Silicon Carbide Nanowire Based Integrated Electrode for High Temperature Supercapacitors
Abstract
:1. Introduction
2. Experiment Section
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usiskin, R.; Lu, Y.X.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Li, Q.Q.; Liu, M.J.; Huang, F.Z.; Zuo, X.Q.; Wei, X.; Li, S.K.; Zhang, H. Co9S8@MnO2 core-shell defective heterostructure for High-Voltage flexible supercapacitor and Zn-ion hybrid supercapacitor. Chem. Eng. J. 2022, 437, 135494. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.L.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric Supercapacitor Electrodes and Devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.Z.; Zhang, M.; Li, X.F. Carbon nanomaterials and their composites for supercapacitors. Carbon Energy 2022, 4, 950–985. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, A.; Zhang, X.R.; Mu, J.B.; Che, H.W.; Wang, Y.M.; Wang, T.T.; Zhang, Z.X.; Kou, Z.K. Fundamentals, advances and challenges of transition metal compounds-based supercapacitors. Chem. Eng. J. 2021, 412, 128611. [Google Scholar] [CrossRef]
- Ma, Y.P.; Xie, X.B.; Yang, W.Y.; Yu, Z.P.; Sun, X.Q.; Zhang, Y.P.; Yang, X.Y.; Kimura, H.; Hou, C.X.; Guo, Z.H.; et al. Recent advances in transition metal oxides with different dimensions as electrodes for high-performance supercapacitors. Adv. Compos. Hybrid Mater. 2021, 4, 906–924. [Google Scholar] [CrossRef]
- Luo, L.; Meng, W.; Wang, G.J.; Qin, J.L.; He, H.Y.; Huang, H.J. MnO2 nanoflowers-decorated MXene nanosheets with enhanced supercapacitor performance. J. Alloys Compd. 2023, 957, 170411. [Google Scholar] [CrossRef]
- Yang, Q.H.; Li, Z.H.; Zhang, R.K.; Zhou, L.; Shao, M.F.; Wei, M. Carbon modified transition metal oxides/hydroxides nanoarrays toward high-performance flexible all-solid-state supercapacitors. Nano Energy 2017, 41, 408–416. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Zhang, J.; Li, J.; Ma, Z.; Li, J.; Leng, B.; Niu, P.; Liu, B. Enhanced optoelectronic performance of 3C-SiC/ZnO heterostructure photodetector based on Piezo-phototronic effect. Nano Energy 2020, 77, 105119. [Google Scholar] [CrossRef]
- Gopika, R.; Arun, K.; Ramesan, M.T. In-situ polymerization of polythiophene/silicon carbide nanocomposites for gas sensing and optoelectronic devices. J. Mater. Res. Technol. 2024, 30, 1288–1300. [Google Scholar] [CrossRef]
- Yu, M.; Wan, P.; Tang, K.; He, S.; Zhao, Q.; Zhai, Y.; Shi, D.; Kan, C.; Jiang, M. Plasmonically-boosted high-performance UV self-biased photodetector based on SiC-based low-dimensional heterojunction via Pt nanostructures deposition. Surf. Interfaces 2024, 51, 104627. [Google Scholar] [CrossRef]
- Yi, A.; Wang, C.; Zhou, L.; Zhu, Y.; Zhang, S.; You, T.; Zhang, J.; Ou, X. Silicon carbide for integrated photonics. Appl. Phys. Rev. 2022, 9, 031302. [Google Scholar] [CrossRef]
- Burk, A.A.; O’Loughlin, M.J.; Siergiej, R.R.; Agarwal, A.K.; Sriram, S.; Clarke, R.C.; MacMillan, M.F.; Balakrishna, V.; Brandt, C.D. SiC and GaN wide bandgap semiconductor materials and devices. Solid-State Electron. 1999, 43, 1459–1464. [Google Scholar] [CrossRef]
- Liang, C.; Wang, S.; Sha, S.; Lv, S.; Wang, G.; Wang, B.; Li, Q.; Yu, J.; Xu, X.; Zhang, L. Novel semiconductor materials for advanced supercapacitors. J. Mater. Chem. C 2023, 11, 4288–4317. [Google Scholar] [CrossRef]
- Ojha, G.P.; Kang, G.W.; Kuk, Y.-S.; Hwang, Y.E.; Kwon, O.H.; Pant, B.; Acharya, J.; Park, Y.W.; Park, M. Silicon Carbide Nanostructures as Potential Carbide Material for Electrochemical Supercapacitors: A Review. Nanomaterials 2023, 13, 150. [Google Scholar] [CrossRef]
- Li, W.J.; Liu, Q.; Chen, S.L.; Fang, Z.; Liang, X.; Wei, G.D.; Wang, L.; Yang, W.Y.; Ji, Y.; Mai, L.Q. Single-crystalline integrated 4H-SiC nanochannel array electrode: Toward high-performance capacitive energy storage for robust wide-temperature operation. Mater. Horiz. 2018, 5, 883–889. [Google Scholar] [CrossRef]
- Li, X.X.; Li, W.J.; Liu, Q.; Chen, S.L.; Wang, L.; Gao, F.M.; Shao, G.; Tian, Y.; Lin, Z.F.; Yang, W.Y. Robust High-Temperature Supercapacitors Based on SiC Nanowires. Adv. Funct. Mater. 2021, 31, 202008901. [Google Scholar] [CrossRef]
- Kang, P.C.; Zhao, Q.Q.; Zhang, T.; Xue, W.; Qian, J.R.; Wei, Z.Y.; Wang, P.P.; Wu, G.H. Synthesis of 3C/2H/6H heterojunction SiC nanowires with high-performance supercapacitors by thermal evaporation. J. Mater. Chem. A 2023, 11, 15347–15358. [Google Scholar] [CrossRef]
- Ran, J.; Liu, Y.F.; Yang, T.T.; Feng, H.X.; Zhan, H.Y.; Shi, H.X. MnO2@MoS2/RGO hollow structure as high-performance supercapacitor electrode materials. J. Energy Storage 2023, 64, 107216. [Google Scholar] [CrossRef]
- Pan, Z.H.; Jin, L.; Yang, C.H.; Ji, X.H.; Liu, M.L. Mo-doped MnO2@CC electrode for high-performance 2.4 V aqueous asymmetric supercapacitors. Chem. Eng. J. 2023, 470, 144084. [Google Scholar] [CrossRef]
- Wang, J.X.; Guo, W.; Liu, Z.X.; Zhang, Q.Y. Engineering of Self-Aggregation-Resistant MnO2 Heterostructure with A Built-in Field for Enhanced High-Mass-Loading Energy Storage. Adv. Energy Mater. 2023, 13, 2300224. [Google Scholar] [CrossRef]
- Martins, V.L.; Torresi, R.M. Ionic liquids in electrochemical energy storage. Curr. Opin. Electrochem. 2018, 9, 26–32. [Google Scholar] [CrossRef]
- Liang, C.; Wang, S.; Tian, G.; Lv, S.; Wang, G.; Xie, X.; Li, L.; Xu, X.; Liu, G.; Zhang, L. Silicon carbide single crystals for high-temperature supercapacitors. Nanoscale 2024, 16, 9536–9544. [Google Scholar] [CrossRef] [PubMed]
- Zeplin, G.; Neiva, E.G.C. One-pot green synthesis of graphene oxide/MnO2/polyaniline nanocomposites applied in aqueous and neutral supercapacitors and sensors. J. Electroanal. Chem. 2021, 902, 115776. [Google Scholar] [CrossRef]
- Chen, D.; Ding, D.; Li, X.; Waller, G.H.; Xiong, X.; El-Sayed, M.A.; Liu, M. Probing the Charge Storage Mechanism of a Pseudocapacitive MnO2 Electrode Using in Operando Raman Spectroscopy. Chem. Mater. 2015, 27, 6608–6619. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, L.; Chen, D.; Ji, X.; Jiang, Z.-j.; Ding, D.; Liu, M. Phase evolution of an alpha MnO2-based electrode for pseudo-capacitors probed by in operando Raman spectroscopy. Nano Energy 2014, 9, 161–167. [Google Scholar] [CrossRef]
- Wang, M.; Xing, Y.; Shi, Q.H.; Ge, Y.S.; Xiang, M.L.; Huang, Z.R.; Xuan, Q.Y.; Fan, Y.Q.; Zhao, Y.F. High Areal Capacity FeS@Fe Foam Anode with Hierarchical Structure for Alkaline Solid-State Energy Storage. Adv. Energy Mater. 2024, 14, 2304060. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Sun, S.S.; Xu, Z.H.; Yin, S.G. Multicomponent Hybridization Transition Metal Oxide Electrode Enriched with Oxygen Vacancy for Ultralong-Life Supercapacitor. Small 2023, 19, 2302479. [Google Scholar] [CrossRef]
- Fu, Y.S.; Gao, X.Y.; Zha, D.S.; Zhu, J.W.; Ouyang, X.P.; Wang, X. Yolk-shell-structured MnO2 microspheres with oxygen vacancies for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 1601–1611. [Google Scholar] [CrossRef]
- Li, C.; Xu, Q.; Zhu, J.; Luo, T.; Yang, M.; Dai, H.; Kosinova, M.L.; Zhang, S.; Tu, R. Laser chemical vapor deposition of nitrogen-doped SiC electrode for electrochemical detection of uric acid. Surf. Interfaces 2024, 29, 104704. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Deng, C.; Yu, C.; Ding, J.; Zhu, H. Controllable synthesis of Si@SiC plate@Si3N4 whisker with core-shell structure and their electrochemical performances. Powder Technol. 2021, 377, 324–335. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Li, K.; Cui, Q.A.; Han, L.; Shen, Q.; Li, H.; Yin, X. Construction of core-shell structured SiC nanowires@carbon nanotubes hybrid conductive network for supercapacitors and electromagnetic interference shielding. Carbon 2024, 228, 119411. [Google Scholar] [CrossRef]
- Zhao, F.; Lin, L.; Zhang, J.; Liu, J. Construction of anchoring MnO2 on wood-derived integral nitrogen-doped carbon electrode for high-performance supercapacitors. J. Energy Storage 2024, 95, 112631. [Google Scholar] [CrossRef]
- Feng, S.; Wang, H.; Ma, J.; Lin, Z.; Wang, C.; Li, X.; Ma, M.; Li, T.; Ma, Y. Fabrication of hollow Ni/NiO/C/MnO2@polypyrrole core-shell structures for high-performance electromagnetic wave absorption. Compos. Part B Eng. 2024, 275, 111344. [Google Scholar] [CrossRef]
- Nongthombam, S.; Laha, S.; Swain, B.P. Chemically synthesized rGO/SiC nanocomposites and their electrochemical performance for supercapacitor electrode. Phys. B Condens. Matter. 2023, 652, 414622. [Google Scholar] [CrossRef]
- Parveen, N.; Ansari, S.A.; Al-Arjan, W.S.; Ansari, M.O. Manganese dioxide coupled with hollow carbon nanofiber toward high-performance electrochemical supercapacitive electrode materials. J. Sci. Adv. Mater. Devices 2021, 6, 472–482. [Google Scholar] [CrossRef]
- Deng, C.; He, Z.; Huang, J.; Liu, L.; Liu, Y.; Wang, T.; Chen, G.; Yi, Y.; Du, K. Pre-activation strategy of α-MnO2 electrode to construct high-performance asymmetric sodium-ion supercapacitors. Appl. Surf. Sci. 2023, 627, 157299. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, X.; Cheng, G.; Li, Y.; Li, C.; He, Z.; Zhu, J.; Meng, W.; Zhou, H.; Dai, L.; et al. Electrocatalytic activity of MnO2 nanosheet array-decorated carbon paper as superior negative electrode for vanadium redox flow batteries. Electrochim. Acta 2019, 322, 134754. [Google Scholar] [CrossRef]
- Bagal, I.V.; Chodankar, N.R.; Waseem, A.; Ali Johar, M.; Patil, S.J.; Abdullah, A.; Afifi Hassan, M.; Han, Y.-K.; Ryu, S.-W. CF4 plasma-treated porous silicon nanowire arrays laminated with MnO2 nanoflakes for asymmetric pseudocapacitors. Chem. Eng. J. 2021, 419, 129515. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, X.; Wang, F.; Xiong, T.; Balogun, M.S.; Zhou, H.; Deng, J. Reduced graphene oxide thin layer induced lattice distortion in high crystalline MnO2 nanowires for high-performance sodium- and potassium-ion batteries and capacitors. Carbon 2021, 174, 556–566. [Google Scholar] [CrossRef]
- Yang, J.; Lian, L.; Ruan, H.; Xie, F.; Wei, M. Nanostructured porous MnO2 on Ni foam substrate with a high mass loading via a CV electrodeposition route for supercapacitor application. Electrochim. Acta 2014, 136, 189–194. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.J.; Zhang, M.; Meng, A.; Li, Q.D. Direct Growth of Ultrathin NiCo2O4/NiO Nanosheets on SiC Nanowires as a Free-Standing Advanced Electrode for High-Performance Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 3598–3608. [Google Scholar] [CrossRef]
- Park, T.; Jang, Y.; Park, J.W.; Kim, H.; Kim, S.J. Quasi-solid-state highly stretchable circular knitted MnO2@CNT supercapacitor. RSC Adv. 2020, 10, 14007–14012. [Google Scholar] [CrossRef]
- Wang, S.T.; Shao, Y.L.; Liu, W.; Wu, Y.Z.; Hao, X.P. Elastic sandwich-type GaN/MnO2/MnON composites for flexible supercapacitors with high energy density. J. Mater. Chem. A 2018, 6, 13215. [Google Scholar] [CrossRef]
- Haque, M.; Li, Q.; Smith, A.D.; Kuzmenko, V.; Köhler, E.; Lundgren, P.; Enoksson, P. Thermal influence on the electrochemical behavior of a supercapacitor containing an ionic liquid electrolyte. Electrochim. Acta 2018, 263, 249–260. [Google Scholar] [CrossRef]
- Huang, Z.H.; Song, Y.; Feng, D.Y.; Sun, Z.; Sun, X.Q.; Liu, X.X. High Mass Loading MnO2 with Hierarchical Nanostructures for Supercapacitors. ACS Nano 2018, 12, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Zeraati, M.; Tahmasebi, K. Supercapacitor behavior of SiC coated copper hydroxide and copper sulfide nano-wires. J. Alloys Compd. 2019, 786, 798–807. [Google Scholar] [CrossRef]
- Gao, F.; Lewes-Malandrakis, G.; Wolfer, M.T.; Müller-Sebert, W.; Gentile, P.; Aradilla, D.; Schubert, T.; Nebel, C.E. Diamond-coated silicon wires for supercapacitor applications in ionic liquids. Diam. Relat. Mater. 2015, 51, 1–6. [Google Scholar] [CrossRef]
- Aradilla, D.; Gao, F.; Lewes-Malandrakis, G.; Müller-Sebert, W.; Gaboriau, D.; Gentile, P.; Iliev, B.; Schubert, T.; Sadki, S.; Bidan, G.; et al. A step forward into hierarchically nanostructured materials for high performance micro-supercapacitors: Diamond-coated SiNW electrodes in protic ionic liquid electrolyte. Electrochem. Commun. 2016, 63, 34–38. [Google Scholar] [CrossRef]
- Alper, J.P.; Kim, M.S.; Vincent, M.; Hsia, B.; Radmilovic, V.; Carraro, C.; Maboudian, R. Silicon carbide nanowires as highly robust electrodes for micro-supercapacitors. J. Power Sources 2013, 230, 298–302. [Google Scholar] [CrossRef]
- Dubal, D.P.; Aradilla, D.; Bidan, G.; Gentile, P.; Schubert, T.J.S.; Wimberg, J.; Sadki, S.; Gomez-Romero, P. 3D hierarchical assembly of ultrathin MnO2 nanoflakes on silicon nanowires for high performance micro-supercapacitors in Li- doped ionic liquid. Sci. Rep. 2015, 5, 9771. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Pan, Z.; Liu, M.; Hou, Y.; Xu, Y.; Deng, H.; Sheng, L.; Zhao, X.; Qiu, Y.; et al. All-Solid-State High-Energy Asymmetric Supercapacitors Enabled by Three-Dimensional Mixed-Valent MnOx Nanospike and Graphene Electrodes. ACS Appl. Mater. Interfaces 2015, 7, 22172–22180. [Google Scholar] [CrossRef]
- Devarapalli, R.R.; Szunerits, S.; Coffinier, Y.; Shelke, M.V.; Boukherroub, R. Glucose-Derived Porous Carbon-Coated Silicon Nanowires as Efficient Electrodes for Aqueous Micro-Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 4298–4302. [Google Scholar] [CrossRef] [PubMed]
- Berton, N.; Brachet, M.; Thissandier, F.; Le Bideau, J.; Gentile, P.; Bidan, G.; Brousse, T.; Sadki, S. Wide-voltage-window silicon nanowire electrodes for micro-supercapacitors via electrochemical surface oxidation in ionic liquid electrolyte. Electrochem. Commun. 2014, 41, 31–34. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M. Spectroscopic studies of the local structure in positive electrodes for lithium batteries. Phys. Chem. Chem. Phys. 2002, 4, 4226–4235. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Y.W.; Fang, Y.J.; Lu, R.; Sha, J. Performance characteristics of supercapacitor electrodes made of silicon carbide nanowires grown on carbon fabric. J. Power Sources 2013, 243, 648–653. [Google Scholar] [CrossRef]

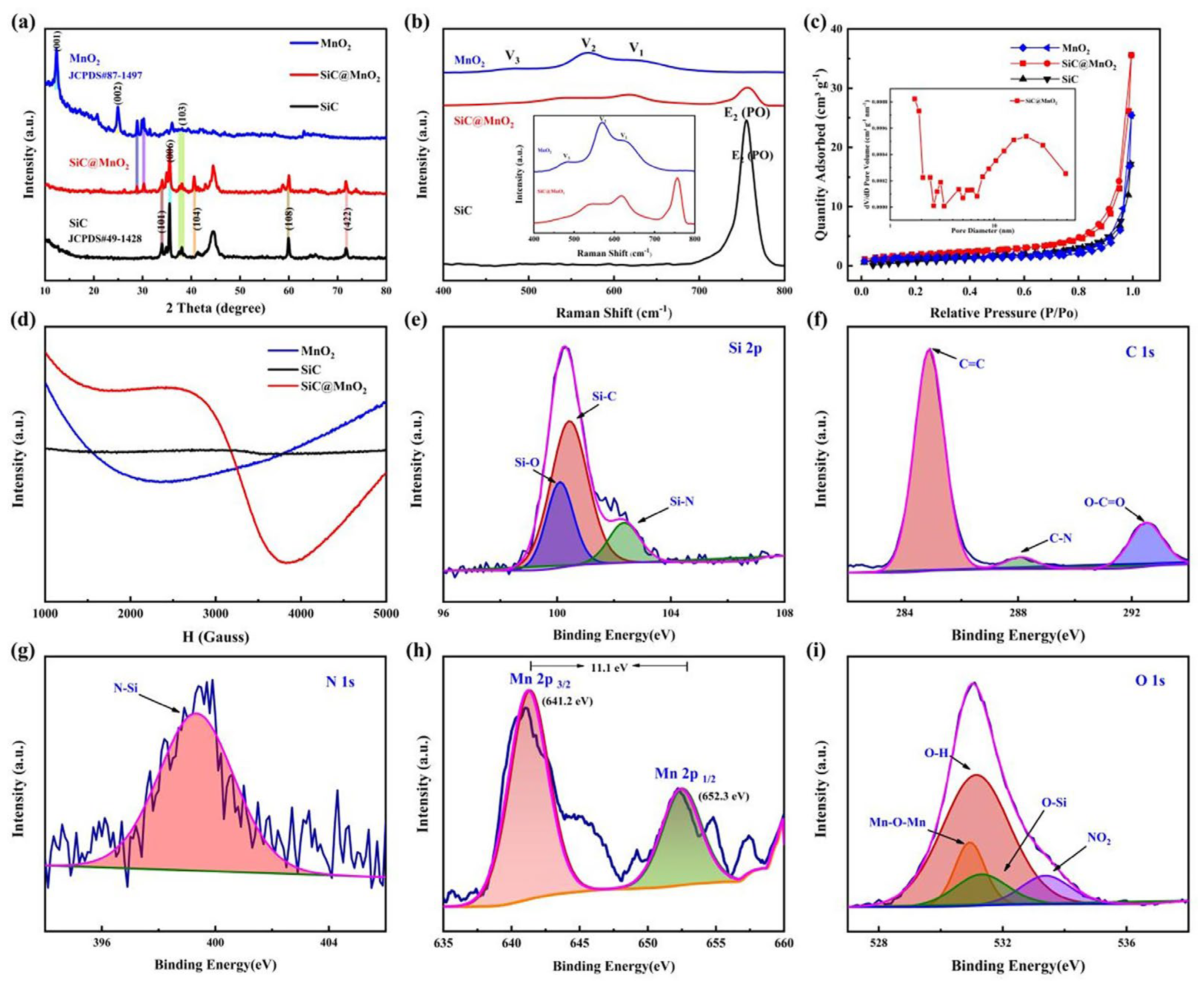
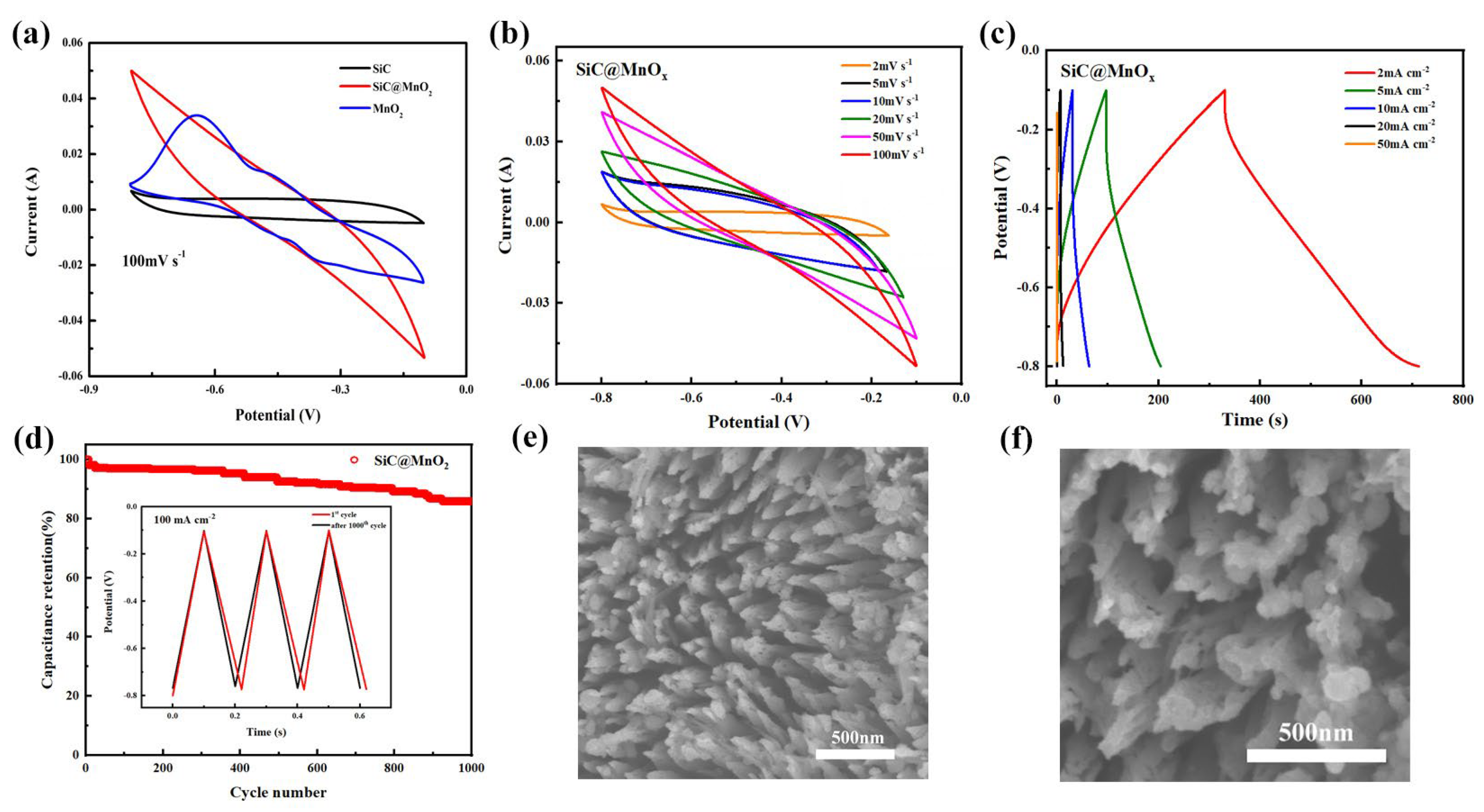
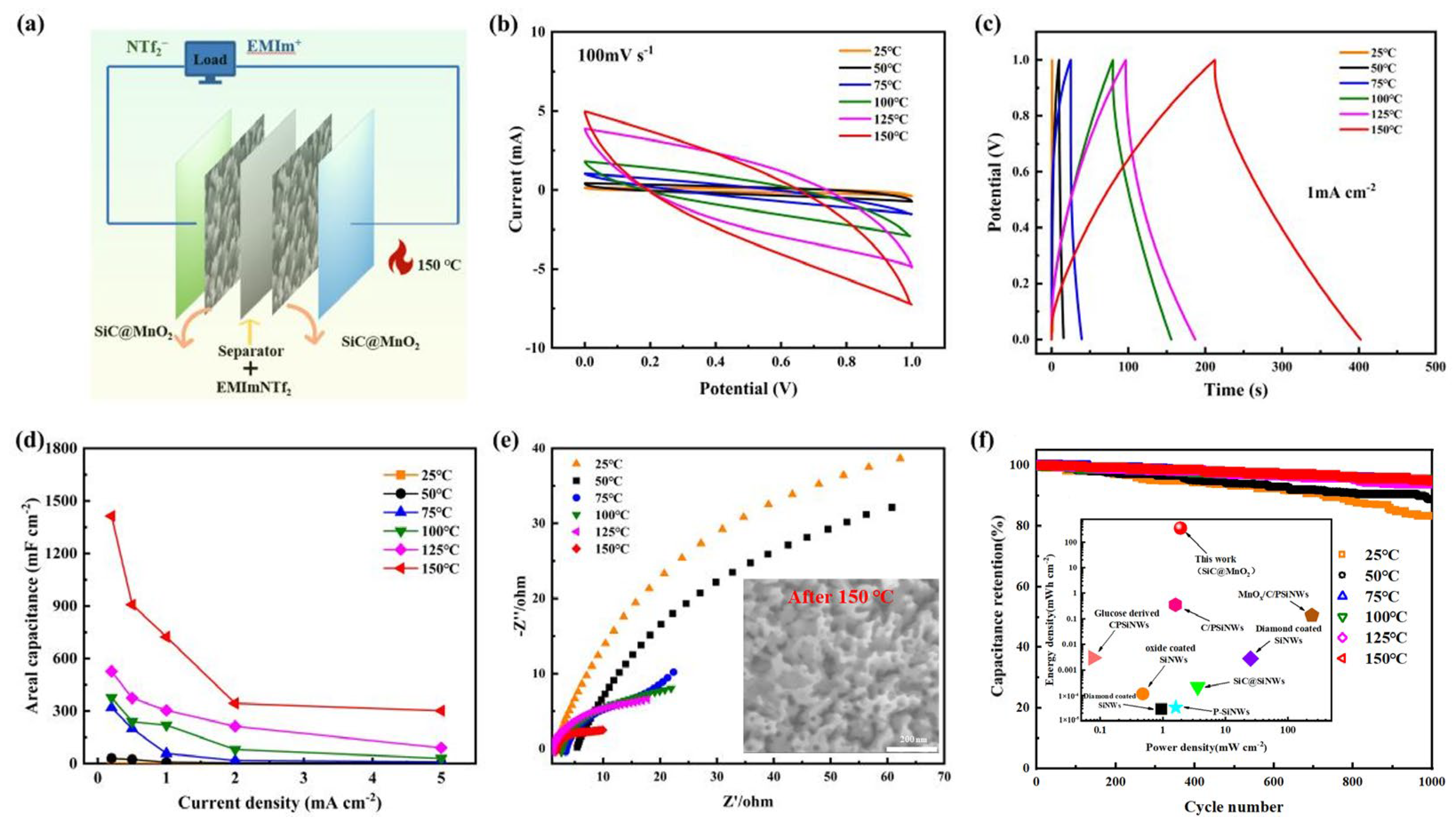
| Electrode Materials | Energy Density | Power Density | Capacity Retained after Cycling | Reference |
|---|---|---|---|---|
| SiC@MnO2 | 362 mWh cm−2 | 1.905 mW cm−2 | 95% after 1000 cycles | This work |
| Diamond-coated Si NWs | 84 μJ cm−2 | 0.94 mW cm−2 | 93.3% after 10,000 cycles | [49] |
| MnO2@ SiNWs | 29.1 μWh cm−2 | 9.1 μWh cm−2 | 91% after 5000 cycles | [52] |
| 3D Al@Ni@MnOx NSP | 23.02 Wh kg−1 | 947.11 Wh kg−1 | 96.3% after 10,000 cycles | [53] |
| C@SiNWs | 17.4 mJ m−2 | 0.351 W m−2 | 75% after 25,000 cycles | [54] |
| SiC nanowires | 1.7 mJ cm−2 | 12 mW cm−2 | 90% after 100,000 cycles | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, S.; Liang, C.; Lv, S.; Xu, L.; Sun, D.; Yang, J.; Zhang, L.; Wang, S. Silicon Carbide Nanowire Based Integrated Electrode for High Temperature Supercapacitors. Materials 2024, 17, 4161. https://doi.org/10.3390/ma17164161
Sha S, Liang C, Lv S, Xu L, Sun D, Yang J, Zhang L, Wang S. Silicon Carbide Nanowire Based Integrated Electrode for High Temperature Supercapacitors. Materials. 2024; 17(16):4161. https://doi.org/10.3390/ma17164161
Chicago/Turabian StyleSha, Shiyu, Chang Liang, Songyang Lv, Lin Xu, Defu Sun, Jiayue Yang, Lei Zhang, and Shouzhi Wang. 2024. "Silicon Carbide Nanowire Based Integrated Electrode for High Temperature Supercapacitors" Materials 17, no. 16: 4161. https://doi.org/10.3390/ma17164161





