Investigation on the Curing and Thermal Properties of Epoxy/Amine/Phthalonitrile Blend
Abstract
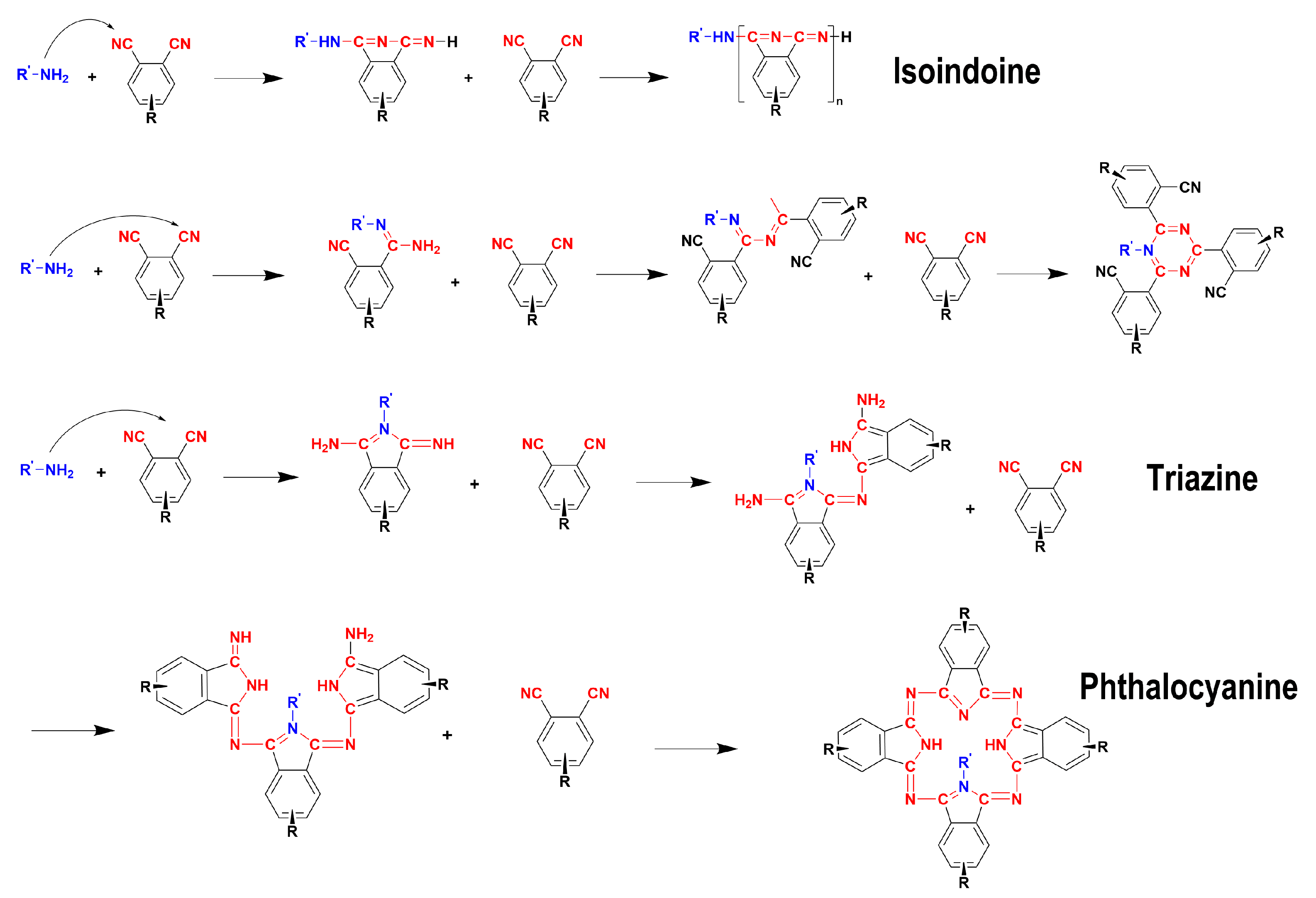
1. Introduction
2. Experiment
2.1. Materials
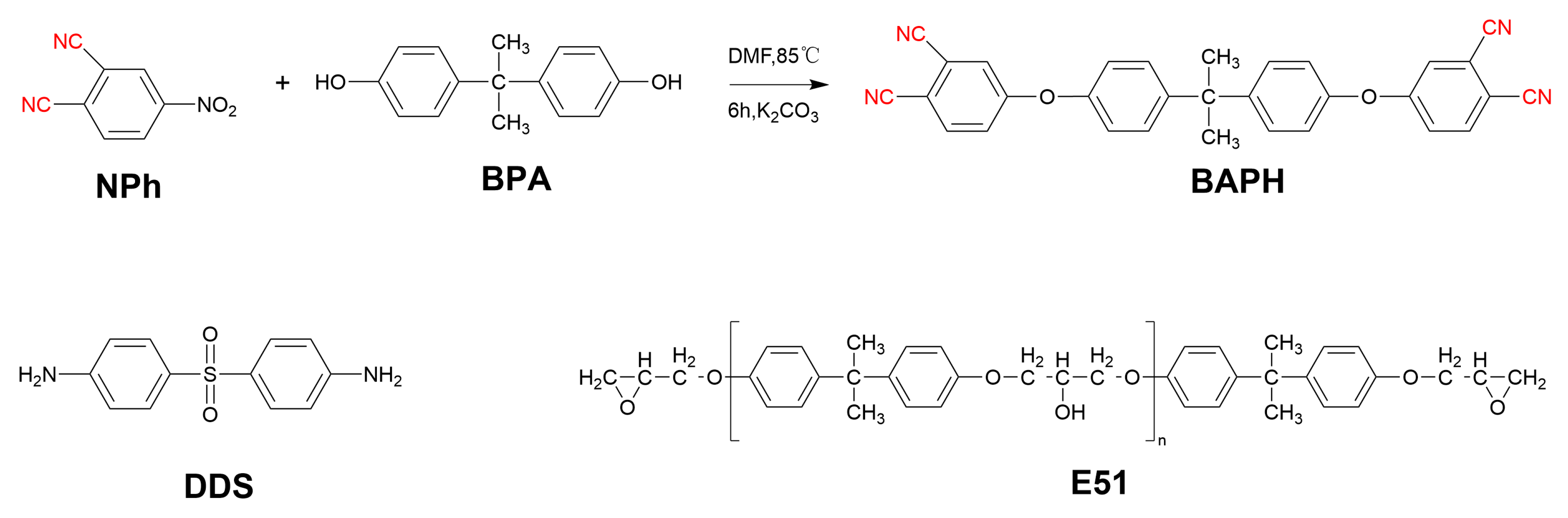
2.2. Preparation of the Bisphenol A-Type Phthalonitrile (BAPH)
2.3. Preparation of the Thermosets
2.4. Characterization
3. Results and Discussion
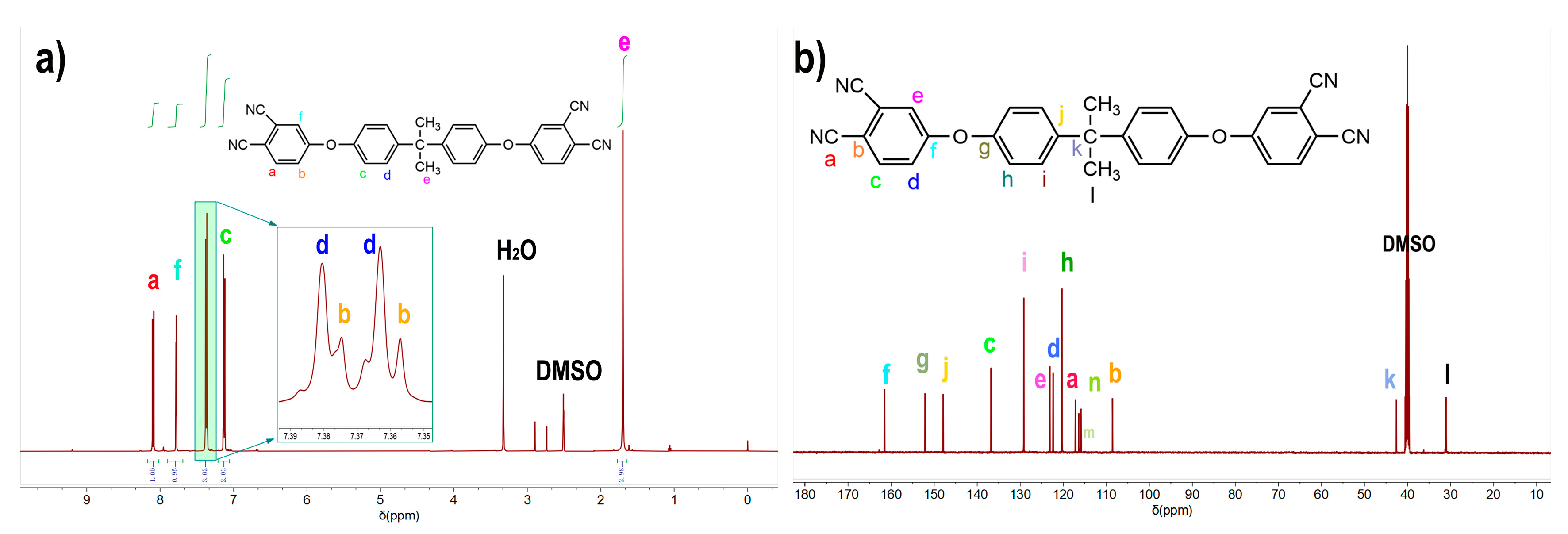
3.1. Characterization of the Synthesized BAPH
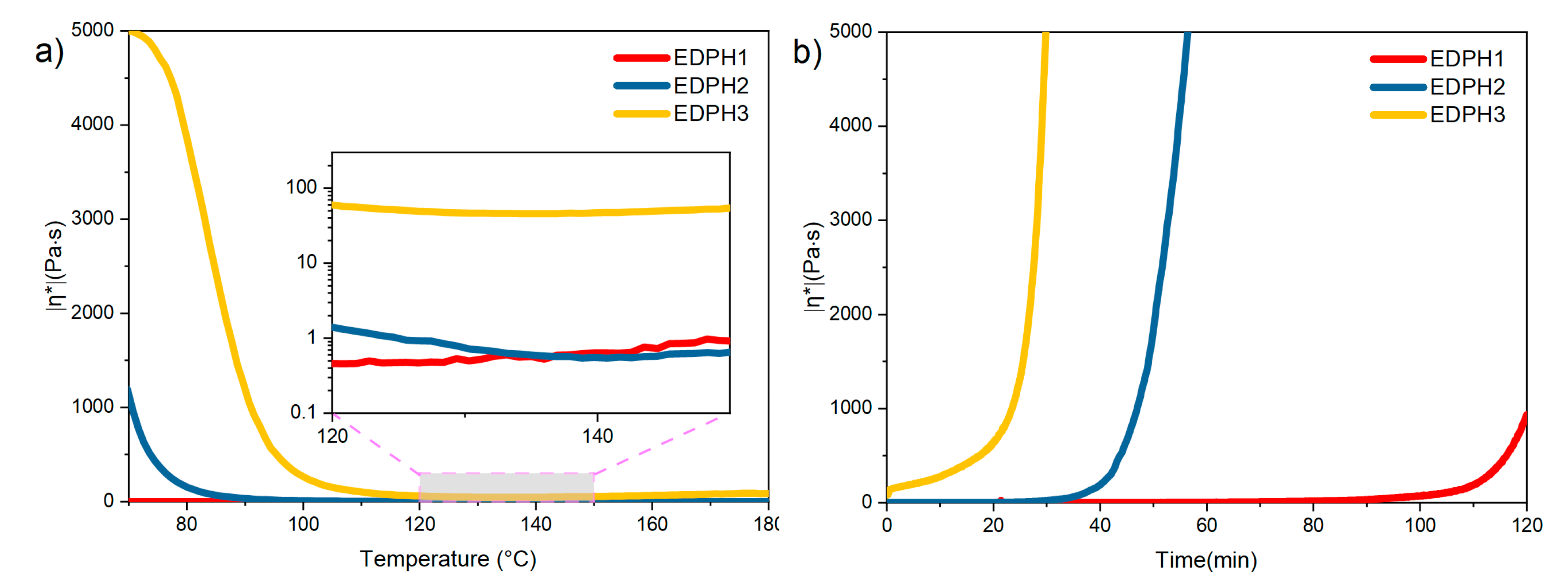
3.2. Rheological Properties of the E51/DDS/BAPH Blend
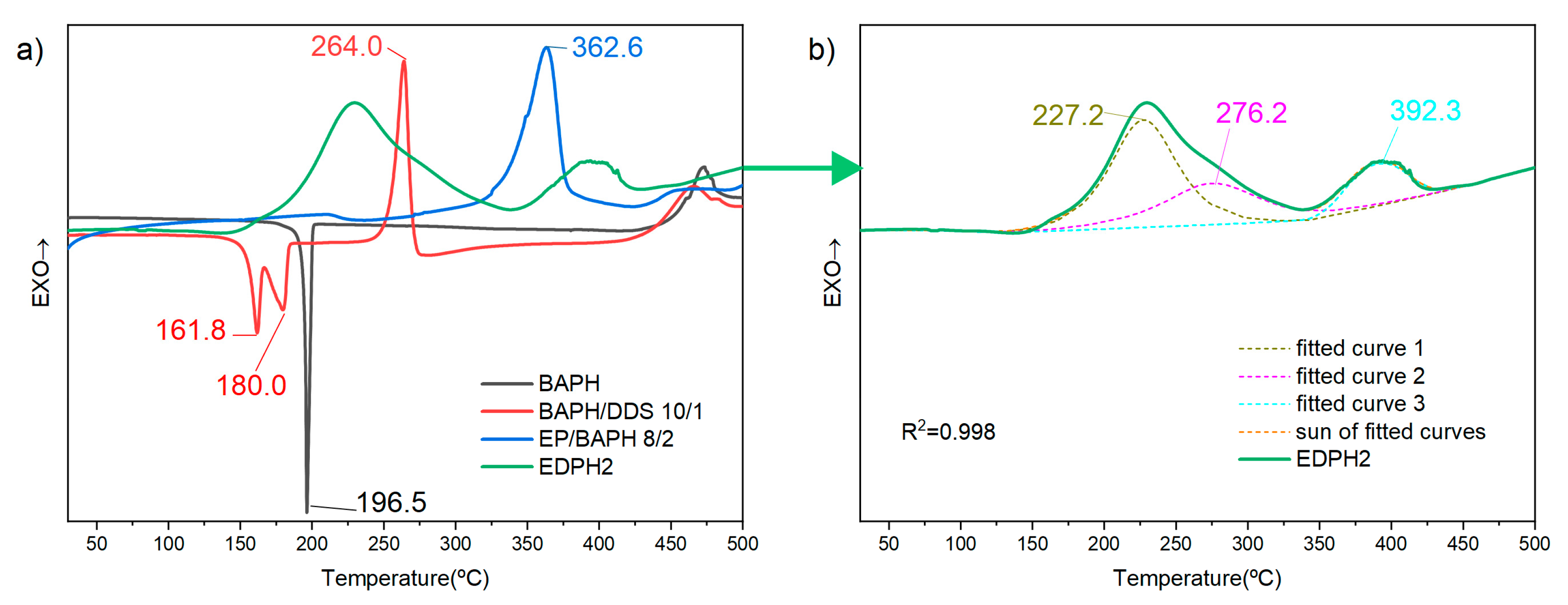
3.3. Curing Process of the E51/DDS/BAPH Blend
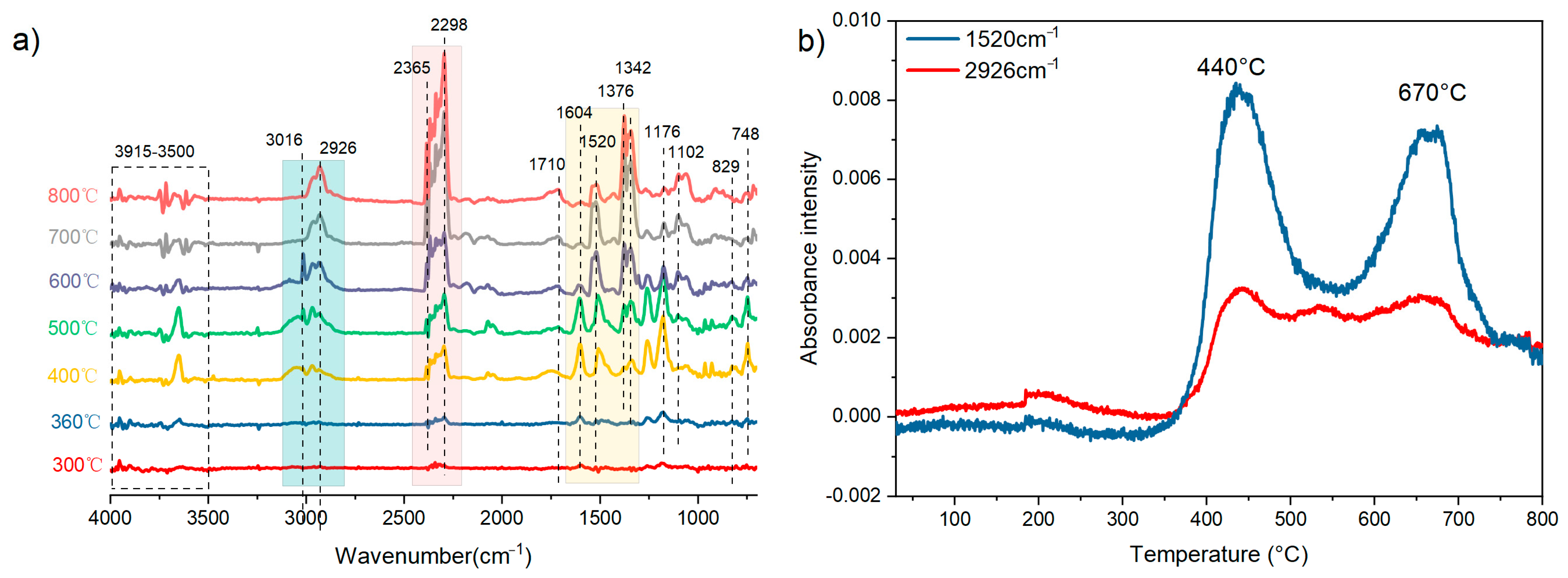
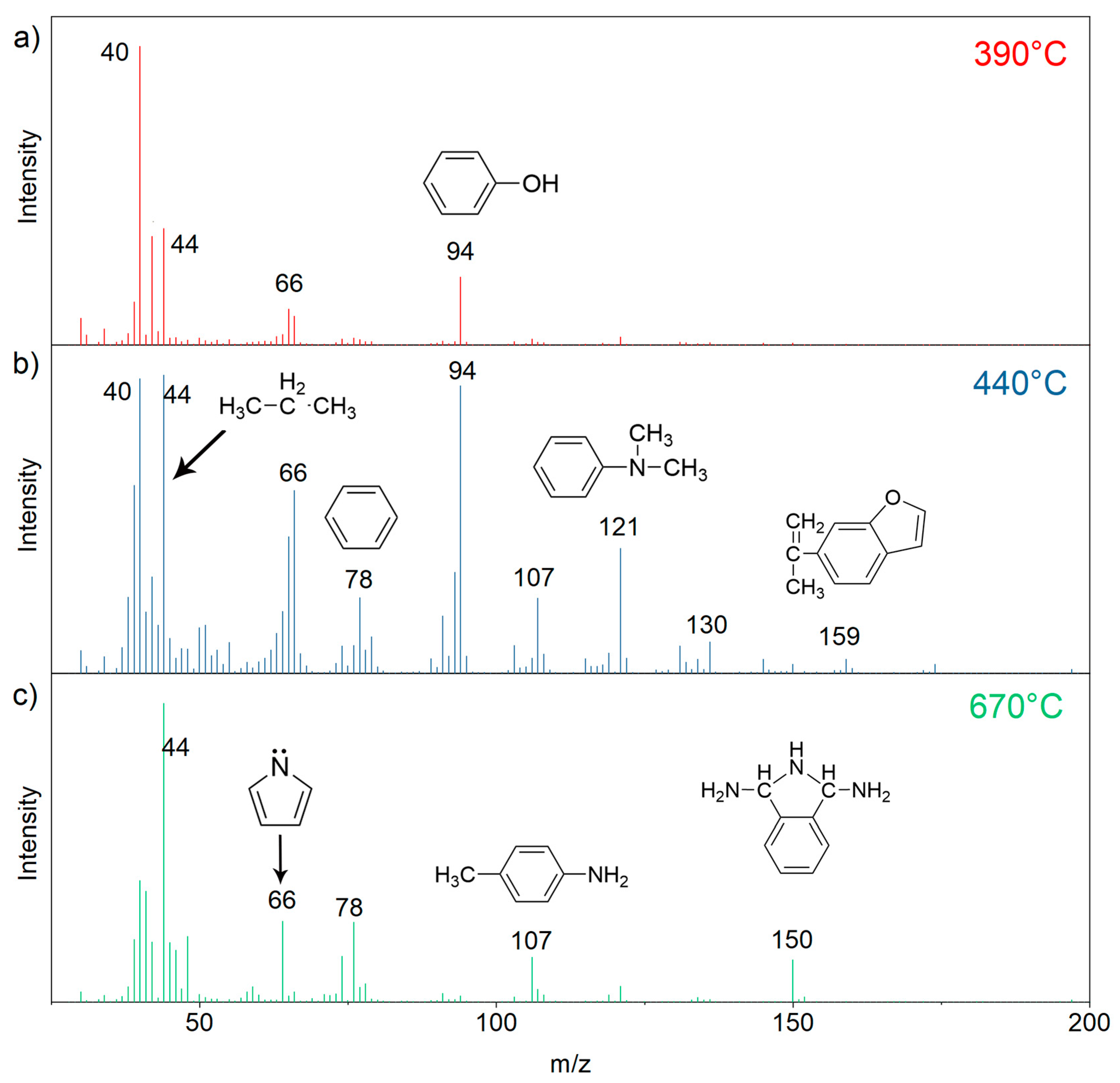
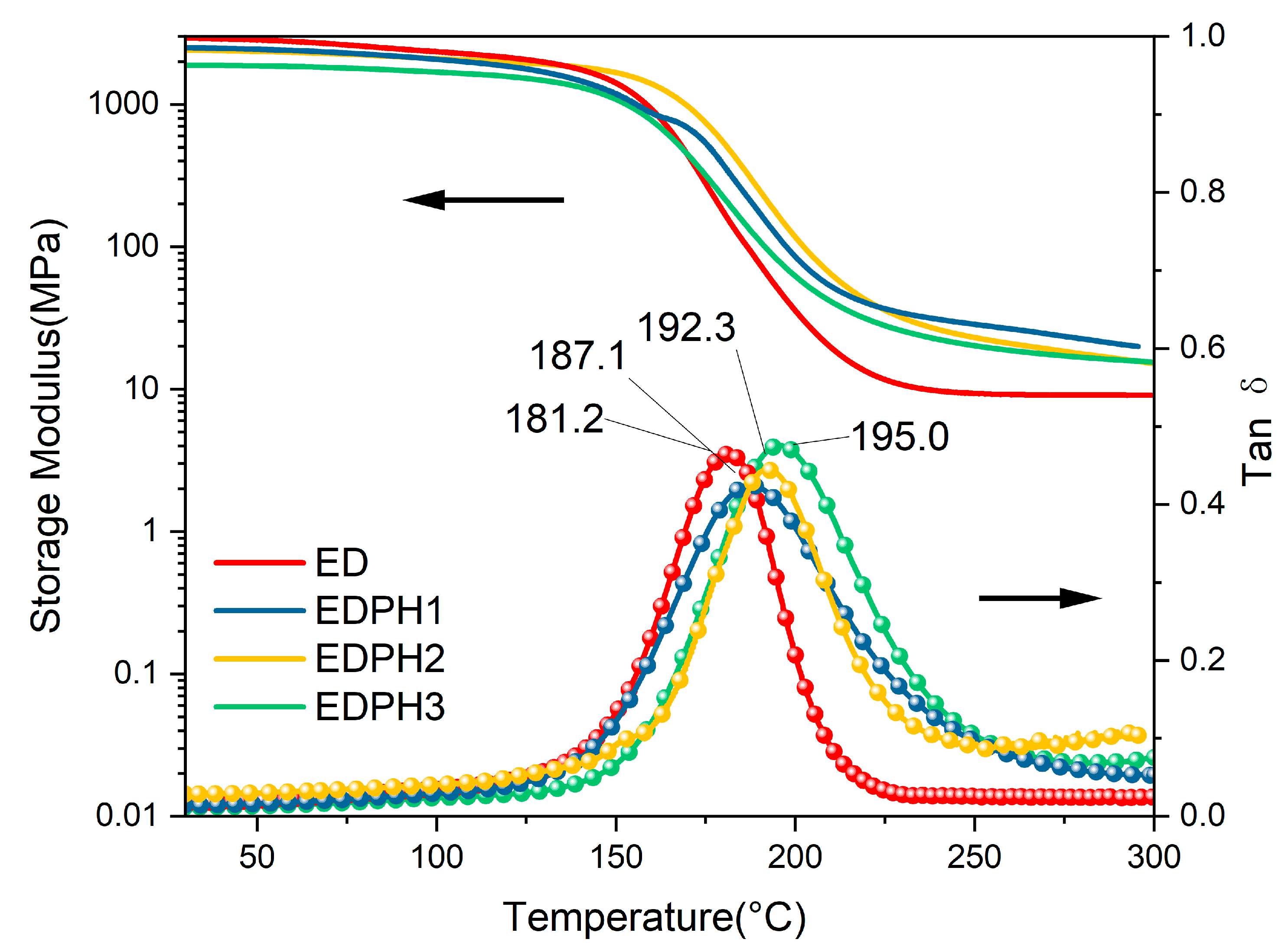
3.4. Thermal Behaviors of the E51/DDS/BAPH Thermosets
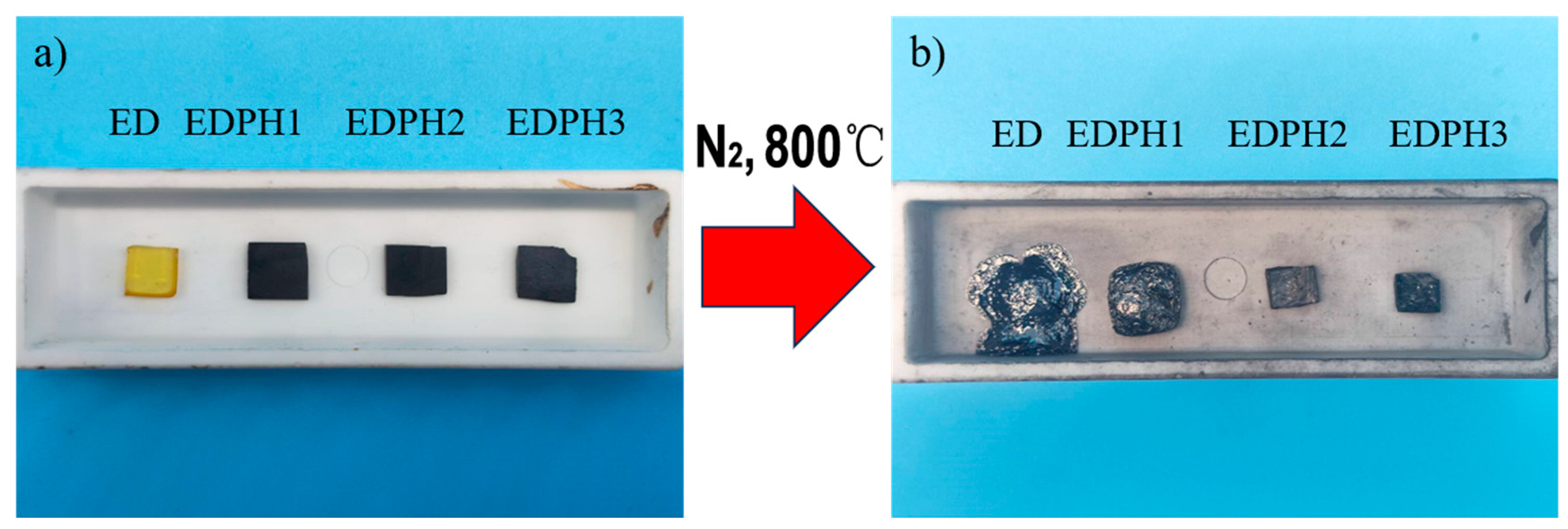
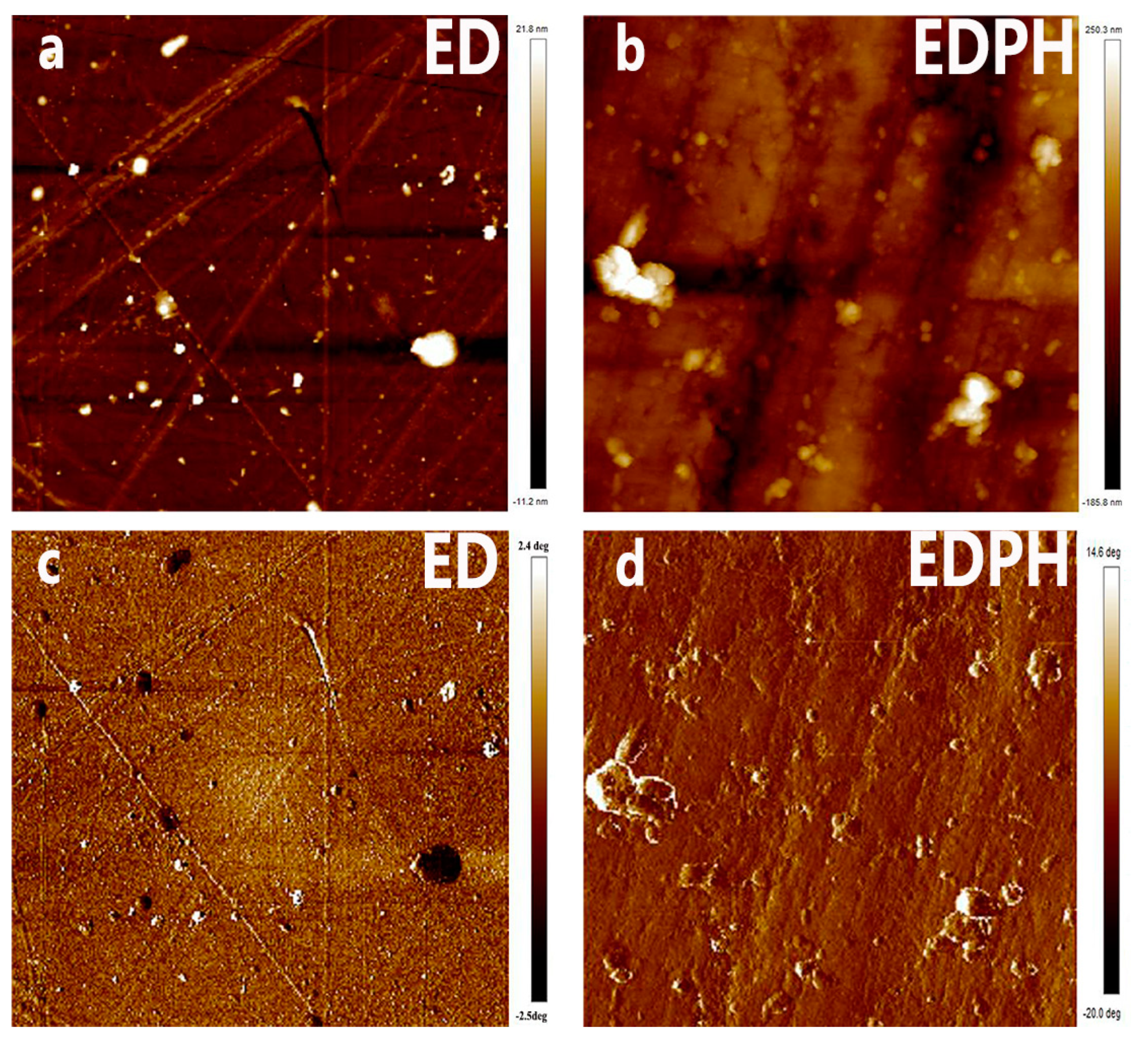
3.5. The Morphology of the Blending Thermoset
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Iqbal, H.M.S.; Bhowmik, S.; Bhatnagar, N.; Mondal, S.; Ahmed, S. High Performance Adhesive Bonding of High Temperature Resistant Polymer. J. Adhes. Sci. Technol. 2012, 26, 955–967. [Google Scholar] [CrossRef]
- Bao, C.; Wang, Y.; Mushtaq, R.T.; Chen, X.; Liu, Z.; Li, X.; Liu, M. Preparation and characterization of elevated and cryogenic temperature-resistant regolith-based epoxy resin composites. Constr. Build. Mater. 2023, 387, 131560. [Google Scholar] [CrossRef]
- Qian, D.; Zhou, J.; Zheng, J.; Cao, J.; Wan, J.; Fan, H. Synthesis, Curing Behaviors and Properties of a Bio-Based Trifunctional Epoxy Silicone Modified Epoxy Thermosets. Polymers 2022, 14, 4391. [Google Scholar] [CrossRef]
- Yoon, S.G.; Kim, E.S.; Kang, N.K.; Lee, W.S.; Kim, Y.H. Synthesis and characterization of high temperature stable epoxy adhesive. J. Ceram. Soc. Jpn. 2008, 116, 550–554. [Google Scholar] [CrossRef]
- Slobodinyuk, A.; Kiselkov, D.; Elchisheva, N.; Slobodinyuk, D. Synthesis and properties of high temperature self-curing epoxy resin. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1181, 012015. [Google Scholar] [CrossRef]
- Wei, M.; Wang, B.; Zhang, X.; Wei, W.; Li, X. Cycloaliphatic epoxy-functionalized polydimethylsiloxanes for comprehensive modifications of epoxy thermosets. Eur. Polym. J. 2024, 202, 112656. [Google Scholar] [CrossRef]
- Mishnev, M.; Korolev, A.; Ulrikh, D.; Gorechneva, A.; Sadretdinov, D.; Grinkevich, D. Solid Particle Erosion of Filled and Unfilled Epoxy Resin at Room and Elevated Temperatures. Polymers 2023, 15, 1. [Google Scholar] [CrossRef]
- Lu, C.; Bian, S.; Hu, K.; Li, C.; Zheng, K.; Sun, Q. Biomass-based epoxy resin derived from resveratrol with high temperature resistance and intrinsic flame retardant properties. Ind. Crop. Prod. 2022, 187, 115500. [Google Scholar] [CrossRef]
- Ning, K.; Zhou, L.-L.; Zhao, B. A novel aminothiazole-based cyclotriphosphazene derivate towards epoxy resins for high flame retardancy and smoke suppression. Polym. Degrad. Stab. 2021, 190, 109651. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Qi, Y.; Zhang, F.; Weng, Z.; Jian, X. Preparation of Novel Epoxy Resins Bearing Phthalazinone Moiety and Their Application as High-Temperature Adhesives. Polymers 2018, 10, 708. [Google Scholar] [CrossRef]
- Huang, S.; Deng, Z.; Lv, Y.; Ding, H.; Liu, X.; Dong, Y.; Huang, Z. Preparation and Properties of Phenolic Epoxy Modified Silicone Resin. J. Macromol. Sci. Part B 2024, 63, 634–648. [Google Scholar] [CrossRef]
- Chen, X.; Dai, C.; Jiang, T.; Paramane, A. Enhanced thermal and dielectric performance of epoxy resin/aluminum nitride nanocomposites at high temperatures. Polym. Compos. 2020, 41, 5375–5386. [Google Scholar] [CrossRef]
- Inamdar, A.; Yang, Y.-H.; Prisacaru, A.; Gromala, P.; Han, B. High temperature aging of epoxy-based molding compound and its effect on mechanical behavior of molded electronic package. Polym. Degrad. Stab. 2021, 188, 109572. [Google Scholar] [CrossRef]
- Rong, L.; Su, J.; Li, Z.; Liu, X.; Zhang, D.; Zhu, J.; Li, X.; Zhao, Y.; Mi, C.; Kong, X.; et al. Silicon Hybridization for the Preparation of Room-Temperature Curing and High-Temperature-Resistant Epoxy Resin. Polymers 2024, 16, 634. [Google Scholar] [CrossRef]
- Yu, C.; Xu, Z.; Ma, X.; Zhang, J.; Chen, S.; Miao, M.; Chen, H.; Zhang, D. Hyperbranched polymers containing epoxy and imide structure. Prog. Org. Coat. 2021, 151, 106031. [Google Scholar] [CrossRef]
- Bao, C.; Wang, Y.; Mushtaq, R.T.; Zhang, K.; Li, X.; Chen, X. Preparation, characterization, and curing kinetics of elevated and cryogenic temperature-resistant epoxy resin composites. Polym. Test. 2022, 116, 107783. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhao, C.H.; Sudo, A.; Endo, T. Storage stability and curing behavior of epoxy-dicyandiamide systems with carbonyldiimidazole-Cu (II) complexes as the accelerator, J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3470–3476. [Google Scholar] [CrossRef]
- Xing, H.; Mao, Y.; Yang, Y.; Qu, C.; Wang, D.; Fan, X.; Zhao, L.; Zhou, D.; Liu, C. Preparation of waterborne polyimide-modified epoxy resin with high thermal properties and adhesion properties. J. Appl. Polym. Sci. 2022, 139, e53103. [Google Scholar] [CrossRef]
- Ji, B.; Pan, Y.; Lyu, J.; Liu, J.; Liao, W.; Yin, C.; Xing, S.; Wu, N. Low defect and high mechanical properties POSS copolymerization phthalonitrile resin prepared by powder hot isostatic pressing. Mater. Lett. 2023, 352, 135140. [Google Scholar] [CrossRef]
- Keller, T.M.; Dominguez, D.D. High temperature resorcinol-based phthalonitrile polymer. Polymer 2005, 46, 4614–4618. [Google Scholar] [CrossRef]
- Laskoski, M.; Dominguez, D.D.; Keller, T.M. Synthesis and properties of a bisphenol A based phthalonitrile resin. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4136–4143. [Google Scholar] [CrossRef]
- Dominguez, D.D.; Keller, T.M. Properties of phthalonitrile monomer blends and thermosetting phthalonitrile copolymers. Polymer 2007, 48, 91–97. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Z.; Zhang, J.; Yang, X.; Zhao, R.; Liu, X. Self-promoted curing phthalonitrile with high glass transition temperature for advanced composites. J. Polym. Res. 2012, 19, 9918. [Google Scholar] [CrossRef]
- Bai, S.; Sun, X.; Zhang, Z.; Chen, X.; Yu, X.; Zhang, Q. Synthesis and Properties of a Low Melting Point Phthalonitrile Resin Containing High Density Nitrile Groups. ChemistrySelect 2020, 5, 265–269. [Google Scholar] [CrossRef]
- Xu, S.; Han, Y.; Guo, Y.; Luo, Z.; Ye, L.; Li, Z.; Zhou, H.; Zhao, Y.; Zhao, T. Allyl phenolic-phthalonitrile resins with tunable properties: Curing, processability and thermal stability. Eur. Polym. J. 2017, 95, 394–405. [Google Scholar] [CrossRef]
- Qi, Y.; Weng, Z.; Wang, J.; Zhang, S.; Zong, L.; Liu, C.; Jian, X. A novel bio-based phthalonitrile resin derived from catechin: Synthesis and comparison of curing behavior with petroleum-based counterpart. Polym. Int. 2018, 67, 322–329. [Google Scholar] [CrossRef]
- Gao, M.; Wu, Q.; Li, T.; Liu, L.; Li, B.; Song, Y.; Liu, M. Rediscovering phthalonitrile resins: A novel liquid monomer towards high-performance resins. Polym. Chem. 2024, 15, 2157–2166. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, H.; Tang, H.; Yang, X.; Xu, M.; Liu, X. Preparation and properties of bisphenol A-based bis-phthalonitrile composite laminates. J. Appl. Polym. Sci. 2013, 129, 2621–2628. [Google Scholar] [CrossRef]
- Gao, M.; Li, T.; Kong, W.; Sun, X.; Liu, L.; Li, B.; Song, Y.; Liu, M. Novel liquid phthalonitrile monomers towards high performance resin. Eur. Polym. J. 2023, 191, 112027. [Google Scholar] [CrossRef]
- Ding, Z.; Zong, L.; Wang, C.; Wang, S.; Liu, R.; Jian, X.; Wang, J. A new approach to enhance the performance of phthalonitrile: Study of carborane curing agents with dual functions. Mol. Syst. Des. Eng. 2023, 8, 1492–1497. [Google Scholar] [CrossRef]
- Brown, L.C.; Richardson, T.J.; Lusk, C.F.; Weise, N.K.; Laskoski, M. Preparation and properties of bisphenol A polyetherketoneketone based phthalonitrile resins. J. Appl. Polym. Sci. 2024, 141, 55080. [Google Scholar] [CrossRef]
- Kong, W.; Sun, J.; Gao, M.; Li, T.; Liu, M.; Song, Y. High-performance boron-containing phthalonitrile resins. Polym. Chem. 2023, 14, 2317–2325. [Google Scholar] [CrossRef]
- Xi, Z.; Chen, X.; Yu, X.; Ma, Y.; Ji, P.; Naito, K.; Ding, H.; Qu, X.; Zhang, Q. Synthesis and properties of a novel high temperature pyridine-containing phthalonitrile polymer. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3819–3825. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Lin, J.; Du, L. A theoretical insight into the curing mechanism of phthalonitrile resins promoted by aromatic amines. Phys. Chem. Chem. Phys. 2021, 23, 17300–17309. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, B.; Sun, M.; Zhang, X. Preparation and characterization of diamine-functional bisphthalonitrile resins with self-promoted cure behavior. Iran Polym. J. 2023, 32, 177–186. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Lei, Y.; Zhong, J.; Liu, X. Effect of different aromatic amines on the crosslinking behavior and thermal properties of phthalonitrile oligomer containing biphenyl ethernitrile. J. Appl. Polym. Sci. 2011, 121, 2331–2337. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Xu, M.; Liu, X. Preparation and properties of bisphenol A-based bisphthalonitrile polymers. High Perform. Polym. 2014, 26, 3–11. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Zong, L.; Li, N.; Wang, J.; Jian, X. Novel phthalonitrile-based composites with excellent processing, thermal, and mechanical properties. High Perform. Polym. 2018, 30, 720–730. [Google Scholar] [CrossRef]
- Peng, W.F.; Liu, Y.; Liu, Z.Z.; Lu, Z.; Hu, J.H.; Zeng, K. Curing kinetics study on highly efficient thermal synergistic polymerization effect between alicyclic imide moiety and phthalonitrile. Thermochim. Acta 2018, 659, 27–33. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, X.; Liu, C.; Qu, C.; Yuan, Z.; Jing, J.; Tang, Y.; Zhao, D.; Xiao, W.; Su, K. Properties of high-temperature epoxy/DDS resin systems for bonding application. High Perform. Polym. 2020, 32, 559–568. [Google Scholar] [CrossRef]
- Xiao, H.; Zhou, T.; Shi, M.; Lv, J.; Li, R.; Zeng, K.; Hu, J.; Yang, G. A molding-sintering method inspired by powder metallurgy for thermosetting resins with narrow processing window: A case study on bio-based adenine containing phthalonitrile. Chem. Eng. J. 2020, 398, 125442. [Google Scholar] [CrossRef]
- Zhao, X.; Lei, Y.; Zhao, R.; Zhong, J.; Liu, X. Preparation and properties of halogen-free flame-retarded phthalonitrile-epoxy blends. J. Appl. Polym. Sci. 2012, 123, 3580–3586. [Google Scholar] [CrossRef]
- Hu, Y.; Weng, Z.; Qi, Y.; Wang, J.; Zhang, S.; Liu, C.; Zong, L.; Jian, X. Self-curing triphenol A-based phthalonitrile resin precursor acts as a flexibilizer and curing agent for phthalonitrile resin. RSC Adv. 2018, 8, 32899–32908. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.F.; Wei, R.B.; Yong, Y. Post Self-Crosslinking of Phthalonitrile-Terminated Polyarylene Ether Nitrile Crystals. Polymers 2018, 10, 640. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Cui, Y.; Li, S.; Yuan, Y.; Peng, C.; Yan, J.; Huang, H.; Wu, Z. Thermal degradation behavior of 10-(2,5-dihydroxyphenyl)-9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide modified epoxy resin with liquid oxygen compatibility. Polym. Degrad. Stab. 2022, 201, 109996. [Google Scholar] [CrossRef]




| Specimen | E51 (g) | BAPH (g) | DDS (g) |
|---|---|---|---|
| ED | 10.00 | 0 | 3.16 |
| EDPH1 | 9.00 | 1.00 | 2.89 |
| EDPH2 | 8.00 | 2.00 | 2.63 |
| EDPH3 | 7.00 | 3.00 | 2.36 |
| Sample | T5% a /°C | Char Yield (Measured) b /% | Char Yield (Calculated) /% | Tmax /°C | (dw/dT)max |
|---|---|---|---|---|---|
| ED | 363 | 16 | / | 389.2 | -1.47 |
| EDPH1 | 368.5 | 44.3 | 21 | 390 | -0.6 |
| EDPH2 | 374.2 | 46.7 | 26.2 | 397 | -0.5 |
| EDPH3 | 390.2 | 59.6 | 31.7 | 411.2 | -0.3 |
| Sample | E′ at 50 °C (MPa) | E′r (MPa) | Tg (°C) | C (mol·cm3) |
|---|---|---|---|---|
| ED | 2827 | 12.8 | 181.2 | 0.0010 |
| EDPH1 | 2437 | 35.6 | 187.1 | 0.0030 |
| EDPH2 | 2358 | 30.2 | 192.3 | 0.0024 |
| EDPH3 | 1864 | 23.7 | 195 | 0.0019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Luo, T.; Wu, Z.; Li, S. Investigation on the Curing and Thermal Properties of Epoxy/Amine/Phthalonitrile Blend. Materials 2024, 17, 4411. https://doi.org/10.3390/ma17174411
Peng C, Luo T, Wu Z, Li S. Investigation on the Curing and Thermal Properties of Epoxy/Amine/Phthalonitrile Blend. Materials. 2024; 17(17):4411. https://doi.org/10.3390/ma17174411
Chicago/Turabian StylePeng, Cong, Tao Luo, Zhanjun Wu, and Shichao Li. 2024. "Investigation on the Curing and Thermal Properties of Epoxy/Amine/Phthalonitrile Blend" Materials 17, no. 17: 4411. https://doi.org/10.3390/ma17174411
APA StylePeng, C., Luo, T., Wu, Z., & Li, S. (2024). Investigation on the Curing and Thermal Properties of Epoxy/Amine/Phthalonitrile Blend. Materials, 17(17), 4411. https://doi.org/10.3390/ma17174411







