna, S.; van Rooijen, N.; Andreas, R.; Richard, L.; Susanne, H.; et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018, 106, 78–89. [Google Scholar]
- Jian, M.H.; Kai, L.; Ji, H.L.; Xian, L.J.; Xue, L.W.; Yun, Z.C.; Shu, G.L.; Hong, Z.; Li, J.P.; Chun, X.L.; et al. CD163 as a marker of M2 macrophage, contribute to predicte aggressiveness and prognosis of Kazakh esophageal squamous cell carcinoma. Oncotarget 2017, 8, 21526–21538. [Google Scholar]
- Tiffany, D.R.; Nestor, P.D.; Pramod, S.G.; Eric, U. Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar]
- Hu, X.; Leak, R.K.; Shi, Y.; Suenaga, J.; Gao, Y.; Zheng, P.; Chen, J. Microglial and macrophage polarization—New prospects for brain repair. Nat. Rev. Neurol. 2015, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nadella, V.; Ranjan, R.; Senthilkumaran, B.; Qadri, S.S.Y.H.; Pothani, S.; Singh, A.K.; Gupta, M.L.; Prakash, H. Podophyllotoxin and Rutin Modulate M1 (iNOS+) Macrophages and Mitigate Lethal Radiation (LR) Induced Inflammatory Responses in Mice. Front. Immunol. 2019, 10, 106. [Google Scholar] [CrossRef]
- Anders, E.; Soren, K.M. CD163 and inflammation: Biological, diagnostic, and therapeutic aspects. Antioxid. Redox Signal 2013, 18, 2352–2363. [Google Scholar]
- Yumi, K.; Yuko, I.; Akiko, I.; Mizuho, N.; Jumpei, M.; Haruki, Y.; Ayumi, K.; Akihiko, K.; Fukumi, F.; Toshikazu, K. Macrophage polarity and wound age determination. Sci. Rep. 2022, 12, 20327. [Google Scholar]
- Marcelo, S.M.; Claudia, C.B.; Raquel, B.P.S.; Ana, C.R.S.; Ana, C.Z.B.; Jordan, L.S.; Maira, C.R.C.; Marco Antônio, H.D.; Joel, F.S.J.; Paulo, S.B.; et al. Inflammatory response and macrophage polarization using different physicochemical biomaterials for oral and maxillofacial reconstruction. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110229. [Google Scholar]
- Richard, J.M.; Dieter, D.B. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2018, 24, 53–65. [Google Scholar]
- Wang, Y.H.; Zhao, C.Z.; Wang, R.Y.; Du, Q.X.; Liu, J.Y.; Pan, J. The crosstalk between macrophages and bone marrow mesenchymal stem cells in bone healing. Stem Cell Res. Ther. 2022, 13, 511. [Google Scholar] [CrossRef] [PubMed]
- Xuening, C.; Menglu, W.; Fuying, C.; Jing, W.; Xiangfeng, L.; Jie, L.; Yujiang, F.; Yumei, X.; Xingdong, Z. Correlations between macrophage polarization and osteoinduction of porous calcium phosphate ceramics. Acta Biomater. 2020, 103, 318–332. [Google Scholar]
- Xiaoshi, J.; Hudi, X.; Richard, J.M.; Chengcheng, Y.; Xiaoxin, Z.; Min, W.; Yufeng, Z. EZH1 Is Associated with TCP-Induced Bone Regeneration through Macrophage Polarization. Stem Cell Int. 2018, 2018, 6310560. [Google Scholar]
- Scott, J.H. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar]
- Vassilis, K.; David, K. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar]
- Ciara, M.M.; Matthew, G.H.; Fergal, J.O.B. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar]
- Susmita, B.; Mangal, R.; Amit, B. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar]
- Reiko, K.; Kazuya, D.; Kaien, W.; Yoshifumi, O.; Koji, M.; Kazuhiro, T. Comparative Implant Stability of a Novel Implant-Porous Titanium Complex and an Implant-Porous Hydroxyapatite Complex for Bone Reconstruction Material. J. Hard Tissue Biol. 2023, 32, 177–182. [Google Scholar]
- Junge, K.; Binnebösel, M.; von Trotha, K.T.; Rosch, R.; Klinge, U.; Neumann, U.P. Mesh biocompatibility: Effects of cellular inflammation and tissue remodelling. Langenbeck’s Arch. Surg. 2012, 397, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, S.B.; Saberski, E.R.; Klueh, D.L.; Kreutzer, D.L.; Novitsky, Y.W. Effects of mast cells modulation on early host response to implanted synthetic meshes. Hernia 2010, 14, 511–516. [Google Scholar] [CrossRef]
- Klinge, U.; Klosterhalfen, B.; Birkenhauer, V.; Junge, K.; Conze, J.; Schumpelick, J. Impact of polymer pore size on the interface scar formation in a rat model. J. Surg. Res. 2002, 103, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Koyal, G.; Nicholas, A.P.; Carole, A.O.; John, J.R.; Gary, L.B. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar]
- Lauran, R.M.; Derek, J.M.; Eric, M.S.; Sarah, K.D.; James, A.F.; Janet, L.C.; Kip, D.H.; Michael, A.L.; Charles, E.M.; Buddy, D.R. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar]
- Yoshinori, K.; Qiming, J.; Masahiro, K.; Javed, M.; Hiroko, T. Geometry of artificial ECM: Sizes of pores controlling phenotype expression in BMP-induced osteogenesis and chondrogenesis. Connect. Tissue Res. 2002, 43, 529–534. [Google Scholar]
- Yasunnori, I.; Kiyofumi, T.; Hidetsugu, T.; Keisuke, N.; Quisheng, S.; Tianyan, P.; Anqi, C.; Hotaka, K.; Hitoshi, N. Novel Artificial Scaffold for Bone Marrow Regeneration: Honeycomb Tricalcium Phosphate. Materials 2023, 16, 1393. [Google Scholar] [CrossRef] [PubMed]
- Koji, I.; Yuka, S.; Yoshiki, A.; Youhei, K.; Kiyonari, M.; Yoshiaki, S.; Tomomi, U.; Satoaki, M.; Hiroyuki, Y.; Mitsuhiko, O.; et al. Macrophages play a unique role in the plaque calcification by enhancing the osteogenic signals exerted by vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 425, 39–44. [Google Scholar]
- Tamas, R. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015, 2015, 816460. [Google Scholar]
- Grinberg, S.; Hasko, G.; Wu, D. Suppression of PLCβ2 by endotoxin plays a role in the adenosine A2A receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. Am. J. Pathol. 2009, 175, 2439–2453. [Google Scholar] [CrossRef] [PubMed]
- Christopher, J.F.; Grace, P.E.; Genie, E.; Bruce, N.C.; Gyorgy, H.; Shalini, O.; Samuel, J.L. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation 2013, 36, 921–931. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takabatake, K.; Tsujigiwa, H.; Nakano, K.; Chang, A.; Piao, T.; Inada, Y.; Arashima, T.; Morimatsu, A.; Tanaka, A.; Kawai, H.; et al. Effect of Scaffold Geometrical Structure on Macrophage Polarization during Bone Regeneration Using Honeycomb Tricalcium Phosphate. Materials 2024, 17, 4108. https://doi.org/10.3390/ma17164108
Takabatake K, Tsujigiwa H, Nakano K, Chang A, Piao T, Inada Y, Arashima T, Morimatsu A, Tanaka A, Kawai H, et al. Effect of Scaffold Geometrical Structure on Macrophage Polarization during Bone Regeneration Using Honeycomb Tricalcium Phosphate. Materials. 2024; 17(16):4108. https://doi.org/10.3390/ma17164108
Chicago/Turabian StyleTakabatake, Kiyofumi, Hidetsugu Tsujigiwa, Keisuke Nakano, Anqi Chang, Tianyan Piao, Yasunori Inada, Takuma Arashima, Ayumi Morimatsu, Ayumi Tanaka, Hotaka Kawai, and et al. 2024. "Effect of Scaffold Geometrical Structure on Macrophage Polarization during Bone Regeneration Using Honeycomb Tricalcium Phosphate" Materials 17, no. 16: 4108. https://doi.org/10.3390/ma17164108
APA StyleTakabatake, K., Tsujigiwa, H., Nakano, K., Chang, A., Piao, T., Inada, Y., Arashima, T., Morimatsu, A., Tanaka, A., Kawai, H., & Nagatsuka, H. (2024). Effect of Scaffold Geometrical Structure on Macrophage Polarization during Bone Regeneration Using Honeycomb Tricalcium Phosphate. Materials, 17(16), 4108. https://doi.org/10.3390/ma17164108





