Enhanced Raman Scattering in CVD-Grown MoS2/Ag Nanoparticle Hybrids
Abstract
1. Introduction
2. Materials and Methods
3. Results
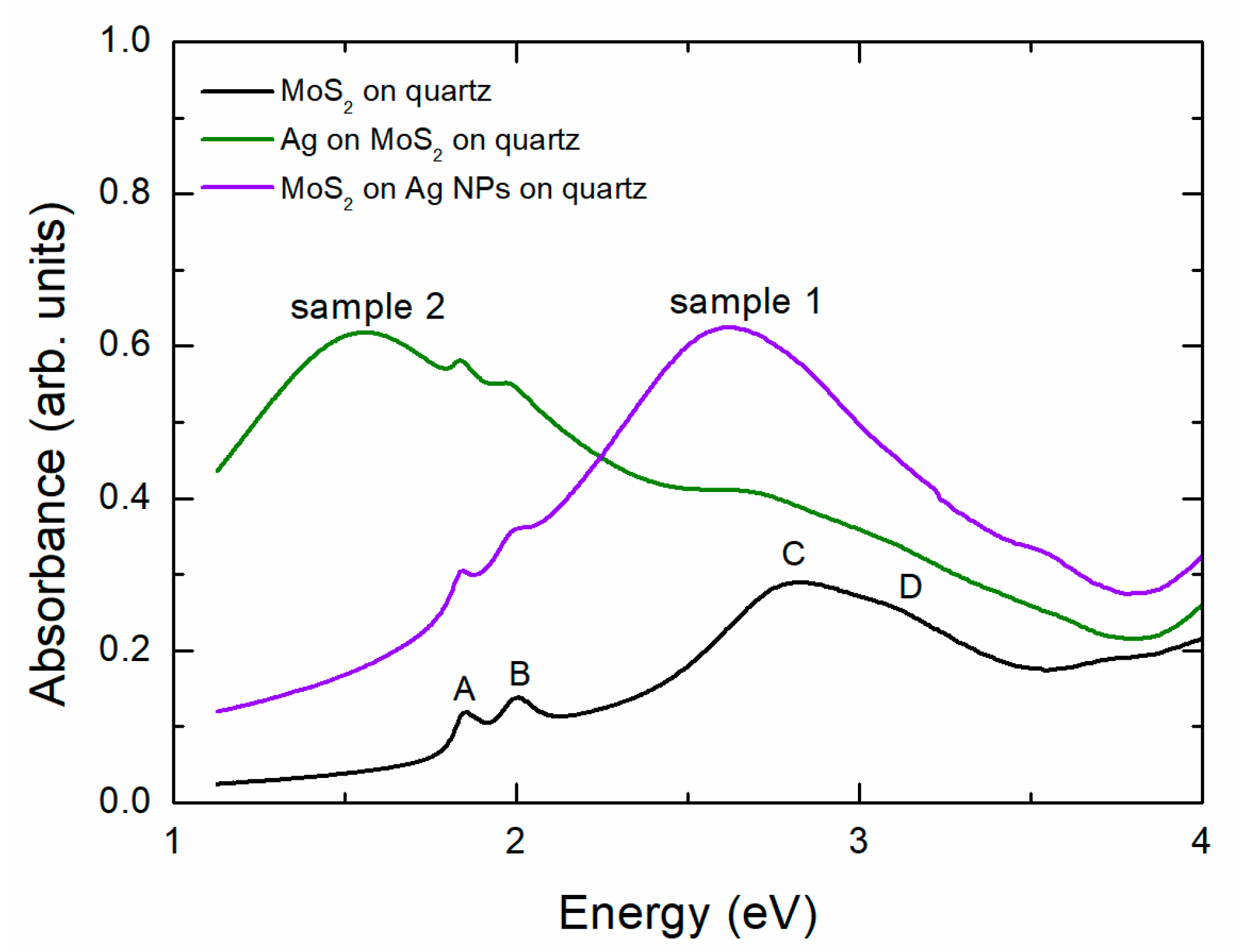
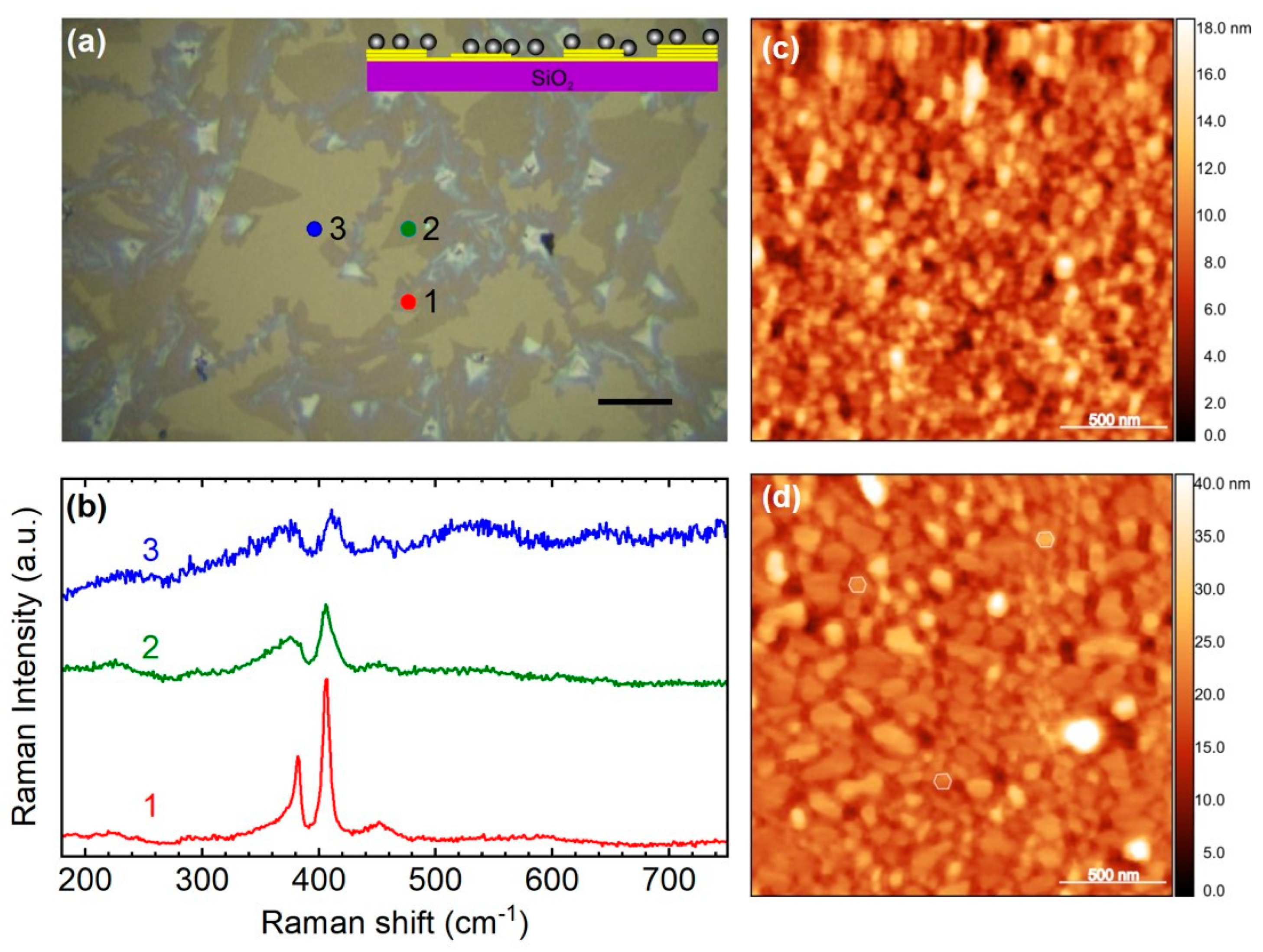
3.1. First Sample: MoS2 Wet-Transferred on Ag NPs Sputtered and Annealed on Quartz
3.2. Sample 2: Ag NPs Grown in Situ on Top of CVD-Produced MoS2 Membrane Transferred on Quartz
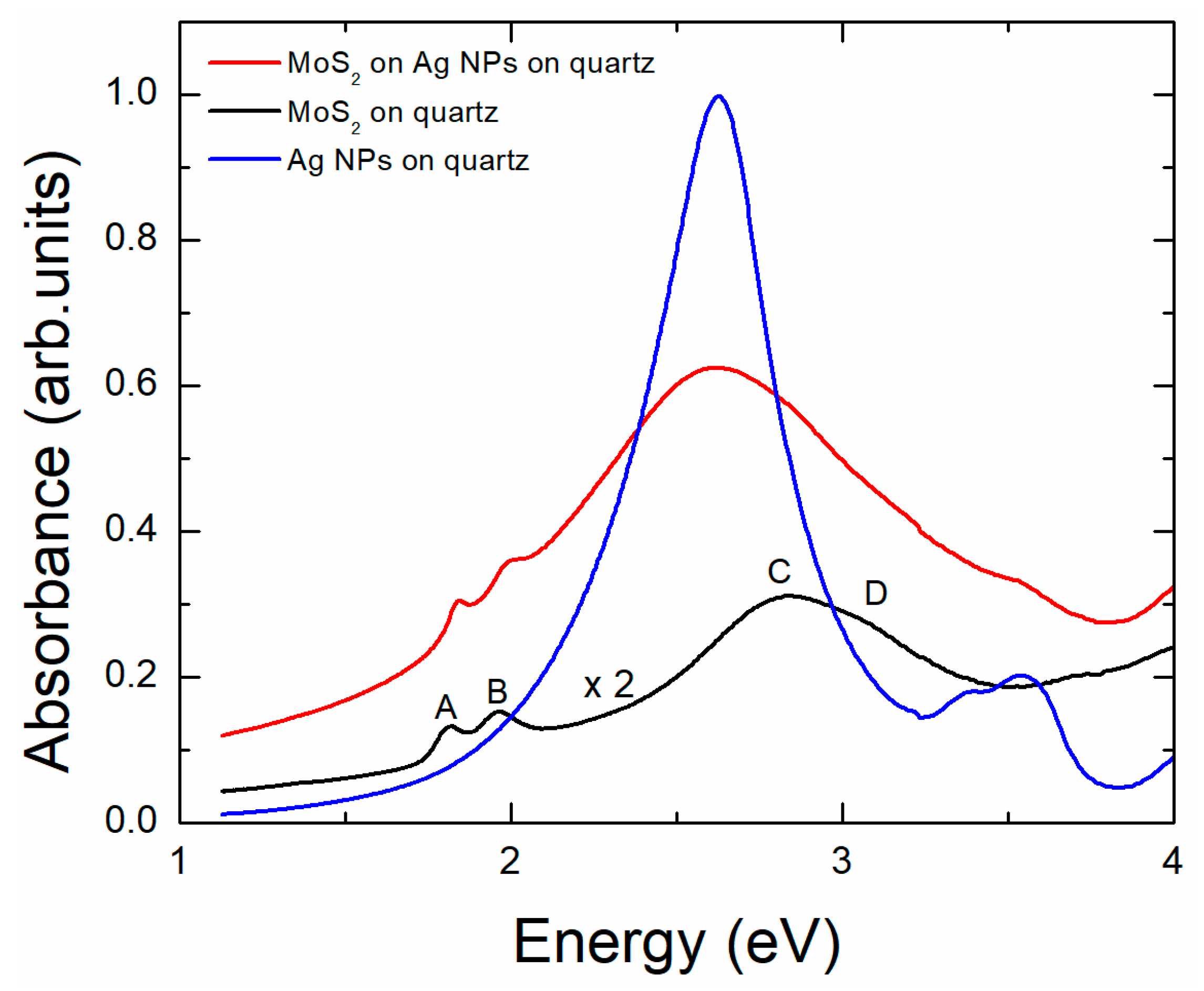
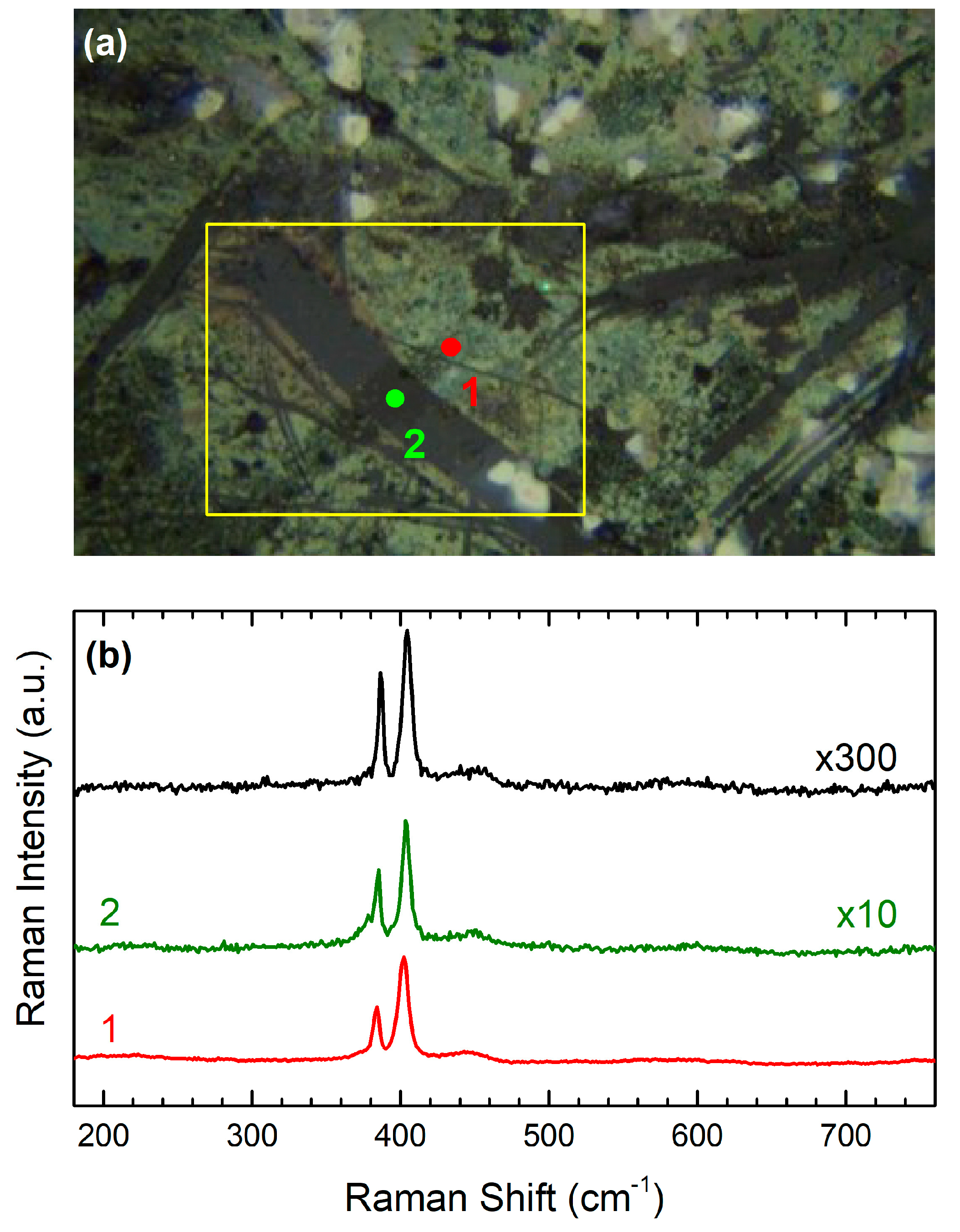
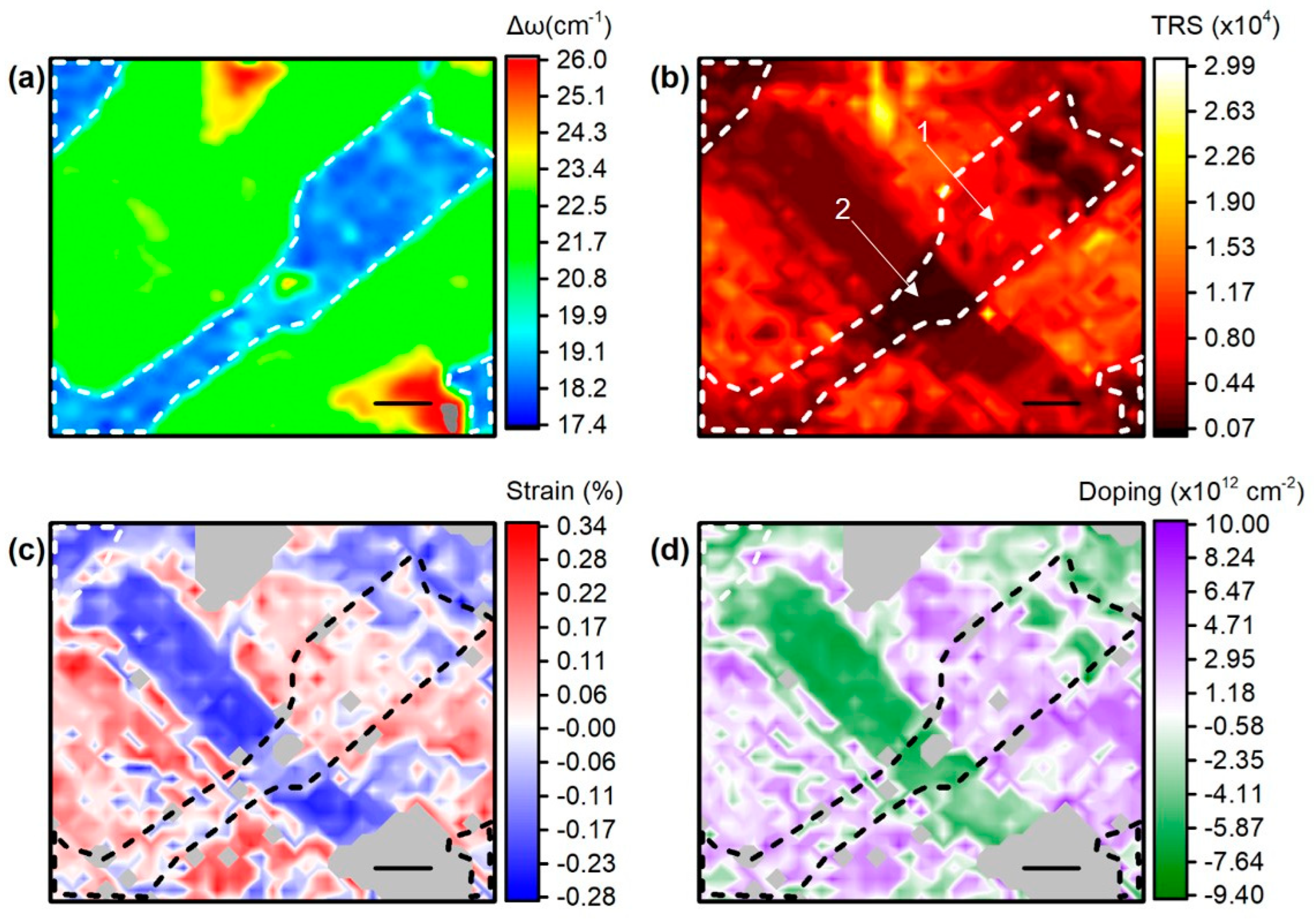
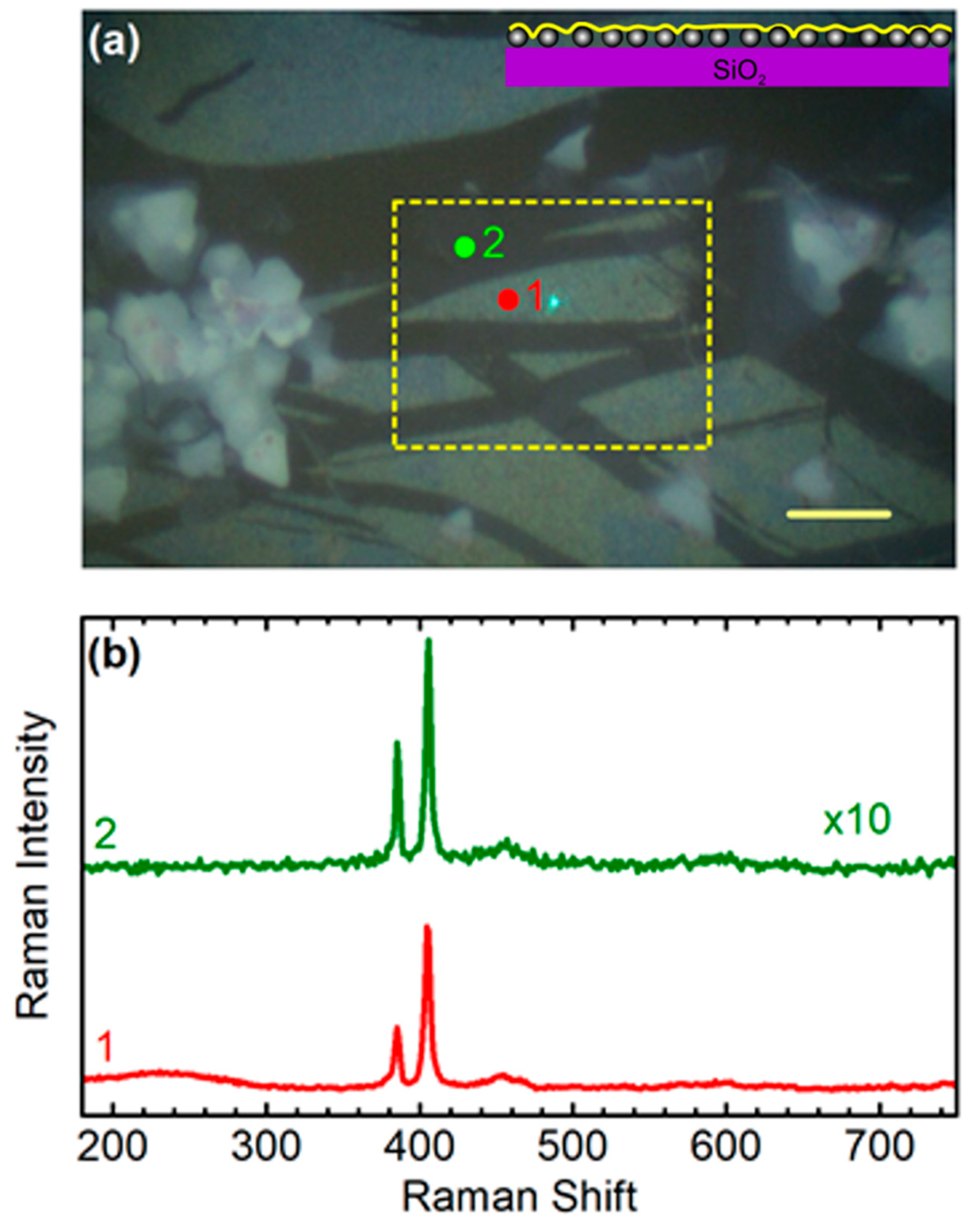
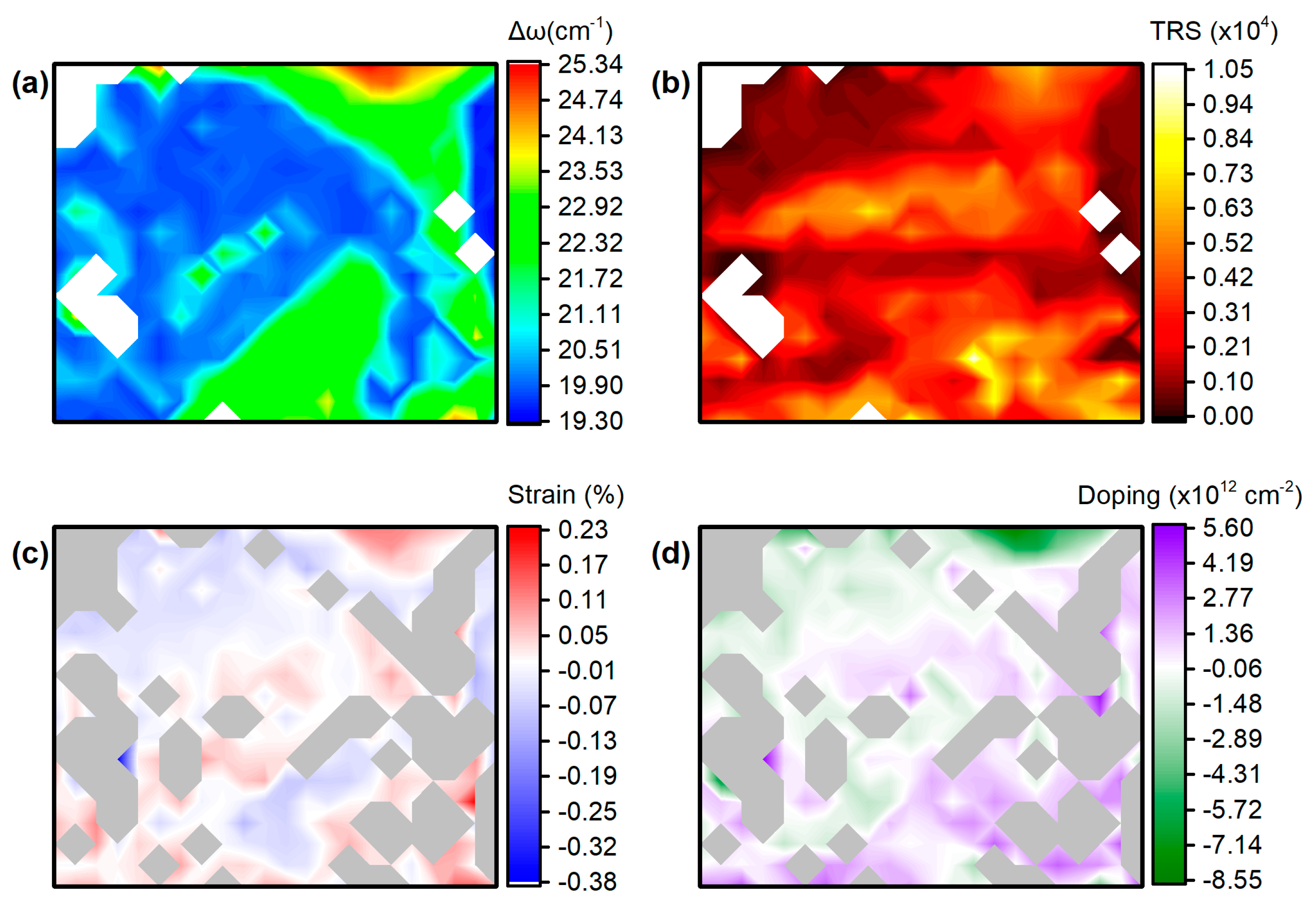
3.3. Sample 3: CVD-Grown MoS2 Membrane Transferred onto Ag NPs Formed in Situ on Quartz
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demirel, G.; Usta, H.; Yilmaz, M.; Celik, M.; Alidagi, H.A.; Buyukserin, F. Surface–enhanced Raman spectroscopy (SERS): An adventure from plasmonic metals to organic semiconductors as SERS platforms. J. Mater. Chem. C 2018, 6, 5314–5335. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Chulhai, D.V.; Hu, Z.; Moore, J.E.; Chen, X.; Jensen, L. Theory of linear and nonlinear surface-enhanced vibrational spectroscopies. Annu. Rev. Phys. Chem. 2016, 67, 541–564. [Google Scholar] [CrossRef] [PubMed]
- Schauble, K.; Zakhidov, D.; Yalon, E.; Deshmukh, S.; Grady, R.W.; Cooley, K.A.; McClellan, C.J.; Vaziri, S.; Passarello, D.; Mohney, S.E. Uncovering the effects of metal contacts on monolayer MoS. ACS Nano 2020, 14, 14798–14808. [Google Scholar] [CrossRef]
- Wang, X.; Kim, S.Y.; Wallace, R.M. Interface chemistry and band alignment study of Ni and Ag contacts on MoS2. ACS Appl. Mater. Interfaces 2021, 13, 15802–15810. [Google Scholar] [CrossRef]
- Aranda, D.; Valdivia, S.; Avila, F.J.; Soto, J.; Otero, J.C.; López–Tocón, I. Charge transfer at the nanoscale and the role of the out–of–plane vibrations in the selection rules of surface–enhanced Raman scattering. Phys. Chem. Chem. Phys. 2018, 20, 29430–29439. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, X.; Yi, Z.; Glushenkov, A.M.; Kong, L. Plasmonic substrates for surface enhanced Raman scattering. Anal. Chim. Acta 2017, 984, 19–41. [Google Scholar] [CrossRef]
- Ling, X.; Fang, W.; Lee, Y.-H.; Araujo, P.T.; Zhang, X.; Rodriguez-Nieva, J.F.; Lin, Y.; Zhang, J.; Kong, J.; Dresselhaus, M.S. Raman Enhancement Effect on Two-Dimensional Layered Materials: Graphene, H-BN and MoS2. Nano Lett. 2014, 14, 3033–3040. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Huang, D.; Sun, H.; Fan, F.; Feng, J.; Wang, Z.; Ning, C.Z. Room-temperature continuous-wave lasing from monolayer molybdenum ditelluride integrated with a silicon nanobeam cavity. Nat. Nanotechnol. 2017, 12, 987–992. [Google Scholar] [CrossRef]
- Michail, A.; Parthenios, J.; Anestopoulos, D.; Galiotis, C.; Christian, M.; Ortolani, L.; Morandi, V.; Papagelis, K. Controllable, eco-friendly, synthesis of highly crystalline 2D- MoS2 and clarification of the role of growth-induced strain. 2D Mater. 2018, 5, 035035. [Google Scholar] [CrossRef]
- Verble, J.L.; Wieting, T.J. Lattice Mode Degeneracy in MoS2 and Other Layer Compounds. Phys. Rev. Lett. 1970, 25, 362. [Google Scholar] [CrossRef]
- Molina-Sanchez, A.; Wirtz, L. Phonons in single-layer and few-layer MoS2 and WS2. Phys. Rev. B 2011, 84, 155413. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single-and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Ray, C.C. Ray Yap, Beng Kang Tay, Teo Hang Tong Edwin, Aurelien Olivier, Dominique Baillargeat. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Zhang, X.; Han, W.P.; Wu, J.B.; Milana, S.; Lu, Y.; Li, Q.Q.; Ferrari, A.C.; Tan, P.H. Raman spectroscopy of shear and layer breathing modes in multilayer MoS2. Phys. Rev. B 2013, 87, 115413. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, A.; Aggarwal, P.; Singh, R. Large-Area Transfer of 2D TMDCs Assisted by a Water-Soluble Layer for Potential Device Applications. ACS Omega 2022, 7, 11731–11741. [Google Scholar] [CrossRef]
- Chronis, A.G.; Stamatelatos, A.; Grammatikopoulos, S.; Sigalas, M.M.; Karoutsos, V.; Maratos, D.M.; Lysandrou, S.P.; Trachylis, D.; Politis, C.; Poulopoulos, P. Microstructure and Plasmonic Behavior of Self-Assembled Silver Nanoparticles and Nanorings. J. Appl. Phys. 2019, 125, 023106. [Google Scholar] [CrossRef]
- Tsarmpopoulou, M.; Ntemogiannis, D.; Stamatelatos, A.; Geralis, D.; Karoutsos, V.; Sigalas, M.; Poulopoulos, P.; Grammatikopoulos, S. Silver Nanoparticles’ Localized Surface Plasmon Resonances Emerged at Polymeric Environments by Theory and Experiment. Micro 2024, 4, 318–333. [Google Scholar] [CrossRef]
- Watson, A.J.; Lu, W.; Guimarães, M.H.D.; Stöhr, M. Transfer of large-scale two-dimensional semiconductors: Challenges and developments. 2d Mater. 2021, 8, 032001. [Google Scholar] [CrossRef]
- Poulopoulos, P.; Baskoutas, S.; Pappas, S.D.; Garoufalis, C.S.; Droulias, S.A.; Zamani, A.; Kapaklis, V. Intense Quantum Confinement Effects in Cu2O Thin Films. J. Phys. Chem. C 2011, 115, 14839–14843. [Google Scholar] [CrossRef]
- Ghayeb Zamharir, S.; Karimzadeh, R.; Aboutalebi, S.H. Laser-assisted tunable optical nonlinearity in liquid-phase exfoliated MoS2 dispersion. Appl. Phys. A 2018, 124, 692. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Beal, A.R.; Knights, J.C.; Liang, W.Y. Transmission spectra of some transition metal dichalcogenides. II. Group VIA: Trigonal prismatic coordination. J. Phys. Solid State Phys. 1972, 5, 3540–3551. [Google Scholar] [CrossRef]
- Ryou, J.; Kim, Y.-S.; Santosh, K.C.; Cho, K. Monolayer MoS2 bandgap modulation by dielectric environments and tunable bandgap transistors. Sci. Rep. 2016, 6, 29184. [Google Scholar] [CrossRef] [PubMed]
- Sousanis, A.; Poulopoulos, P.; Karoutsos, V.; Trachylis, D.; Politis, C. Giant enhancement of small photoluminescent signals on glass surfaces covered by self-assembled silver nanorings. J. Nanosci. Nanotechnol. 2017, 17, 1428–1433. [Google Scholar] [CrossRef]
- Ntemogiannis, D.; Tsarmpopoulou, M.; Stamatelatos, A.; Grammatikopoulos, S.; Karoutsos, V.; Anyfantis, D.I.; Barnasas, A.; Alexopoulos, V.; Giantzelidis, K.; Ndoj, E.A.; et al. ZnO Matrices as a Platform for Tunable Localized Surface Plasmon Resonances of Silver Nanoparticles. Coatings 2024, 14, 69. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Van Velson, N.; Zobeiri, H.; Wang, X. Thickness-Dependent Raman Scattering from Thin-Film Systems. J. Phys. Chem. C 2023, 127, 2995–3004. [Google Scholar] [CrossRef]
- Michail, A.; Delikoukos, N.; Parthenios, J.; Galiotis, C.; Papagelis, K. Optical detection of strain and doping inhomogeneities in single layer MoS2. Appl. Phys. Lett. 2016, 108, 173102. [Google Scholar] [CrossRef]
- Yamamoto, M.; Pierre-Louis, O.; Huang, J.; Fuhrer, M.S.; Einstein, T.L.; Cullen, W.G. “The Princess and the Pea” at the Nanoscale: Wrinkling and Delamination of Graphene on Nanoparticles. Phys. Rev. X 2012, 2, 041018. [Google Scholar] [CrossRef]
- Kayal, A.; Dey, S.; Harikrishnan; Nadarajan, R.; Chattopadhyay, S.; Mitra, J. Mobility Enhancement in CVD-Grown Monolayer MoS2 Via Patterned Substrate-Induced Nonuniform Straining. Nano Lett. 2023, 23, 6629–6636. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulos, S.; Pappas, S.D.; Dracopoulos, V.; Poulopoulos, P.; Fumagalli, P.; Velgakis, M.J.; Politis, C. Self-assembled au nanoparticles on heated corning glass by dc magnetron sputtering: Size-dependent surface plasmon resonance tuning. J. Nanoparticle Res. 2013, 15, 1446. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, M.; Zhang, J.; Wang, Z.; Huang, W.; Zhao, Y.; Nshimiyimana, J.P.; Hu, X.; Chi, X.; Hou, G.; et al. Thickness-dependent morphologies of Ag on n-layer MoS2 and its surface-enhanced Raman scattering. Nano Res. 2016, 9, 1682–1688. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maratos, D.M.; Michail, A.; Stamatelatos, A.; Grammatikopoulos, S.; Anestopoulos, D.; Tangoulis, V.; Papagelis, K.; Parthenios, J.; Poulopoulos, P. Enhanced Raman Scattering in CVD-Grown MoS2/Ag Nanoparticle Hybrids. Materials 2024, 17, 4396. https://doi.org/10.3390/ma17174396
Maratos DM, Michail A, Stamatelatos A, Grammatikopoulos S, Anestopoulos D, Tangoulis V, Papagelis K, Parthenios J, Poulopoulos P. Enhanced Raman Scattering in CVD-Grown MoS2/Ag Nanoparticle Hybrids. Materials. 2024; 17(17):4396. https://doi.org/10.3390/ma17174396
Chicago/Turabian StyleMaratos, Dionysios M., Antonios Michail, Alkeos Stamatelatos, Spyridon Grammatikopoulos, Dimitris Anestopoulos, Vassilis Tangoulis, Konstantinos Papagelis, John Parthenios, and Panagiotis Poulopoulos. 2024. "Enhanced Raman Scattering in CVD-Grown MoS2/Ag Nanoparticle Hybrids" Materials 17, no. 17: 4396. https://doi.org/10.3390/ma17174396
APA StyleMaratos, D. M., Michail, A., Stamatelatos, A., Grammatikopoulos, S., Anestopoulos, D., Tangoulis, V., Papagelis, K., Parthenios, J., & Poulopoulos, P. (2024). Enhanced Raman Scattering in CVD-Grown MoS2/Ag Nanoparticle Hybrids. Materials, 17(17), 4396. https://doi.org/10.3390/ma17174396











