Damage and Recovery Behavior of Low-Replacement-Rate Fly Ash Concrete after Different High-Temperature Exposures
Abstract
:1. Introduction
2. Materials and Methods
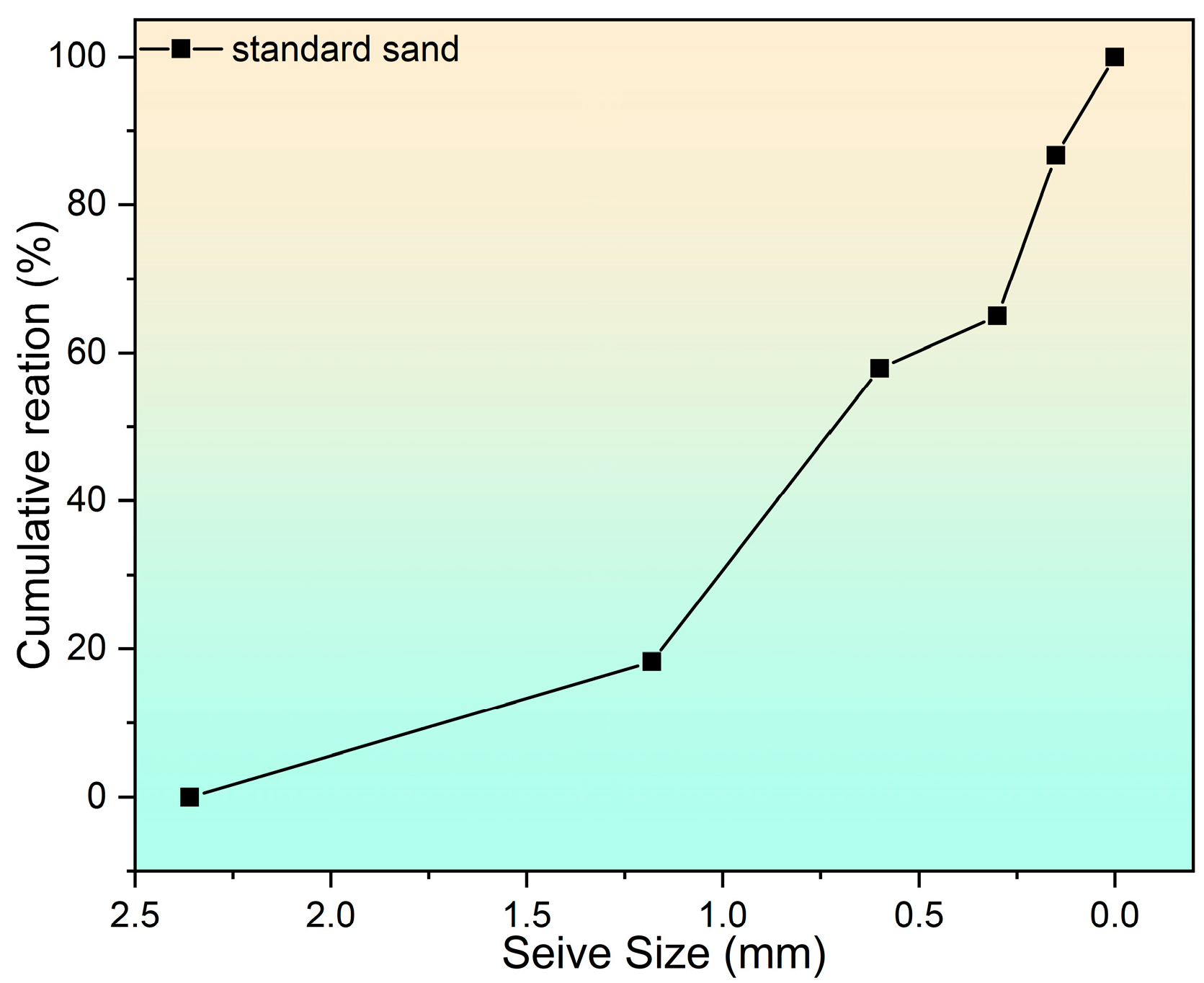
2.1. Raw Materials and Mix Design
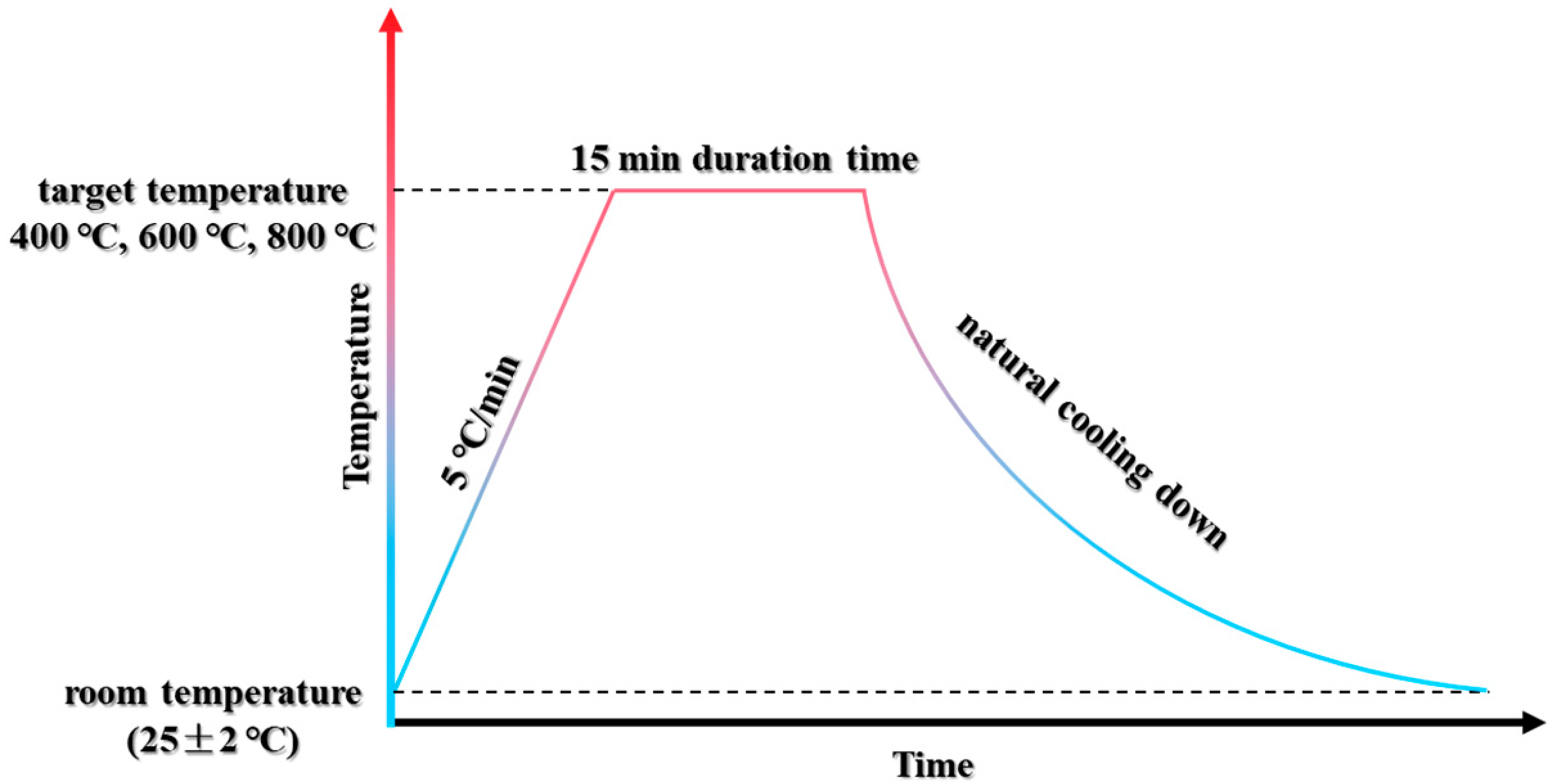
2.2. High-Temperature Exposure and Recuring
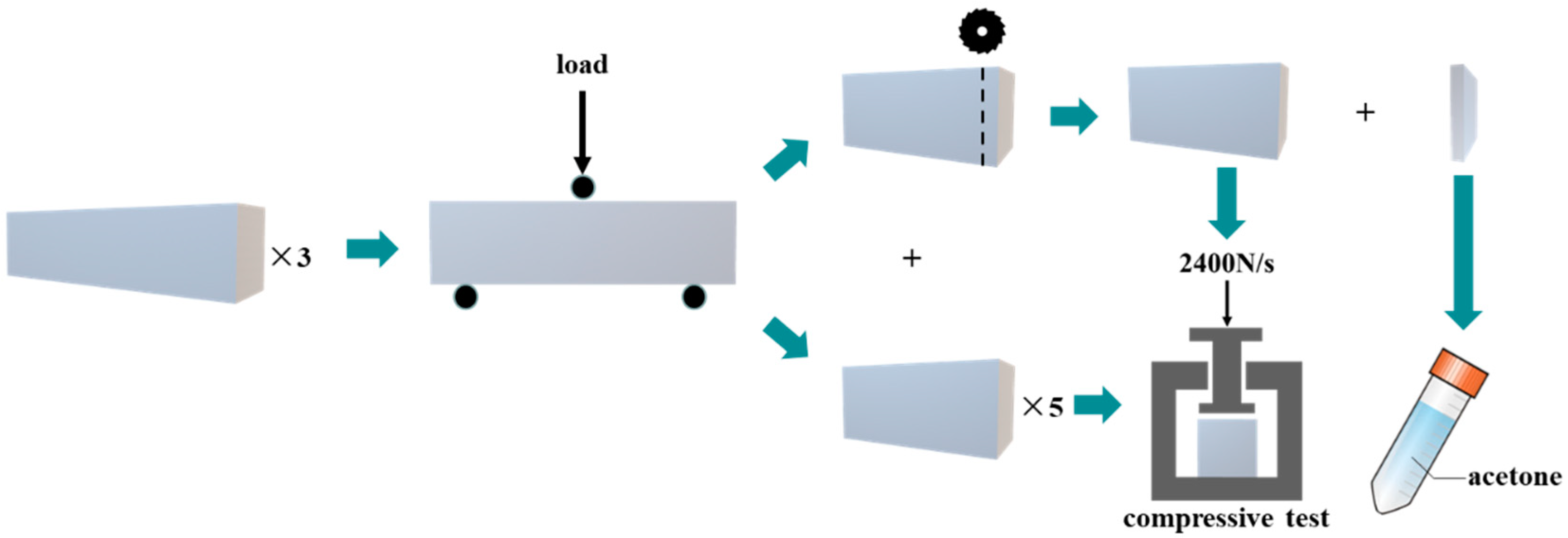
2.3. Test and Sampling Methods
3. Results and Discussion
3.1. Raw Materials
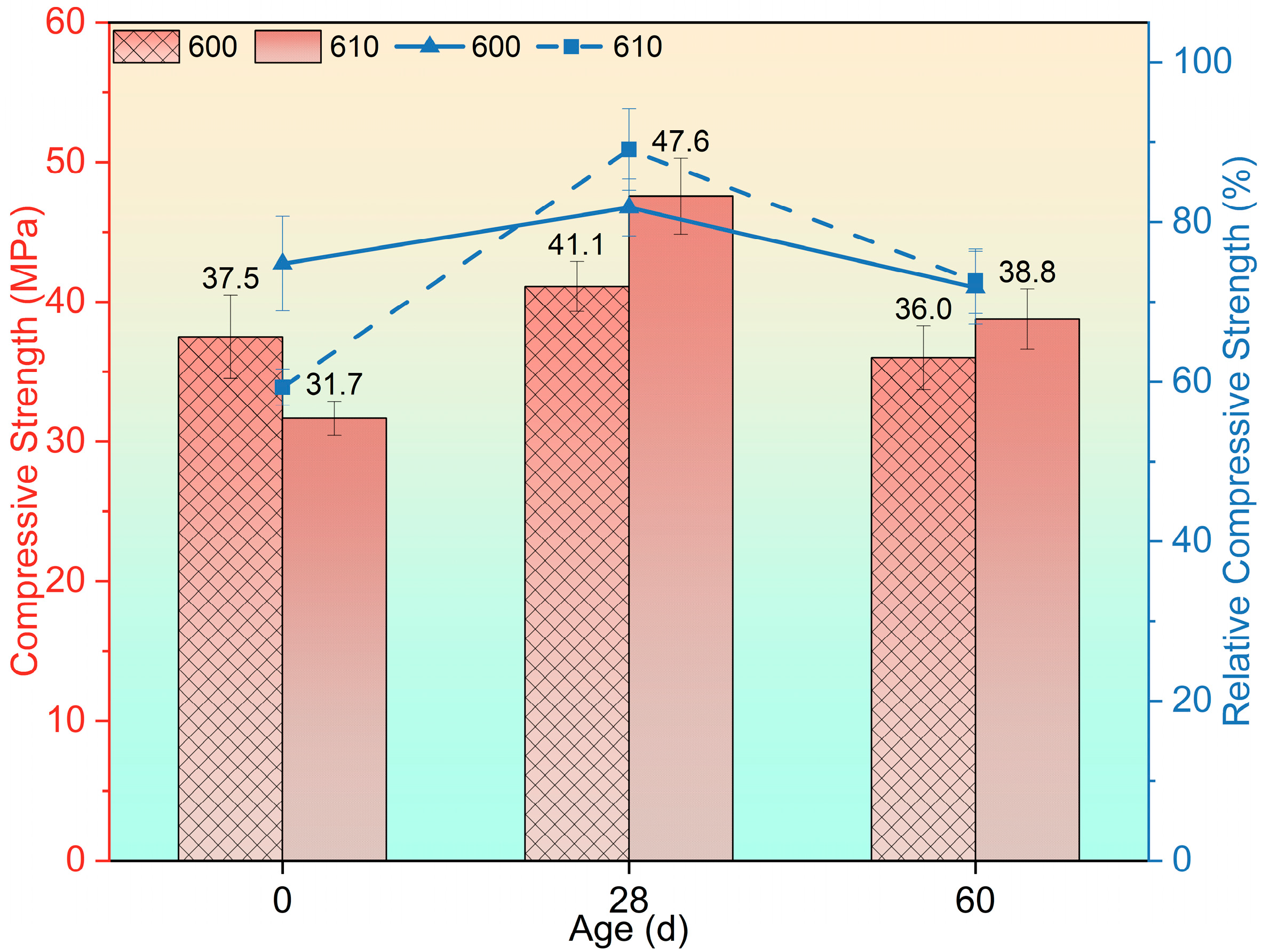
3.2. Flexural and Compressive Tests
3.3. Microstructural and Chemical Composition Studies
3.3.1. XRD Studies
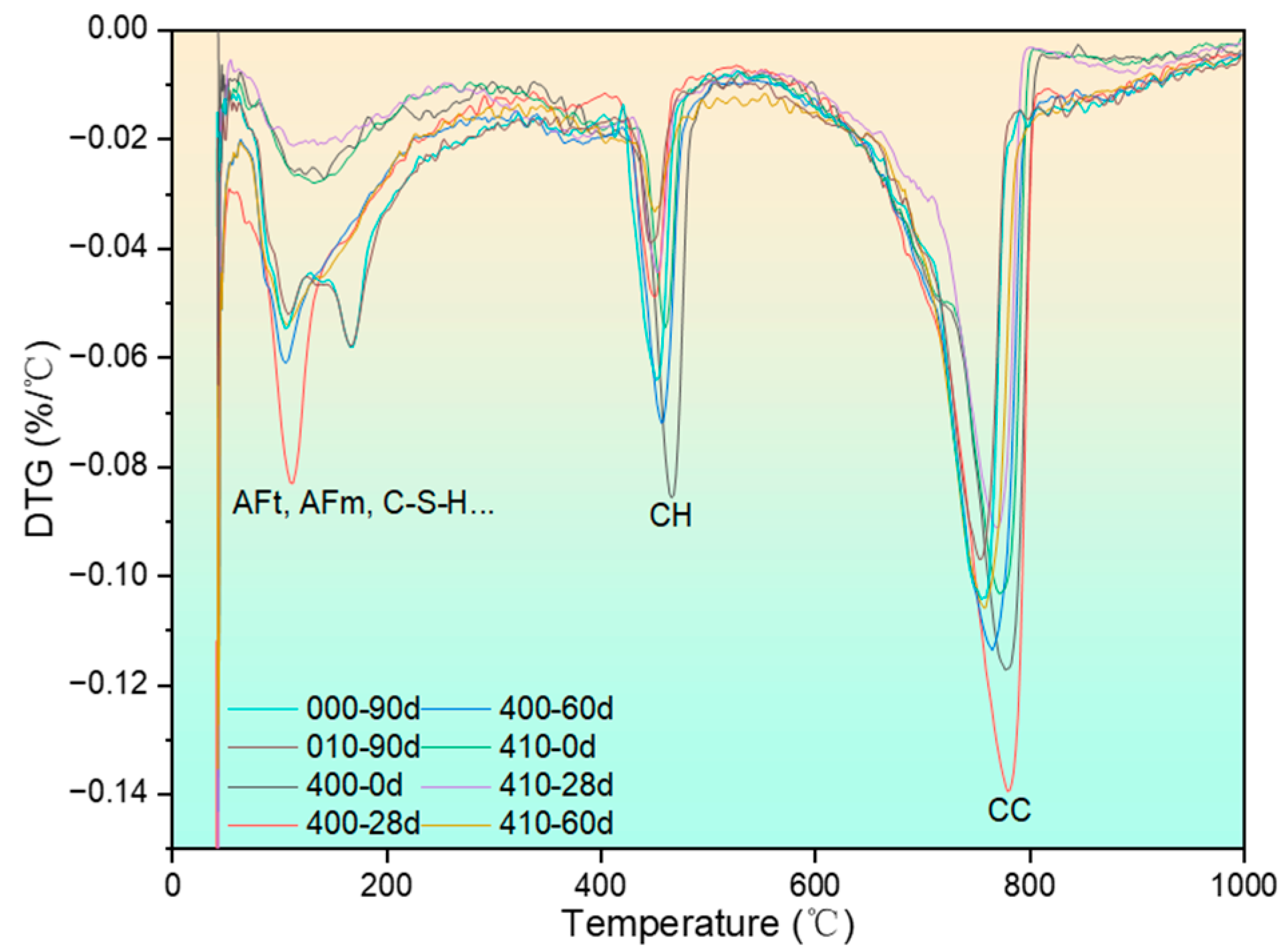
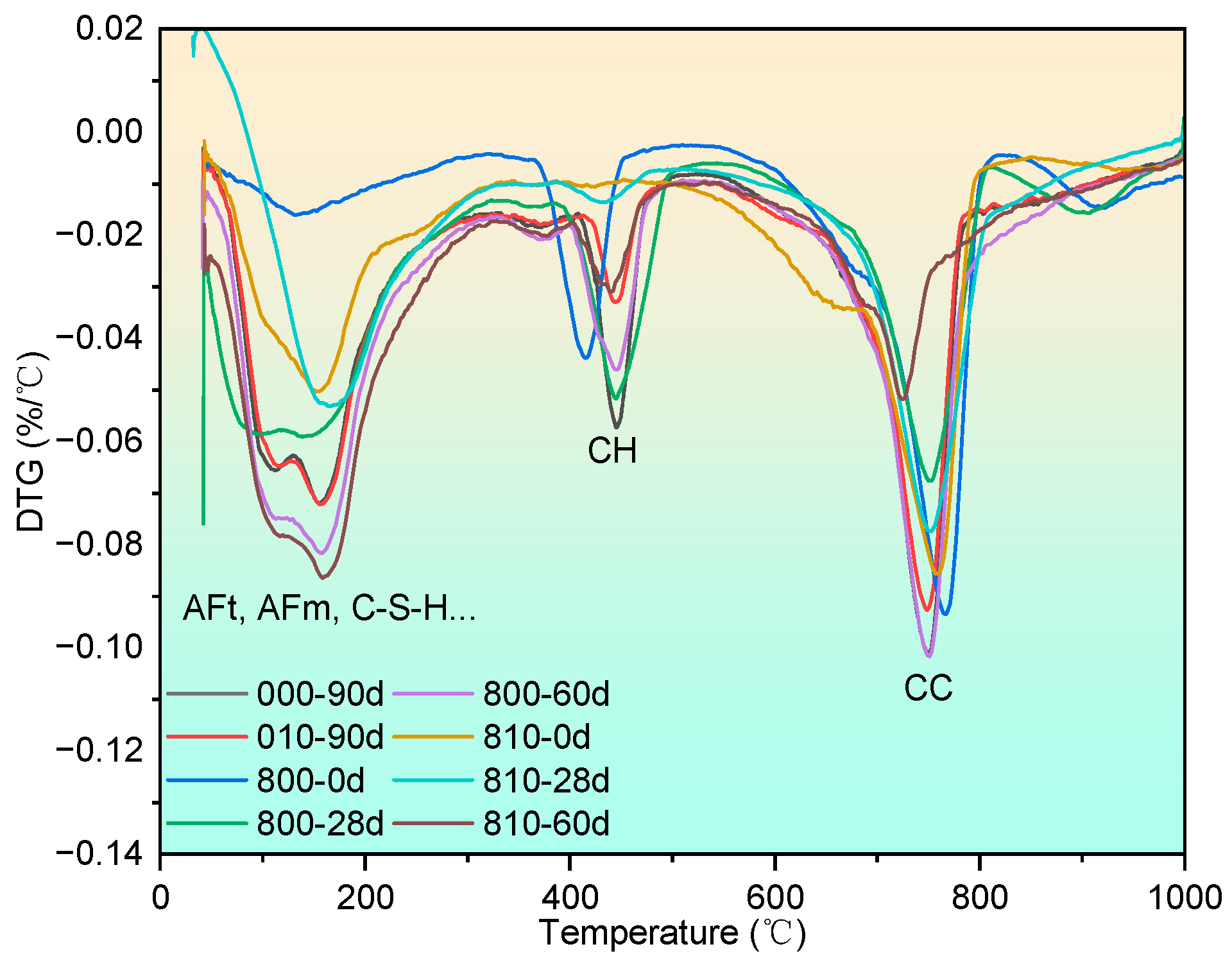
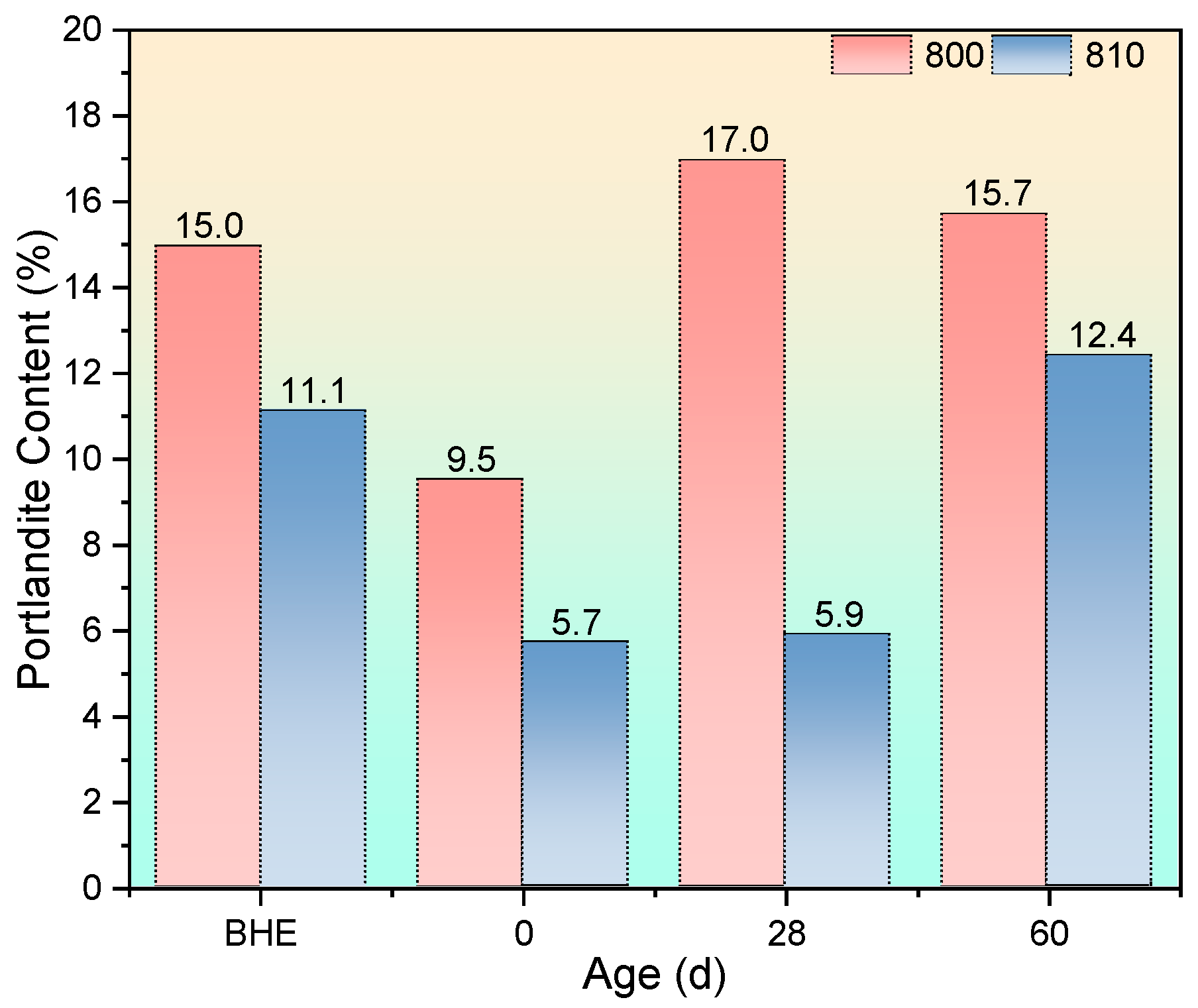
3.3.2. Thermogravimetric Analysis
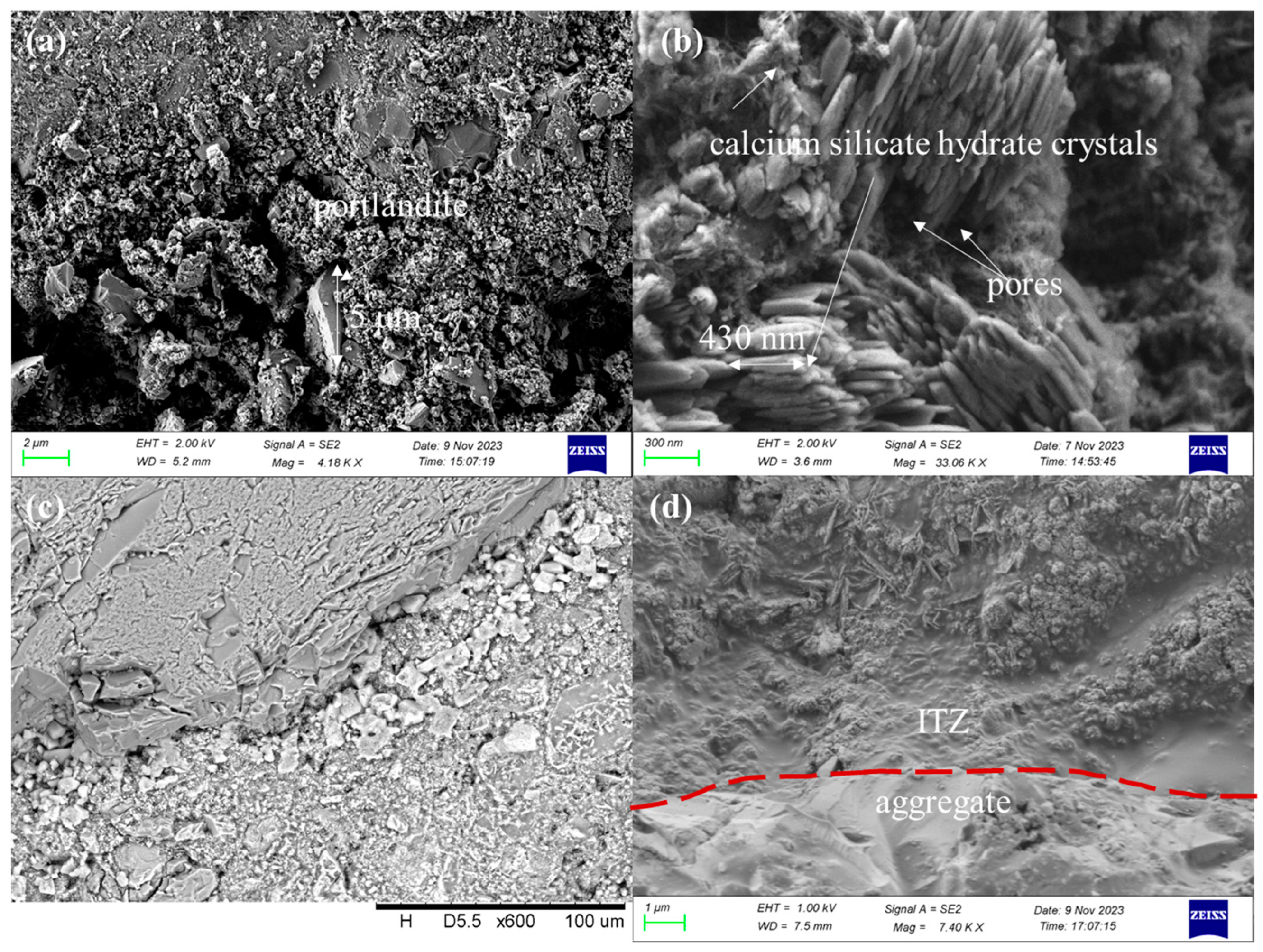
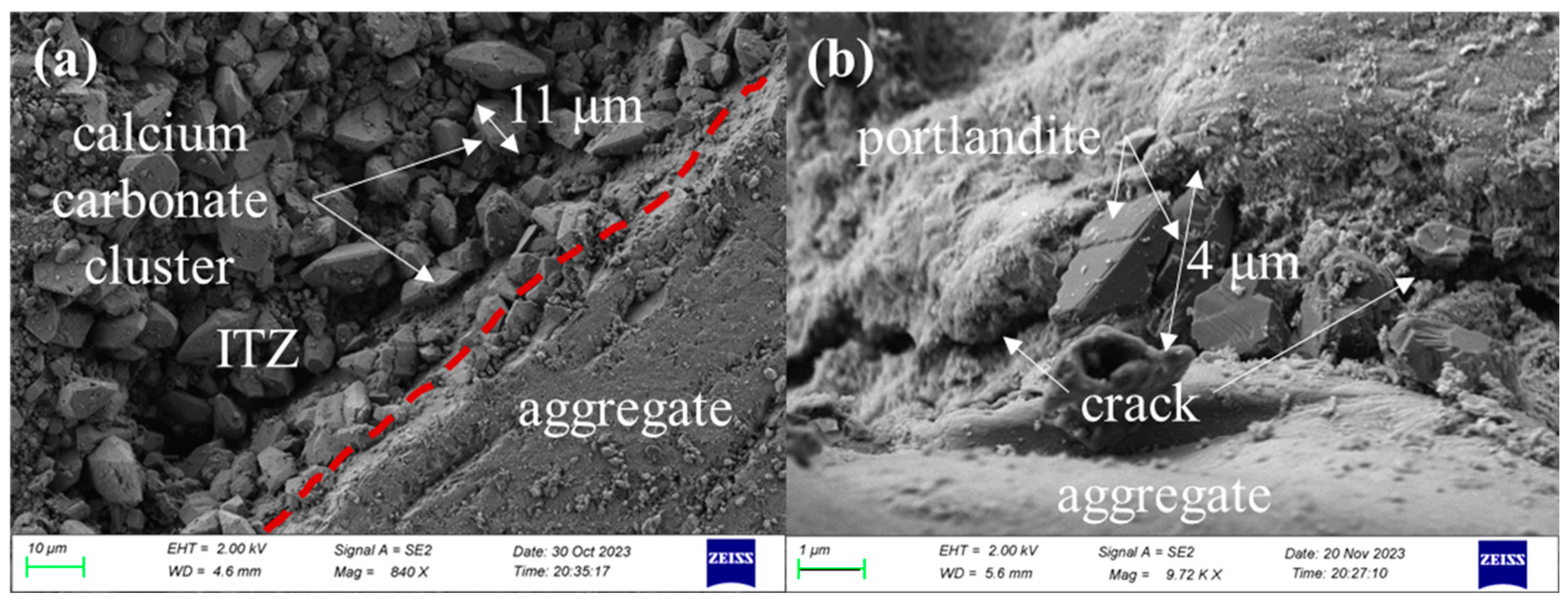
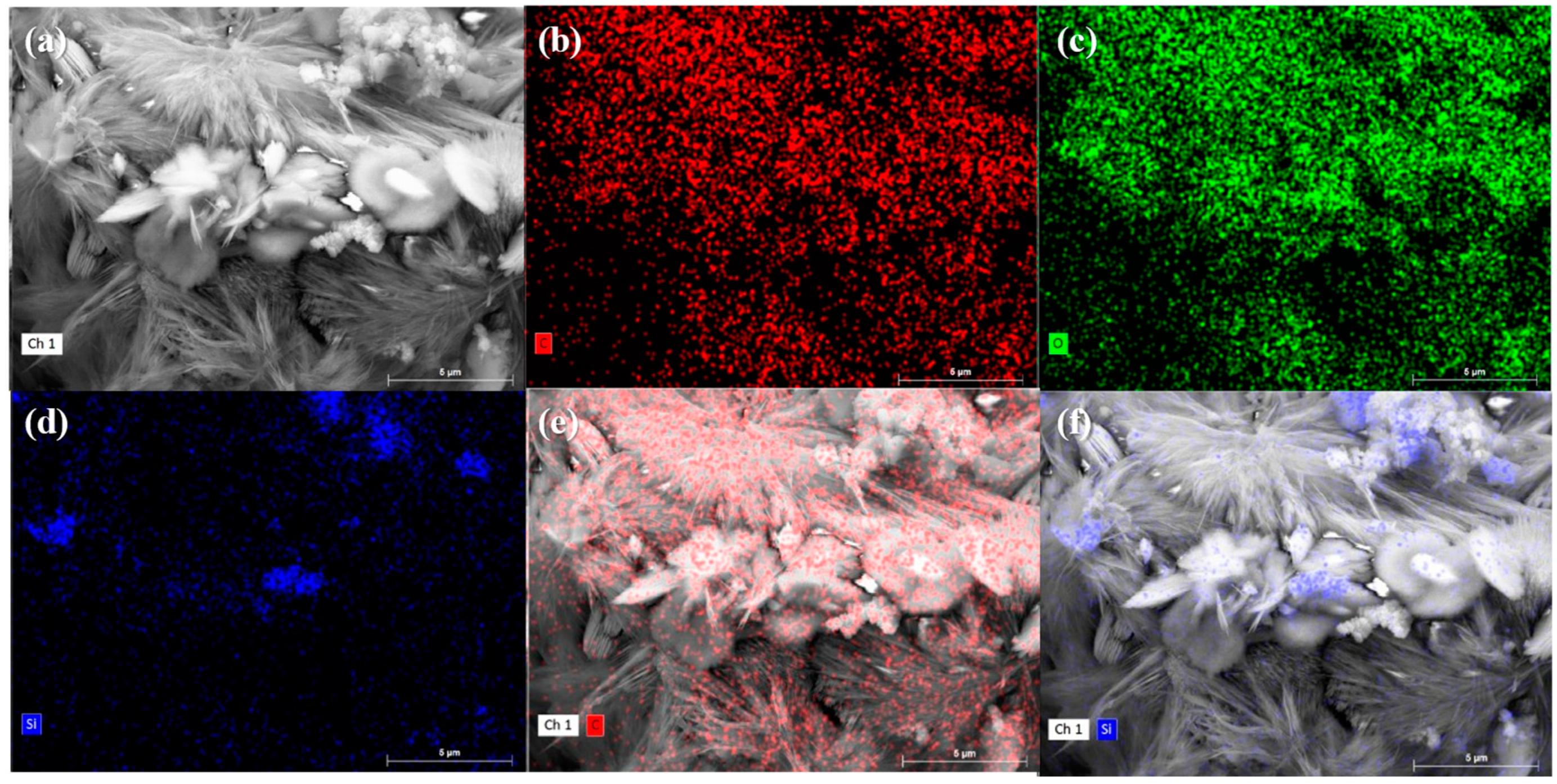
3.3.3. SEM Observation and the EDS Test
3.3.4. Nitrogen Adsorption Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Q.; Guo, R.; Zhao, Z.; Lin, Z.; He, K. Mechanical Properties of Concrete at High Temperature—A Review. Constr. Build. Mater. 2015, 93, 371–383. [Google Scholar] [CrossRef]
- Akca, A.H.; Özyurt, N. Deterioration and Recovery of FRC after High Temperature Exposure. Cem. Concr. Compos. 2018, 93, 260–273. [Google Scholar] [CrossRef]
- Mendes, A.; Sanjayan, J.G.; Gates, W.P.; Collins, F. The Influence of Water Absorption and Porosity on the Deterioration of Cement Paste and Concrete Exposed to Elevated Temperatures, as in a Fire Event. Cem. Concr. Compos. 2012, 34, 1067–1074. [Google Scholar] [CrossRef]
- Valente Monteiro, A.; Vieira, M. Effect of Elevated Temperatures on the Residual Durability-Related Performance of Concrete. Mater. Struct. Mater. Constr. 2021, 54, 232. [Google Scholar] [CrossRef]
- Klimek, A.; Stelzner, L.; Hothan, S.; Zehfuß, J. Influence of Thermal Strain on Concrete Spalling. Mater. Struct. Mater. Constr. 2024, 57, 15. [Google Scholar] [CrossRef]
- Fernandes, B.; Khodeir, M.; Perlot, C.; Carré, H.; Mindeguia, J.C.; La Borderie, C. Durability of Concrete Made with Recycled Concrete Aggregates after Exposure to Elevated Temperatures. Mater. Struct. Mater. Constr. 2023, 56, 25. [Google Scholar] [CrossRef]
- Khoury, G.A. Compressive Strength of Concrete at High Temperatures: A Reassessment. Mag. Concr. Res. 1992, 44, 291–309. [Google Scholar] [CrossRef]
- Fu, Y.F.; Wong, Y.L.; Poon, C.S.; Tang, C.A.; Lin, P. Experimental Study of Micro/Macro Crack Development and Stress–Strain Relations of Cement-Based Composite Materials at Elevated Temperatures. Cem. Concr. Res. 2004, 34, 789–797. [Google Scholar] [CrossRef]
- Piasta, J.; Sawicz, Z.; Rudzinski, L. Changes in the Structure of Hardened Cement Paste Due to High Temperature. Matériaux Constr. 1984, 17, 291–296. [Google Scholar] [CrossRef]
- Englert, G.; Wittmann, F. Water in Hardened Cement Paste. Matériaux Constr. 1968, 1, 535–546. [Google Scholar] [CrossRef]
- Saad, M.; Abo-El-Eneinf, S.A.; Hanna, G.B.; Kotkata, M.F. Effect of Temperature on Physical and Mechanical Properties of Concrete Containing Silica Fume. Cem. Concr. Res. 1996, 26, 669–675. [Google Scholar] [CrossRef]
- Alonso, C.; Fernandez, L. Dehydration and Rehydration Processes of Cement Paste Exposed to High Temperature Environments. J. Mater. Sci. 2004, 39, 3015–3024. [Google Scholar] [CrossRef]
- Kharrazi, H.; Ahmadi, B.; Shekarchi, M.; Farhoudi, N. A Comprehensive Study of the Effects of Re-Curing on Macro- and Microstructure of Zeolite Incorporating Concretes after Exposure to High Temperature. Mater. Struct. Mater. Constr. 2023, 56, 50. [Google Scholar] [CrossRef]
- Peng, G.F.; Huang, Z.S. Change in Microstructure of Hardened Cement Paste Subjected to Elevated Temperatures. Constr. Build. Mater. 2008, 22, 593–599. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The Use of Thermal Analysis in Assessing the Effect of Temperature on a Cement Paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Handoo, S.K.; Agarwal, S.; Agarwal, S.K. Physicochemical, Mineralogical, and Morphological Characteristics of Concrete Exposed to Elevated Temperatures. Cem. Concr. Res. 2002, 32, 1009–1018. [Google Scholar] [CrossRef]
- Pei, Y.; Agostini, F.; Skoczylas, F. Rehydration on Heat-Treated Cementitious Materials up to 700 °C-Coupled Transport Properties Characterization. Constr. Build. Mater. 2017, 144, 650–662. [Google Scholar] [CrossRef]
- Serpell, R.; Zunino, F. Recycling of Hydrated Cement Pastes by Synthesis of A′H-C2S. Cem. Concr. Res. 2017, 100, 398–412. [Google Scholar] [CrossRef]
- Henry, M.; Hashimot, K.; Darma, I.S.; Sugiyama, T.; Vichit-Vadakan, W.; Kerr, E.A.; Promentilla, M.A.B.; Promentilla, M.; Hashimoto, K. Cracking and Chemical Composition of Cement Paste Subjected to Heating and Water Re-Curing. J. Adv. Concr. Technol. 2016, 14, 134–143. [Google Scholar] [CrossRef]
- Bett, J.B.; Klingner, R.E.; Jirsa, J.O. Lateral Load Response of Strengthened and Repaired Reinforced Concrete Columns. Struct. J. 1988, 85, 499–508. [Google Scholar] [CrossRef]
- Wang, L.; Su, R.K.-L. Repair of Fire-Exposed Preloaded Rectangular Concrete Columns by Postcompressed Steel Plates. J. Struct. Eng. 2013, 140, 04013083. [Google Scholar] [CrossRef]
- Tovey, A.K. Assessment and Repair of Fire-Damaged Concrete Structures-an Update. Spec. Publ. 1986, 92, 47–62. [Google Scholar] [CrossRef]
- Abdullah, M.A.H.; Zahid, M.Z.A.M.; Bakar, B.H.A.; Nazri, F.M.; Ayob, A. UHPFRC as Repair Material for Fire-Damaged Reinforced Concrete Structure—A Review. Appl. Mech. Mater. 2015, 802, 283–289. [Google Scholar] [CrossRef]
- Noman, M.; Yaqub, M.; Abid, M.; Musarat, M.A.; Vatin, N.I.; Usman, M. Effects of Low-Cost Repair Techniques on Restoration of Mechanical Properties of Fire-Damaged Concrete. Front. Mater. 2022, 8, 801464. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Chen, W.; Yu, R.; Zhang, R. Cementitious Characteristics of Hydrated Cement Paste Subjected to Various Dehydration Temperatures. Constr. Build. Mater. 2009, 23, 531–537. [Google Scholar] [CrossRef]
- Poon, C.S.; Azhar, S.; Anson, M.; Wong, Y.L. Strength and Durability Recovery of Fire-Damaged Concrete after Post-Fire-Curing. Cem. Concr. Res. 2001, 31, 1307–1318. [Google Scholar] [CrossRef]
- Serpell, R.; Lopez, M. Reactivated Cementitious Materials from Hydrated Cement Paste Wastes. Cem. Concr. Compos. 2013, 39, 104–114. [Google Scholar] [CrossRef]
- Xuan, D.X.; Shui, Z.H. Rehydration Activity of Hydrated Cement Paste Exposed to High Temperature. Fire Mater. 2011, 35, 481–490. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; Zhang, B.; Li, Q.; Shui, Z. Study on the High-Temperature Behavior and Rehydration Characteristics of Hardened Cement Paste. Fire Mater. 2015, 39, 741–750. [Google Scholar] [CrossRef]
- Mendes, A.; Sanjayan, J.G.; Collins, F. Long-Term Progressive Deterioration Following Fire Exposure of OPC versus Slag Blended Cement Pastes. Mater. Struct. Mater. Constr. 2009, 42, 95–101. [Google Scholar] [CrossRef]
- Barnes, P.; Bensted, J. Structure and Performance of Cements; CRC Press: London, UK, 2002. [Google Scholar] [CrossRef]
- Hernández, M.S.; Goñi, S.; Puertas, F.; Guerrero, A.; Palacios, M.; Dolado, J.S. Synergy of T1-C3S and β-C2S Hydration Reactions. J. Am. Ceram. Soc. 2011, 94, 1265–1271. [Google Scholar] [CrossRef]
- Nayak, D.K.; Abhilash, P.P.; Singh, R.; Kumar, R.; Kumar, V. Fly Ash for Sustainable Construction: A Review of Fly Ash Concrete and Its Beneficial Use Case Studies. Clean. Mater. 2022, 6, 100143. [Google Scholar] [CrossRef]
- Sahmaran, M.; Yildirim, G.; Erdem, T.K. Self-Healing Capability of Cementitious Composites Incorporating Different Supplementary Cementitious Materials. Cem. Concr. Compos. 2013, 35, 89–101. [Google Scholar] [CrossRef]
- Termkhajornkit, P.; Nawa, T.; Yamashiro, Y.; Saito, T. Self-Healing Ability of Fly Ash–Cement Systems. Cem. Concr. Compos. 2009, 31, 195–203. [Google Scholar] [CrossRef]
- Xu, Y.; Wong, Y.L.; Poon, C.S.; Anson, M. Influence of PFA on Cracking of Concrete and Cement Paste after Exposure to High Temperatures. Cem. Concr. Res. 2003, 33, 2009–2016. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Wan, H.; Cao, B. Rehydration Reactivity of Recycled Mortar from Concrete Waste Experienced to Thermal Treatment. Constr. Build. Mater. 2008, 22, 1723–1729. [Google Scholar] [CrossRef]
- JGJ 55-2011; Specification for Mix Proportion Design of Ordinary Concrete. Standardization Administration of China: Beijing, China, 2011.
- GB 175-2023; Common Portland Cement. Standardization Administration of China: Beijing, China, 2023.
- GB/T 14684-2011; Sand for Construction. Standardization Administration of China: Beijing, China, 2011.
- GB/T 17671-2021; Test Method of Cement Mortar Strength. (ISO Method). Standardization Administration of China: Beijing, China, 2021.
- ISO-ISO 679:2009; Cement—Test Methods—Determination of Strength. International Organization for Standardization: Geneva, Switzerland, 2009.
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018; ISBN 1498738672. [Google Scholar]
- Ming, X.; Cao, M.; Lv, X.; Yin, H.; Li, L.; Liu, Z. Effects of High Temperature and Post-Fire-Curing on Compressive Strength and Microstructure of Calcium Carbonate Whisker-Fly Ash-Cement System. Constr. Build. Mater. 2020, 244, 118333. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration Mechanisms of Ternary Portland Cements Containing Limestone Powder and Fly Ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Kurihara, R.; Maruyama, I. Surface Area Development of Portland Cement Paste during Hydration: Direct Comparison with 1H NMR Relaxometry and Water Vapor/Nitrogen Sorption. Cem. Concr. Res. 2022, 157, 106805. [Google Scholar] [CrossRef]
- Xu, Y.; Wong, Y.L.; Poon, C.S.; Anson, M. Impact of High Temperature on PFA Concrete. Cem. Concr. Res. 2001, 31, 1065–1073. [Google Scholar] [CrossRef]
- Li, L.; Shi, L.; Wang, Q.; Liu, Y.; Dong, J.; Zhang, H.; Zhang, G. A Review on the Recovery of Fire-Damaged Concrete with Post-Fire-Curing. Constr. Build. Mater. 2020, 237, 117564. [Google Scholar] [CrossRef]
- Xiao, J.; König, G. Study on Concrete at High Temperature in China—An Overview. Fire Saf. J. 2004, 39, 89–103. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Q.; Mao, M.; Li, J. Carbonation Performance of Concrete with Fly Ash as Fine Aggregate after Stress Damage and High Temperature Exposure. Constr. Build. Mater. 2020, 242, 118125. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C.; Li, Y.; Yuan, G.; Li, Q. Effects of Stress and High Temperature on the Carbonation Resistance of Fly Ash Concrete. Constr. Build. Mater. 2017, 138, 486–495. [Google Scholar] [CrossRef]
- Ollivier, J.P.; Maso, J.C.; Bourdette, B. Interfacial Transition Zone in Concrete. Adv. Cem. Based Mater. 1995, 2, 30–38. [Google Scholar] [CrossRef]
- Guo, X.; Meng, F.; Shi, H. Microstructure and Characterization of Hydrothermal Synthesis of Al-Substituted Tobermorite. Constr. Build. Mater. 2017, 133, 253–260. [Google Scholar] [CrossRef]
- Zhao, L. Morphology Control and Surface Modification of Calcium Carbonate; Jilin University: Changchun, China, 2009. [Google Scholar]







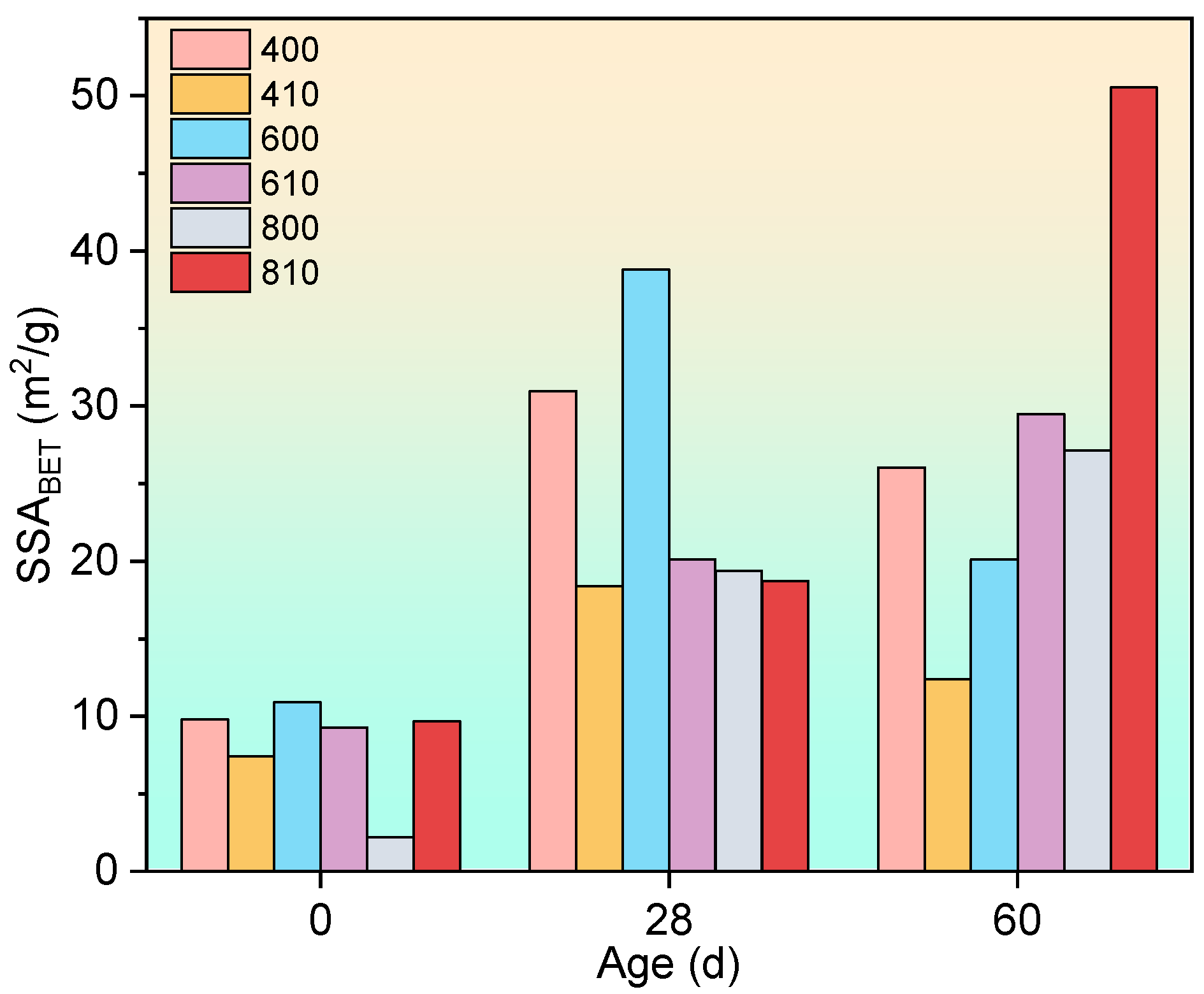
| Group Name a | Cement | Fly Ash | Water | Fine Aggregate |
|---|---|---|---|---|
| X00 | 100 | 0 | 50 | 300 |
| X10 | 90 | 10 | 50 | 300 |
| Type | SiO2 | Al2O3 | K2O | CaO | Fe2O3 | SO3 | MgO | Others |
|---|---|---|---|---|---|---|---|---|
| Cement | 15.08 | 3.73 | 0.92 | 70.45 | 3.10 | 4.06 | 1.80 | 0.86 |
| Fly ash | 66.48 | 14.92 | 3.32 | 5.41 | 9.86 | - | - | - |
| Element | O | C | Ca | Si |
|---|---|---|---|---|
| Atom percentage (%) | 44.11 | 20.88 | 19.43 | 5.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, L.; Kuang, B.; Fu, D.; Li, L.; Liu, Y.; Wang, C.; He, C.; Chen, Y.; Zhang, H.; Liu, F.; et al. Damage and Recovery Behavior of Low-Replacement-Rate Fly Ash Concrete after Different High-Temperature Exposures. Materials 2024, 17, 4330. https://doi.org/10.3390/ma17174330
Mi L, Kuang B, Fu D, Li L, Liu Y, Wang C, He C, Chen Y, Zhang H, Liu F, et al. Damage and Recovery Behavior of Low-Replacement-Rate Fly Ash Concrete after Different High-Temperature Exposures. Materials. 2024; 17(17):4330. https://doi.org/10.3390/ma17174330
Chicago/Turabian StyleMi, Lin, Bowen Kuang, Daixin Fu, Lang Li, Yongjie Liu, Chong Wang, Chao He, Yao Chen, Hong Zhang, Fulin Liu, and et al. 2024. "Damage and Recovery Behavior of Low-Replacement-Rate Fly Ash Concrete after Different High-Temperature Exposures" Materials 17, no. 17: 4330. https://doi.org/10.3390/ma17174330
APA StyleMi, L., Kuang, B., Fu, D., Li, L., Liu, Y., Wang, C., He, C., Chen, Y., Zhang, H., Liu, F., & Wang, Q. (2024). Damage and Recovery Behavior of Low-Replacement-Rate Fly Ash Concrete after Different High-Temperature Exposures. Materials, 17(17), 4330. https://doi.org/10.3390/ma17174330








