Molten Aluminum-Induced Corrosion and Wear-Resistance Properties of ZrB2-Based Cermets Improved by Sintering-Temperature Manipulation
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization and Mechanical Tests
3. Results and Discussion
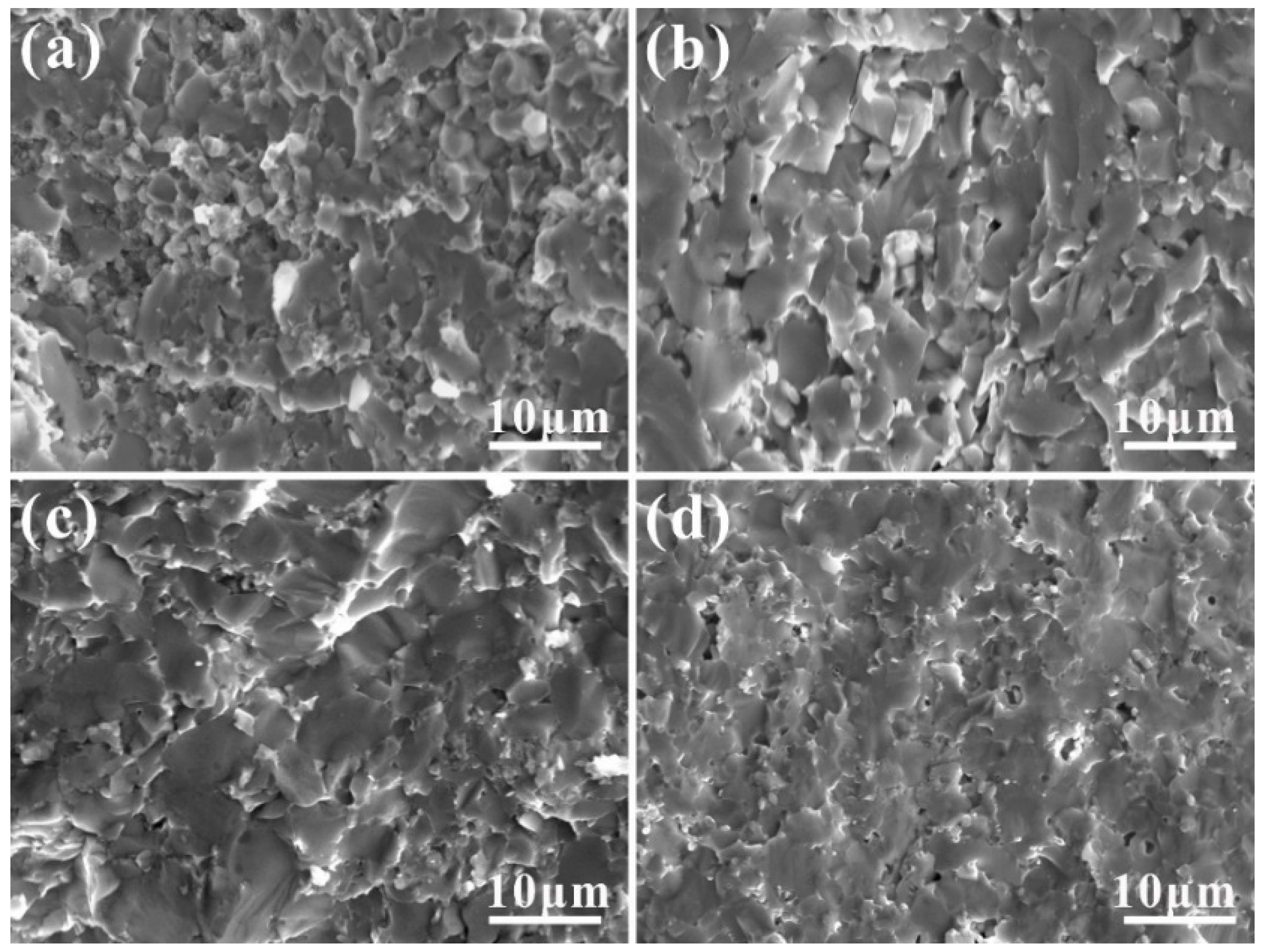
3.1. Microstructure
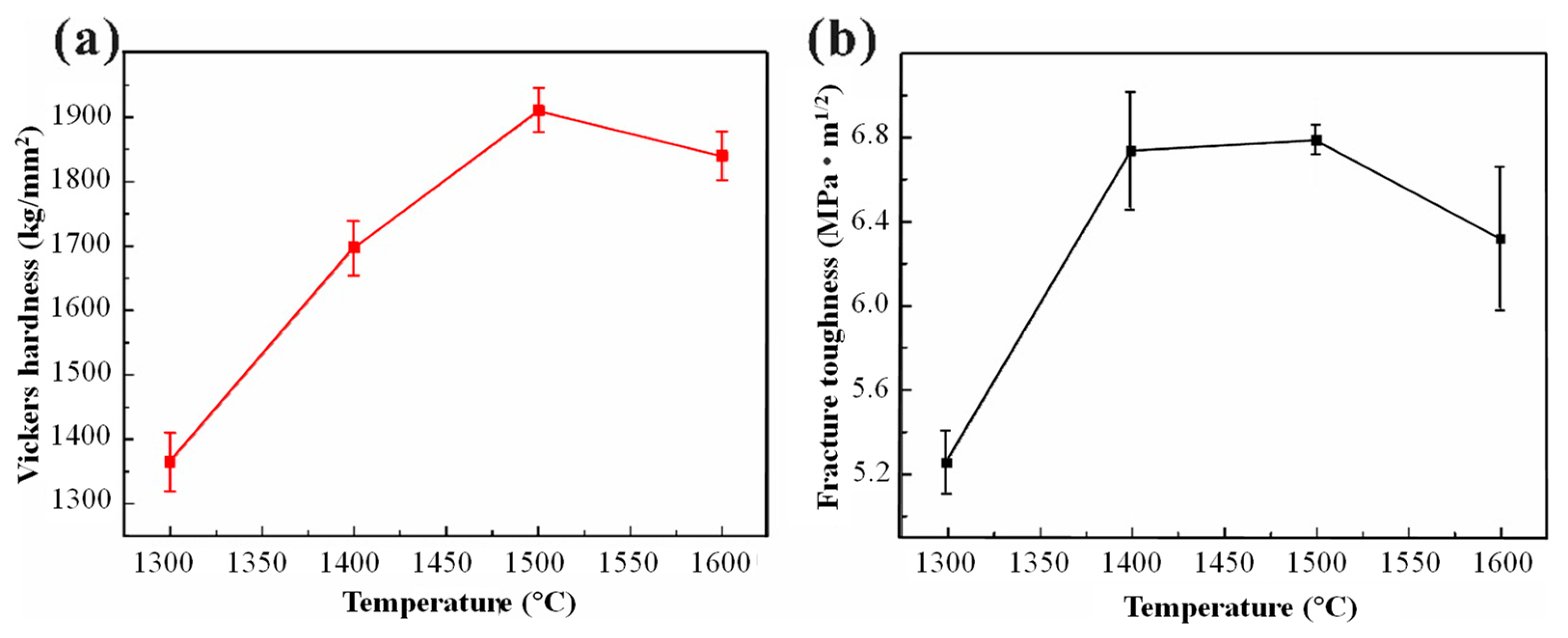
3.2. Mechanical Properties
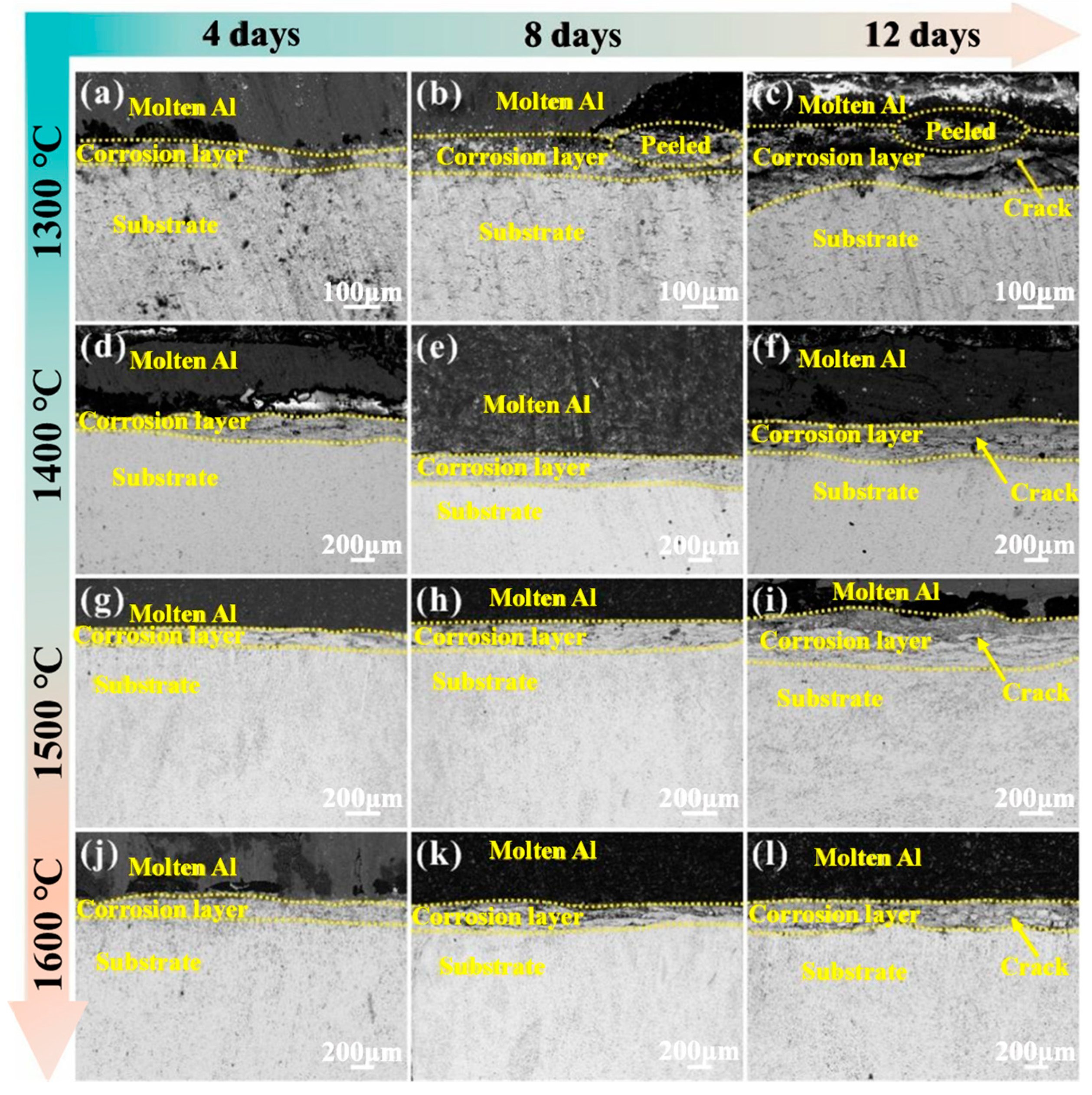
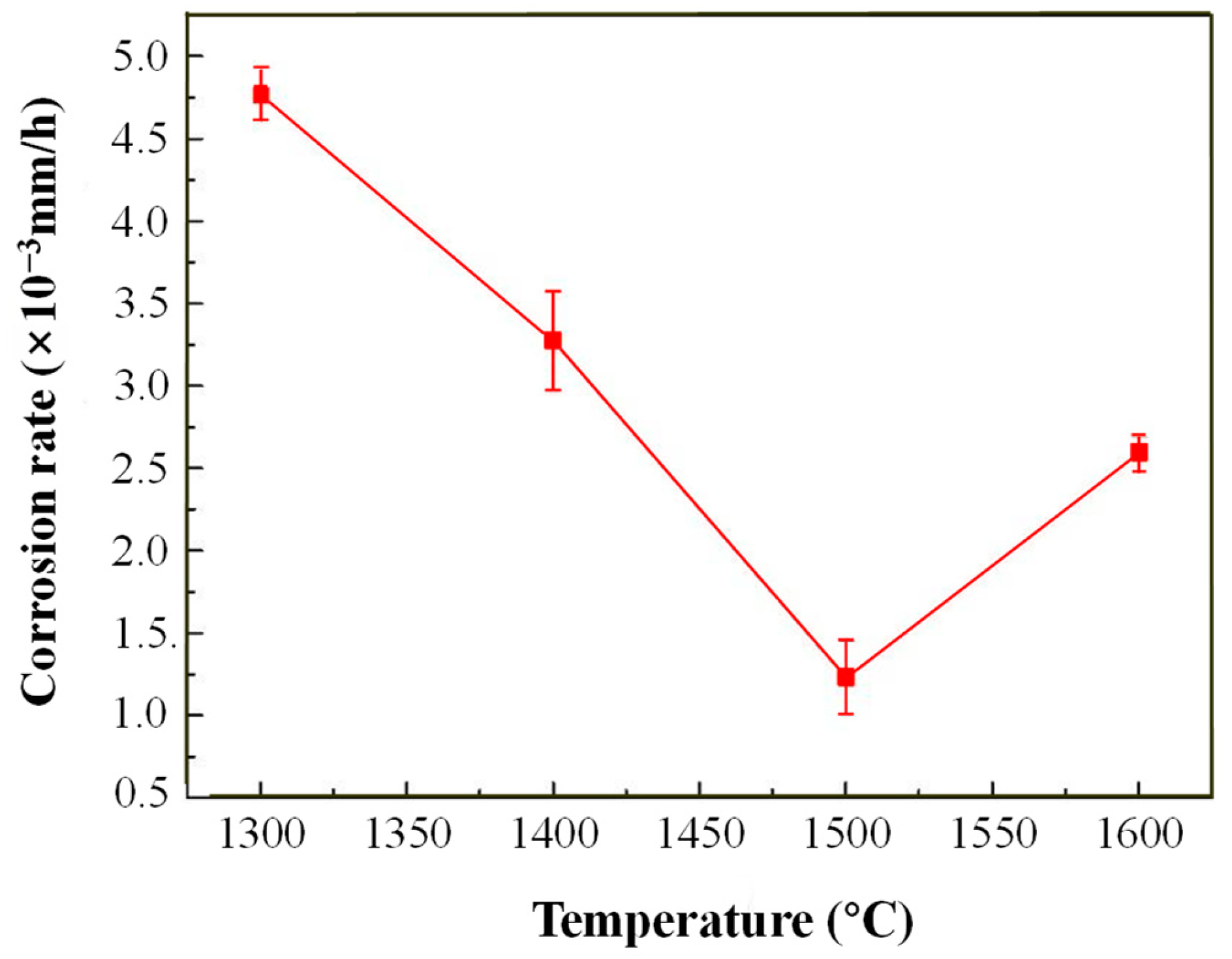
3.3. Exposure to Molten Aluminum
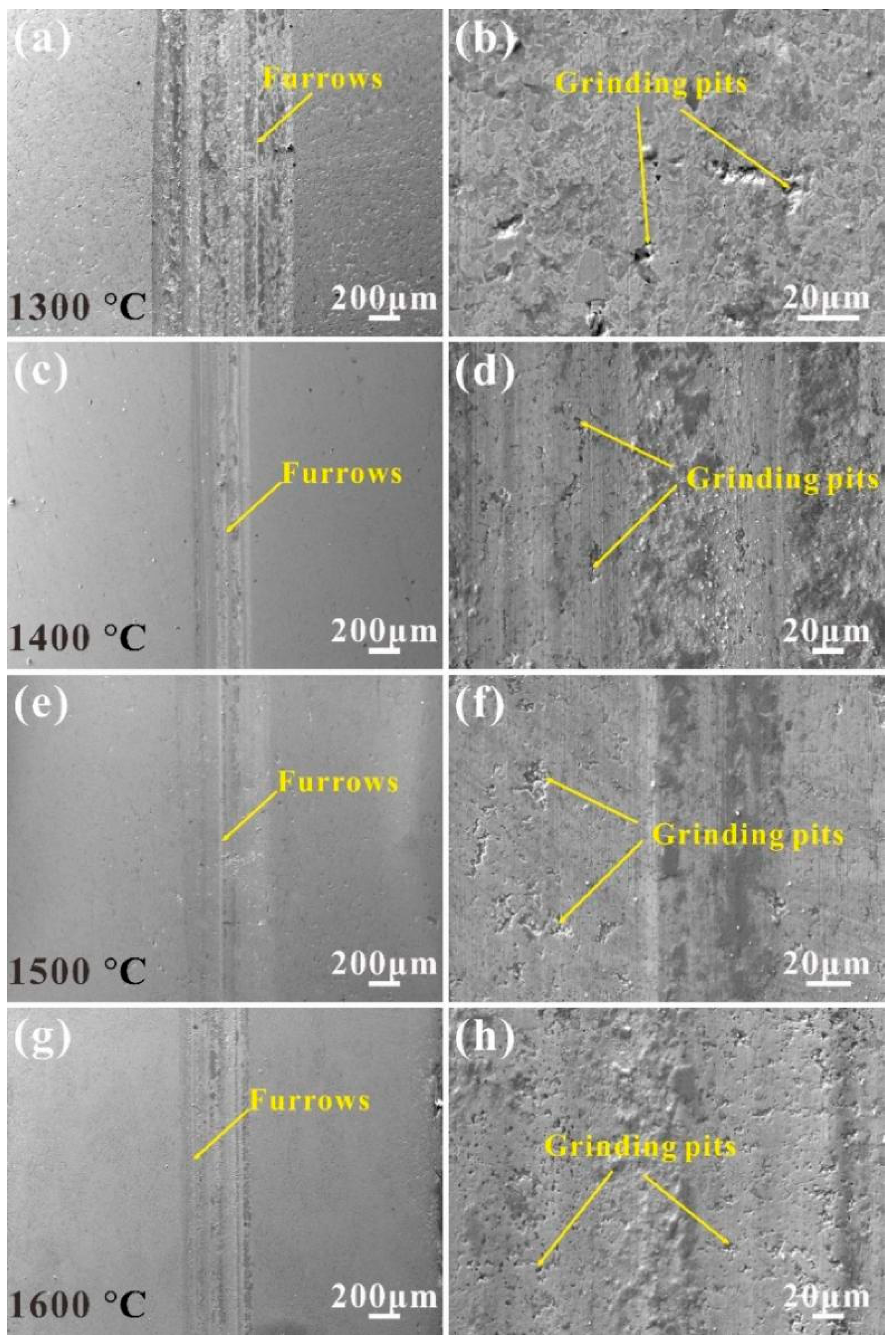
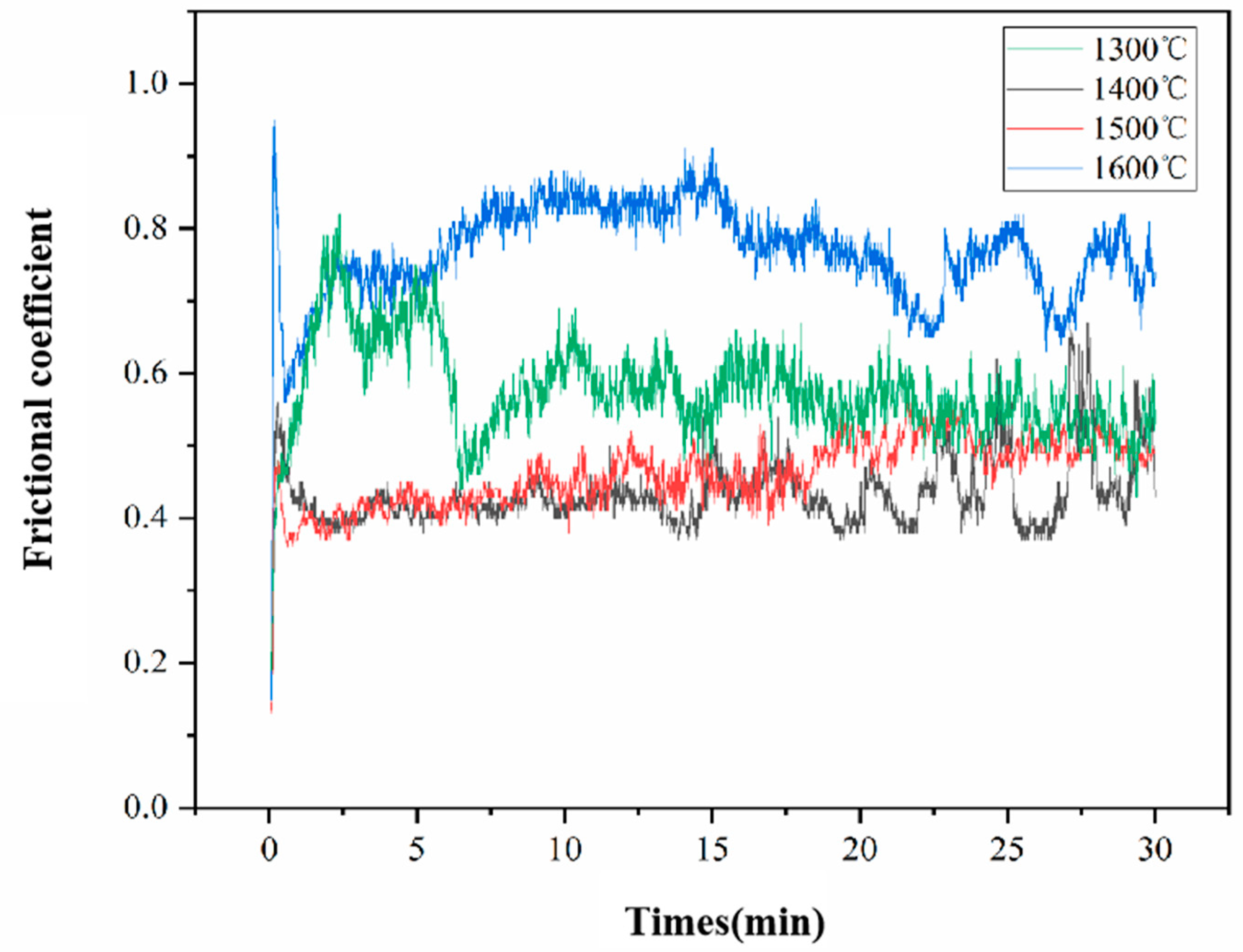
3.4. Wear Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, M.; Fan, Z. Review durability of materials in molten aluminum alloys. J. Mater. Sci. 2001, 36, 285–295. [Google Scholar] [CrossRef]
- Bastidas, D.M. Corrosion and protection of metals. Metals 2020, 10, 458. [Google Scholar] [CrossRef]
- Samsonov, G.V.; Panasyuk, A.D.; Borovikova, M.S. Contact reaction between refractory compounds and liquid metals: IV. Reaction between refractory borides and liquid iron group metals. Sov. Powder Metall. Met. Ceram. 1973, 12, 476–480. [Google Scholar] [CrossRef]
- Tsipas, D.; Triantafyllidis, G.; Kiplagat, J.K.; Psillaki, P. Degradation behaviour of boronized carbon and high alloy steels in molten aluminium and zinc. Mater. Lett. 1998, 37, 128–131. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, W.; Yang, X.; Li, B.; Lu, T. Interfacial morphologies and corrosion behaviours of novel Fe-Cr-B alloys immersed in molten aluminium. Mater. Res. Express 2019, 6, 046557. [Google Scholar] [CrossRef]
- Yadav, K.K.; Guchhait, S.K.; Sunaina; Ankush; Hussain, C.M.; Ganguli, A.K.; Jha, M. Synthesis of zirconium diboride and its application in the protection of stainless steel surface in harsh environment. J. Solid State Electrochem. 2019, 23, 3243–3253. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Tong, Y.; Jiao, Z.B.; Cai, J.X.; Han, X.D.; Chen, D.; Hu, A.; Liu, Y.; Liu, C.T. Multicomponent intermetallic nanoparticles and superb mechanical behaviors ofcomplex alloys. Science 2018, 362, 933–937. [Google Scholar] [CrossRef]
- Binner, J.; Porter, M.; Baker, B.; Zou, J.; Venkatachalam, V.; Diaz, V.R.; D’Angio, A.; Ramanujam, P.; Zhang, T.; Murthy, T.S.R.C. Selection, processing, properties and applications of ultra-high temperature ceramic matrix composites, UHTCMCs—A review. Int. Mater. Rev. 2020, 65, 389–444. [Google Scholar] [CrossRef]
- Monteverde, F.; Bellosi, A.; Scatteia, L. Processing and properties of ultra-high temperature ceramics for space applications. Mater. Sci. Eng. A 2008, 485, 415–421. [Google Scholar] [CrossRef]
- Murthy, T.S.R.C.; Sonber, J.K.; Sairam, K.; Majumdar, S.; Kain, V. Boron-based ceramics and composites for nuclear and space applications: Synthesis and consolidation. In Handbook of Advanced Ceramics and Composites: Defense, Security, Aerospace and Energy Applications; Springer: Cham, Switzerland, 2020; pp. 703–738. [Google Scholar]
- Pierson, J.F.; Belmonte, T.; Michel, H. Effect of ZrCl4 addition on ZrB2 film synthesis in flowing Ar–BCl3 post-discharges. Surf. Coat. Technol. 2000, 133, 301–306. [Google Scholar] [CrossRef]
- Sharma, A.; Karunakar, D.B. Development and investigation of densification behavior of ZrB2–SiC composites through microwave sintering. Mater. Res. Express 2019, 6, 105072. [Google Scholar] [CrossRef]
- Kashyap, S.K.; Mitra, R. Densification behavior involving creep during spark plasma sintering of ZrB2-SiC based ultra-high temperature ceramic composites. Ceram. Int. 2020, 46, 5028–5036. [Google Scholar] [CrossRef]
- Kumar, S.; Manasa, V.; Singla, A.; Tyagi, L.K.; Srivastava, A.P.; Kareem, H. Green Microwave-Assisted Cladding: Enhancing SS-304 with Ni-ZrB2 Composite Coatings. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2024; p. 01005. [Google Scholar]
- Sun, X.; Han, W.; Liu, Q.; Hu, P.; Hong, C. ZrB2-ceramic toughened by refractory metal Nb prepared by hot-pressing. Mater. Des. 2010, 31, 4427–4431. [Google Scholar] [CrossRef]
- Meléndez-Martınez, J.; Domınguez-Rodrıguez, A.; Monteverde, F.; Melandri, C.; De Portu, G. Characterisation and high temperature mechanical properties of zirconium boride-based materials. J. Eur. Ceram. Soc. 2002, 22, 2543–2549. [Google Scholar] [CrossRef]
- Sonber, J.K.; Murthy, T.C.R.C.; Sairam, K.; Nagaraj, A.; Majumdar, S.; Kain, V. ZrB2 based novel composite with NiAl as reinforcement phase. Int. J. Refract. Met. Hard Mater. 2018, 70, 56–65. [Google Scholar] [CrossRef]
- Monteverde, F.; Bellosi, A.; Guicciardi, S. Processing and properties of zirconium diboride-based composites. J. Eur. Ceram. Soc. 2002, 22, 279–288. [Google Scholar] [CrossRef]
- Ghaderi, A.; Dehghani, K. Mechanical Behavior of Al0. 5CoCrFeNi HEA During Warm Deformation. JOM 2024, 76, 1962–1972. [Google Scholar] [CrossRef]
- Mamnooni, S.; Borhani, E.; Asl, M.S. Feasibility of using Ni25Co20Cu10Fe25Mn20 high entropy alloy as a novel sintering aid in ZrB2 ceramics. Ceram. Int. 2024, 50, 19791–19805. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Y.; Ye, J. Early high-temperature oxidation behavior of Ti (C. N)-based cermets with multi-component AlCoCrFeNi high-entropy alloy binder. Int. J. Refract. Met. Hard Mater. 2014, 44, 35–41. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of Al xCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Nayebi, B.; Parvin, N.; Mohandesi, J.A.; Asl, M.S. Effect of Zr and C co-addition on the characteristics of ZrB2-based ceramics: Role of spark plasma sintering temperature. Ceram. Int. 2020, 46, 24975–24985. [Google Scholar] [CrossRef]
- Gupta, N.; Mukhopadhyay, A.; Pavani, K.; Basu, B. Spark plasma sintering of novel ZrB2–SiC–TiSi2 composites with better mechanical properties. Mater. Sci. Eng. A 2012, 534, 111–118. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Jing, K.; Liu, R.; Xie, Z.-M.; Yang, J.-F.; Li, G.; Wang, X.-P.; Wu, X.-B.; Fang, Q.-F.; Liu, C.-S. Mechanical property and thermal shock behavior of tungsten-yttria-stabilized zirconia cermet fabricated by spark plasma sintering. Tungsten 2024, 6, 134–140. [Google Scholar] [CrossRef]
- Chou, Y.; Wang, Y.; Yeh, J.; Shih, H. Pitting corrosion of the high-entropy alloy Co1.5CrFeNi1.5Ti0.5Mo0.1 in chloride-containing sulphate solutions. Corros. Sci. 2010, 52, 3481–3491. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A. Microstructure and wear behavior of a refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2016, 57, 50–63. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Tsai, M.-H.; Wang, W.-R.; Lin, S.-J.; Yeh, J.-W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Mishra, S.K.; Das, S.K.; Ray, A.K.; Ramachandrarao, P. Effect of Fe and Cr addition on the sintering behavior of ZrB2 produced by self-propagating high-temperature synthesis. J. Am. Ceram. Soc. 2002, 85, 2846–2848. [Google Scholar] [CrossRef]
- Ghasali, E.; Asl, M.S. Microstructural development during spark plasma sintering of ZrB2–SiC–Ti composite. Ceram. Int. 2018, 44, 18078–18083. [Google Scholar] [CrossRef]
- Purwar, A.; Mukherjee, R.; Ravikumar, K.; Ariharan, S.; Gopinath, N.K.; Basu, B. Development of ZrB2–SiC–Ti by multi stage spark plasma sintering at 1600 °C. J. Ceram. Soc. Jpn. 2016, 124, 393–402. [Google Scholar] [CrossRef]
- Evans, A.G.; Charles, E.A. Fracture toughness determinations by indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Wang, M.; Xue, J.; Gao, R.; Gao, H.; Zhou, Y.; Zhao, Y.; Liu, Y.; Kang, M.; Wang, J. Interface morphology and corrosion behavior of bulk Fe2B in liquid Al. Mater. Charact. 2019, 152, 1–11. [Google Scholar] [CrossRef]


| ZrB2 | Al | Fe | Ni | Co | Cr | |
|---|---|---|---|---|---|---|
| ZrB2-30 | 70 | 3.21 | 6.63 | 6.99 | 6.99 | 6.18 |
| Elements | Al | Fe | Ni | Co | Cr | Zr | B |
|---|---|---|---|---|---|---|---|
| Point1 | 13.2 | 21.8 | 18.5 | 22.6 | 23.9 | - | - |
| Point 2 | - | 1.5 | 0.4 | 0.1 | 0.2 | 46.4 | 51.4 |
| Point 3 | 0.1 | 8.2 | 1.1 | 2.7 | 81.7 | 1.5 | 4.7 |
| Point 4 | 12.8 | 23.3 | 19.5 | 21.3 | 23.1 | - | - |
| Point 5 | 0.1 | 1.3 | 0.3 | 0.3 | 0.4 | 47.7 | 49.9 |
| Point 6 | 0.1 | 8.8 | 1.7 | 2.1 | 82.5 | 1.1 | 3.7 |
| Point 7 | 11.9 | 22.5 | 17.8 | 23.1 | 24.7 | - | - |
| Point 8 | - | 1.9 | 0.3 | 0.2 | 0.3 | 48.3 | 49.0 |
| Point 9 | - | 8.6 | 1.5 | 2.2 | 82.3 | 1.3 | 4.1 |
| Point 10 | 12.1 | 22.3 | 19.2 | 22.7 | 23.7 | - | - |
| Point 11 | - | 1.4 | 0.4 | 0.5 | 0.4 | 45.9 | 51.4 |
| Point 12 | 0.3 | 9.3 | 1.6 | 3.5 | 79.0 | 2.5 | 3.8 |
| 1300 °C | 1400 °C | 1500 °C | 1600 °C | |
|---|---|---|---|---|
| Point 1 | 4.754 | 4.701 | 4.730 | 4.875 |
| Point 2 | 4.831 | 4.641 | 4.787 | 4.704 |
| Point 3 | 4.853 | 4.617 | 4.884 | 4.558 |
| Average | 4.813 | 4.686 | 4.800 | 4.746 |
| 1300 °C | 1400 °C | 1500 °C | 1600 °C | |
|---|---|---|---|---|
| Point 1 | 4.027 | 4.132 | 4.478 | 4.487 |
| Point 2 | 3.970 | 4.273 | 4.606 | 4.316 |
| Point 3 | 4.213 | 4.249 | 4.776 | 4.226 |
| Average | 4.129 | 4.218 | 4.620 | 4.386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, H.; Ren, K.; Chen, H.; Cheng, X.; Xie, X.; Liang, M.; Yin, B.; Yang, Y. Molten Aluminum-Induced Corrosion and Wear-Resistance Properties of ZrB2-Based Cermets Improved by Sintering-Temperature Manipulation. Materials 2024, 17, 4451. https://doi.org/10.3390/ma17184451
Yi H, Ren K, Chen H, Cheng X, Xie X, Liang M, Yin B, Yang Y. Molten Aluminum-Induced Corrosion and Wear-Resistance Properties of ZrB2-Based Cermets Improved by Sintering-Temperature Manipulation. Materials. 2024; 17(18):4451. https://doi.org/10.3390/ma17184451
Chicago/Turabian StyleYi, Huaqing, Kezhu Ren, Hao Chen, Xiang Cheng, Xiaolong Xie, Mengtian Liang, Bingbing Yin, and Yi Yang. 2024. "Molten Aluminum-Induced Corrosion and Wear-Resistance Properties of ZrB2-Based Cermets Improved by Sintering-Temperature Manipulation" Materials 17, no. 18: 4451. https://doi.org/10.3390/ma17184451
APA StyleYi, H., Ren, K., Chen, H., Cheng, X., Xie, X., Liang, M., Yin, B., & Yang, Y. (2024). Molten Aluminum-Induced Corrosion and Wear-Resistance Properties of ZrB2-Based Cermets Improved by Sintering-Temperature Manipulation. Materials, 17(18), 4451. https://doi.org/10.3390/ma17184451







