“Water-in-Salt” Electrolyte Suppressed MnVOPO4·2H2O Cathode Dissolution for Stable High-Voltage Platform and Cycling Performance for Aqueous Zinc Metal Battery
Abstract
:1. Introduction
2. Materials and Methods
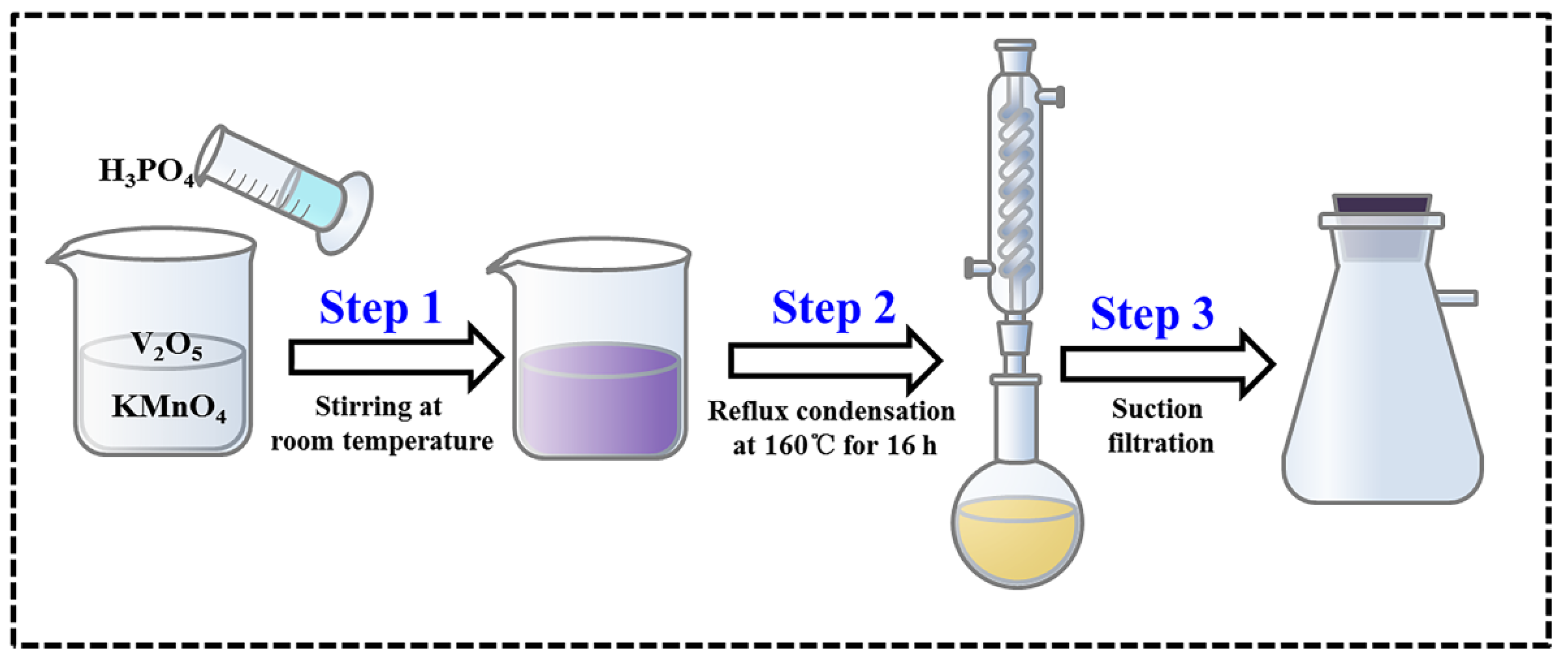
2.1. Synthesis of Materials
2.2. Preparation of Electrolyte
2.2.1. High-Concentration Electrolyte
2.2.2. Phosphate Buffer-Type Electrolyte Additives
2.2.3. MnSO4 Electrolyte Additive
2.3. Material Characterization
2.4. Electrochemical Measurements
2.5. Operation before Ex Situ XRD and XPS Analysis
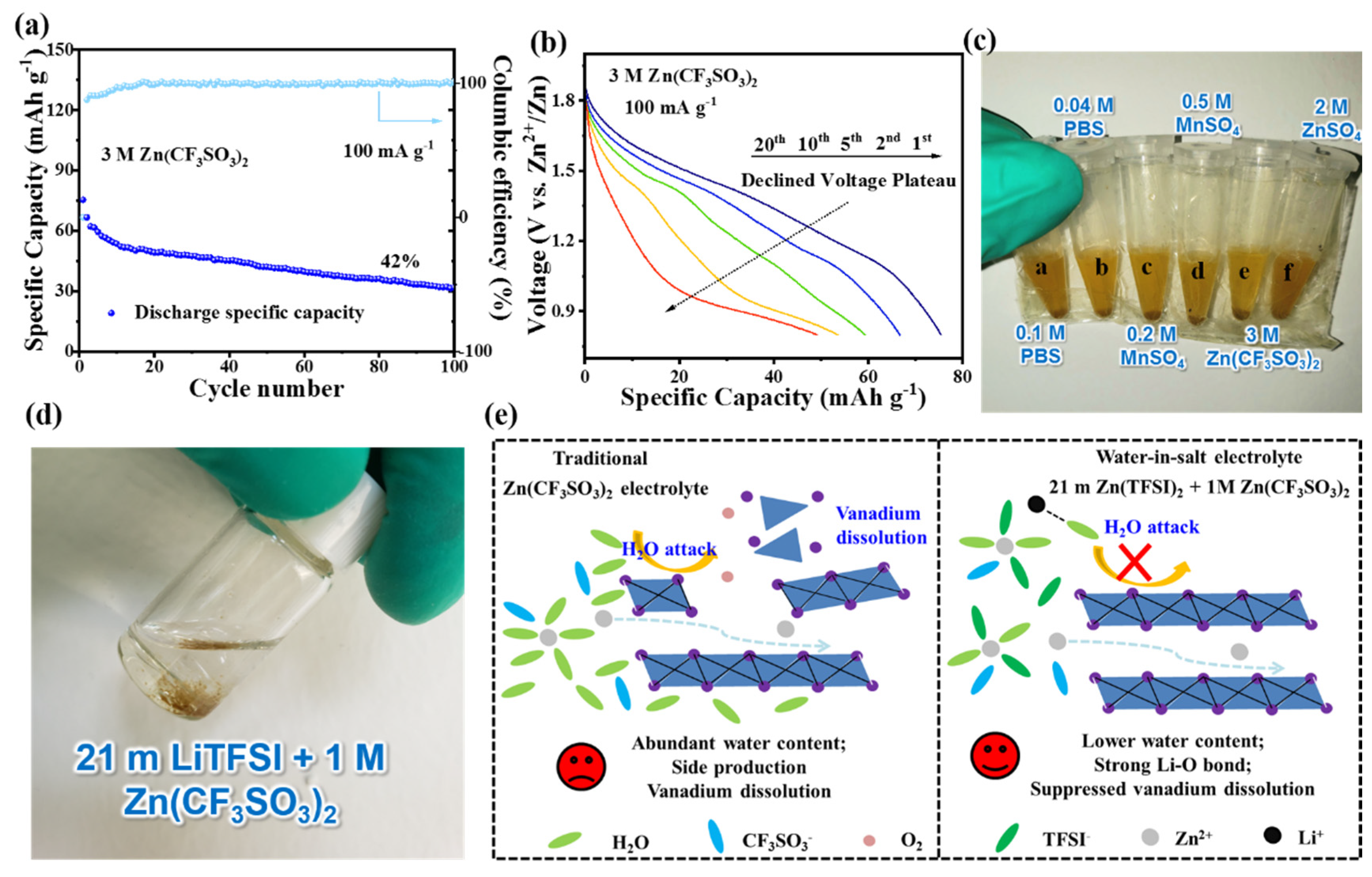
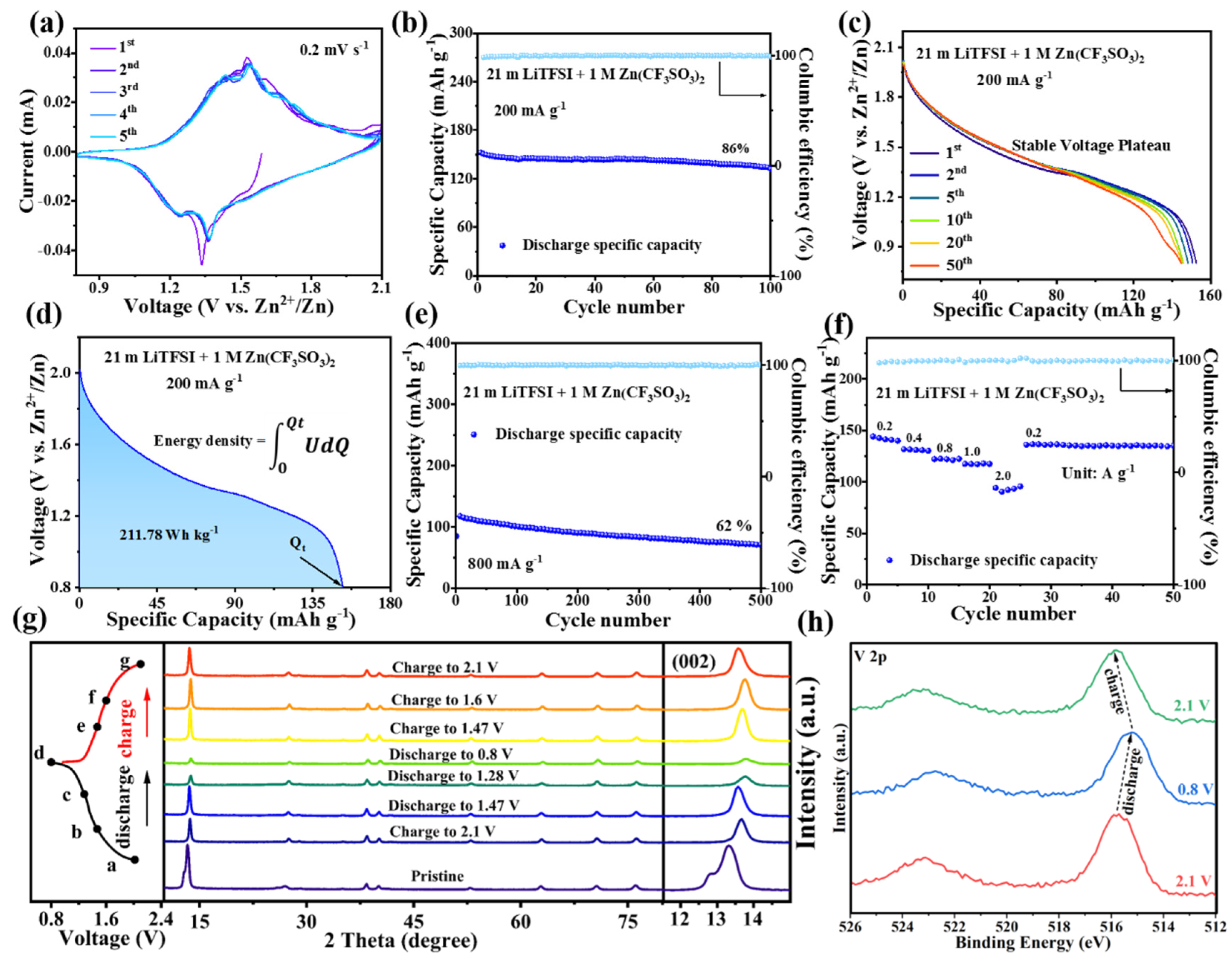
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Jin, S.; Archer, L.A.; Nazar, L.F. Toward practical aqueous zinc-ion batteries for electrochemical energy storage. Joule 2022, 6, 1733–1738. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.; Liu, J.; Zhang, J.; Wang, X.; Pu, X.; Wang, J.; Yan, C.; Cao, Y.; Yang, H.; et al. Recent advances on challenges and strategies of manganese dioxide cathodes for aqueous zinc-ion batteries. Energy Environ. Mater. 2023, 6, e12575. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Kim, J.M.; Chun, Y.T.; Zhang, J.; Jun, S.C. Recent Advances in Vanadium-Based Aqueous Rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2020, 10, 2000477. [Google Scholar] [CrossRef]
- Mathew, V.; Sambandam, B.; Kim, S.; Kim, S.; Park, S.; Lee, S.; Alfaruqi, M.H.; Soundharrajan, V.; Islam, S.; Putro, D.Y.; et al. Manganese and Vanadium Oxide Cathodes for Aqueous Rechargeable Zinc-Ion Batteries: A Focused View on Performance, Mechanism, and Developments. ACS Energy Lett. 2020, 5, 2376–2400. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, W.; Yang, T.; Gao, R.; Luo, D.; Park, H.W.; Hu, Y.; Yu, A. Vacancy and Growth Modulation of Cobalt Hexacyanoferrate by Porous MXene for Zinc Ion Batteries. Adv. Energy Mater. 2024, 14, 2400543. [Google Scholar] [CrossRef]
- Zhong, W.; Shen, Z.; Mao, J.; Zhang, S.; Cheng, H.; Kim, Y.; Lu, Y. Mitigating cathodic dissolution through interfacial water masking to enhance the longevity of aqueous zinc–ion batteries. Energy Environ. Sci. 2024, 17, 2059–2068. [Google Scholar] [CrossRef]
- Liao, M.; Wang, J.; Ye, L.; Sun, H.; Wen, Y.; Wang, C.; Sun, X.; Wang, B.; Peng, H. A Deep-Cycle Aqueous Zinc-Ion Battery Containing an Oxygen-Deficient Vanadium Oxide Cathode. Angew. Chem. Int. Ed. 2020, 59, 2273–2278. [Google Scholar] [CrossRef]
- Wang, K.; Li, S.; Chen, X.; Shen, J.; Zhao, H.; Bai, Y. Trifunctional Rb+-Intercalation Enhancing the Electrochemical Cyclability of Ammonium Vanadate Cathode for Aqueous Zinc lon Batteries. ACS Nano 2024, 18, 7311–7323. [Google Scholar] [CrossRef]
- Zhao, D.; Pu, X.; Tang, S.; Ding, M.; Zeng, Y.; Cao, Y.; Chen, Z. delta-VOPO4 as a high-voltage cathode material for aqueous zinc-ion batteries. Chem. Sci. 2023, 14, 8206–8213. [Google Scholar] [CrossRef]
- Shi, H.Y.; Song, Y.; Qin, Z.; Li, C.; Guo, D.; Liu, X.X.; Sun, X. Inhibiting VOPO4⋅xH2O Decomposition and Dissolution in Rechargeable Aqueous Zinc Batteries to Promote Voltage and Capacity Stabilities. Angew. Chem. Int. Edit. 2019, 58, 16057–16061. [Google Scholar] [CrossRef]
- Zhang, M.; Pei, Y.; Liang, R.; Gao, R.; Deng, Y.-P.; Hu, Y.; Chen, Z.; Yu, A. Ionic interaction-mediated interlayer repulsion force promotes steadily shuttling of Zn2+ ions within VOPO4. Nano Energy 2022, 98, 107268. [Google Scholar] [CrossRef]
- Huang, Q.; Shao, L.; Shi, X.; Guan, J.; Xu, J.; Wu, Y.; Sun, Z. Na3V2O2(PO4)2F nanoparticles@reduced graphene oxide: A high-voltage polyanionic cathode with enhanced reaction kinetics for aqueous zinc-ion batteries. Chem. Eng. J. 2023, 468, 143738. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Q.; Wang, D.; Liang, G.; Zhu, Y.; Mo, F.; Huang, Z.; Li, X.; Ma, L.; Tang, T.; et al. A Flexible Solid-State Aqueous Zinc Hybrid Battery with Flat and High-Voltage Discharge Plateau. Adv. Energy Mater. 2019, 9, 1902473. [Google Scholar] [CrossRef]
- Xing, Z.; Xu, G.; Han, J.; Chen, G.; Lu, B.; Liang, S.; Zhou, J. Facing the capacity fading of vanadium-based zinc-ion batteries. Trends Chem. 2023, 5, 380–392. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, K.; Yang, W. Critical Issues of Vanadium-Based Cathodes Towards Practical Aqueous Zn-Ion Batteries. Chem. Eur. J. 2023, 29, e202301769. [Google Scholar] [CrossRef]
- Nian, Q.; Luo, X.; Ruan, D.; Li, Y.; Xiong, B.Q.; Cui, Z.; Wang, Z.; Dong, Q.; Fan, J.; Jiang, J.; et al. Highly reversible zinc metal anode enabled by strong Bronsted acid and hydrophobic interfacial chemistry. Nat. Commun. 2024, 15, 4303. [Google Scholar] [CrossRef]
- Zhang, F.; Liao, T.; Peng, H.; Xi, S.; Qi, D.C.; Micallef, A.; Yan, C.; Jiang, L.; Sun, Z. Outer Sphere Electron Transfer Enabling High-Voltage Aqueous Electrolytes. J. Am. Chem. Soc. 2024, 146, 10812–10821. [Google Scholar] [CrossRef]
- Han, M.C.; Zhang, J.H.; Yu, C.Y.; Yu, J.C.; Wang, Y.X.; Jiang, Z.G.; Yao, M.; Xie, G.; Yu, Z.Z.; Qu, J. Constructing Dynamic Anode/Electrolyte Interfaces Coupled with Regulated Solvation Structures for Long-Term and Highly Reversible Zinc Metal Anodes. Angew. Chem. Int. Ed. 2024, 63, e202403695. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Chi, X.; Liu, J.; Wen, B.; Liu, Y. Ultralow-water-activity electrolyte endows vanadium-based zinc-ion batteries with durable lifespan exceeding 30000 cycles. Energy Storage Mater. 2022, 53, 774–782. [Google Scholar] [CrossRef]
- Liang, G.; Gan, Z.; Wang, X.; Jin, X.; Xiong, B.; Zhang, X.; Chen, S.; Wang, Y.; He, H.; Zhi, C. Reconstructing Vanadium Oxide with Anisotropic Pathways for a Durable and Fast Aqueous K-Ion Battery. ACS Nano 2021, 15, 17717–17728. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nagaiah, T.C. High energy density aqueous rechargeable sodium-ion/sulfur batteries in ‘water in salt” electrolyte. Energy Storage Mater. 2022, 49, 390–400. [Google Scholar] [CrossRef]
- Gomez Vazquez, D.; Pollard, T.P.; Mars, J.; Yoo, J.M.; Steinrück, H.-G.; Bone, S.E.; Safonova, O.V.; Toney, M.F.; Borodin, O.; Lukatskaya, M.R. Creating water-in-salt-like environment using coordinating anions in non-concentrated aqueous electrolytes for efficient Zn batteries. Energy Environ. Sci. 2023, 16, 1982–1991. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Q.; Xiao, L.; Rong, Y.; Liu, Y.; Chen, Z.; Ai, X.; Cao, Y.; Yang, H.; Xie, J.; et al. A Fully Sodiated NaVOPO4 with Layered Structure for High-Voltage and Long-Lifespan Sodium-Ion Batteries. Chem 2018, 4, 1167–1180. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, E.; Hu, T.; Li, Z.; Liu, T. Effect of synthetic methods on electrochemical performances of VOPO4·2H2O supercapacitor. Ionics 2014, 21, 289–294. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Zhang, L.; Jiang, L.; Tian, W.; Cai, C.; Price, J.; Gu, Q.; Hu, L. A Layered Zn0.4VOPO4·0.8H2O Cathode for Robust and Stable Zn Ion Storage. ACS Appl. Energy Mater. 2020, 3, 3919–3927. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.; Fang, G.; Wang, Z.; Cai, Y.; Tang, B.; Zhou, J.; Liang, S. V2O5 Nanospheres with Mixed Vanadium Valences as High Electrochemically Active Aqueous Zinc-Ion Battery Cathode. Nano-Micro Lett. 2019, 11, 25. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, C.; Lan, B.; Chen, L.; Tang, W.; Zuo, C.; Dong, S.; An, Q.; Luo, P. K0.23V2O5 as a promising cathode material for rechargeable aqueous zinc ion batteries with excellent performance. J. Alloys Compd. 2020, 819, 152971. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, C.; Wang, Y.; Zhang, L.; Jiang, L.; Tian, W.; Cai, C.; Gu, Q.; Sun, Z.; Hu, L. Ultrathin VSe2 Nanosheets with Fast Ion Diffusion and Robust Structural Stability for Rechargeable Zinc-Ion Battery Cathode. Small 2020, 16, e2000698. [Google Scholar] [CrossRef]
- Jiao, T.; Yang, Q.; Wu, S.; Wang, Z.; Chen, D.; Shen, D.; Liu, B.; Cheng, J.; Li, H.; Ma, L.; et al. Binder-free hierarchical VS2 electrodes for high-performance aqueous Zn ion batteries towards commercial level mass loading. J. Mater. Chem. A 2019, 7, 16330–16338. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, Y.; Zhang, L.; Liu, D.; Wang, C.; Song, L.; Niu, Z.; Chen, J. Reversible Oxygen Redox Chemistry in Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 7062–7067. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Zhang, L.; Wang, X.; Bi, S.; Niu, Z.; Chen, J. An Aqueous Rechargeable Zinc-Organic Battery with Hybrid Mechanism. Adv. Funct. Mater. 2018, 28, 1804975. [Google Scholar] [CrossRef]
- Lv, Z.; Kang, Y.; Tang, R.; Yang, J.; Chen, G.; Hu, Y.; Lin, P.; Yang, H.; Wu, Q.; Zhang, M.; et al. Stabilizing layered superlattice MoSe2 anodes by the rational solvation structure design for low-temperature aqueous zinc-ion batteries. Electron 2023, 1, e5. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, W.; Cai, W.; Huang, Z.; Liu, G.; Liu, C.; Wang, S.; Chen, F.; Xia, L.; Zhao, Y.; et al. Accelerated ion/electron transport kinetics and increased active sites via local internal electric fields in heterostructured VO2-carbon cloth for enhanced zinc-ion storage. Nano Res. 2022, 16, 503–512. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, T.; Wang, X.; Zhou, X.; Wei, X.; Yao, X.; Luo, W.; Shi, C.; Owusu, K.A.; Zhou, L.; et al. Aqueous Zn//Zn(CF3SO3)2//Na3V2(PO4)3 batteries with simultaneous Zn2+/Na+ intercalation/de-intercalation. Nano Energy 2019, 58, 492–498. [Google Scholar] [CrossRef]
- Hu, P.; Zou, Z.; Sun, X.; Wang, D.; Ma, J.; Kong, Q.; Xiao, D.; Gu, L.; Zhou, X.; Zhao, J.; et al. Uncovering the Potential of M1-Site-Activated NASICON Cathodes for Zn-Ion Batteries. Adv. Mater. 2020, 32, e1907526. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, W.; Shadike, Z.; Hu, E.; Ji, X.; Gao, T.; Yang, X.Q.; Xu, K.; Wang, C. How Water Accelerates Bivalent Ion Diffusion at the Electrolyte/Electrode Interface. Angew. Chem. Int. Ed. 2018, 57, 11978–11981. [Google Scholar] [CrossRef] [PubMed]

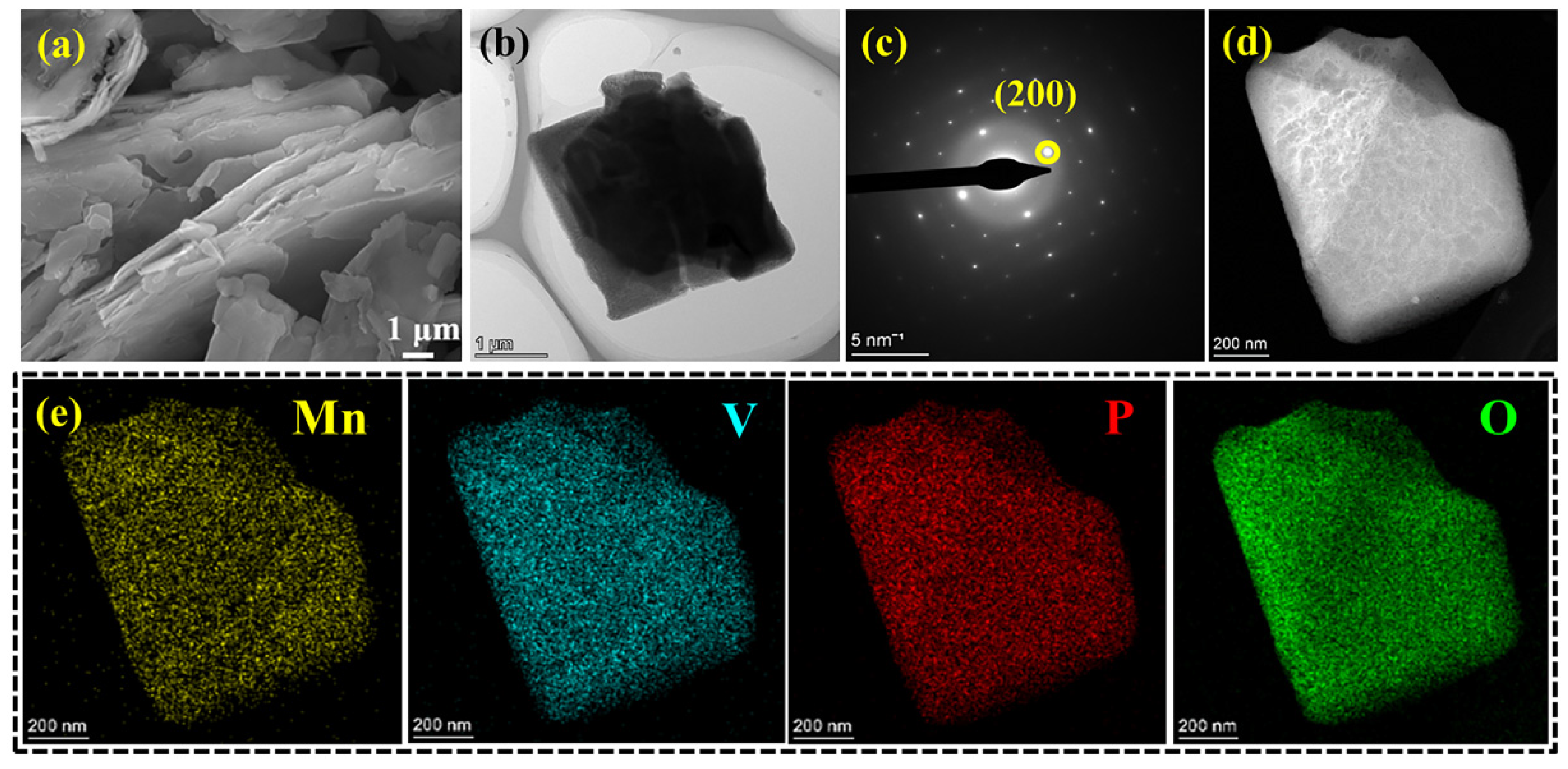
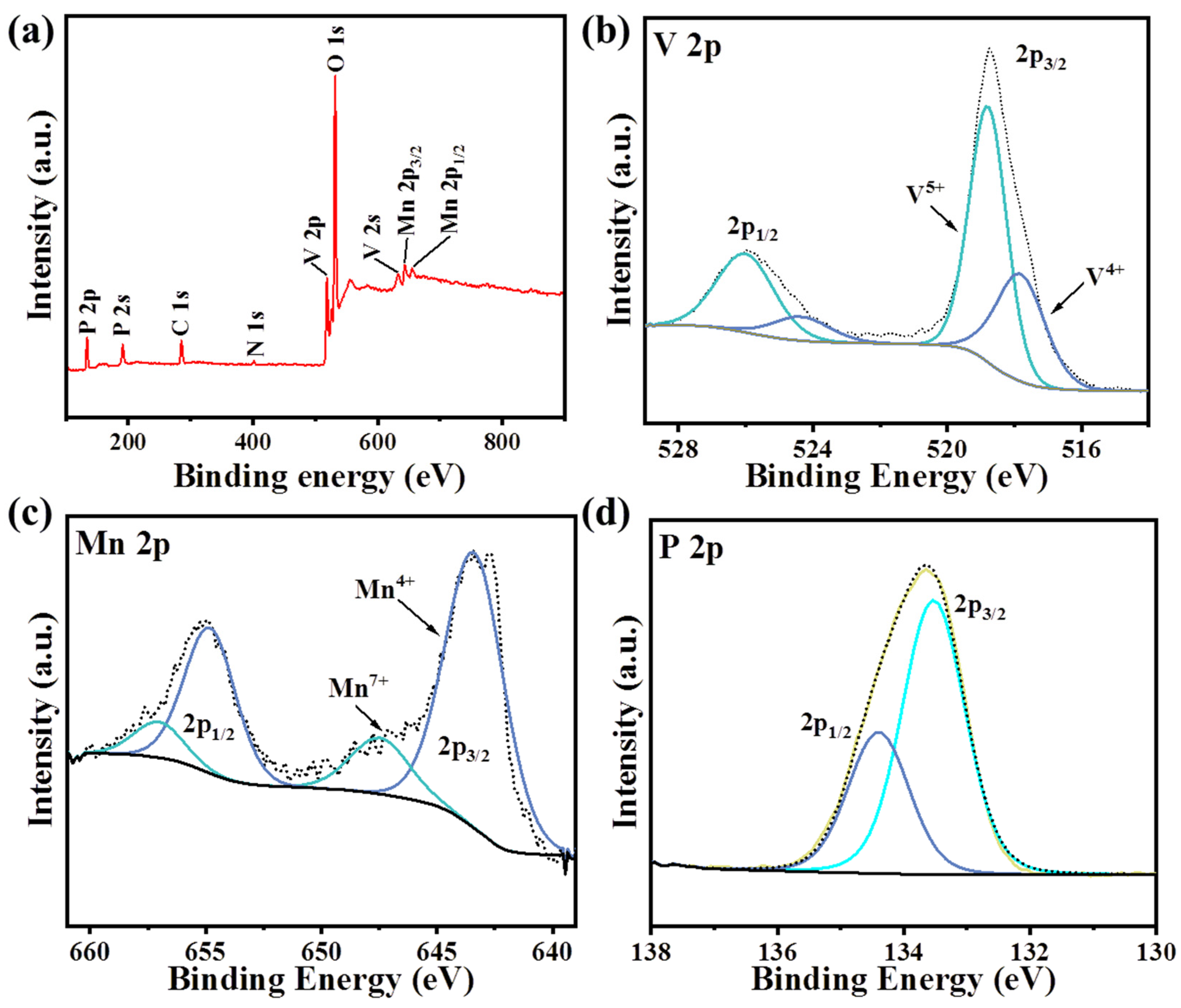
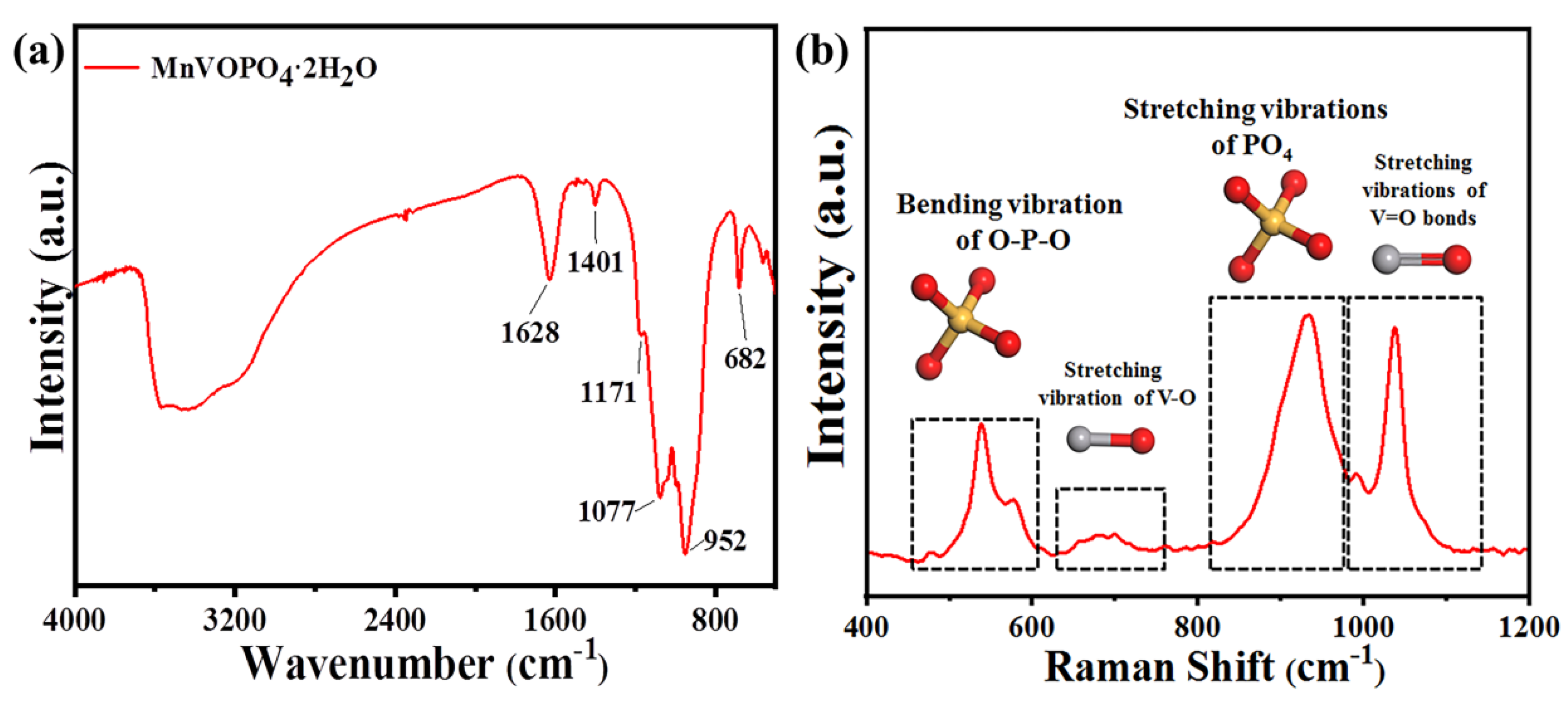
| Number | hkl | Distance in PDF Card | Distance | I % in PDF Card | I % in Result | D/nm |
|---|---|---|---|---|---|---|
| 1 | 002 | 6.9034 | 6.9091 | 100 | 100 | 58.6 |
| 2 | 102 | 4.6125 | 4.6171 | 11 | 7 | 57.4 |
| 3 | 004 | 3.4535 | 3.4506 | 10 | 12.7 | 57.6 |
| 4 | 200 | 3.1013 | 3.1071 | 36 | 25.9 | 56.4 |
| 5 | 202 | 2.8285 | 2.8317 | 15 | 12.2 | 50.6 |
| Cathode | Discharge Medium Voltage | Ref. |
|---|---|---|
| MnVOPO4·2H2O | 1.39 V | This work |
| K0.23V2O5 | Approximately 0.7 V | [28] |
| VS2 | Approximately 0.65 V | [30] |
| VSe2 | Approximately 0.75 V | [29] |
| PANI | Approximately 1.0 V | [32] |
| MoSe2 | Approximately 0.3 V | [33] |
| VO2 | Approximately 0.5 V | [34] |
| Na3V2(PO4)3 | At 1.23 V | [35] |
| Na3V2(PO4)3 | Approximately 1.1 V | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Zhang, W.; Liao, X.; Zhang, L.; An, Q.; Wang, X. “Water-in-Salt” Electrolyte Suppressed MnVOPO4·2H2O Cathode Dissolution for Stable High-Voltage Platform and Cycling Performance for Aqueous Zinc Metal Battery. Materials 2024, 17, 4456. https://doi.org/10.3390/ma17184456
Zhu S, Zhang W, Liao X, Zhang L, An Q, Wang X. “Water-in-Salt” Electrolyte Suppressed MnVOPO4·2H2O Cathode Dissolution for Stable High-Voltage Platform and Cycling Performance for Aqueous Zinc Metal Battery. Materials. 2024; 17(18):4456. https://doi.org/10.3390/ma17184456
Chicago/Turabian StyleZhu, Shaohua, Wenwei Zhang, Xiaobin Liao, Lei Zhang, Qinyou An, and Xuanpeng Wang. 2024. "“Water-in-Salt” Electrolyte Suppressed MnVOPO4·2H2O Cathode Dissolution for Stable High-Voltage Platform and Cycling Performance for Aqueous Zinc Metal Battery" Materials 17, no. 18: 4456. https://doi.org/10.3390/ma17184456







