Corrosion of Chromium Coating Fabricated on Zircaloy-4 Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Autoclave Testing
2.3. Methods for Corrosion Assessment
3. Results
3.1. Gravimetric Analysis of Cr-Coated Zircaloy-4 Alloy, Examined under Primary Circuit Situations
3.2. Morphological and Structural Characterization of Cr Coating Post-Autoclave Testing
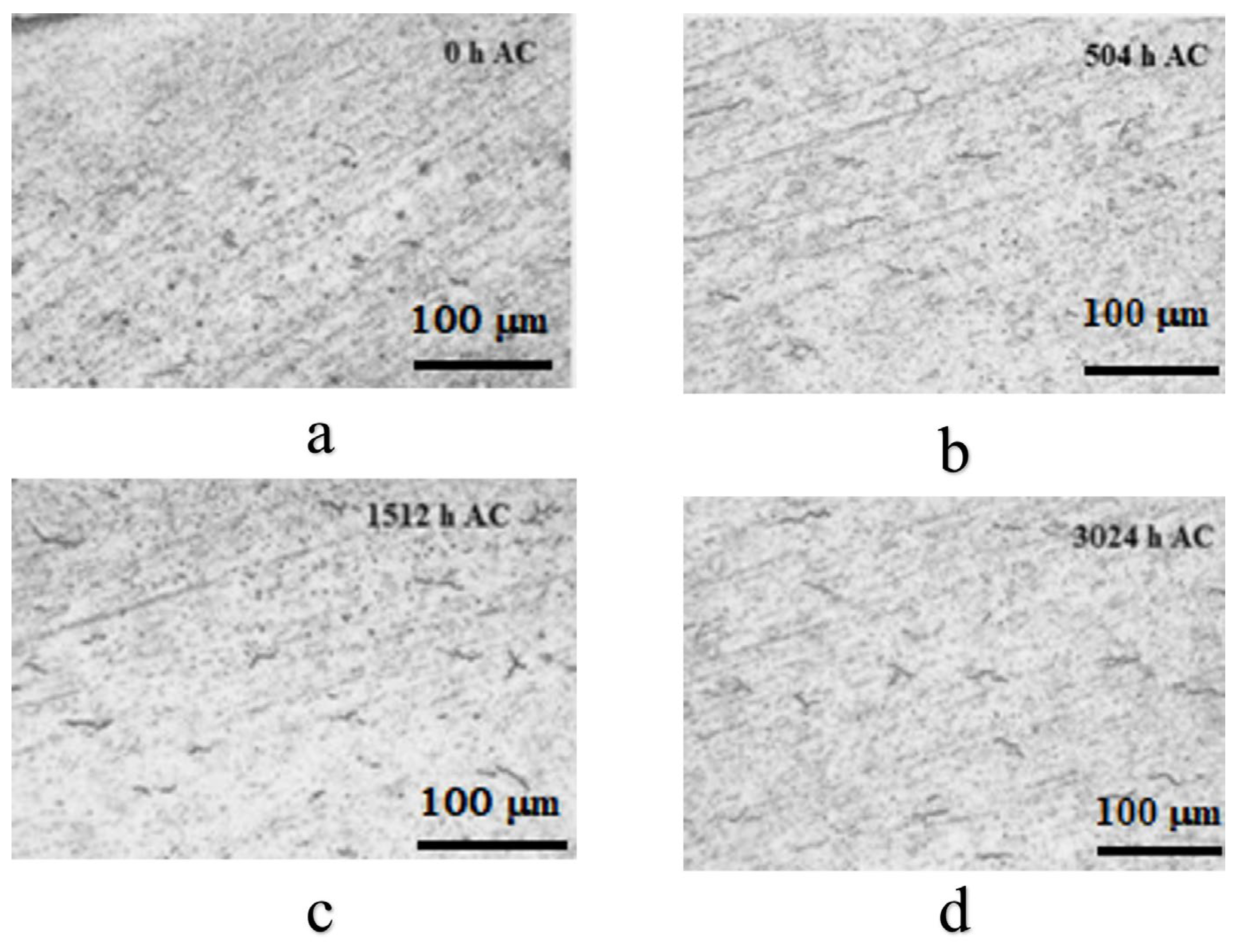
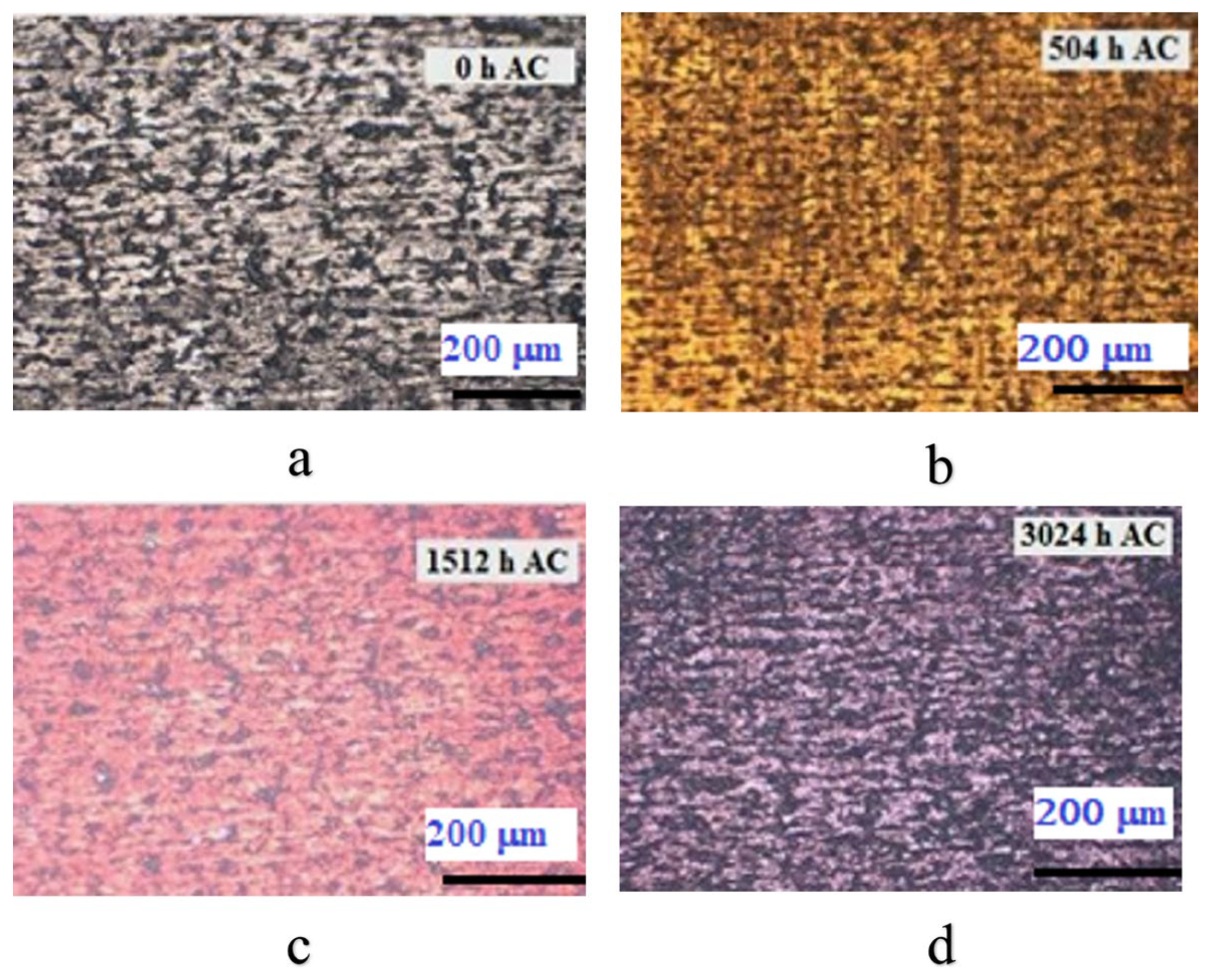
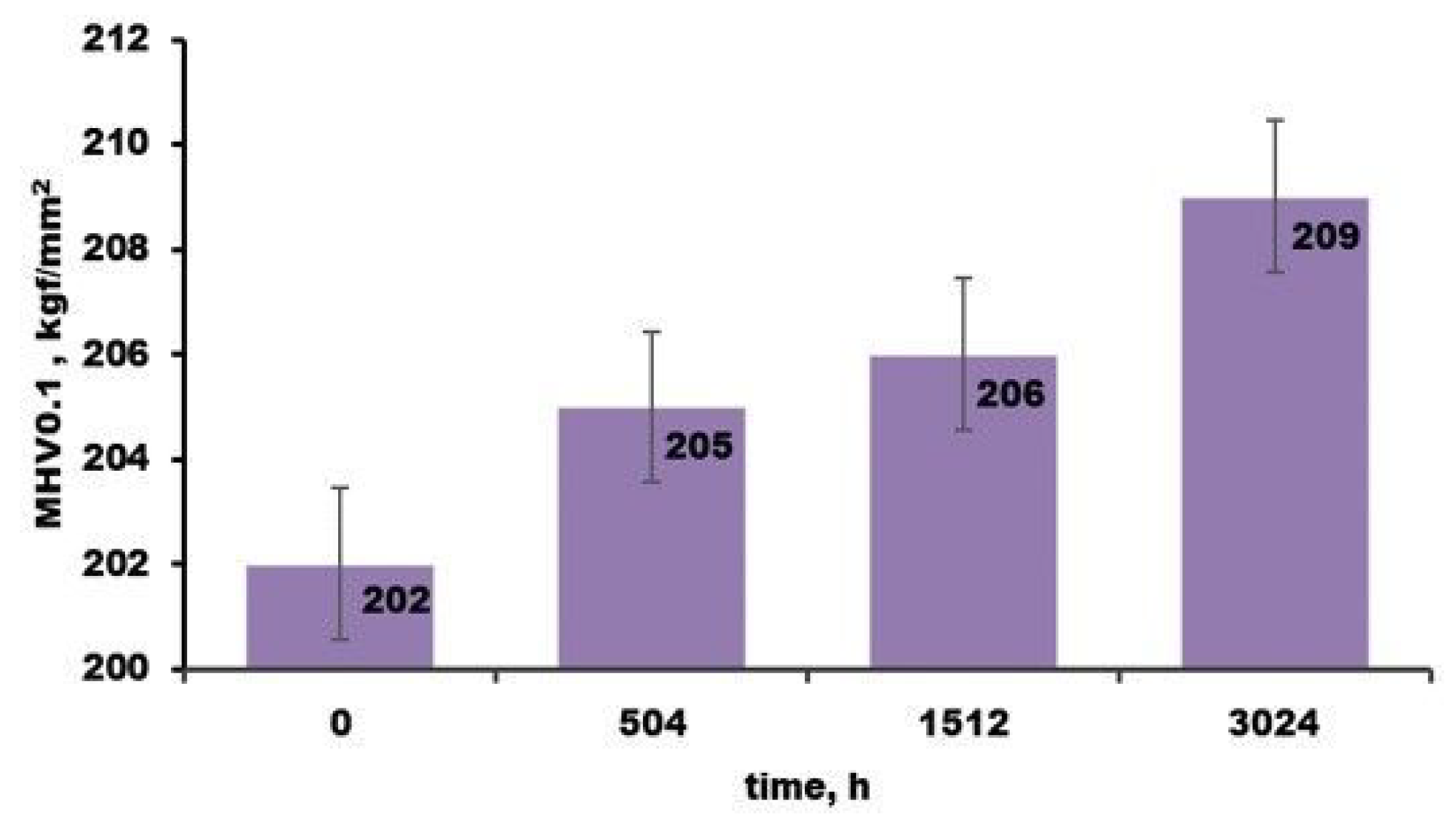
3.2.1. Metallographic Analysis
3.2.2. Scanning Electron Microscopy (SEM) Measurements
3.2.3. XRD Measurements
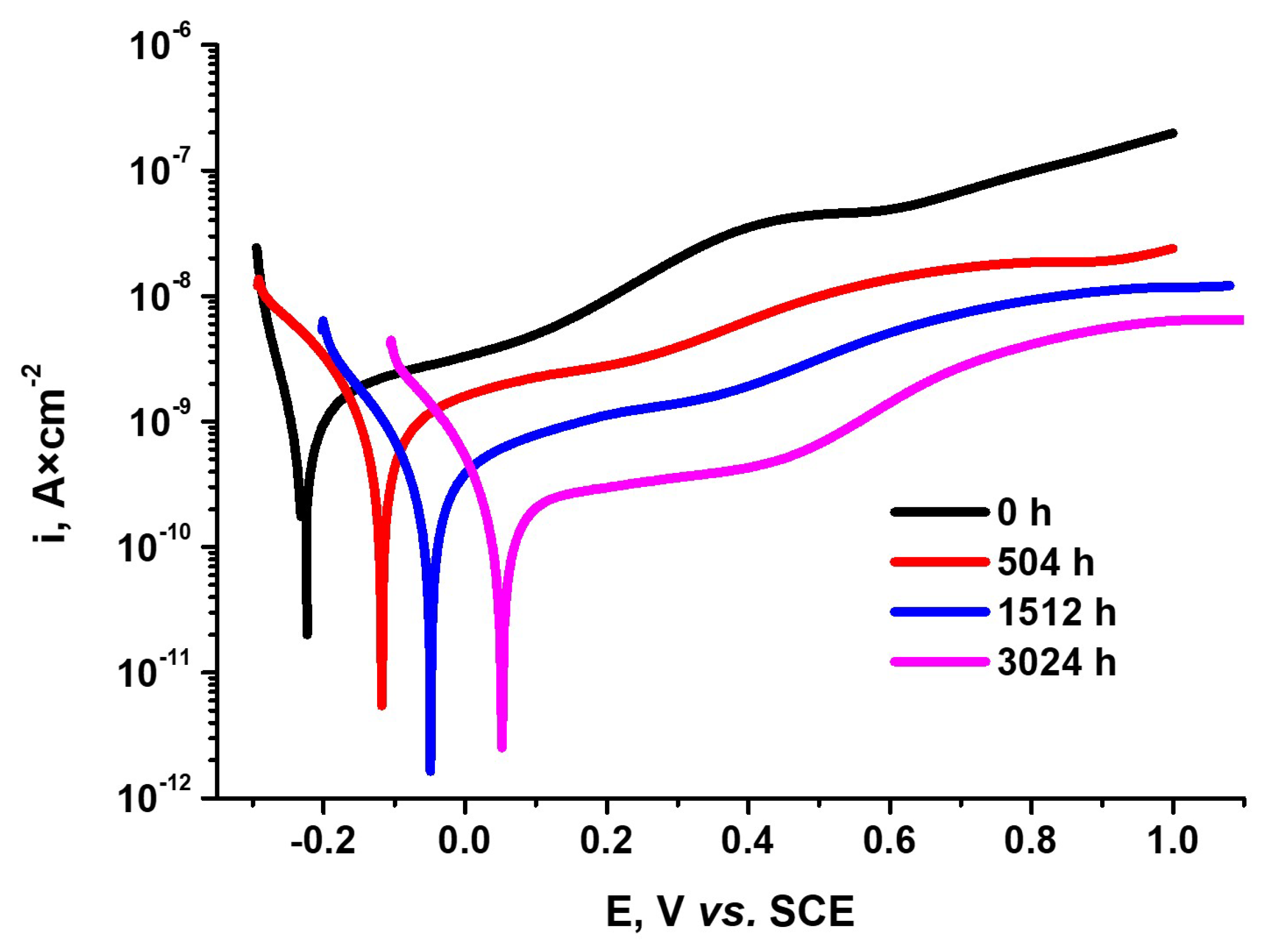
3.3. Evaluation of Susceptibility to Corrosion by Electrochemical Methods
3.3.1. Open-Circuit Potential Tests
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3.3. Potentiodynamic Polarization Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawat, M.; Das, A.; Shukla, D.K.; Rajput, P.; Chettah, A.; Phase, D.M.; Ramola, R.C.; Singh, F. Micro-Raman and electronic structure study on kinetics of electronic excitations induced monoclinic-to-tetragonal phase transition in zirconium oxide films. RSC Adv. 2016, 6, 104425–104432. [Google Scholar] [CrossRef]
- Wang, Y.B.; Zheng, Y.F.; Wei, S.C.; Li, M. In vitro study on Zr-based bulk metallic glasses as potential biomaterials. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ramonti, D.; Gomez Sanchez, A.; Milosev, I.; Demetrescu, I.; Cere, S. Effect of anodization on the surface characteristics and electrochemical behaviour of zirconium in artificial saliva. Mater. Sci. Eng. C 2016, 62, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Kashkarov, E.; Gusey, K.; Kudijarov, V.; Kurdyumov, N.; Sidelev, D. Hydrogenation Behavior of Cr-Coated Resistance Upset Welds of E110 Zirconium Alloy. Coatings 2023, 13, 339. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Zhang, R. Application and Development Progress of Cr-Based Surface Coatings in Nuclear Fuel Element: I.Selection, Preparation, and Characteristics of Coating Materials. Coatings 2020, 10, 808. [Google Scholar] [CrossRef]
- Hussain, D. Accident Tolerant Barriers for Fuel Rod Cladding in Nuclear Reactors. Ph.D. Thesis, Department of Materials Science and Engineering, The University of Sheffield, Sheffield, UK, 2020. [Google Scholar]
- Kashkarov, E.; Afornu, B.; Sidelev, D.; Krinitcyn, M.; Gouws, V.; Lider, A. Recent advances in protective coatings for accident tolerant Zr-based fuel claddings. Coatings 2021, 1, 557. [Google Scholar] [CrossRef]
- Ma, H.-B.; Yan, J.; Zhao, Y.-H.; Liu, T.; Ren, Q.-S.; Liao, Y.-H.; Zuo, J.-D.; Liu, G.; Yao, M.-Y. Oxidation behaviour of Cr-coated zirconium alloy cladding in high-temperature steam above 1200C. Mater. Degrad. 2021, 5, e7. [Google Scholar] [CrossRef]
- Brachet, J.; Idarraga-Trujillo, I.; Le Flem, M.; Le Saux, M.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon Masclet, C.; Tupin, M. Early studies on Cr-Coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W.; et al. AREVA NP’s Enhanced Accident-Tolerant Fuel Developments: Focus on Cr-coated M5 Cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 1080077. [Google Scholar] [CrossRef]
- Syrtanov, M.; Kashkarov, E.; Abdulmenova, A.; Gusev, K.; Sidelev, D. High-Temperature Steam Oxidation of Accident-Tolerant Cr/Mo-Coated Zr Alloy at 1200–1400 °C. Coatings 2023, 13, 191. [Google Scholar] [CrossRef]
- Sevecek, M.; Gurgen, A.; Phillips, B.; Che, Y.; Wagih, M.; Shirvan, K. Development of Cold spray Cr coated fuel cladding with enhanced accident tolerance. Nucl. Eng. Technol. 2017, 50, 229–236. [Google Scholar] [CrossRef]
- Huang, J.; Zou, S.; Xiao, W.; Yang, C.; Tang, D.; Yu, H.; Zhang, L.; Zhang, K. Influence of arc current on microstructure of Cr coating for Zr-4 alloy prepared by multi-arc ion plating via EBSD. Mater. Charact. 2021, 178, 11211. [Google Scholar] [CrossRef]
- Krejci, J.; Kabatova, J.; Manoch, F.; Koci, J.; Cvercek, L.; Malek, J.; Krum, S.; Sutta, P.; Bublikova, P.; Halodova, P.; et al. Development and testing of multicomponent fuel cladding with enhanced accidental performance. Nucl. Eng. Technol. 2020, 52, 597–609. [Google Scholar] [CrossRef]
- Yang, J.; Steinbruck, M.; Tang, C.; Grobe, M.; Liu, J.; Zhang, J.; Yun, D.; Wang, S. Review on chromium coated zirconium alloy accident tolerant fuel cladding. J. Alloys Compd. 2021, 895, 162450. [Google Scholar] [CrossRef]
- Duarte, T.; Meyer, Y.A.; Osório, W.R. The Holes of Zn Phosphate and Hot Dip Galvanizing on Electrochemical Behaviors of Multicoatings on Steel Substrates. Metals 2022, 12, 863. [Google Scholar] [CrossRef]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS investigation of the corrosion behavior of steel bars embedded into modified concretes with eggshell contentes. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-Phase-Element Behavior Caused by Resistivity Distributions in Films. J. Electrochem. Soc. 2010, 157, C458–C463. [Google Scholar] [CrossRef]
- Diniasi, D.; Golgovici, F.; Marin, A.H.; Negrea, A.D.; Fulger, M.; Demetrescu, I. Long-term corrosion testing of Zy-4 in a LiOH solution under high pressure and temperature conditions. Materials 2021, 14, 4586. [Google Scholar] [CrossRef]
- Yung, T.; Lu, W.; Tsai, K.; Chuang, C.; Chen, P. Oxidation behaviour characterization of Zircaloy-4 cladding with different hydrogen concentrations at 500–800C in an ambient atmosphere. Materials 2022, 15, 2997. [Google Scholar] [CrossRef] [PubMed]
- Zino, R.; Chosson, R.; Ollivier, M.; Serris, E.; Favergeon, L. Parallel mechanism of growth of the oxide and α-Zr(O) layers on Zircaloy-4 oxidized in steam at high temperatures. Corr. Sci. 2021, 179, 109178. [Google Scholar] [CrossRef]
- Bossis, P.; Lelievre, G.; Barberis, P.; Ilitis, X.; LeFebvre, F. Multi-Scale Characterization of the Metal-Oxide Interface of Zirconium Alloys; ASTM Special Technical Publication: Philadelphia, PA, USA, 2000; pp. 918–945. [Google Scholar]
- De Oliveira, L.; da Silva, R.; dos Santos, D.; Ribeiro, R. Hydrogen kinetics and hydride formation effect on Zr-1Nb and Zr-1Nb-1Sn-0.1Fe alloys for nuclear application. Mater. Res. 2017, 20, 786–791. [Google Scholar] [CrossRef]
- Maric, M.; Thomas, R.; Nunez-Iglesias, J.; Atkinson, M.; Bertsch, J.; Frankel, P.; Race, C.; Barberis, P.; Bourlier, F.; Preuss, M.; et al. A novel method for radial hydride analysis in zirconium alloys: HAPPy. J. Nucl. Mater. 2022, 559, e153442. [Google Scholar] [CrossRef]
- Wang, Z.; Garbe, U.; Li, H.; Harrison, R.; Kaestner, A.; Lehmann, E. Observations on the Zirconium Hydride Precipitation and Distribution in Zircaloy-4. Metall. Mater. Trans. B 2013, 45, 532–539. [Google Scholar] [CrossRef]
- ASTM E 384; Standard Test Method for Microhardness of Materials. ASTM: Philadelphia, PA, USA, 2022.
- Ogawa, Y.; Takakuwa, O.; Tsuzaki, K. Solid-solution hardening by hydrogen in Fe-Cr-Ni based austenitic steel: Temperature and strain rate effects. J. Mater. Sci. 2023, 879, e145281. [Google Scholar] [CrossRef]
- Diniasi, D.; Fulger, M.; Butoi, B.; Dinca, P.; Golgovici, F. Accident tolerant barriers for fuel rod cladding of CANDU nuclear reactor. Coatings 2023, 13, 1739. [Google Scholar] [CrossRef]
- Umretiya, R.; Elward, B.; Lee, D.; Anderson, M.; Rebak, R.; Rojas, J. Mechanical and chemical properties of PVD and Cold Spray Cr-coatings on Zircaloy-4. J. Nucl. Mater. 2020, 541, e152420. [Google Scholar] [CrossRef]
- Hazan, J.; Gauthier, A.; Pouillier, E.; Shirvan, K. Semi-integral LOCA test of cold-spray chromium coated zircaloy-4 accident tolerant fuel cladding. J. Nucl. Mater. 2021, 550, e152940. [Google Scholar] [CrossRef]
- OECD. State-of-the-Art Report on Light Water Reactor Accident-Tolerant Fuels; NEA No. 7317; OECD: Paris, France, 2018. [Google Scholar]

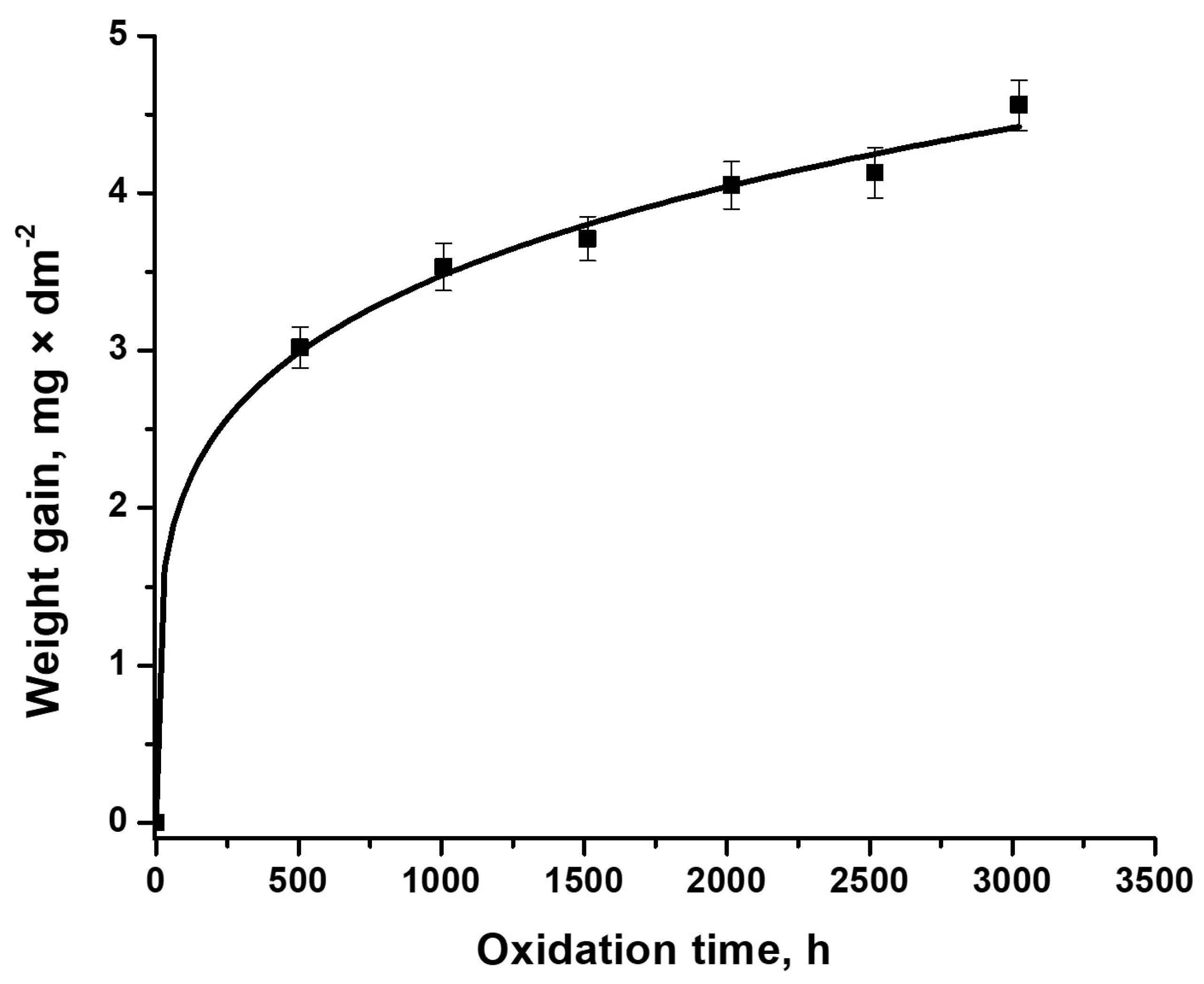

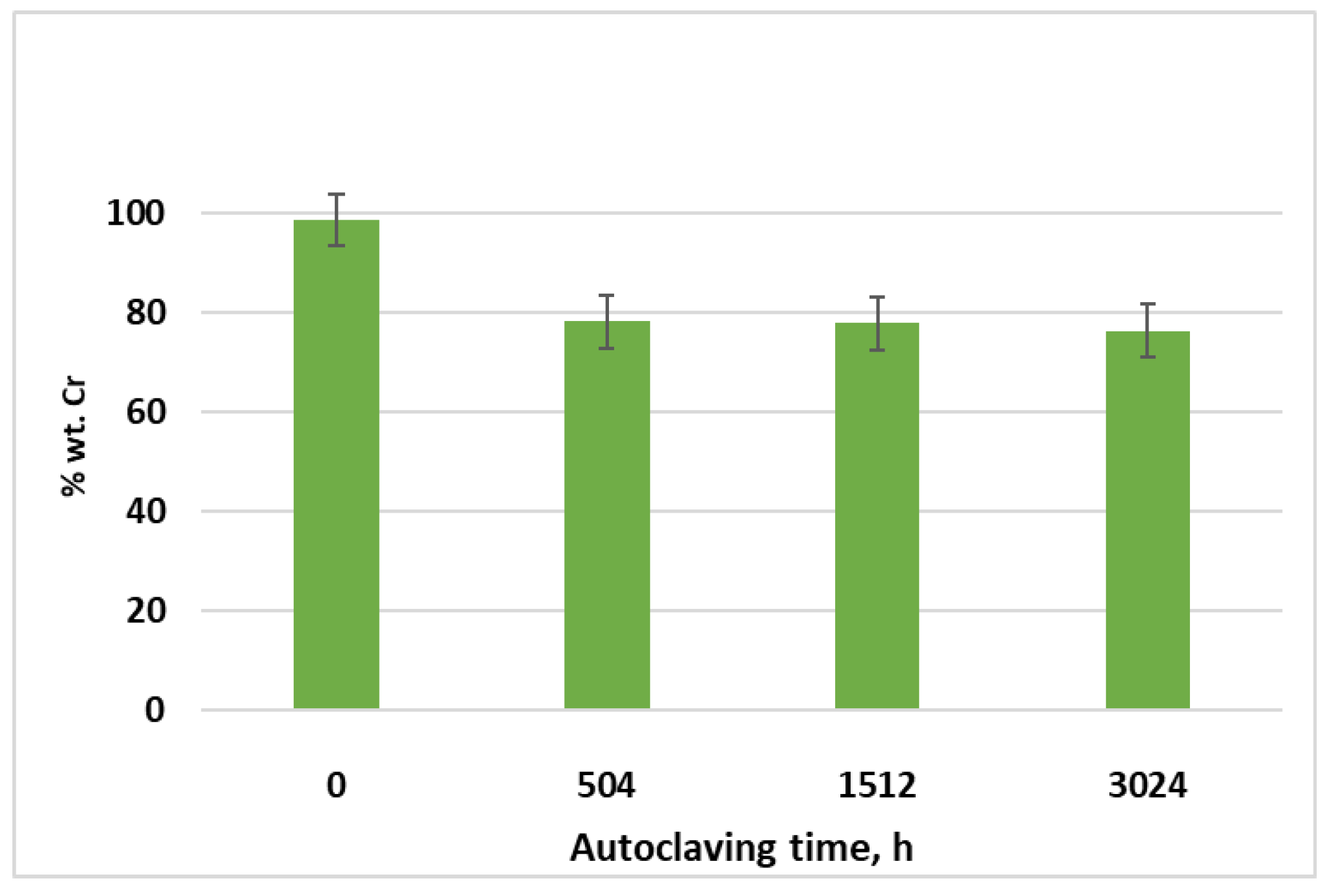



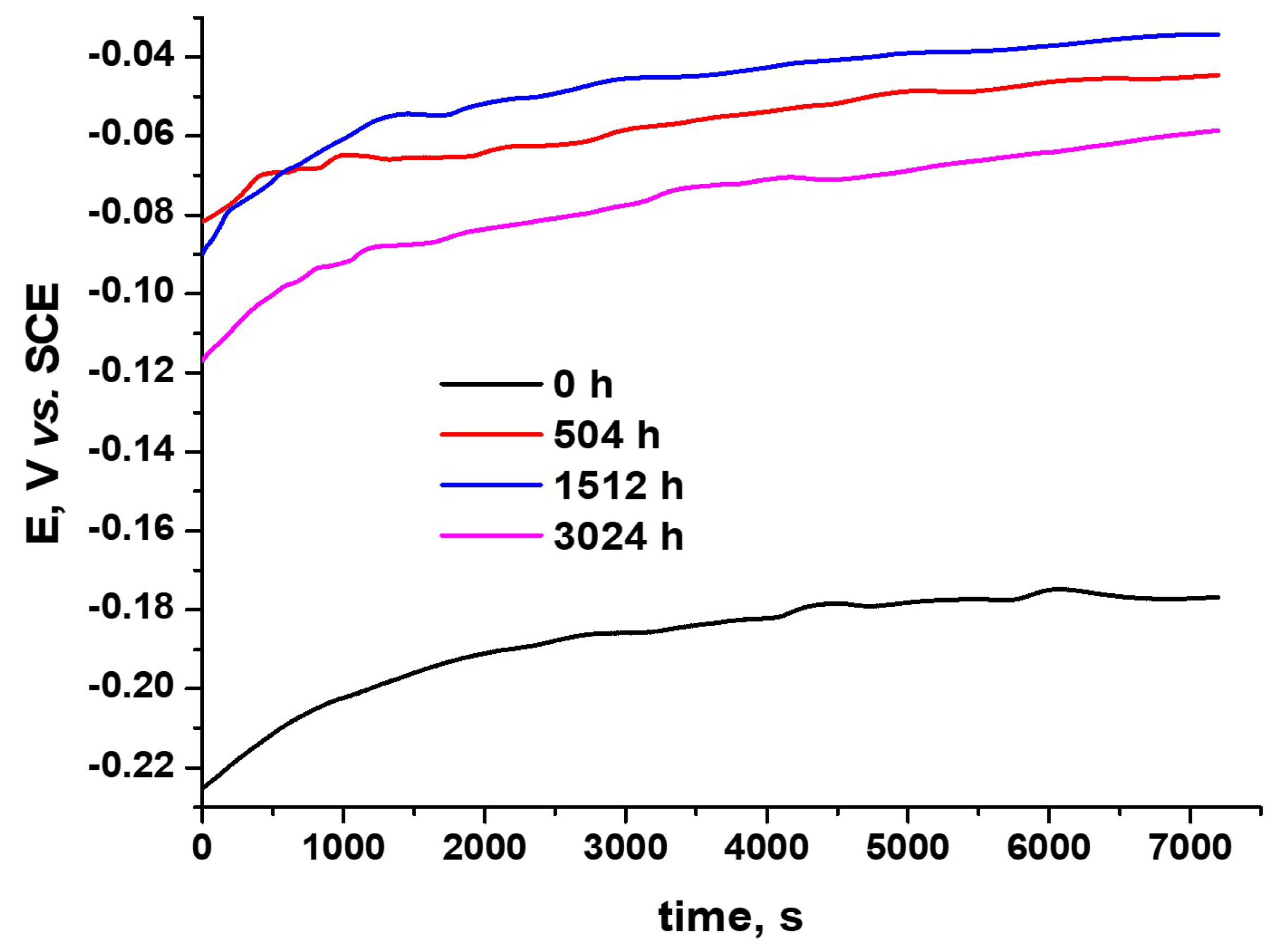
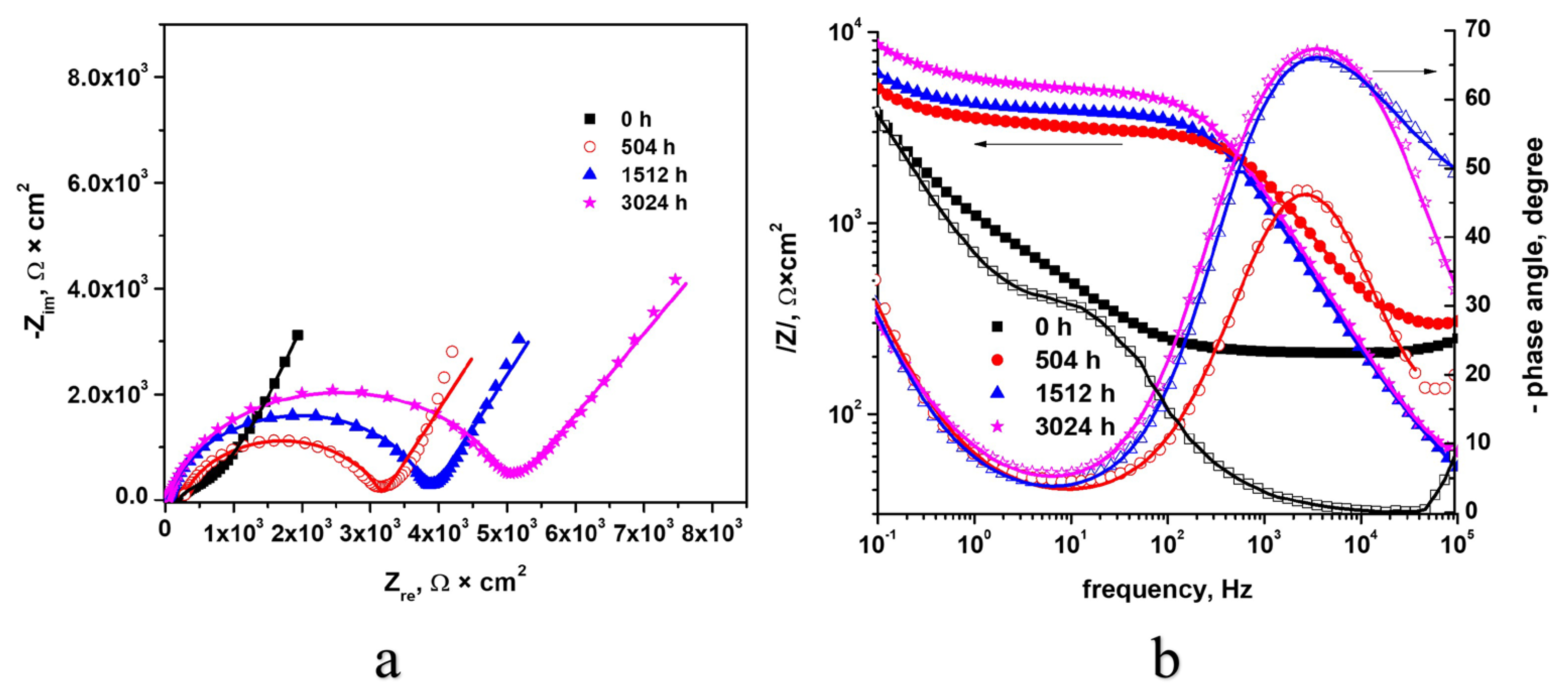
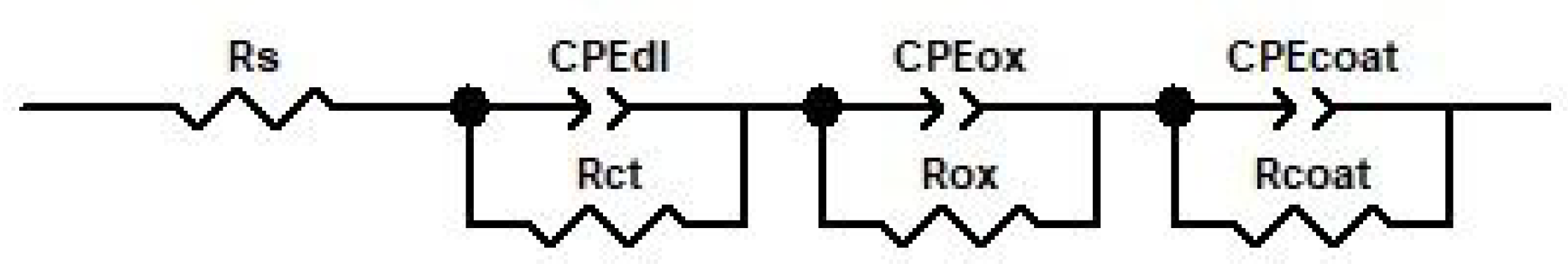
| Kinetic Equation | kp | n | R2 |
|---|---|---|---|
| y = 1.376 × t0.167 | 1.376 | 0.167 | 0.997 |
| Autoclave Testing, h | 0 | 504 | 1512 | 3024 |
|---|---|---|---|---|
| Rs, Ω × cm2 | 20.2 ± 0.2 | 17.3 ± 0.1 | 18.7 ± 0.1 | 19.3 ± 0.21 |
| CPEdl-T, F × cm−2 | 3.7 × 10−4 ± 0.3 | 3.2 × 10−7 ± 0.2 | 2.6 × 10−7 ± 0.3 | 2.7 × 10−7 ± 0.1 |
| CPEdl-P | 0.64 ± 0.05 | 0.84 ± 0.08 | 0.89 ± 0.075 | 0.87 ± 0.05 |
| Rct, Ω × cm2 | 1.8 × 1014 ± 0.5 | 2881 ± 0.3 | 3723 ± 0.2 | 4920 ± 0.1 |
| CPEox-T, F × cm−2 | - | 4.6 × 10−4 ± 0.4 | 4.1 × 10−4 ± 0.3 | 2.7 × 10−4 ± 0.6 |
| CPEox-P | - | 0.71 ± 0.03 | 0.78 ± 0.05 | 0.64 ± 0.05 |
| Rox, Ω × cm2 | - | 1.9 × 1014 | 7.5 × 1018 | 7.8 × 1019 |
| CPEcoat-T, F × cm−2 | 7.1 × 10−6 ± 0.1 | 7.8 × 10−6 ± 0.1 | 4.2 × 10−5 ± 0.2 | 1.1 × 10−4 ± 0.2 |
| CPEcoat-P | 0.79 ± 0.03 | 0.99 ± 0.07 | 0.87 ± 0.05 | 0.69 ± 0.02 |
| Rcoat, Ω × cm2 | 201.2 ± 12.4 | 267.1 ± 12.5 | 109.1 ± 11 | 57.6 ± 8.2 |
| Chi-Squared | 2.3 × 10−3 | 1.3 × 10−3 | 2.5 × 10−4 | 3 × 10−4 |
| Autoclaving Time, h | Tafel Slope Method | Polarization Resistance Method | Pi (%) | P (%) | |||
|---|---|---|---|---|---|---|---|
| Ecorr, mV | icorr, nA × cm−2 | Vcorr, nm × year−1 | Rp, MΩ × cm2 | icorr, nA × cm−2 | |||
| 0 | −232 ± 0.4 | 0.797 ± 0.003 | 18.3 ± 0.04 | 31.2 ± 0.2 | 0.787 ± 0.004 | - | - |
| 504 | −117 ± 0.3 | 0.515 ± 0.003 | 11.82 ± 0.03 | 55.1 ± 0.4 | 0.476 ± 0.003 | 35.38 ± 0.03 | 0.0166 ± 0.02 |
| 1512 | −54 ± 0.5 | 0.216 ± 0.002 | 4.96 ± 0.03 | 210 ± 0.5 | 0.229 ± 0.002 | 72.89 ± 0.02 | 6.3 × 10−4 ± 0.01 |
| 3024 | −56 ± 0.3 | 0.121 ± 0.002 | 2.77 ± 0.02 | 375 ± 0.6 | 0.122 ± 0.002 | 84.82 ± 0.02 | 0.12 × 10−4 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golgovici, F.; Diniași, D.; Dincă, P.P.; Butoi, B.; Demetrescu, I. Corrosion of Chromium Coating Fabricated on Zircaloy-4 Substrate. Materials 2024, 17, 4445. https://doi.org/10.3390/ma17184445
Golgovici F, Diniași D, Dincă PP, Butoi B, Demetrescu I. Corrosion of Chromium Coating Fabricated on Zircaloy-4 Substrate. Materials. 2024; 17(18):4445. https://doi.org/10.3390/ma17184445
Chicago/Turabian StyleGolgovici, Florentina, Diana Diniași, Paul Pavel Dincă, Bogdan Butoi, and Ioana Demetrescu. 2024. "Corrosion of Chromium Coating Fabricated on Zircaloy-4 Substrate" Materials 17, no. 18: 4445. https://doi.org/10.3390/ma17184445
APA StyleGolgovici, F., Diniași, D., Dincă, P. P., Butoi, B., & Demetrescu, I. (2024). Corrosion of Chromium Coating Fabricated on Zircaloy-4 Substrate. Materials, 17(18), 4445. https://doi.org/10.3390/ma17184445








