Corncob-Derived Activated Carbon as Electrode Material for High-Performance Supercapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of CACx
2.3. Characterization
3. Results and Discussion
3.1. Structural Characterization
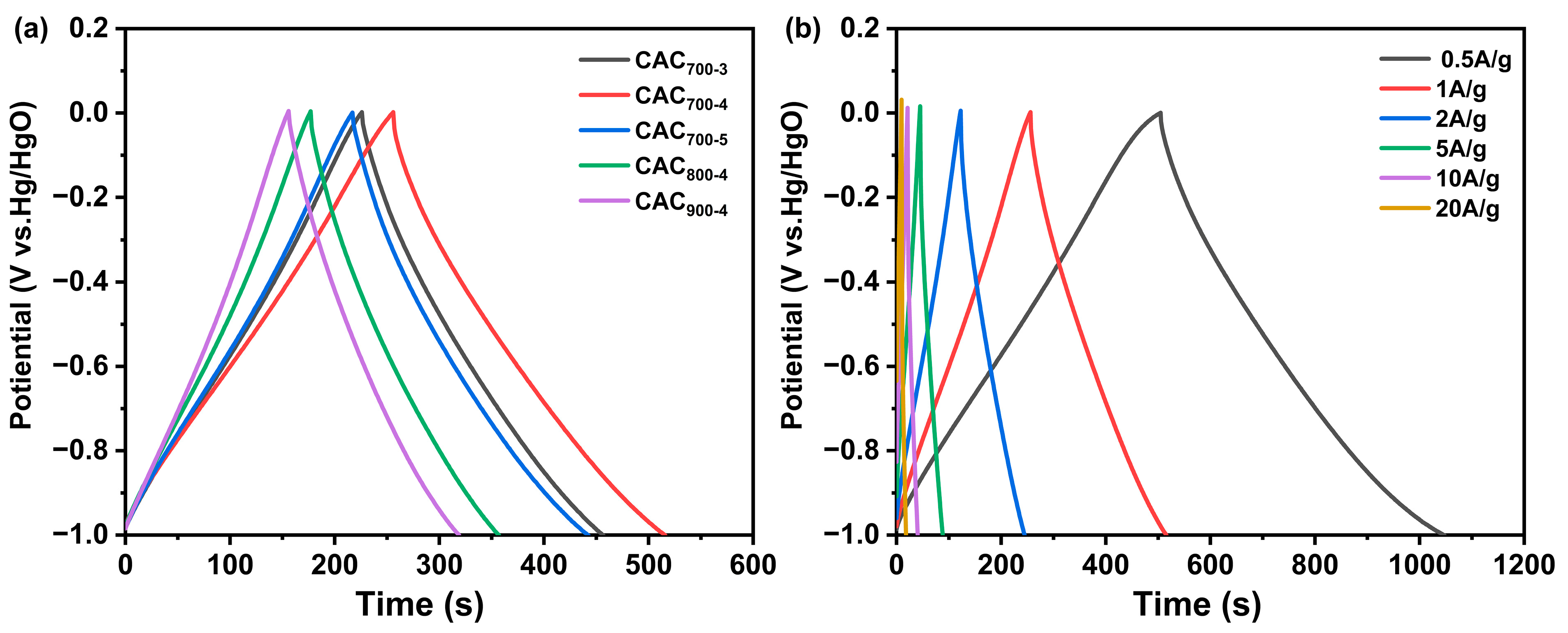
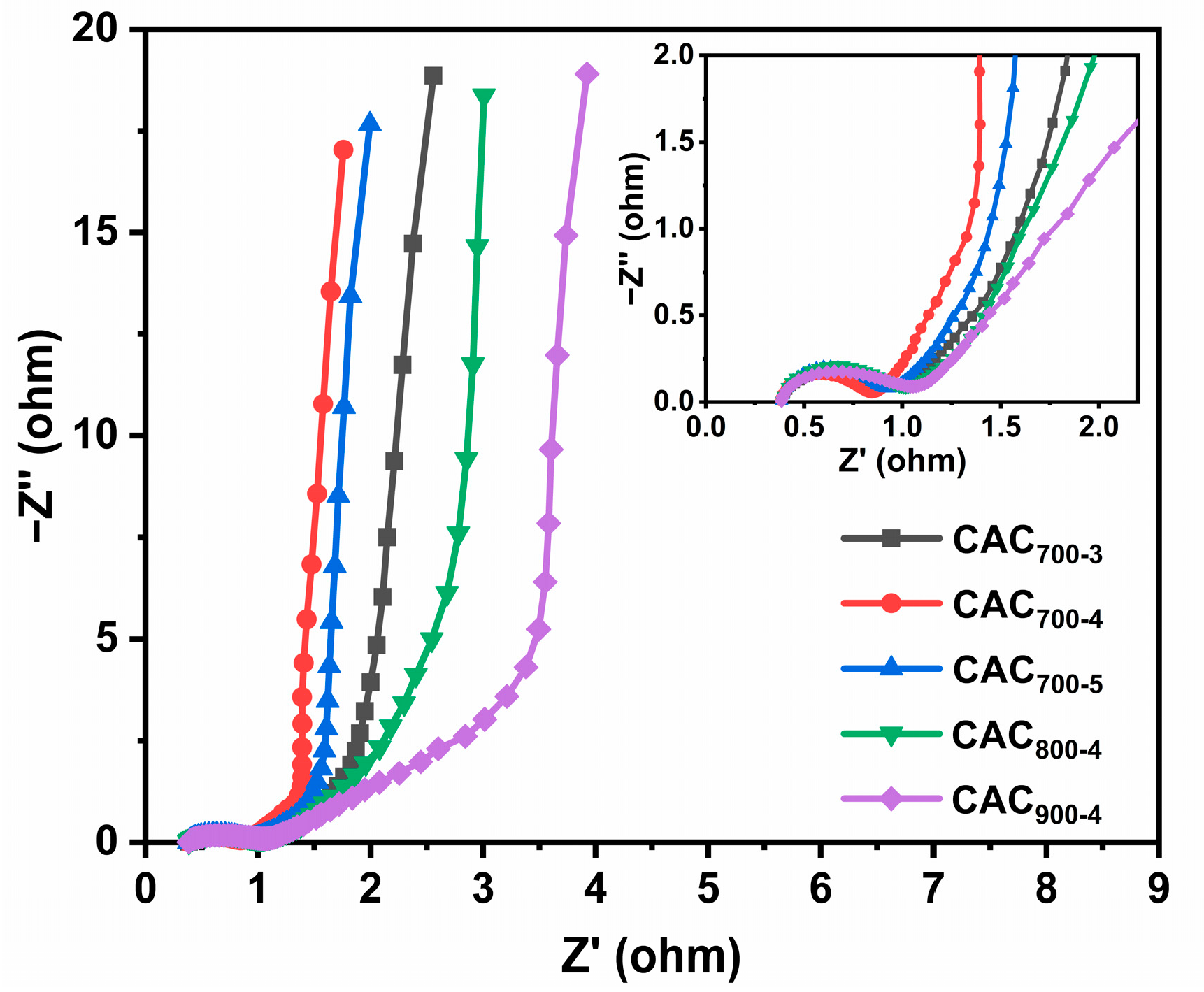
3.2. Electrochemical Performance Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhat, M.Y.; Hashmi, S.A.; Khan, M.; Choi, D.; Qurashi, A. Frontiers and recent developments on supercapacitor’s materials, design, and applications: Transport and power system applications. J. Energy Storage 2023, 58, 106104. [Google Scholar] [CrossRef]
- Manasa, P.; Sambasivam, S.; Ran, F. Recent progress on biomass waste derived activated carbon electrode materials for supercapacitors applications-A review. J. Energy Storage 2022, 54, 105290. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.L.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis strategies of porous carbon for supercapacitor applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Wang, R.; Jayakumar, A.; Xu, C.; Lee, J.M. Ni(OH)2 nanoflowers/graphene hydrogels: A new assembly for supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 3736–3742. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.; Liu, X.; Ma, L.; Wang, J.; Yang, S. Facile construction of 3D graphene/MoS2 composites as advanced electrode materials for supercapacitors. J. Power Sources 2016, 331, 180–188. [Google Scholar] [CrossRef]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An efficient way for energy storage application. Materials 2024, 17, 702. [Google Scholar] [CrossRef]
- Li, H.L.; Cao, L.H.; Zhang, H.J.; Tian, Z.W.; Zhang, Q.; Yang, F.; Yang, H.Q.; He, S.J.; Jiang, S.H. Intertwined carbon networks derived from Polyimide/Cellulose composite as porous electrode for symmetrical supercapacitor. J. Colloid Interface Sci. 2022, 609, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Baykara, E.A.; Zeybek, B. The preparation of polypyrrole/carboxyl functionalized multi-walled carbon nanotube@cobalthexacyanoferrate hybrid composite for high-performance supercapacitor application. J. Energy Storage 2023, 73, 109091. [Google Scholar] [CrossRef]
- Hwang, H.C.; Woo, J.S.; Park, S.Y. Flexible carbonized cellulose/single-walled carbon nanotube films with high conductivity. Carbohyd. Polym. 2018, 196, 168–175. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Liu, Y.X.; Wang, D.H.; Wang, C.; Guo, L.; Yi, T.F. Free-standing honeycomb-like N doped carbon foam derived from coal tar pitch for high-performance supercapacitor. Appl. Surf. Sci. 2020, 506, 145014. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, Q.; Zhao, Y.; Chen, T.; Wang, J.; Gui, L.; Lu, C. Recent progress and future directions of biomass-derived hierarchical porous carbon: Designing, preparation, and supercapacitor applications. Energy Fuels 2023, 37, 3523–3554. [Google Scholar] [CrossRef]
- Zeng, L.; Thiruppathi, A.R.; Zalm, J.v.d.; Li, X.; Chen, A. Biomass-derived amorphous carbon with localized active graphite defects for effective electrocatalytic N2 reduction. Appl. Surf. Sci. 2022, 575, 151630. [Google Scholar] [CrossRef]
- Yan, D.; Liu, L.; Wang, X.; Xu, K.; Zhong, J. Biomass-derived activated carbon nanoarchitectonics with hibiscus flowers for high-performance supercapacitor electrode applications. Chem. Eng. Technol. 2022, 45, 649–657. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Jin, T.; Su, J.; Luo, Q.; Zhu, W.; Lai, H.; Huang, D.; Wang, C. Preparation of N, P co-doped porous carbon derived from daylily for supercapacitor applications. ACS Omega 2022, 7, 37564–37571. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Yang, W.; Jian, S.; Zhang, C.; Duan, G. One step activation by ammonium chloride toward N-doped porous carbon from camellia oleifera for supercapacitor with high specific capacitance and rate capability. Diam. Relat. Mater. 2022, 130, 109526. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Novotortsev, R.Y.; Ivanov, A.S.; Maslakov, K.I.; Savilov, S.V. Rice husk-derived activated carbon electrode in redox-active electrolyte—New approach for enhancing supercapacitor performance. J. Energy Storage 2022, 55, 105699. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.P.; Qiu, L.L.; Xiao, J.; Wu, F.P.; Cao, J.P.; Bai, Y.H.; Liu, F.J. Insights into the KOH activation parameters in the preparation of corncob-based microporous carbon for high-performance supercapacitors. Diam. Relat. Mater. 2022, 129, 109331. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Shahi, S.; Gnawali, C.L.; Adhikari, M.P.; Rajbhandari, R.; Pokharel, B.P.; Ma, R.; Shrestha, R.G.; Ariga, K. Phyllanthus emblica seed-derived hierarchically porous carbon materials for high-performance supercapacitor applications. Materials 2022, 15, 8335. [Google Scholar] [CrossRef]
- Merin, P.; Jimmy Joy, P.; Muralidharan, M.N.; Veena Gopalan, E.; Seema, A. Biomass-derived activated carbon for high-performance supercapacitor electrode applications. Chem. Eng. Technol. 2021, 44, 844–851. [Google Scholar] [CrossRef]
- Ortiz-Olivares, R.D.; Lobato-Peralta, D.R.; Arias, D.M.; Okolie, J.A.; Cuentas-Gallegos, A.K.; Sebastian, P.J.; Mayer, A.R.; Okoye, P.U. Production of nanoarchitectonics corncob activated carbon as electrode material for enhanced supercapacitor performance. J. Energy Storage 2022, 55, 105447. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Arias, D.M.; Okoye, P.U. Polymer superabsorbent from disposable diaper as a sustainable precursor for the development of stable supercapacitor electrode. J. Energy Storage 2021, 40, 102760. [Google Scholar] [CrossRef]
- Shi, F.Y.; Tong, Y.; Li, H.S.; Li, J.J.; Cong, Z.Y.; Zhai, S.R.; An, Q.D.; Wang, K. Synthesis of oxygen/nitrogen/sulfur codoped hierarchical porous carbon from enzymatically hydrolyzed lignin for high-performance supercapacitors. J. Energy Storage 2022, 52, 104992. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.G.; Li, P.F.; Wei, H.Q.; Wei, K.X.; Chen, X.R. Lignin-derived carbon aerogels with high surface area for supercapacitor applications. Chem. Eng. J. 2023, 466, 143118. [Google Scholar] [CrossRef]
- Genovese, M.; Jiang, J.H.; Lian, K.; Holm, N. High capacitive performance of exfoliated biochar nanosheets from biomass waste corn cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Yang, S.R.; Zhang, K.L. Converting corncob to activated porous carbon for supercapacitor application. Nanomaterials 2018, 8, 181. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, F.Q.; Yang, J.M.; Ma, B.L.; Qing, Y.; Chu, F.X.; Wu, Y.Q. Preparation of size-controlled all-lignin based carbon nanospheres and their electrochemical performance in supercapacitor. Ind. Crop. Prod. 2022, 179, 114689. [Google Scholar] [CrossRef]
- Fu, F.; Yang, D.; Zhang, W.; Wang, H.; Qiu, X. Green self-assembly synthesis of porous lignin-derived carbon quasi-nanosheets for high-performance supercapacitors. Chem. Eng. J. 2020, 392, 123721. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.Y.; Yang, S.W.; Zhao, G.Z.; Han, L.; Li, Y.J.; Zhu, G. Biomass-derived S, P, Cl tri-doped porous carbon for high-performance supercapacitor. Diam. Relat. Mater. 2022, 126, 109061. [Google Scholar] [CrossRef]
- Feng, T.; Wang, S.; Hua, Y.; Zhou, P.; Liu, G.; Ji, K.; Lin, Z.; Shi, S.; Jiang, X.; Zhang, R. Synthesis of biomass-derived N, O-codoped hierarchical porous carbon with large surface area for high-performance supercapacitor. J. Energy Storage 2021, 44, 103286. [Google Scholar] [CrossRef]
- Hao, J.Y.; Wang, B.X.; Xu, H.; Du, J.C.; Wu, C.; Qin, W.; Wu, X.Q. Interfacial regulation of biomass-derived carbon towards high-performance supercapacitor. J. Energy Storage 2024, 86, 111301. [Google Scholar] [CrossRef]
- Yuan, Y.D.; Sun, Y.; Liu, C.G.; Yang, L.; Zhao, C.Z. Influence of KHCO3 Activation on characteristics of biomass-derived carbons for supercapacitor. Coatings 2023, 13, 1326. [Google Scholar] [CrossRef]
- Liu, Z.T.; Li, L.S.; Wang, M.L.; Wu, F.Z.; Jin, H.X.; Wang, Y. 3D hierarchical porous N, O co-doped carbon derived from the waste walnut shell via one-step carbonization for high-performance supercapacitor. Electroanalysis 2024, 36, e202400002. [Google Scholar] [CrossRef]
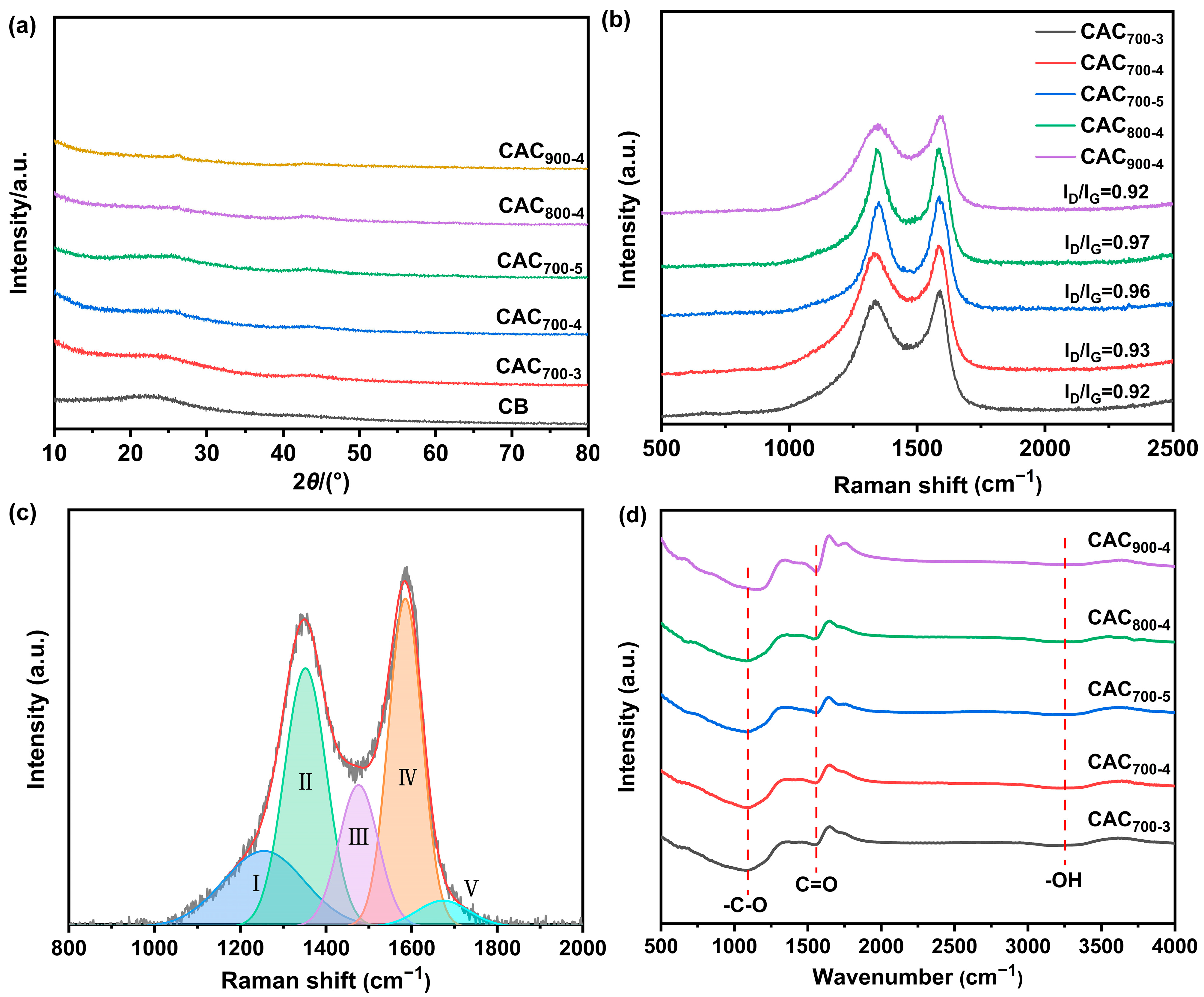
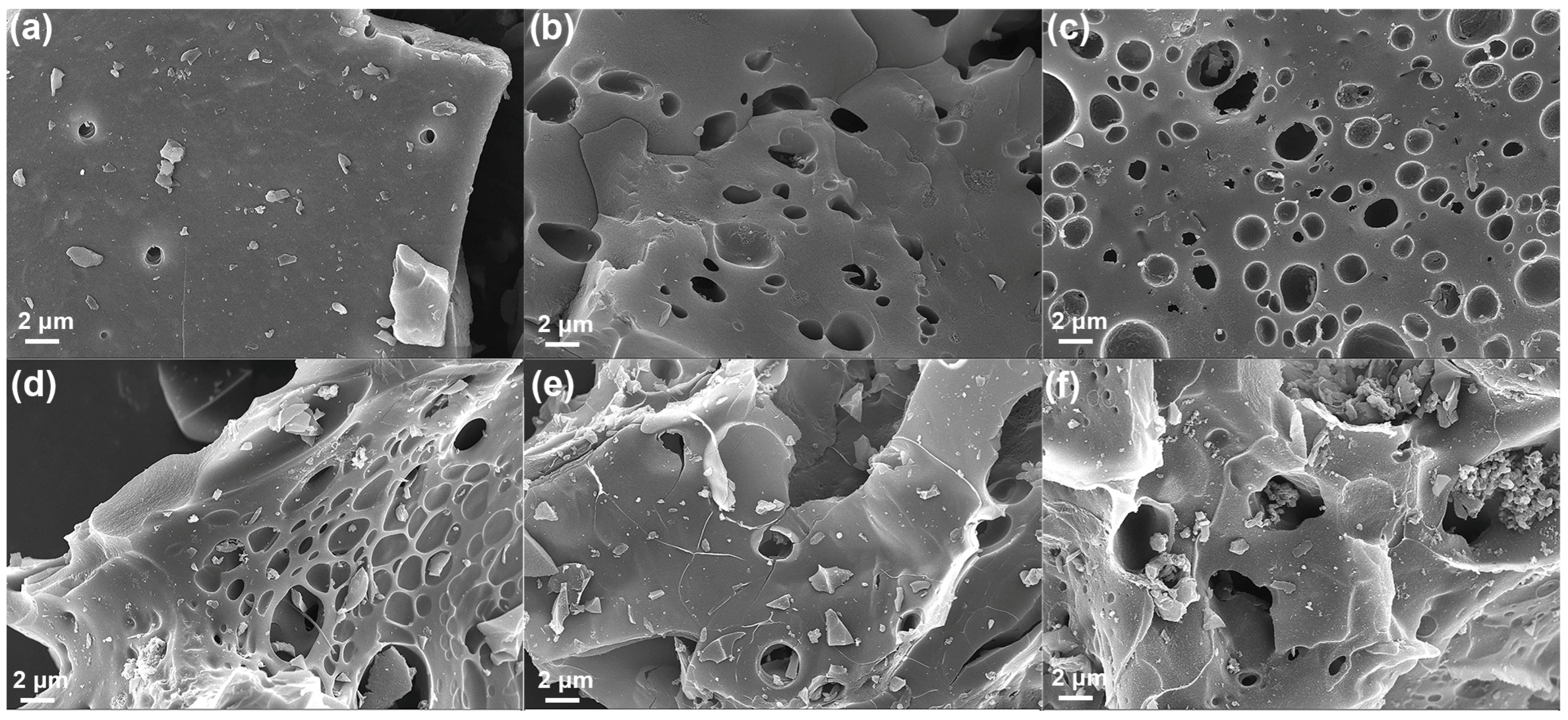
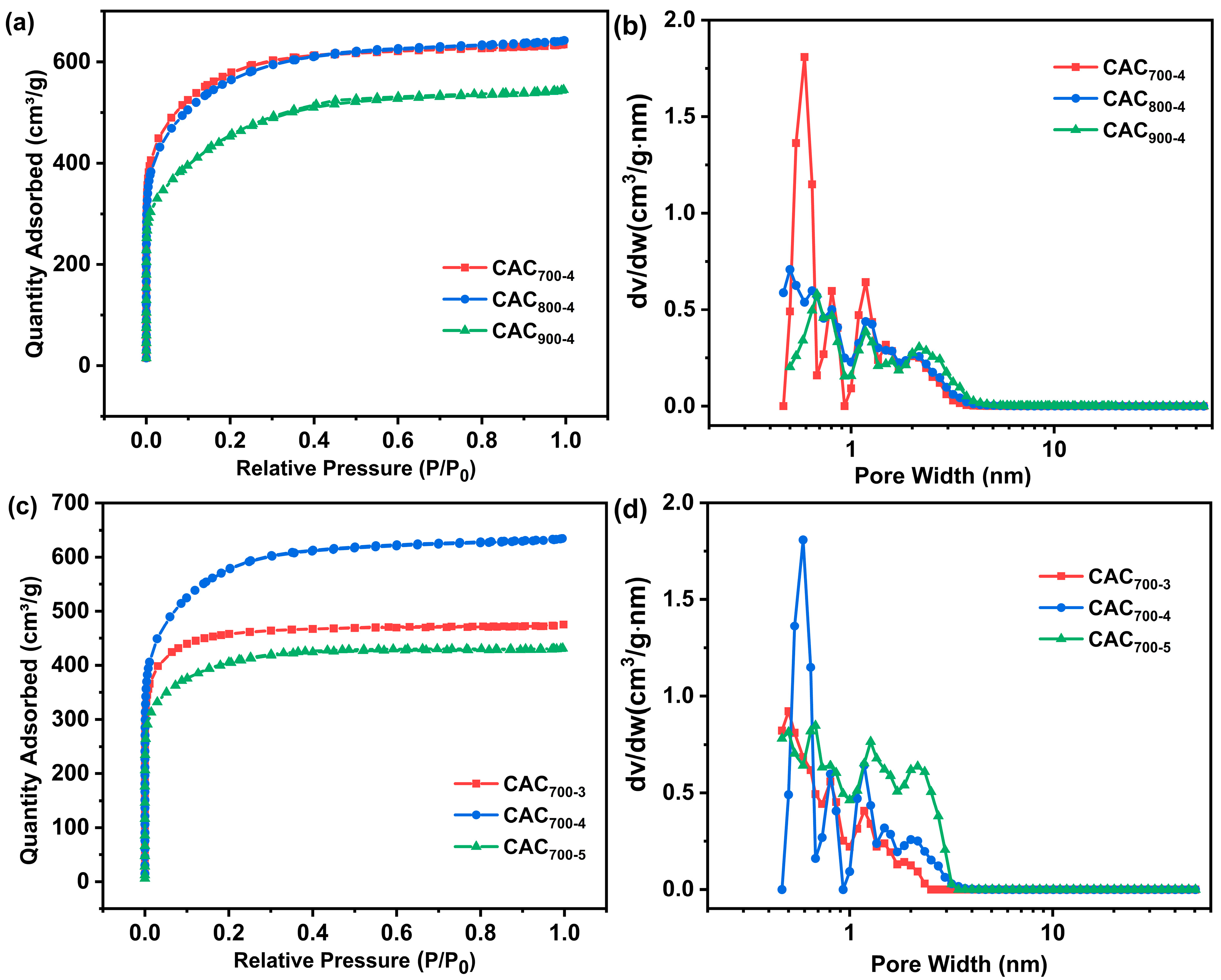
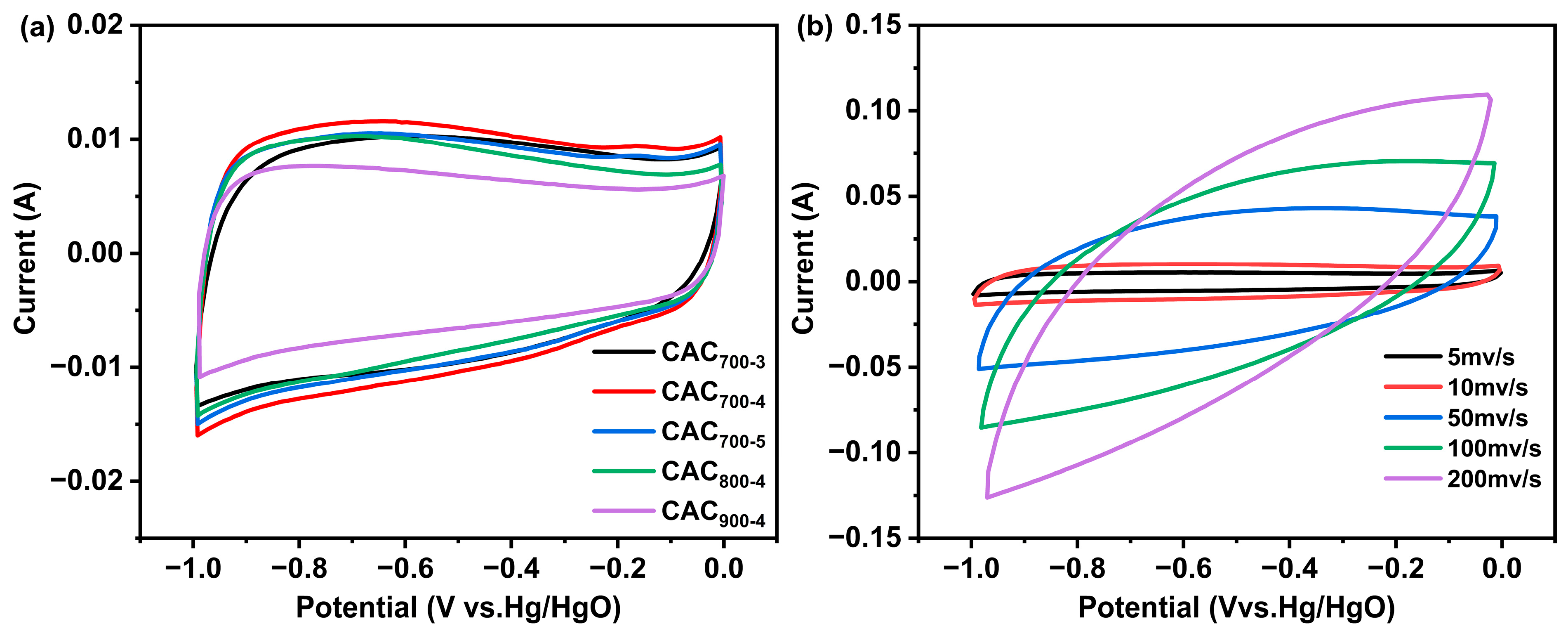

| Samples | SBET a | Vtotal b | Vmicro c | Vmicro/Vtotal | Dave (nm) |
|---|---|---|---|---|---|
| (m2/g) | (m3/g) | (m3/g) | |||
| CAC700-4 | 1945.70 | 0.9812 | 0.9122 | 92.96% | 2.0173 |
| CAC800-4 | 1919.16 | 0.9932 | 0.8965 | 90.26% | 2.0701 |
| CAC900-4 | 1627.58 | 0.7534 | 0.5002 | 66.39% | 2.3861 |
| CAC700-3 | 1482.41 | 0.7347 | 0.6971 | 94.88% | 1.9825 |
| CAC700-5 | 1470.09 | 0.6671 | 0.3785 | 56.73% | 2.3912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Pan, C.; Ji, Y.; Ren, S.; Lei, T. Corncob-Derived Activated Carbon as Electrode Material for High-Performance Supercapacitor. Materials 2024, 17, 4341. https://doi.org/10.3390/ma17174341
Dong L, Pan C, Ji Y, Ren S, Lei T. Corncob-Derived Activated Carbon as Electrode Material for High-Performance Supercapacitor. Materials. 2024; 17(17):4341. https://doi.org/10.3390/ma17174341
Chicago/Turabian StyleDong, Lili, Chenghao Pan, Yongfeng Ji, Suxia Ren, and Tingzhou Lei. 2024. "Corncob-Derived Activated Carbon as Electrode Material for High-Performance Supercapacitor" Materials 17, no. 17: 4341. https://doi.org/10.3390/ma17174341
APA StyleDong, L., Pan, C., Ji, Y., Ren, S., & Lei, T. (2024). Corncob-Derived Activated Carbon as Electrode Material for High-Performance Supercapacitor. Materials, 17(17), 4341. https://doi.org/10.3390/ma17174341






