Influence of SiO2 Nanoparticles Extracted from Biomass on the Properties of Electrodeposited Ni Matrix Composite Films on Si(100) Substrate
Abstract
1. Introduction
2. Materials and Methods
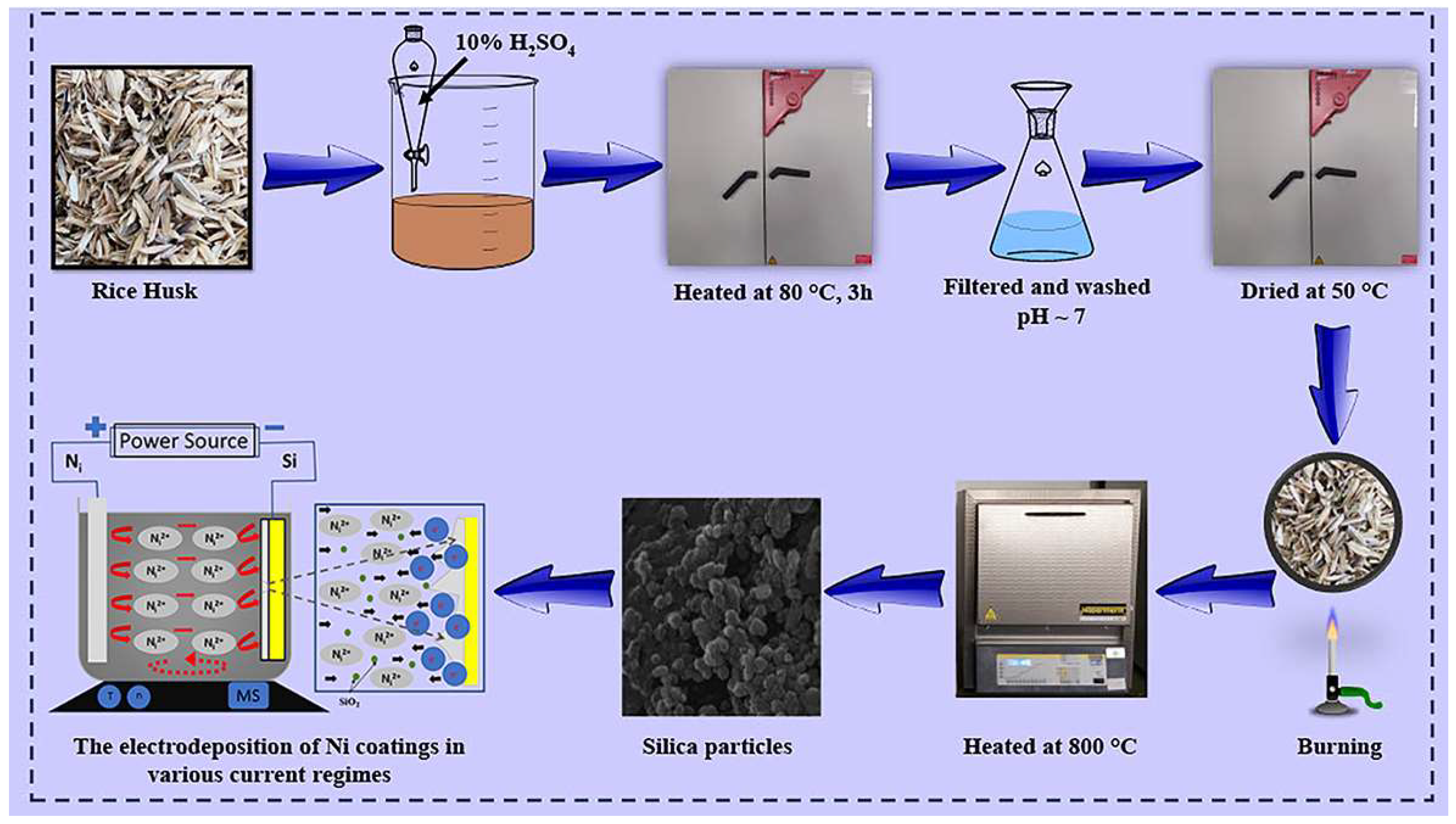
2.1. Materials and Methods for the Synthesis of SiO2 Nanoparticles from RH Biomass
2.2. Materials and Methods for the Synthesis of Ni Films
2.3. Materials and Methods for the Synthesis of Ni/SiO2 Films
2.4. Characterization Methods for SiO2 Nanoparticles
2.5. Characterization Methods for Si(100) Cathode, Pure Ni, and Ni/SiO2 Films
3. Results and Discussion
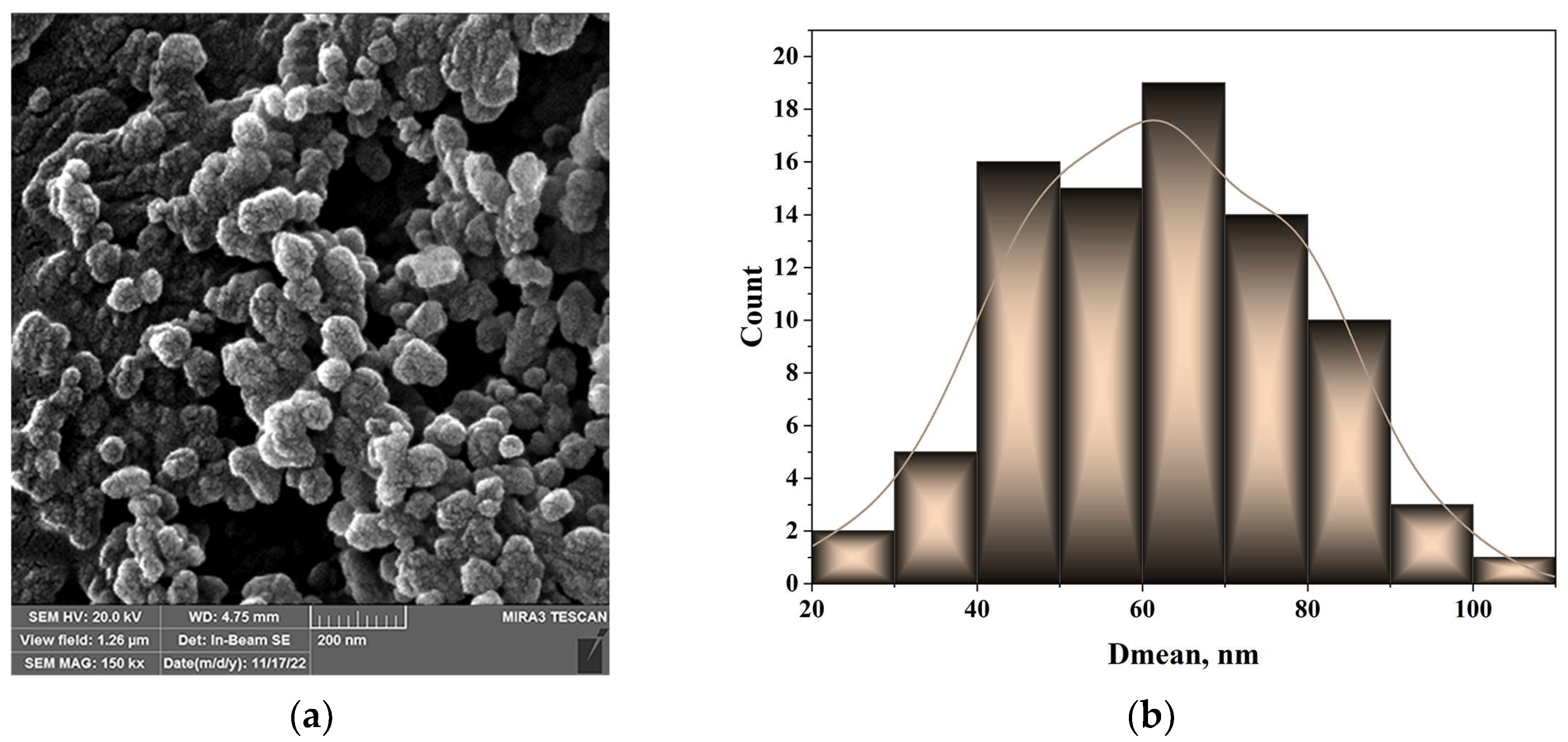
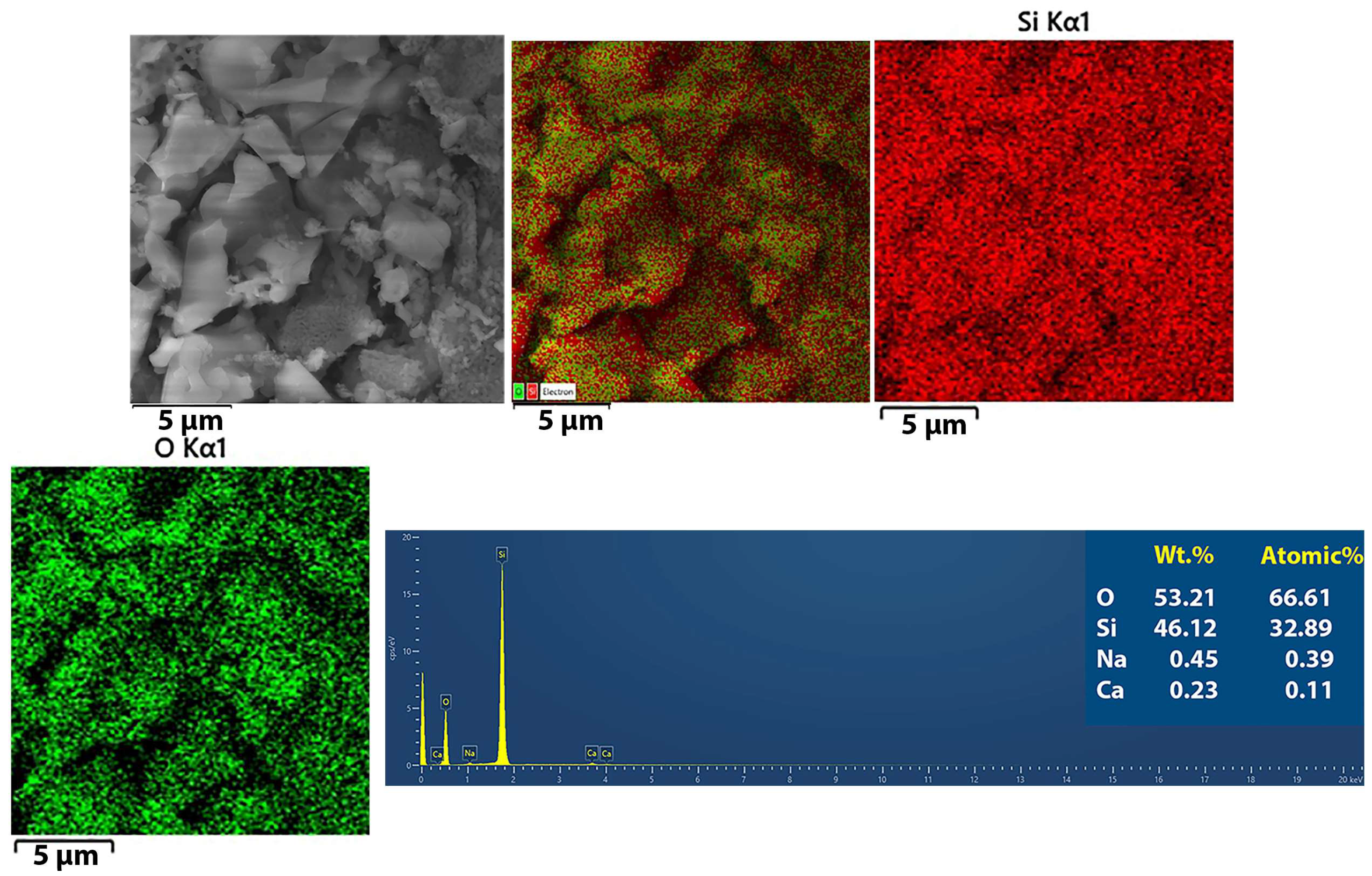
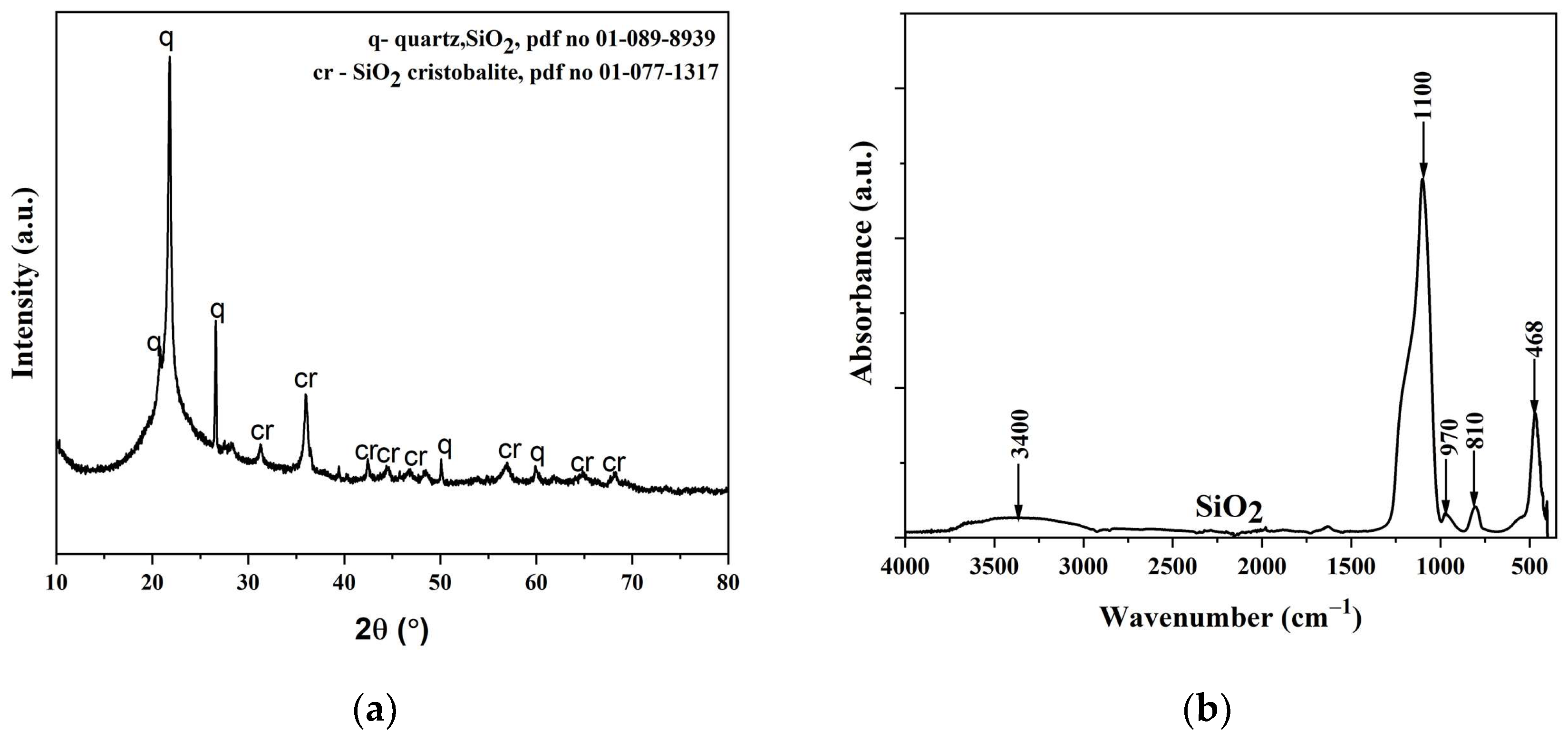
3.1. Characterization of SiO2 Nanoparticles
3.1.1. Morphology of the SiO2 Nanoparticles
3.1.2. Elemental Analysis of the SiO2 Particles
3.1.3. XRD Analysis of the SiO2 Particles
3.2. Characterization of Ni and Ni/SiO2 Films
3.2.1. The Basic Facts for Understanding the Current Regimes
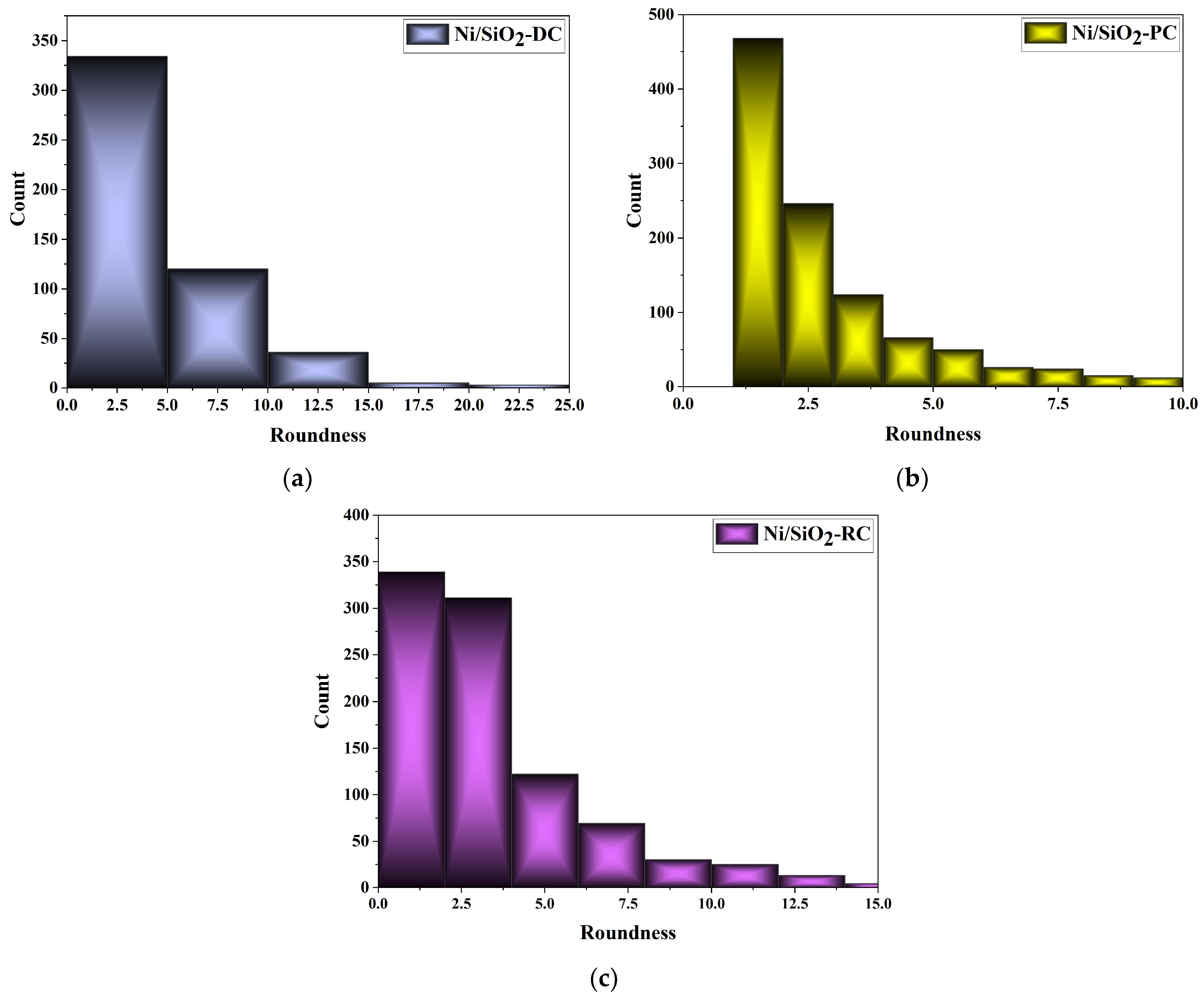
3.2.2. Morphology of the Ni and Ni/SiO2 Films
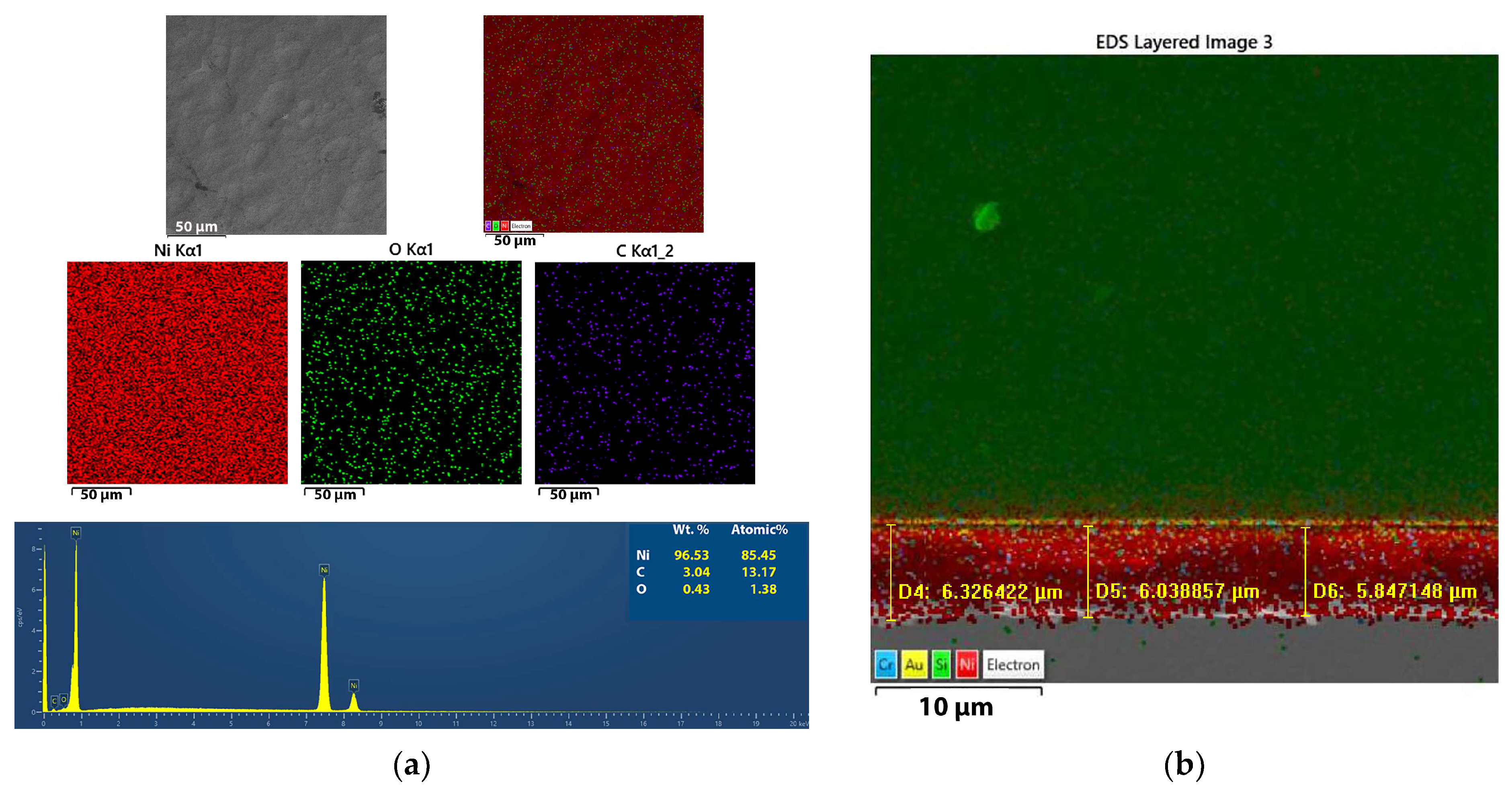
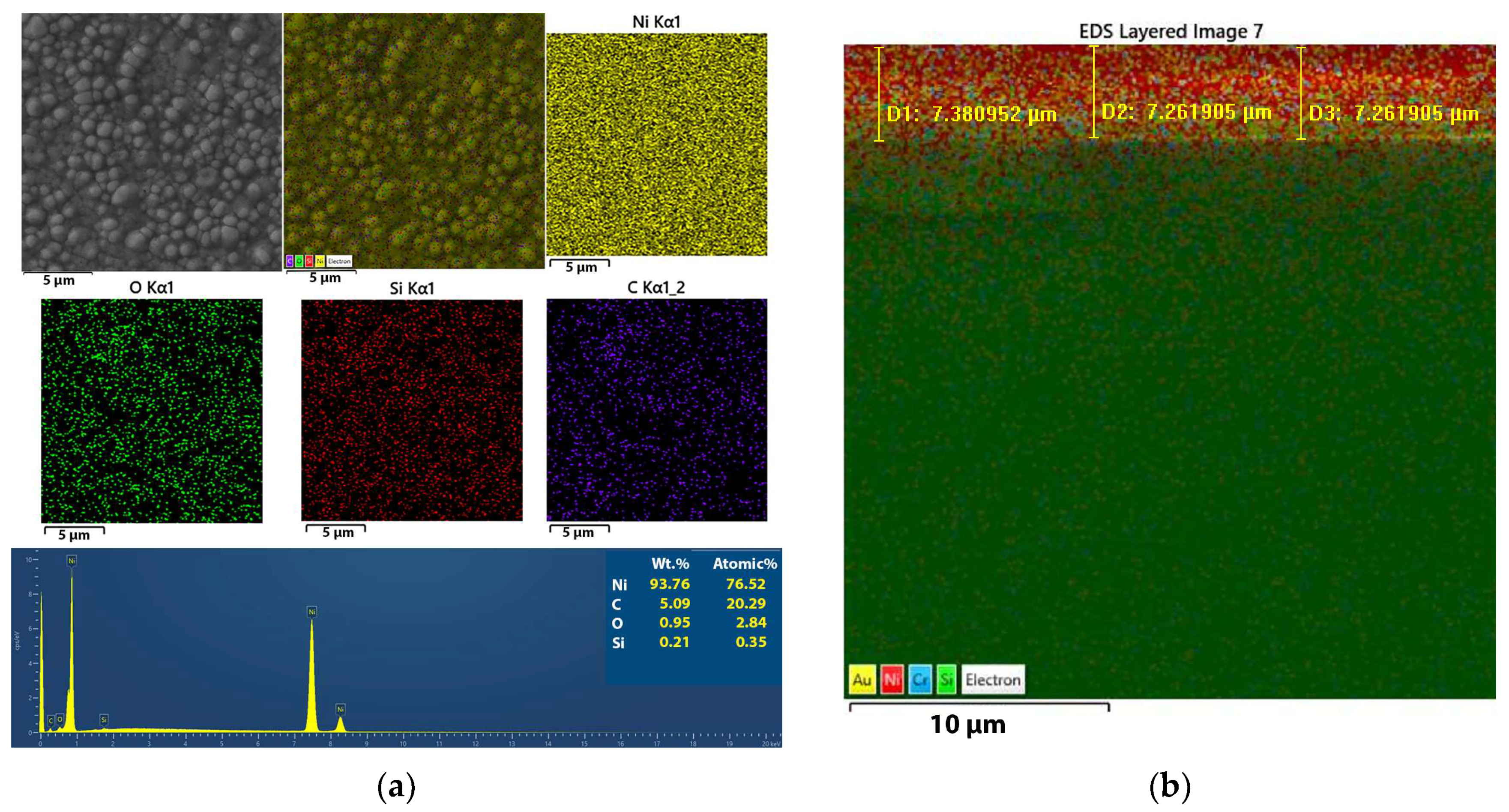
3.2.3. Mapping of the Ni and Ni/SiO2 Films—EDS
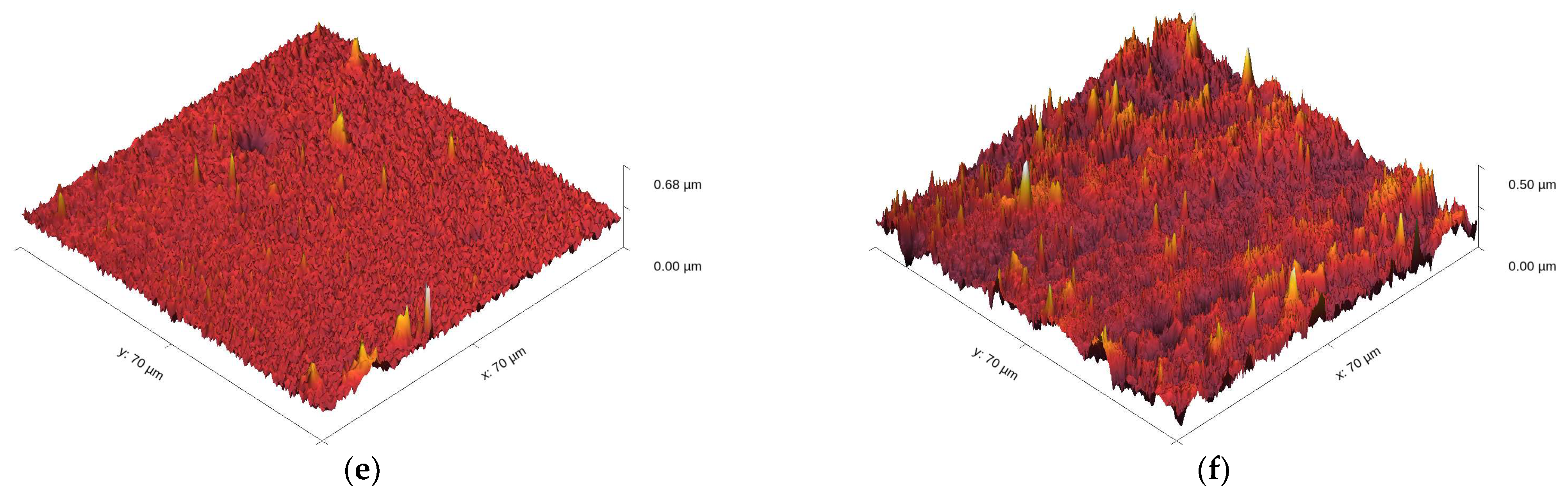
3.2.4. Topography of the Ni and Ni/SiO2 Films
3.2.5. Microhardness Properties of the Ni and Ni/SiO2 Films Using the Chen-Gao Composite Hardness Model
3.2.6. Electrical Properties of the Ni and Ni/SiO2 Films—Sheet Resistance
3.3. Mechanism of Formation of Nickel Matrix Composite (NiMC) Films and Comparison with Existing Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Xu, R.; Zhang, Y. Influence of SiO2 Nano-Particles on Microstructures and Properties of Ni-W-P/CeO2-SiO2 Composites Prepared by Pulse Electrodeposition. Trans. Nonferrous Met. Soc. China 2010, 20, 839–843. [Google Scholar] [CrossRef]
- Singh, N.; Mehta, A.; Vasudev, H.; Samra, P.S. A Review on the Design and Analysis for the Application of Wear and Corrosion Resistance Coatings. Int. J. Interact. Des. Manuf. 2023, 1–25. [Google Scholar] [CrossRef]
- Shozib, I.A.; Ahmad, A.; Abdul-Rani, A.M.; Beheshti, M.; Aliyu, A.A. A Review on the Corrosion Resistance of Electroless Ni-P Based Composite Coatings and Electrochemical Corrosion Testing Methods. Corros. Rev. 2022, 40, 1–37. [Google Scholar] [CrossRef]
- Poddar, N.P.; Chelvane, J.A.; Prasad, N.D.V.; Raja, M.M. Magnetic and Electrical Properties of Sputtered Ni Films. J. Supercond. Nov. Magn. 2024, 37, 1133–1140. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.; Sinha, M.K.; Kumar, R. Ferrous-Metal Matrix Composites: A Review on Status, Scope and Challenges. Int. J. Interact. Des. Manuf. 2023, 17, 2807–2829. [Google Scholar] [CrossRef]
- Natarajan, N.; Krishnaraj, V.; Davim, J.P. Metal Matrix Composites; Springer Briefs in Applied Sciences and Technology; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-02984-9. [Google Scholar]
- Djouan, R.; Qian, X. Mechanism of Electrodeposition of Nickel in Aqueous Solution. Int. J. Curr. Res. 2018, 10, 64228–64239. [Google Scholar]
- Banovic, S.W.; Barmak, K.; Marder, A.R. Microstructural Characterization and Hardness of Electrodeposited Nickel Coatings from a Sulphamate Bath. J. Mater. Sci. 1998, 33, 639–645. [Google Scholar]
- Bigos, A.; Wolowicz, M.; Janusz-Skuza, M.; Starowicz, Z.; Szczerba, M.J.; Bogucki, R.; Beltowska-Lehman, E. Citrate-Based Baths for Electrodeposition of Nanocrystalline Nickel Coatings with Enhanced Hardness. J. Alloys Compd. 2021, 850, 156857. [Google Scholar] [CrossRef]
- Kamel, M.M.; Anwer, Z.M.; Abdel-Salam, I.T.; Ibrahim, I.S. Nickel Electrodeposition from Novel Lactate Bath. Trans. IMF 2010, 88, 191–197. [Google Scholar] [CrossRef]
- Srinivasan, R.; Ramesh Bapu, G.N.K. Effect of Additives on Electrodeposition of Nickel from Acetate Bath: Cyclic Voltammetric Study. Trans. IMF 2013, 91, 52–56. [Google Scholar] [CrossRef]
- Salehikahrizsangi, P.; Raeissi, K.; Karimzadeh, F.; Calabrese, L.; Proverbio, E. Highly Hydrophobic Nickel and Nickel-Tungsten Coatings: Microstructural and Surface Properties. Appl. Surf. Sci. 2020, 520, 146319. [Google Scholar] [CrossRef]
- Salehikahrizsangi, P.; Raeissi, K.; Karimzadeh, F.; Calabrese, L.; Patane, S.; Proverbio, E. Erosion-Corrosion Behavior of Highly Hydrophobic Hierarchical Nickel Coatings. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 558, 446–454. [Google Scholar] [CrossRef]
- Priyadarshi, P.; Katiyar, P.K.; Maurya, R. A Review on Mechanical, Tribological and Electrochemical Performance of Ceramic Particle-Reinforced Ni-Based Electrodeposited Composite Coatings. J. Mater. Sci. 2022, 57, 19179–19211. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Vuksanović, M.M.; Dimitrijević, S.P.; Vasilić, R.; Radojević, V.J.; Vasiljević-Radović, D.G.; Nikolić, N.D. Mechanical Properties of Electrolytically Produced Copper Coatings Reinforced with Pigment Particles. Metals 2023, 13, 1979. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Vuksanović, M.M.; Jovanov, V.; Radovanović, Ž.; Obradov, M.; Nikolić, N.D.; Vasiljević-Radović, D.G. Metal Matrix Composite Coatings Based on Ni Matrix and Biosilica Filers Obtained from Rice Husks. In Proceedings of the 2023 IEEE 33rd International Conference on Microelectronics (MIEL), Nis, Serbia, 16–18 October 2023; pp. 1–4. [Google Scholar]
- Jenczyk, P.; Grzywacz, H.; Milczarek, M.; Jarząbek, D.M. Mechanical and Tribological Properties of Co-Electrodeposited Particulate-Reinforced Metal Matrix Composites: A Critical Review with Interfacial Aspects. Materials 2021, 14, 3181. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, L.; Zhang, M.; Xu, X.; Wang, Z.; Zhang, L. Mechanical Properties of Nanoparticle-Reinforced Ni-Based Composite Coatings Prepared by Jet Electrodeposition. J. Mater. Eng. Perform. 2023, 1–16. [Google Scholar] [CrossRef]
- Alnassar, S.I.; Kadhim, H.M.; Hassan, S.R.; Mahmoud, A.K.; Alrubaiy, A.A.A.G. Synthesis of Nano-SiC Reinforced Nickel-Based Nanocomposite Coating Using Electroless Deposition Technique. Mater. Today Proc. 2023, in press. [CrossRef]
- Sharma, D.K.; Mahant, D.; Upadhyay, G. Manufacturing of Metal Matrix Composites: A State of Review. Mater. Today Proc. 2020, 26, 506–519. [Google Scholar] [CrossRef]
- Kumar, N.M.S.; Shashank, T.N.; Dheeraj, N.U.; Dhruthi; Kordijazi, A.; Rohatgi, P.K.; Sadashiva, M. Coatings on Reinforcements in Aluminum Metal Matrix Composites. Int. J. Met. 2023, 17, 1049–1064. [Google Scholar] [CrossRef]
- Li, R.; Hou, Y.; Liang, J. Electro-Codeposition of Ni-SiO2 Nanocomposite Coatings from Deep Eutectic Solvent with Improved Corrosion Resistance. Appl. Surf. Sci. 2016, 367, 449–458. [Google Scholar] [CrossRef]
- Guglielmi, N. Kinetics of the Deposition of Inert Particles from Electrolytic Baths. J. Electrochem. Soc. 1972, 119, 1009. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, X.Z.; Zhi, C.C. Kinetics of Ni/Nano-SiO2 Codeposition on the Sintered NdFeB Surface. Strength Mater. 2021, 53, 134–144. [Google Scholar] [CrossRef]
- Cherrington, R.; Marshall, J.; Alexander, A.T.; Goodship, V. Exploring the Circular Economy through Coatings in Transport. Sustain. Prod. Consum. 2022, 32, 136–146. [Google Scholar] [CrossRef]
- Singh, J.; Boddula, R.; Digambar Jirimali, H. Utilization of Secondary Agricultural Products for the Preparation of Value Added Silica Materials and Their Important Applications: A Review. J. Sol-Gel Sci. Technol. 2020, 96, 15–33. [Google Scholar] [CrossRef]
- Ekwenna, E.B.; Wang, Y.; Roskilly, A. The Production of Bio-Silica from Agro-Industrial Wastes Leached and Anaerobically Digested Rice Straws. Bioresour. Technol. Rep. 2023, 22, 101452. [Google Scholar] [CrossRef]
- Morales-Paredes, C.A.; Rodríguez-Linzán, I.; Saquete, M.D.; Luque, R.; Osman, S.M.; Boluda-Botella, N.; Joan Manuel, R.-D. Silica-Derived Materials from Agro-Industrial Waste Biomass: Characterization and Comparative Studies. Environ. Res. 2023, 231, 116002. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and Structural Properties of Coconut Husk as Potential Silica Source. Results Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Ramasamy, S.P.; Veeraswamy, D.; Ettiyagounder, P.; Arunachalam, L.; Devaraj, S.S.; Krishna, K.; Oumabady, S.; Sakrabani, R. New Insights Into Method Development and Characterization of Amorphous Silica From Wheat Straw. Silicon 2023, 15, 5049–5063. [Google Scholar] [CrossRef]
- Castaño, F.C.; Igal, K.; Arreche, R.; Vázquez, P. Synthesis of Silica-Based Solids by Sol-Gel Technique Using Lemon Bio-Waste: Juice, Peels and Ethanolic Extract. Curr. Res. Green Sustain. Chem. 2022, 5, 100322. [Google Scholar] [CrossRef]
- Ratajski, T.; Kalemba-Rec, I.; Indyka, P.; Kąc, S.; Kot, M.; Dubiel, B. Microstructural Characterization of SiO2/Ni Nanocomposites Electrodeposited from a Sulphate Bath Modified by PEI. Mater. Charact. 2018, 142, 478–491. [Google Scholar] [CrossRef]
- Cihlářová, P.; Švejcar, J.; Sklenička, V. Microstructure and Mechanical Properties of Electrodeposited Nickel and Its Particle-Reinforced Nanocomposite. Mater. Sci. Forum 2007, 567–568, 205–208. [Google Scholar] [CrossRef]
- Kasturibai, S.; Kalaignan, G.P. Physical and Electrochemical Characterizations of Ni-SiO2 Nanocomposite Coatings. Ionics 2013, 19, 763–770. [Google Scholar] [CrossRef]
- Vidrich, G.; Castagnet, J.-F.; Ferkel, H. Dispersion Behavior of Al2O3 and SiO2 Nanoparticles in Nickel Sulfamate Plating Baths of Different Compositions. J. Electrochem. Soc. 2005, 152, C294. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Bathini, L.; Ramakrishna, L.; Rao, D.S.; Padmanabham, G. Pulsed Electrodeposition, Mechanical Properties and Wear Mechanism in Ni-W/SiC Nanocomposite Coatings Used for Automotive Applications. Appl. Surf. Sci. 2020, 527, 146896. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Bathini, L.; Sundararajan, G. Tribological Behavior of Pulsed Electrodeposited Ni-W/SiC Nanocomposites. J. Mater. Eng. Perform. 2018, 27, 5236–5245. [Google Scholar] [CrossRef]
- Karthik, R.; Mani, R.; Manikandan, P. Tribological Studies of Ni-SiC and Ni-Al2O3 Composite Coatings by Pulsed Electrodeposition. Mater. Today Proc. 2021, 37, 701–706. [Google Scholar] [CrossRef]
- Vuksanovic, M.; Mladenovic, I.; Tomic, N.; Petrovic, M.; Radojevic, V.; Marinkovic, A.; Jancic-Heinemann, R. Mechanical Properties of Biomass-Derived Silica Nanoparticles Reinforced PMMA Composite Material. Sci. Sinter. 2022, 54, 211–221. [Google Scholar] [CrossRef]
- Embirsh, H.S.A.; Stajčić, I.; Gržetić, J.; Mladenović, I.O.; Anđelković, B.; Marinković, A.; Vuksanović, M.M. Synthesis, Characterization and Application of Biobased Unsaturated Polyester Resin Reinforced with Unmodified/Modified Biosilica Nanoparticles. Polymers 2023, 15, 3756. [Google Scholar] [CrossRef] [PubMed]
- Lamovec, J.; Jovic, V.; Aleksic, R.; Radojevic, V. Micromechanical and Structural Properties of Nickel Coatings Electrodeposited on Two Different Substrates. J. Serbian Chem. Soc. 2009, 74, 817–831. [Google Scholar] [CrossRef]
- Du, C.; Zhao, Y.; Li, Y. Effect of Surface Cleaning Process on the Wafer Bonding of Silicon and Pyrex Glass. J. Inorg. Organomet. Polym. Mater. 2023, 33, 673–679. [Google Scholar] [CrossRef]
- Available online: http://gwyddion.net/ (accessed on 23 May 2024).
- Broitman, E. Indentation Hardness Measurements at Macro-, Micro-, and Nanoscale: A Critical Overview. Tribol. Lett. 2017, 65, 23. [Google Scholar] [CrossRef]
- Hou, Q.R.; Gao, J.; Li, S.J. Adhesion and Its Influence on Micro-Hardness of DLC and SiC Films. Eur. Phys. J. B 1999, 8, 493–496. [Google Scholar] [CrossRef]
- He, J.L. Hardness Measurement of Thin Films: Separation from Composite Hardness. Appl. Phys. Lett. 1996, 25, 2002. [Google Scholar] [CrossRef]
- Chen, M.; Gao, J. The Adhesion of Copper Films Coated on Silicon and Glass Substrates. Mod. Phys. Lett. B 2000, 14, 103–108. [Google Scholar] [CrossRef]
- Magagnin, L.; Maboudian, R.; Carraro, C. Adhesion Evaluation of Immersion Plating Copper Films on Silicon by Microindentation Measurements. Thin Solid Films 2003, 434, 100–105. [Google Scholar] [CrossRef]
- Magagnin, L.; Cojocaru, P.; Raygani, A.; Brivio, D.; Secundo, F.; Turolla, A.; Ottolina, G. Galvanic Displacement of Nanostructured Gold for Flavoenzyme Adsorption in Biotechnology. ECS Trans. 2011, 33, 59–66. [Google Scholar] [CrossRef]
- Algellai, A.A.; Tomić, N.; Vuksanović, M.M.; Dojčinović, M.; Volkov-Husović, T.; Radojević, V.; Heinemann, R.J. Adhesion Testing of Composites Based on Bis-GMA/TEGDMA Monomers Reinforced with Alumina Based Fillers on Brass Substrate. Compos. Part B Eng. 2018, 140, 164–173. [Google Scholar] [CrossRef]
- Tomić, N.Z.; Saleh, M.N.; Vuksanović, M.M.; Egelja, A.; Obradović, V.; Marinković, A.; Jančić Heinemann, R. Tailored Adhesion Properties of Acrylate Adhesives on Al Alloys by the Addition of Mn-Al–LDH. Polymers 2021, 13, 1525. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Bošković, M.V.; Vuksanović, M.M.; Nikolić, N.D.; Lamovec, J.S.; Vasiljević-Radović, D.G.; Radojević, V.J. Structural, Mechanical and Electrical Characteristics of Copper Coatings Obtained by Various Electrodeposition Processes. Electronics 2022, 11, 443. [Google Scholar] [CrossRef]
- Mladenović, I.O.; Nikolić, N.D.; Lamovec, J.S.; Vasiljević-Radović, D.; Radojević, V. Application of the Composite Hardness Models in the Analysis of Mechanical Characteristics of Electrolytically Deposited Copper Coatings: The Effect of the Type of Substrate. Metals 2021, 11, 111. [Google Scholar] [CrossRef]
- Li, H.; Bradt, R.C. The Microhardness Indentation Load/Size Effect in Rutile and Cassiterite Single Crystals. J. Mater. Sci. 1993, 28, 917–926. [Google Scholar] [CrossRef]
- Lamovec, J.; Jović, V.; Randjelović, D.; Aleksić, R.; Radojević, V. Analysis of the Composite and Film Hardness of Electrodeposited Nickel Coatings on Different Substrates. Thin Solid Films 2008, 516, 8646–8654. [Google Scholar] [CrossRef]
- Available online: https://Mediacy.Com/Image-Pro/ (accessed on 12 June 2024).
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Silica Extraction from Rice Husk: Comprehensive Review and Applications. Hybrid Adv. 2023, 4, 100111. [Google Scholar] [CrossRef]
- Yan, N.; Wang, F.; Zhong, H.; Li, Y.; Wang, Y.; Hu, L.; Chen, Q. Hollow Porous SiO2 Nanocubes Towards High-Performance Anodes for Lithium-Ion Batteries. Sci. Rep. 2013, 3, 1568. [Google Scholar] [CrossRef]
- Kwon, S.H.; Park, I.H.; Vu, C.M.; Choi, H.J. Fabrication and Electro-Responsive Electrorheological Characteristics of Rice Husk-Based Nanosilica Suspension. J. Taiwan Inst. Chem. Eng. 2019, 95, 432–437. [Google Scholar] [CrossRef]
- Popov, K.I.; Djokić, S.S.; Nikolić, N.D.; Jović, V.D. Morphology of Electrochemically and Chemically Deposited Metals; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-26071-6. [Google Scholar]
- Mladenović, I.O.; Lamovec, J.S.; Vasiljević Radović, D.G.; Vasilić, R.; Radojević, V.J.; Nikolić, N.D. Morphology, Structure and Mechanical Properties of Copper Coatings Electrodeposited by Pulsating Current (PC) Regime on Si(111). Metals 2020, 10, 488. [Google Scholar] [CrossRef]
- Kelly, J.J.; Goods, S.H.; Talin, A.A.; Hachman, J.T. Electrodeposition of Ni from Low-Temperature Sulfamate Electrolytes. J. Electrochem. Soc. 2006, 153, C318. [Google Scholar] [CrossRef]
- Godon, A.; Creus, J.; Feaugas, X.; Conforto, E.; Pichon, L.; Armand, C.; Savall, C. Characterization of Electrodeposited Nickel Coatings from Sulphamate Electrolyte without Additive. Mater. Charact. 2011, 62, 164–173. [Google Scholar] [CrossRef]
- Qu, N.; Zhu, D.; Chan, K.; Lei, W. Pulse Electrodeposition of Nanocrystalline Nickel Using Ultra Narrow Pulse Width and High Peak Current Density. Surf. Coat. Technol. 2003, 168, 123–128. [Google Scholar] [CrossRef]
- Rashidi, A.M.; Amadeh, A. The Effect of Saccharin Addition and Bath Temperature on the Grain Size of Nanocrystalline Nickel Coatings. Surf. Coat. Technol. 2009, 204, 353–358. [Google Scholar] [CrossRef]
- Tang, L.; Han, S.; Chen, P.; Hang, T.; Ling, H.; Wu, Y.; Li, M. Influence of Artificial Exchange Current Density on Microstructure of Ni Films by Pulse-Reverse Electroplating. Mater. Chem. Phys. 2022, 288, 126338. [Google Scholar] [CrossRef]
- Qin, B.; Zhou, S.; Chen, H.; Wang, M. Superior Corrosion and Wear Resistance of AZ91D Mg Alloy via Electrodeposited SiO2–Ni-Based Composite Coating. Mater. Chem. Phys. 2022, 283, 126001. [Google Scholar] [CrossRef]
- Xijing, L.; Yong, C. Effect of SiO2 Nanoparticles on the Hardness and Corrosion Resistance of NiW/SiO2 Nano Composite Coating Prepared by Electrodeposition. Int. J. Electrochem. Sci. 2023, 18, 100138. [Google Scholar] [CrossRef]
- Zhang, W.; Brongersma, S.H.; Clarysse, T.; Terzieva, V.; Rosseel, E.; Vandervorst, W.; Maex, K. Surface and Grain Boundary Scattering Studied in Beveled Polycrystalline Thin Copper Films. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. Process. Meas. Phenom. 2004, 22, 1830–1833. [Google Scholar] [CrossRef]
- Suhir, E.; Lee, Y.C.; Wong, C.P. (Eds.) Micro- and Opto-Electronic Materials and Structures: Physics, Mechanics, Design, Reliability, Packaging; Springer US: Boston, MA, USA, 2007; ISBN 978-0-387-27974-9. [Google Scholar]
- Chang, S.-C.; Shieh, J.-M.; Dai, B.-T.; Feng, M.-S.; Li, Y.-H. The Effect of Plating Current Densities on Self-Annealing Behaviors of Electroplated Copper Films. J. Electrochem. Soc. 2002, 149, G535. [Google Scholar] [CrossRef][Green Version]
- Lee, H.; Chen, C.-M. Impurity Effects in Electroplated-Copper Solder Joints. Metals 2018, 8, 388. [Google Scholar] [CrossRef]
- Luo, J.K.; Pritschow, M.; Flewitt, A.J.; Spearing, S.M.; Fleck, N.A.; Milne, W.I. Effects of Process Conditions on Properties of Electroplated Ni Thin Films for Microsystem Applications. J. Electrochem. Soc. 2006, 153, D155. [Google Scholar] [CrossRef]
- Liu, H.-D.; Zhao, Y.-P.; Ramanath, G.; Murarka, S.; Wang, G.-C. Thickness Dependent Electrical Resistivity of Ultrathin (<40 Nm) Cu Films. Thin Solid Films 2001, 384, 151–156. [Google Scholar] [CrossRef]
- Dordsheikh Torkamani, A.; Velashjerdi, M.; Abbas, A.; Bolourchi, M.; Maji, P. Electrodeposition of Nickel Matrix Composite Coatings via Various Boride Particles: A Review. J. Compos. Compd. 2021, 3, 91–98. [Google Scholar] [CrossRef]




| Film Type/Regime | j/mA·cm−2 | jA or jc/ja/mA·cm−2 | δ/μm | tc/ms | tp/ms (in PC), and ta (in RC) | νPC or νRC/Hz | jav/mA·cm−2 |
|---|---|---|---|---|---|---|---|
| Ni/DC | 50 | / | 6.5 ± 0.3 | / | / | / | / |
| Ni/SiO2/DC | 50 | / | 6.8 ± 0.4 | / | / | / | / |
| Ni/PC | / | 100 | 6.3 ± 0.2 | 10 | 10 | 50 | 50 |
| Ni/SiO2/PC | / | 100 | 7.2 ± 0.6 | 10 | 10 | 50 | 50 |
| Ni/RC | / | 100/100 | 5.3 ± 0.2 | 15 | 5 | 50 | 50 |
| Ni/SiO2/RC | / | 100/100 | 7.5 ± 0.7 | 15 | 5 | 50 | 50 |
| Film Type/Regime | Ni-DC | Ni/SiO2-DC | Ni-PC | Ni/SiO2-PC | Ni-RC | Ni/SiO2-RC |
|---|---|---|---|---|---|---|
| Rq/nm | 78.36 ± 3.13 | 105.4 ± 5.13 | 37.87 ± 2.03 | 49.02 ± 2.95 | 32.26 ± 2.26 | 46.66 ± 3.21 |
| Sq/nm | 73.67 ± 3.13 | 106.1 ± 5.13 | 32.45 ± 1.13 | 48.28 ± 2.95 | 27.87 ± 1.26 | 46.90 ± 3.01 |
| Film Type/Regime | Ni-DC | Ni/SiO2-DC | Ni-PC | Ni/SiO2-PC | Ni-RC | Ni/SiO2-RC |
|---|---|---|---|---|---|---|
| A ± SE | 3.225 ± 0.4 | 4.971 ± 0.4 | 3.956 ± 0.6 | 5.405 ± 0.3 | 3.029 ± 0.6 | 5.741 ± 0.3 |
| B ± SE | 9.663 ± 0.8 | −1.139 ± 1.3 | 9.396 ± 1.2 | −1.439 ± 0.8 | 11.913 ± 1.0 | −4.724 ± 1.1 |
| C ± SE | −4.293 ± 0.4 | −3.37 ± 1.3 | −3.444 ± 0.4 | −2.251 ± 0.7 | −2.409 ± 0.3 | −0.209 ± 1.2 |
| H | 3.542 | 4.861 | 4.308 | 5.562 | 3.519 | 6.880 |
| Film Type/Regime | Ni-DC | Ni/SiO2-DC | Ni-PC | Ni/SiO2-PC | Ni-RC | Ni/SiO2-RC |
|---|---|---|---|---|---|---|
| U1/mV | 394 | 330 | 383 | 156 | 360 | 212 |
| U2/mV | 429 | 320 | 410 | 170 | 355 | 180 |
| U3/mV | 360 | 296 | 460 | 180 | 360 | 180 |
| Uav/(mV) | 394.3 | 315.3 | 417.7 | 168.7 | 358.3 | 190.7 |
| R/(Ω/□) | 39.43 | 31.53 | 41.77 | 16.87 | 35.83 | 19.07 |
| σ/(S/cm) | 39.02 | 46.64 | 38.00 | 82.52 | 52.66 | 69.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenović, I.O.; Nikolić, N.D.; Jovanov, V.; Radovanović, Ž.M.; Obradov, M.M.; Vasiljević-Radović, D.G.; Vuksanović, M.M. Influence of SiO2 Nanoparticles Extracted from Biomass on the Properties of Electrodeposited Ni Matrix Composite Films on Si(100) Substrate. Materials 2024, 17, 4138. https://doi.org/10.3390/ma17164138
Mladenović IO, Nikolić ND, Jovanov V, Radovanović ŽM, Obradov MM, Vasiljević-Radović DG, Vuksanović MM. Influence of SiO2 Nanoparticles Extracted from Biomass on the Properties of Electrodeposited Ni Matrix Composite Films on Si(100) Substrate. Materials. 2024; 17(16):4138. https://doi.org/10.3390/ma17164138
Chicago/Turabian StyleMladenović, Ivana O., Nebojša D. Nikolić, Vladislav Jovanov, Željko M. Radovanović, Marko M. Obradov, Dana G. Vasiljević-Radović, and Marija M. Vuksanović. 2024. "Influence of SiO2 Nanoparticles Extracted from Biomass on the Properties of Electrodeposited Ni Matrix Composite Films on Si(100) Substrate" Materials 17, no. 16: 4138. https://doi.org/10.3390/ma17164138
APA StyleMladenović, I. O., Nikolić, N. D., Jovanov, V., Radovanović, Ž. M., Obradov, M. M., Vasiljević-Radović, D. G., & Vuksanović, M. M. (2024). Influence of SiO2 Nanoparticles Extracted from Biomass on the Properties of Electrodeposited Ni Matrix Composite Films on Si(100) Substrate. Materials, 17(16), 4138. https://doi.org/10.3390/ma17164138










