Effect of Stearyl Methacrylate Comonomer on the Mechanical and Physical Properties of Dimethacrylate-Based Dental Resins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Resins
2.3. Double Bond Conversion Test
2.4. Vickers Microhardness Test
2.5. Three Point Bending Test
2.6. Water Sorption and Water Solubility Test
2.7. Thermal Analysis Test
2.8. Statistical Analysis
3. Results
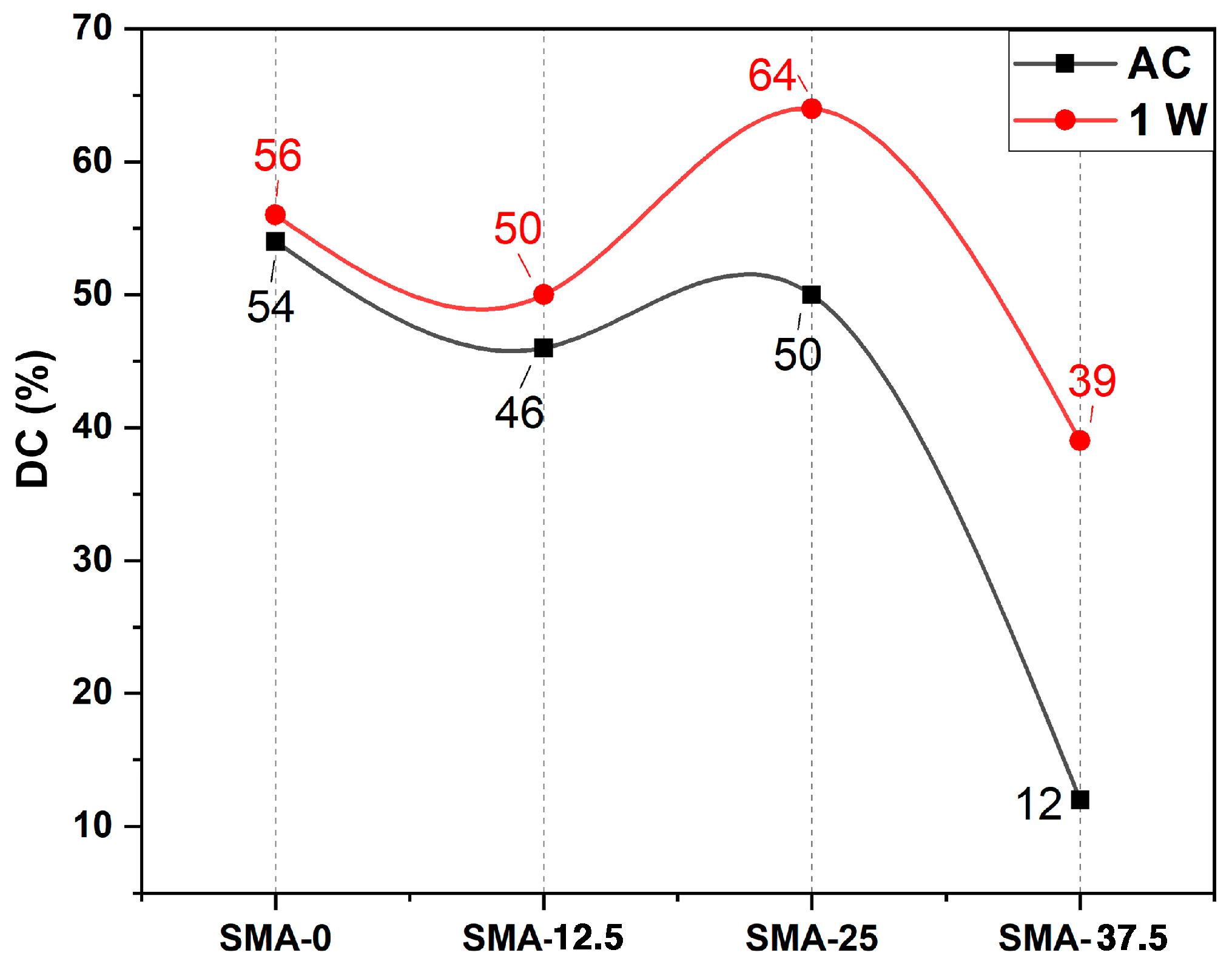
3.1. Double Bond Conversion
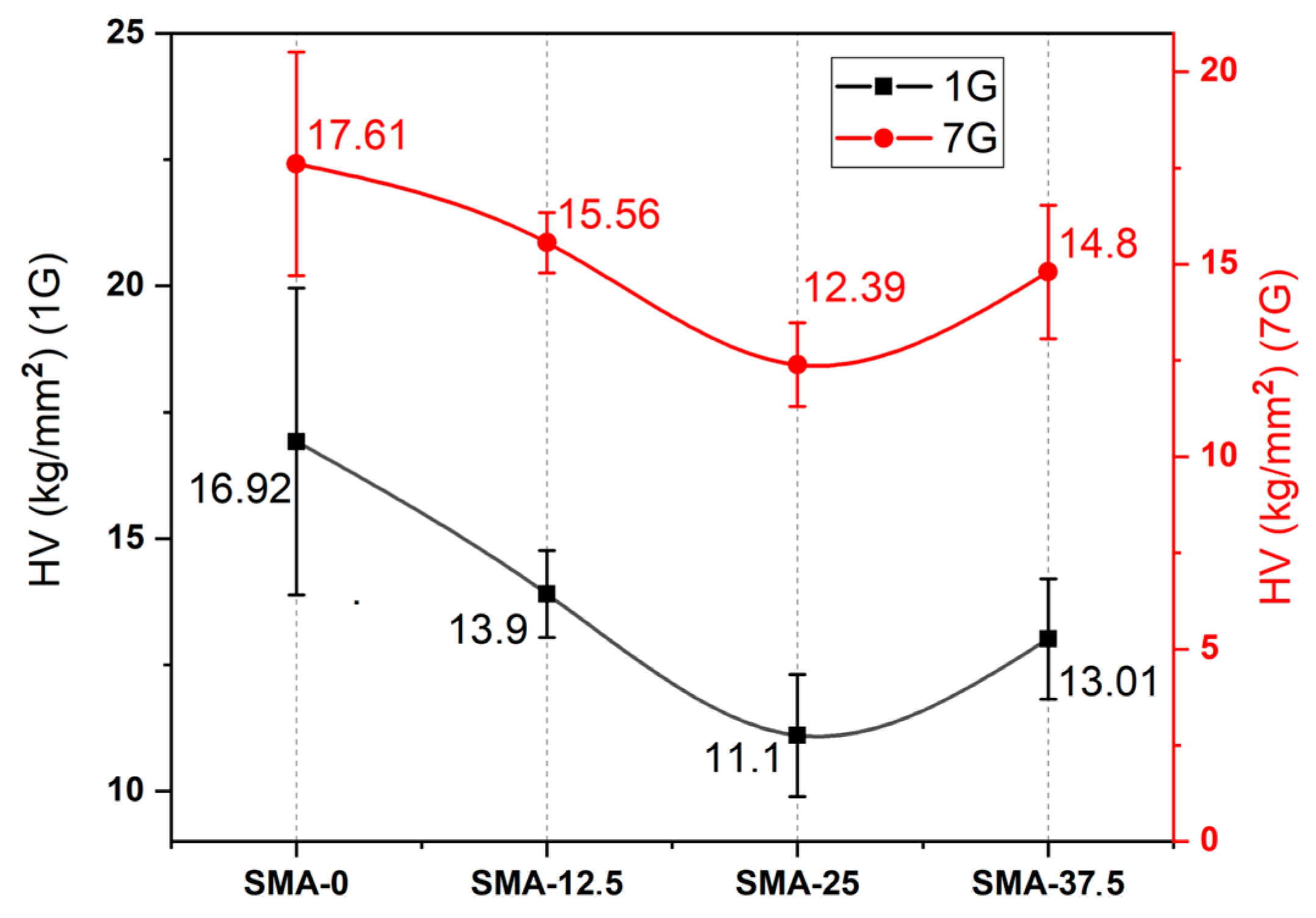
3.2. Vickers Microhardness
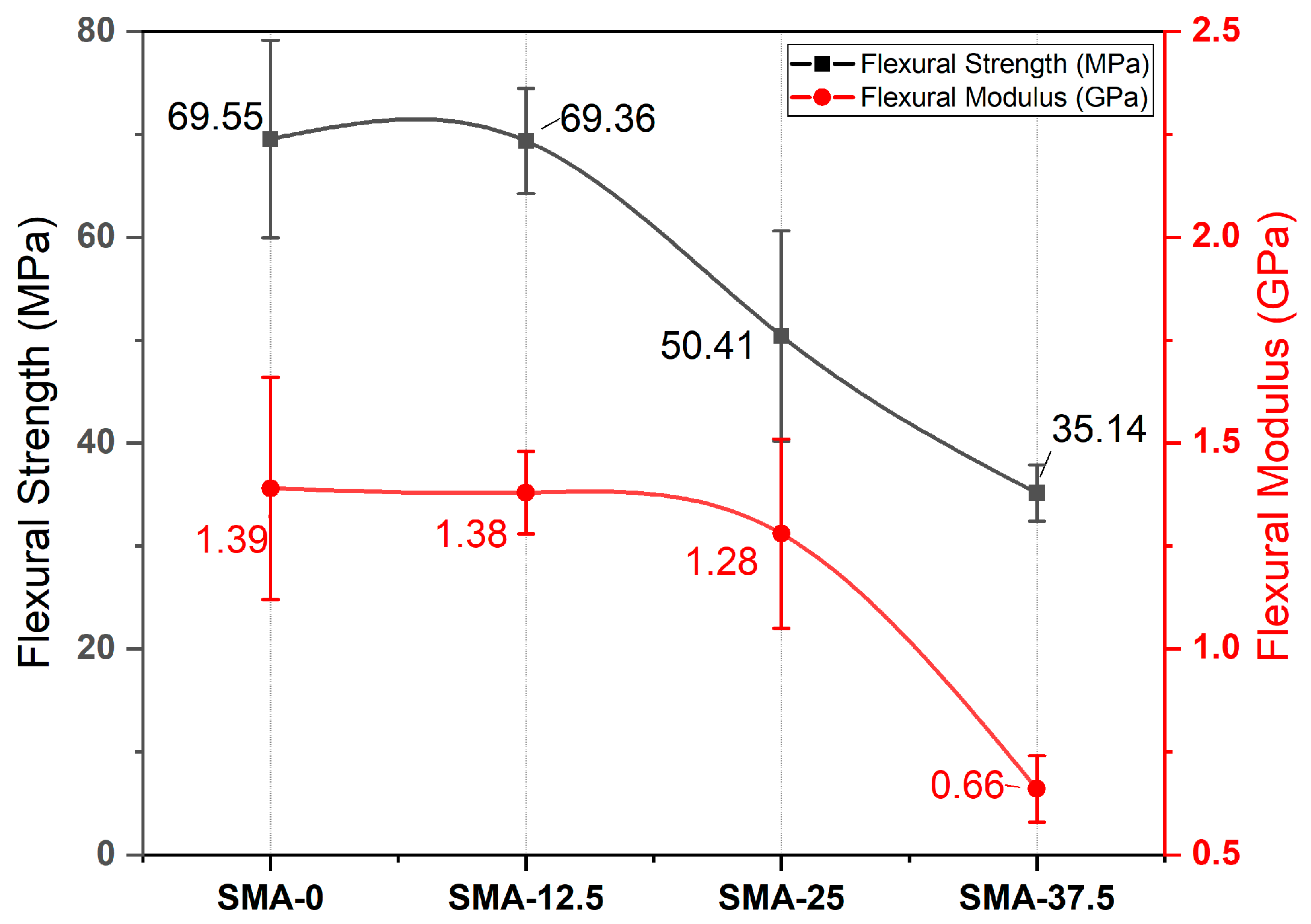
3.3. Three Point Bending
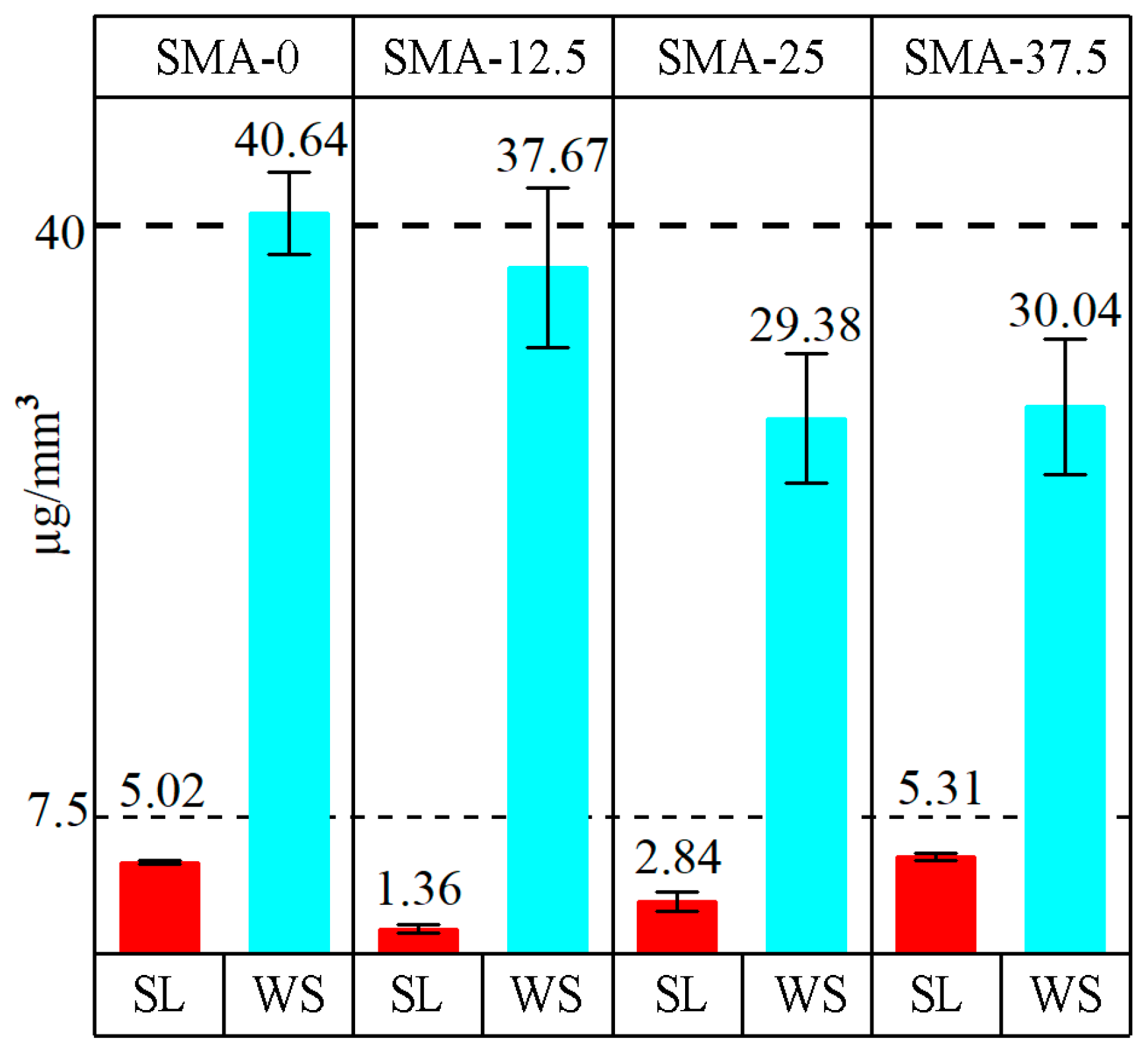
3.4. Water Sorption and Water Solubility
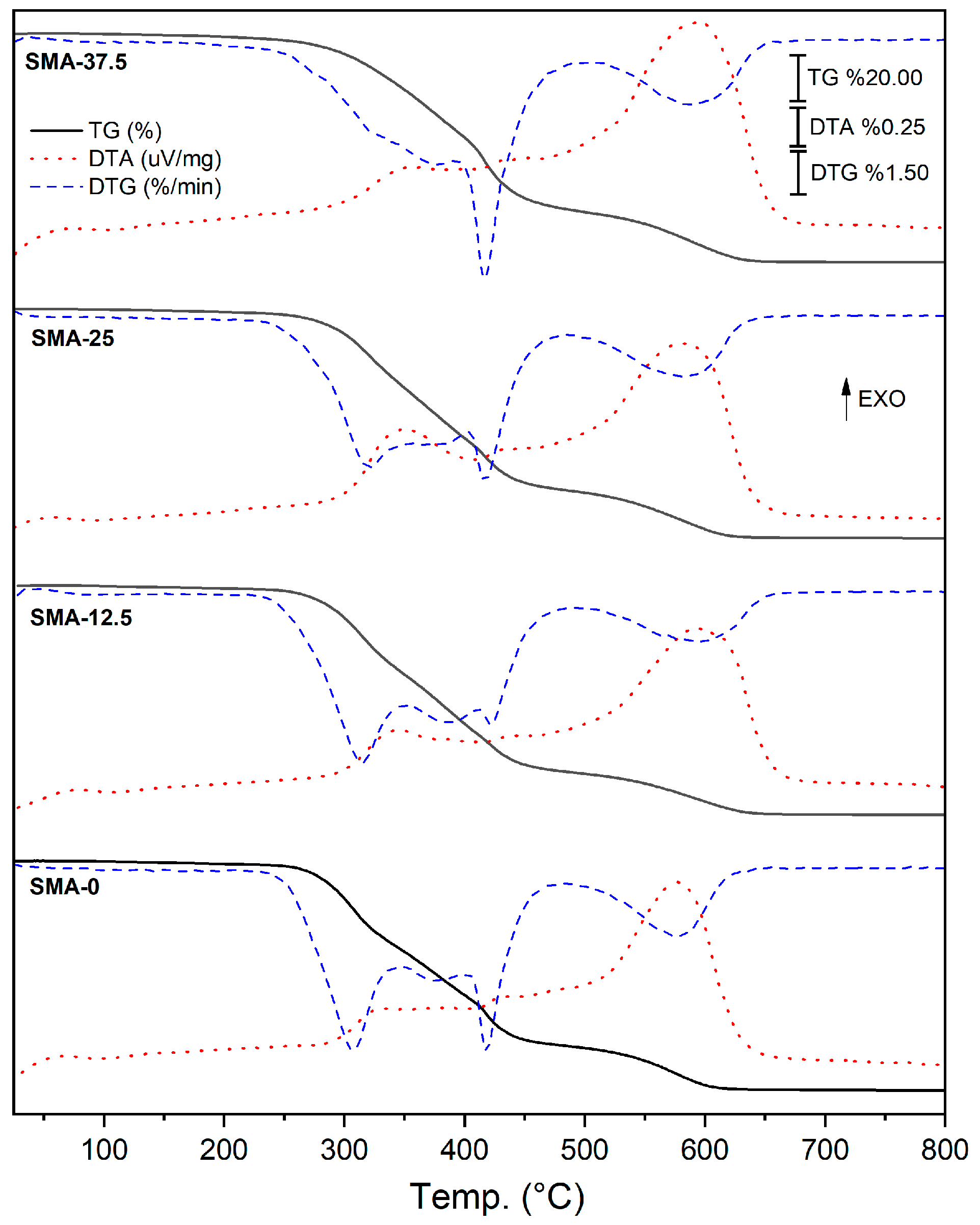
3.5. Thermal Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward Dental Caries: Exploring Nanoparticle-Based Platforms and Calcium Phosphate Compounds for Dental Restorative Materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Czarnecka, B. Materials for the Direct Restoration of Teeth; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-08-100491-3. [Google Scholar]
- Bors, A.; Antoniac, I.; Cotrut, C.; Antoniac, A.; Szekely, M. Surface Analysis of Contemporary Aesthetic Dental Filling Materials after Storage in Erosive Solutions. Mater. Plast. 2016, 53, 607–611. [Google Scholar] [CrossRef]
- Esteves, R.A.; Boaro, L.C.C.; Gonçalves, F.; Campos, L.M.P.; Silva, C.M.; Rodrigues-Filho, L.E. Chemical and Mechanical Properties of Experimental Dental Composites as a Function of Formulation and Postcuring Thermal Treatment. Biomed. Res. Int. 2018, 2018, 9845427. [Google Scholar] [CrossRef]
- Bowen, R.L. Use of Epoxy Resins in Restorative Materials. J. Dent. Res. 1956, 35, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Kent, B.E. The Glass-Ionomer Cement, a New Translucent Dental Filling Material. J. Appl. Chem. Biotechnol. 1971, 21, 313. [Google Scholar] [CrossRef]
- Wilson, A.D.; Kent, B.E. Surgical Cement. British Patent No.1316129, 15 December 1969. [Google Scholar]
- Wilson, A.D. Dental Silicate Cements: VII. Alternative Liquid Cement Formers. J. Dent. Res. 1968, 47, 1133–1136. [Google Scholar] [CrossRef]
- McCabe, J.F.; Walls, A. Applied Dental Materials, 9th ed.; Blackwell Publishing: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-3961-8. [Google Scholar]
- Alsari, A.; Ghilotti, J.; Sanz, J.L.; Llena, C.; Folguera, S.; Melo, M. Comparative Evaluation of the Microleakage of Glass Ionomers as Restorative Materials: A Systematic Review of In Vitro Studies. Appl. Sci. 2024, 14, 1729. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial Quaternary Ammonium Compounds in Dental Materials: A Systematic Review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Meereis, C.T.W.; Münchow, E.A.; de Oliveira da Rosa, W.L.; da Silva, A.F.; Piva, E. Polymerization Shrinkage Stress of Resin-Based Dental Materials: A Systematic Review and Meta-Analyses of Composition Strategies. J. Mech. Behav. Biomed. Mater. 2018, 82, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Rosa de Lacerda, L.; Bossardi, M.; Silveira Mitterhofer, W.J.; Galbiatti de Carvalho, F.; Carlo, H.L.; Piva, E.; Münchow, E.A. New Generation Bulk-Fill Resin Composites: Effects on Mechanical Strength and Fracture Reliability. J. Mech. Behav. Biomed. Mater. 2019, 96, 214–218. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, M. Dental Restorative Composite Materials: A Review. J. Oral Biosci. 2019, 61, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Luo, S.H.; Li, X.; Wang, Z.; Zhang, X.; Lei, X.; Yan, S.X. Effect of Silica Nanospheres Silanized by Functional Silanes on the Physicochemical and Mechanical Properties of Bis-GMA/TEGDMA Dental Composite Resin by Photocuring Synthesis. Int. J. Adhes. Adhes. 2024, 132, 103724. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, P.; Dumur, F.; Lalevée, J. Organic Dye-Based Photoinitiating Systems for Visible-Light-Induced Photopolymerization. J. Polym. Sci. 2021, 59, 1338–1389. [Google Scholar] [CrossRef]
- Dumur, F. Recent Advances on Visible Light Pyrrole-Derived Photoinitiators of Polymerization. Eur. Polym. J. 2022, 173, 111254. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of Water Sorption, Solubility and Modulus of Elasticity of Light-Cured Dimethacrylate-Based Dental Resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Vouvoudi, E.C. Overviews on the Progress of Flowable Dental Polymeric Composites: Their Composition, Polymerization Process, Flowability and Radiopacity Aspects. Polymers 2022, 14, 4182. [Google Scholar] [CrossRef]
- Ok, I.; Aykac, A. Enhancement of the Mechanical and Antibacterial Properties of Bis-GMA/TEGDMA Dental Composite Incorporated with ZnO/CS and Si/PMMA Core–Shell Nanostructures. Chem. Pap. 2023, 77, 6959–6973. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, Y.; Wang, R.; Zhu, M. Synthesis of Polymerizable Betulin Maleic Diester Derivative for Dental Restorative Resins with Antibacterial Activity. Dent. Mater. 2024, 40, 941–950. [Google Scholar] [CrossRef]
- Bouzidi, A.; Bayou, S.; Khier, N.; Dehamchia, M. Photoinitiated Polymerization of a Dental Formulation, Part 2: Kinetic Studies. Polym. Bull. 2024, 81, 4221–4235. [Google Scholar] [CrossRef]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin Based Restorative Dental Materials: Characteristics and Future Perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef]
- Eltahlah, D.; Lynch, C.D.; Chadwick, B.L.; Blum, I.R.; Wilson, N.H.F. An Update on the Reasons for Placement and Replacement of Direct Restorations. J. Dent. 2018, 72, 1–7. [Google Scholar] [CrossRef]
- Bhadila, G.Y.; Baras, B.H.; Balhaddad, A.A.; Williams, M.A.; Oates, T.W.; Weir, M.D.; Xu, H.H.K. Recurrent Caries Models to Assess Dental Restorations: A Scoping Review. J. Dent. 2023, 136, 104604. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, L.S.; Scotti, C.K.; Mondelli, R.F.L.; Vega, H.A.; Faggion, C.M.; Bombonatti, J.F.S.; Velo, M.M. de A.C. Effect of Nanofibers as Reinforcement on Resin-Based Dental Materials: A Systematic Review of in Vitro Studies. Jpn. Dent. Sci. Rev. 2023, 59, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Podgórski, M.; Becka, E.; Claudino, M.; Flores, A.; Shah, P.K.; Stansbury, J.W.; Bowman, C.N. Ester-Free Thiol-Ene Dental Restoratives—Part B: Composite Development. Dent. Mater. 2015, 31, 1263–1270. [Google Scholar] [CrossRef][Green Version]
- Sideridou, I.; Achilias, D.S.; Spyroudi, C.; Karabela, M. Water Sorption Characteristics of Light-Cured Dental Resins and Composites Based on Bis-EMA/PCDMA. Biomaterials 2004, 25, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Wang, T.P.; Li, Y.T.; Tu, I.H. Synthesis, Characterization and Ocular Biocompatibility of Potential Keratoprosthetic Hydrogels Based on Photopolymerized Poly(2-Hydroxyethyl Methacrylate)-Co-Poly(Acrylic Acid). J. Mater. Chem. 2012, 22, 1812–1823. [Google Scholar] [CrossRef]
- Kim, J.G.; Chung, C.M. Trifunctional Methacrylate Monomers and Their Photocured Composites with Reduced Curing Shrinkage, Water Sorption, and Water Solubility. Biomaterials 2003, 24, 3845–3851. [Google Scholar] [CrossRef]
- van Noort, R. Introduction to Dental Materials, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-7234-3659-1. [Google Scholar]
- Benkeser, S.M.; Karlin, S.; Rohr, N. Effect of Curing Mode of Resin Composite Cements on Water Sorption, Color Stability, and Biaxial Flexural Strength. Dent. Mater. 2024, 40, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.; Dobson, A.; Mbiya, W.; Navarro, O.; Ferracane, J.L.; Pfeifer, C.S. Use of (Meth)Acrylamides as Alternative Monomers in Dental Adhesive Systems. Dent. Mater. 2019, 35, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A Review of Dental Composites: Challenges, Chemistry Aspects, Filler Influences, and Future Insights. Compos. B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Ito, S.; Hashimoto, M.; Wadgaonkar, B.; Svizero, N.; Carvalho, R.M.; Yiu, C.; Rueggeberg, F.A.; Foulger, S.; Saito, T.; Nishitani, Y.; et al. Effects of Resin Hydrophilicity on Water Sorption and Changes in Modulus of Elasticity. Biomaterials 2005, 26, 6449–6459. [Google Scholar] [CrossRef]
- Sankarapandian, M.; Shobha, H.; Kalachandra, S.; Mcgrath, J. Characterization of Some Aromatic Dimethacrylates for Dental Composite Applications. J. Mater. Sci. Mater. Med. 1997, 8, 465–468. [Google Scholar] [CrossRef]
- Shekofteh, K.; Kashi, T.J.; Behroozibakhsh, M.; Sadr, A.; Najafi, F.; Bagheri, H. Evaluation of Physical/Mechanical Properties of an Experimental Dental Composite Modified with a Zirconium-Based Metal-Organic Framework (MOF) as an Innovative Dental Filler. Dent. Mater. 2023, 39, 790–799. [Google Scholar] [CrossRef]
- Varghese, J.T.; Cho, K.; Raju; Farrar, P.; Prentice, L.; Prusty, B.G. Effect of Silane Coupling Agent and Concentration on Fracture Toughness and Water Sorption Behaviour of Fibre-Reinforced Dental Composites. Dent. Mater. 2023, 39, 362–371. [Google Scholar] [CrossRef]
- Saini, S.; Meena, A.; Yadav, R.; Patnaik, A. Investigation of Physical, Mechanical, Thermal, and Tribological Characterization of Tricalcium Phosphate and Zirconia Particulate Reinforced Dental Resin Composite Materials. Tribol. Int. 2023, 181, 108322. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Zhang, H.; Hang, R.; Wang, Y. Preparation of Al-Doped Mesoporous Silica Spheres (Al-MSSs) for the Improvement of Mechanical Properties and Aging Resistance of Dental Resin Composites. J. Mech. Behav. Biomed. Mater. 2024, 157, 106624. [Google Scholar] [CrossRef] [PubMed]
- Althahban, S.; Alomari, A.S.; Sallam, H.E.-D.M.; Jazaa, Y. An Investigation of Wear, Mechanical, and Water Sorption/Solubility Behaviors of a Commercial Restorative Composite Containing Nano-Additives. J. Mater. Res. Technol. 2023, 23, 491–502. [Google Scholar] [CrossRef]
- Tihan, T.G.; Ionita, M.D.; Popescu, R.G.; Iordachescu, D. Effect of Hydrophilic-Hydrophobic Balance on Biocompatibility of Poly(Methyl Methacrylate) (PMMA)-Hydroxyapatite (HA) Composites. Mater. Chem. Phys. 2009, 118, 265–269. [Google Scholar] [CrossRef]
- Örtengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I.E. Water Sorption and Solubility of Dental Composites and Identification of Monomers Released in an Aqueous Environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Thanoon, H.; Silikas, N.; Watts, D.C. Effect of Polymerisation Protocols on Water Sorption, Solubility and Hygroscopic Expansion of Fast-Cure Bulk-Fill Composite. Dent. Mater. 2024, 40, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Rattanawongwiboon, T.; Haema, K.; Pasanphan, W. Stearyl Methacrylate-Grafted-Chitosan Nanoparticle as a Nanofiller for PLA: Radiation-Induced Grafting and Characterization. Radiat. Phys. Chem. 2014, 94, 205–210. [Google Scholar] [CrossRef]
- Atai, M.; Watts, D.C.; Atai, Z. Shrinkage Strain-Rates of Dental Resin-Monomer and Composite Systems. Biomaterials 2005, 26, 5015–5020. [Google Scholar] [CrossRef] [PubMed]
- ISO 4049; Dentistry—Polymer-Based Restorative Materials. ISO: Geneva, Switzerland, 2019.
- Yiu, C.K.Y.; King, N.M.; Carrilho, M.R.O.; Sauro, S.; Rueggeberg, F.A.; Prati, C.; Carvalho, R.M.; Pashley, D.H.; Tay, F.R. Effect of Resin Hydrophilicity and Temperature on Water Sorption of Dental Adhesive Resins. Biomaterials 2006, 27, 1695–1703. [Google Scholar] [CrossRef]
- Rueggeberg, F.; Tamareselvy, K. Resin Cure Determination by Polymerization Shrinkage. Dent. Mater. 1995, 11, 265–268. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Influence of Selected Components on Crosslink Density in Polymer Structures. Eur. J. Oral Sci. 2001, 109, 282–285. [Google Scholar] [CrossRef]
- Asmussen, E. Factors Affecting the Quantity of Remaining Double Bonds in Restorative Resin Polymers. Eur. J. Oral Sci. 1982, 90, 490–496. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Greener, E.H. The Effect of Resin Formulation on the Degree of Conversion and Mechanical Properties of Dental Restorative Resins. J. Biomed. Mater. Res. 1986, 20, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Alabdali, Z.N.; Reiter, M.P.; Lynch-Branzoi, J.K.; Mann, A.B. Compositional Effects on Mechanical Properties and Viscosity in UDMA-MMA Blends. J. Adhes. Sci. Technol. 2021, 35, 610–625. [Google Scholar] [CrossRef]
- Fugolin, A.P.; de Paula, A.B.; Dobson, A.; Huynh, V.; Consani, R.; Ferracane, J.L.; Pfeifer, C.S. Alternative Monomer for BisGMA-Free Resin Composites Formulations. Dent. Mater. 2020, 36, 884–892. [Google Scholar] [CrossRef]
- Suzuki, Y.; Mishima, R.; Kato, E.; Matsumoto, A. Analysis of the Glass Effect and Trommsdorff Effect during Bulk Polymerization of Methyl Methacrylate, Ethyl Methacrylate, and Butyl Methacrylate. Polym. J. 2023, 55, 229–238. [Google Scholar] [CrossRef]
- González-López, J.A.; Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; Trejo-Carbajal, N.; Herrera-González, A.M. Evaluation of Dental Composites Resins Formulated with Non-Toxic Monomers Derived from Catechol. J. Mech. Behav. Biomed. Mater. 2020, 104, 103613. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, F.; Luo, Y.; Jia, D. Synthesis and Characterization of a Dimethacrylates Monomer with Low Shrinkage and Water Sorption for Dental Application. J. Appl. Polym. Sci. 2012, 125, 114–120. [Google Scholar] [CrossRef]
- Aromaa, M.K.; Vallittu, P.K. Delayed Post-Curing Stage and Oxygen Inhibition of Free-Radical Polymerization of Dimethacrylate Resin. Dent. Mater. 2018, 34, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mondragón, A.A.; Cuevas-Suárez, C.E.; González-López, J.A.; Trejo-Carbajal, N.; Meléndez-Rodríguez, M.; Herrera-González, A.M. Preparation and Evaluation of a BisGMA-Free Dental Composite Resin Based on a Novel Trimethacrylate Monomer. Dent. Mater. 2020, 36, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.D.; Park, C.H.; Moon, J.I.; Kim, H.J.; Masubuchi, T. UV-Curing Behavior and Physical Properties of Waterborne UV-Curable Polycarbonate-Based Polyurethane Dispersion. Prog. Org. Coat. 2011, 72, 663–675. [Google Scholar] [CrossRef]
- Liu, H.; Lin, F.; Chen, M.; Xu, K. Preparation and Properties of New UV-Curable Naphtyl Epoxy Acrylates. Iran. Polym. J. 2010, 19, 219–227. [Google Scholar]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Garoushi, S.; Säilynoja, E.; Vallittu, P.K.; Lassila, L. The Effect of Adding a New Monomer “Phene” on the Polymerization Shrinkage Reduction of a Dental Resin Composite. Dent. Mater. 2019, 35, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Soucek, M.D. Investigation of Non-Isocyanate Urethane Dimethacrylate Reactive Diluents for UV-Curable Polyurethane Coatings. Prog. Org. Coat. 2013, 76, 1057–1067. [Google Scholar] [CrossRef]
- Kerby, R.E.; Knobloch, L.A.; Schricker, S.; Gregg, B. Synthesis and Evaluation of Modified Urethane Dimethacrylate Resins with Reduced Water Sorption and Solubility. Dent. Mater. 2009, 25, 302–313. [Google Scholar] [CrossRef]
- Da Silva Tanaka, A.C.; Dos Santos, G.C.; Alarcon, R.T.; Da Silva Filho, L.C.; Rezende, M.C.R.A. Effect of the Incorporation of EDB Co-Initiator in the Resin in Halogen and LED Light. Mater. Res. 2021, 24, e20210297. [Google Scholar] [CrossRef]
| Abbreviation | Composition of Resins by Weight Percent (%) | ||
|---|---|---|---|
| Bis-GMA | TEGDMA | SMA | |
| SMA-0 | 50 | 50 | 0 |
| SMA-12.5 | 50 | 37.5 | 12.5 |
| SMA-25 | 50 | 25 | 25 |
| SMA-37.5 | 50 | 12.5 | 37.5 |
| Samples | Volatilization Step | First Decomposition (FD) | Second Decomposition (SD) | Units |
|---|---|---|---|---|
| SMA-0 | 25–231 | 231–482 | 482–653 | °C |
| 1.6 | 78.6 | 19.8 | Δm/% | |
| SMA-12.5 | 25–232 | 232–490 | 490–669 | °C |
| 1.8 | 79.6 | 18.6 | Δm/% | |
| SMA-25 | 25–236 | 236–486 | 486–676 | °C |
| 1.8 | 77 | 21.2 | Δm/% | |
| SMA-37.5 | 25–242 | 242–508 | 508–686 | °C |
| 2.7 | 75.7 | 21.6 | Δm/% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadag, M.; Dolekcekic, E.; Erdem, M.; Özcan, M. Effect of Stearyl Methacrylate Comonomer on the Mechanical and Physical Properties of Dimethacrylate-Based Dental Resins. Materials 2024, 17, 4136. https://doi.org/10.3390/ma17164136
Karadag M, Dolekcekic E, Erdem M, Özcan M. Effect of Stearyl Methacrylate Comonomer on the Mechanical and Physical Properties of Dimethacrylate-Based Dental Resins. Materials. 2024; 17(16):4136. https://doi.org/10.3390/ma17164136
Chicago/Turabian StyleKaradag, Mecit, Emrah Dolekcekic, Murat Erdem, and Mutlu Özcan. 2024. "Effect of Stearyl Methacrylate Comonomer on the Mechanical and Physical Properties of Dimethacrylate-Based Dental Resins" Materials 17, no. 16: 4136. https://doi.org/10.3390/ma17164136
APA StyleKaradag, M., Dolekcekic, E., Erdem, M., & Özcan, M. (2024). Effect of Stearyl Methacrylate Comonomer on the Mechanical and Physical Properties of Dimethacrylate-Based Dental Resins. Materials, 17(16), 4136. https://doi.org/10.3390/ma17164136







